Abstract
Background
Susceptibility to rhinovirus (RV) induced early wheezing episode has been recognized as an important risk factor for asthma but the data on different RV species is limited. Our aim was to investigate the risk for recurrences in first-time wheezing children with special focus on RV species.
Methods
First-time wheezing children (88/23 in-/outpatients) were prospectively followed at 2-week, 2-month and 12-month time-points, and at first recurrence within 12 months. The respiratory virus etiology was analyzed using PCR. RV positive samples were sequenced. The primary outcomes were time to a new physician-confirmed wheezing episode and time to the initiation of regular controller medication for asthma symptoms.
Results
The median age of the children was 12 months (SD 6.0), 67% were males and 23% were sensitized. RV dominated in symptomatic and asymptomatic infections. Different virus strains caused 97 % of the consecutive RV infections. First-time wheezing children with RV-C (respectively 3.6; p < 0.001) and RV-A (hazard ratio 3.6, p = 0.002) had an increased risk for a new wheezing episode than non-RV group. Also, the risk for the initiation of regular controller medication was increased in RV-A and RV-C groups (both p < 0.05), respectively. There was no difference in response on oral prednisolone between RV-A and RV-C.
Conclusions
RV causes reinfections with different strains in small children after the first wheezing episode. Both RV-A and RV-C affected children have an increased risk for recurrence and initiation of regular controller medication than those with other viruses.
Introduction
A rhinovirus (RV) induced first wheezing episode is an important risk factor for recurrent wheezing and asthma, and is associated with decreased lung function at school-age (1–5). These associations may be explained by pre-existing airway inflammation (epithelial barrier dysfunction and T helper type 2 polarized immune responses promoting virus replication), low interferon levels (impaired antiviral responses) and genetic susceptibility (specific variations increase susceptibility to RV) (4, 6–13).
Currently, over 160 different RV types have been identified and they are classified into A, B and C species (14). Different RV strains cause short-lasting (<14 days) illnesses in non-immunocompromised individuals (15–20). RV-A and RV-C have been shown to cause more severe illnesses than RV-B (21–23). However, data on the role of RV species associated with recurrent wheezing is scarce.
To establish new preventive strategies for asthma, it is important to better understand the role of different RV species in early wheezing episodes. Therefore, the aim of this study was to investigate the etiology of respiratory virus infections during a one-year follow-up period after the first wheezing episode. We hypothesized that the RV species causing the first wheezing episode would be associated with different rates of subsequent infections and illnesses.
Methods
Patients
The Vinku2 trial was carried out in the Department of Pediatrics, Turku University Hospital, Turku, Finland, from the June 2007 to March 2009 (Vinku means “wheeze” in Finnish) (11, 13). The inclusion criteria consisted of children ages 3–23 months, a history of delivery at ≥36 gestational age, and a first wheezing episode (parental report and confirmed from medical records). Main exclusion criteria consisted of chronic non-atopic illness (such as inflammatory bowel disease, juvenile rheumatoid arthritis, heart disease), previous use of systemic or inhaled corticosteroid medication, or need for treatment in intensive care unit. The study was commenced after obtaining written informed consent from the parents. The study was approved by the Ethics Committee of the Turku University Hospital.
Study protocol
All study children were included in the long-term follow-up. In addition, children with RV infection participated in a randomized controlled trial with oral prednisolone (13). The scheduled follow-up visits were arranged at 2-week, 2-month and 12-month time-points by the study physicians. Nasopharyngeal samples (NPS), aspirate at study entry and thereafter swabs at each visit (nylon flocked dry swab, 520CS01, Copan, Brescia, Italy) were taken for viral diagnostics using standardized procedures as previously described (11). Blood was drawn at study entry. A standard questionnaire on host and environmental risk factors for asthma was completed by the guardian at study entry (Appendix 1, 13).
Of 111 children, 79% needed treatment in the hospital ward. The guardian was asked to fill in daily symptom (cough, expiratory breathing difficulty, noisy breathing, rhinitis, and nocturnal wakening for breathing difficulties) and to complete a medication diary assessing symptom severity on a 4-point graded scale for the first 2 weeks. Thereafter, they were asked to fill in dates of symptoms, breathing difficulties, outpatient clinic visits, hospitalizations, oral corticosteroid courses, and initiations of inhaled corticosteroids for wheezing up to 2 months (Appendix 2–4, 13). The guardians were asked to bring the child to the study physician each time the child had breathing difficulties during the follow-up period.
Clinical outcomes
There were 3 primary clinical outcomes in the 12-month follow-up: time to the occurrence of the first physician-confirmed wheezing episode; time to a new RV-associated wheezing episode; and time to the initiation of regular controller medication for asthma symptoms. Secondary clinical outcomes included the occurrence and severity of respiratory symptoms (cough, expiratory breathing difficulty, noisy breathing, rhinitis, and nocturnal wakening for breathing difficulties) on a 4-point scale recorded by the parents on a 2-week daily symptom diary. Within 2 months after discharge, outpatient clinic visits; hospitalizations, oral corticosteroid courses, and initiations of inhaled corticosteroids for wheezing were also recorded. As an exploratory outcome, the efficacy of prednisolone on the primary clinical outcomes was analyzed between RV-A and RV-C.
Definitions and laboratory data
Wheezing was defined as a high-pitched whistling sound in expiration with breathing difficulty. Atopy was defined as allergen-specific immunoglobulin E (IgE) level of 0.35 kU/L or more to any of the following common allergens at study entry: codfish, cow’s milk, egg, peanut, soybean, wheat, cat, dog, horse, birch, mugwort, timothy, Cladosporium herbarum and Dermatophagoides pteronyssinus (Phadiatop Combi®, Phadia, Uppsala, Sweden). Aeroallergen sensitization was defined as positive IgE antibodies to any of the latter 8 allergens. Blood eosinophil count was analyzed by the routine diagnostics of Central Laboratory, Turku University Hospital, Turku, Finland. The diagnosis of eczema was based on typical symptoms including pruritus, typical morphology and chronicity of illness. It was defined as atopic if any sensitization was found. Atopic illness included sensitization, eczema and/or elevated blood eosinophil count.
Virology data
Virologic tests were performed for all nasal samples taken during the 12-month follow-up. Samples were handled and stored as previously described (11). The baseline respiratory samples were analyzed for RV, respiratory syncytial virus (RSV) and enteroviruses (EV) within 3 days (4 times a week) by RT-PCR and for respiratory viruses from frozen samples by Multiplex-PCR (Seeplex RV12 ACE Detection; Seegene, Seoul, Korea) as previously described (11). Bocavirus (HBoV) was analyzed using quantitative (q) RT-PCR in follow-up samples and by both qRT-PCR and serology in acute phase samples (24).
The partial VP4/VP2 and 5′ non-coding region (NCR) of RV genome from RV positive samples were amplified and sequenced using four different primer pairs (14, 25–27). The sequencing of 5′ NCR was performed as previously described (25). The amplification and sequencing method for VP4/VP2 region is also described in a recent publication (28).
The type RV assignment was based on the ≥96% (5′ NCR) or ≥90% (VP4/VP2) nucleotide identity with reference sequences in GenBank by BLAST analysis tool (29). For the RVs that were successfully sequenced in both VP4/VP2 region and 5′ NCR at the study entry, the typing results were merged. There were a total of 17 (out of 84) non-typeable RVs that were not sequenced presumably due to a low virus load in samples leading to poor yield/quality of the PCR amplicons (n = 6) or insufficient sensitivity of the primers used in sequencing reactions (n = 11).
Statistics
Statistical power calculation was carried out for the randomized trial but not specifically for the current analysis (13). The baseline patient characteristics are precisely described in previous publication (11). Symptomatic infections included cough and rhinitis as symptoms. In basic statistics, one-way analysis of variance (ANOVA), Kruskall-Wallis test, Pearson’s chi square test or Fischer exact test (when count <5) were used when appropriate to compare children with RV-A, RV-C and other respiratory virus (non-RV) infection. Parametric variables were expressed as mean and standard deviation (SD) and nonparametric variables as median and interquartile range (IQR). Univariate Cox regression analysis was used to analyze the risk factors, including viral etiology (RV-A, RV-C and non-RV), any sensitization, eczema, parental asthma, parental smoking, age and sex, for primary outcomes. Major comparisons were carried out between RV-A, RV-C, and non-RV groups. Separate grouping of other respiratory viruses was not done due to the lower number of positive findings. Non-typeable RV cases (17 of 84 RV infections) and the single RV-B case were excluded from the analyses. The multivariable Cox regression was carried out including factors (any sensitization) that were significantly (p < 0.05) associated with primary outcomes. The modifying effect of RV-A or RV-C induced first wheezing episode at study entry on the effect of prednisolone was tested including the RV-A and RV-C infection as interaction effect on Cox regression model. Statistical significance was established at the level of p < 0.05. Data was analyzed using SPSS software (Version 23, SPSS Inc, Chicago, Ill, USA).
Results
Study population
Of the 125 consecutive eligible children, 111 were enrolled and included in the analysis (Online Supplementary, Figure S1). Median age was 12 months (SD 6.0), 67% were male and 23% were atopic (Table 1). At study entry, 17 children had RV-A infection, 1 had RV-B, 49 had RV-C infection and 27 had other respiratory virus (non-RV) infection. One or more viruses were detected in each of the 111 children. Coinfections occurred in 42/111 (38%) of children.
Table 1.
Patient characteristics.
| Factor | N = 111 |
|---|---|
| At study entry | |
|
| |
| Age | 12 (sd 6.0) |
| Male | 74 (67%) |
| Female | 37 (33%) |
| Eczema | 32 (29%) |
| Atopic eczema | 17/108 (15%) |
| Atopic illness* | 62 (56%) |
| Allergen sensitization# | 25/108 (23%) |
| Blood eosinophilia ≥0.4 × 109/L | 45/107 (41%) |
| Parental asthma | 22 (20%) |
| Parental smoking | 45 (41%) |
Values are presented as number of cases (%) and mean (standard deviation).
The term ‘atopic illness’ included specific sensitization, eczema, and/or blood eosinophil count ≥0.4 9 109/L
Allergen sensitization was defined as a positive IgE antibody result (>0.35 kU/L) to any of the following allergens: codfish, cow’s milk, egg, peanut, soybean, wheat, cat, dog, horse, birch, mugwort, timothy grass, Cladosporium herbarum, and Dermatophagoides pteronyssinus (Phadiatop Combi; Phadia, Uppsala, Sweden).
Recurrence data
Of 92 children who fulfilled the one-year follow-up, 82% (72) had an additional physician diagnosed wheezing episode within 12 months (Table 1). The loss of follow-up was due to the moving to another city, inability to get contact with the patient and/or unwillingness to continue the study. Of the patients with recurrent wheezing episodes, 90% (65/72) were the first recurrences. Of the first recurrences, 92% (60/65) were seen by the physician at the study clinic and 8% (5/65) were identified by medical record review.
Rhinovirus divergence
RVs were successfully detected in 48% (49/102) of children with virus infection at 2 weeks visit, in 23% (23/101) at 2 months visit, in 39% (36/92) at 12 months visit and in 62% (40/65) of recurrent wheezing episodes, respectively. RVs were successfully typed from 47% (23/49) at 2 weeks, 87% (20/23) at 2 months, from 67% (24/36) at 12 months and from 83% (33/40) of the first recurrent wheezing episodes. Overall, 35% (54/153) of RVs were non-typeable. In nasal samples taken from the same subjects at study entry, 2 weeks, 2 months, 12 months and at a new physician-confirmed wheezing episode, different RV strains were observed in 97% (67/69) of consecutive samples. Interestingly, only 2 children had the same RV strain at study entry and 2 week visit (99% and 100% sequence identity).
Virus etiology of symptomatic visits
At least one virus was detected in 75% of all symptomatic illnesses during the one-year follow-up. RVs dominated and were detected in 48% of all symptomatic infections. RV-C was the most common RV species in symptomatic infections, followed by RV-A (Online Supplementary, Table S1). RV-B was detected in 2.4% of the follow-up samples. During symptomatic infections, respiratory virus coinfections (≥ 2 viruses detected) occurred in 24% of children (Online Supplementary, Table S1). Besides RV, the most common viruses detected were HBoV (13%) followed by RSV (11%). The frequency of infection with other viruses was ≤ 7% each (Table 2).
Table 2.
Primary clinical outcomes.
| Outcome | RV-A vs non-RV | RV-C vs non-RV | RV-C vs RV-A |
|---|---|---|---|
|
| |||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| A new physician-confirmed wheezing episode within 12 months after discharge |
3.9 (1.7, 9.0) p = 0.009 |
3.9 (1.9, 7.9) p = 0.001 |
0.99 (0.53, 1.9) p = 0.98 |
| A new physician-confirmed RV induced wheezing episode within 12 months after discharge |
4.5 (1.4, 14) p = 0.001 |
5.0 (1.9, 13) p < 0.001 |
1.1 (0.50, 2.5) p = 0.79 |
| Initiation of controller medication for asthma symptoms within 12 months after discharge |
4.7 (1.6, 14) p = 0.004 |
3.2 (1.2, 8.5) p = 0.017 |
0.69 (0.34, 1.4) p = 0.31 |
Univariable Cox regression analysis was used to analyze the risk for primary clinical outcomes. HR for the difference between RV-A vs non-RV, RV-C vs non-RV and RV-C vs RV-A. HR, hazard ratio; CI, confidence interval; RV, rhinovirus. Statistically significant results are marked with bold.
Ninety-four percent of children had virus detected during recurrent wheezing episode (Online Supplementary, Table S1). RV was detected in 62% of samples (Online Supplementary, Table S1). RV-induced recurrent wheezing episodes were most likely to occur (25/40, 63%) in children who were initially infected at study entry with RV-C, followed by 8/40 (20%) RV reinfection following RV-A, and 5/40 (13%) children were infected with RV following an initial non-RV infection. Coinfections were detected in 28% of recurrent wheezing episodes.
Virus etiology of asymptomatic visits
In asymptomatic virus infections, at least one virus was present in the total of 49% of children during the 1-year follow-up (Online Supplementary, Table S1). RV was the most common pathogen (31%) detected during asymptomatic infections (Online Supplementary, Table S1). Of the RV species, only RV-C was detected less frequently in asymptomatic infections than in symptomatic infections (10% vs 28%). In contrast, RV-A was more common in symptomatic infections than in asymptomatic during the follow-up (10% vs 6.7%). Coinfections were present in 10% of asymptomatic infections. Other viruses that commonly caused asymptomatic infections included HBoV (12%) and RSV (5.3%) (Online Supplementary, Table S1). HBoV infections were not serologically confirmed. Overall, other viruses than RV were detected in 51% of symptomatic infections and in 28% of asymptomatic infections.
Primary clinical outcomes
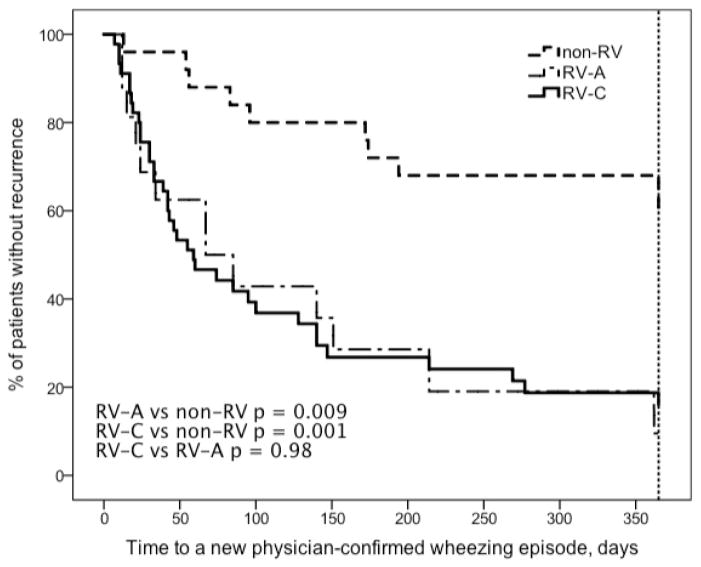
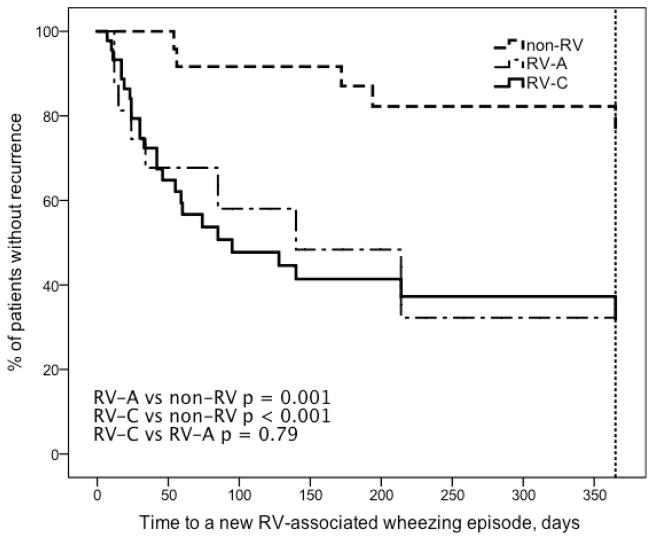
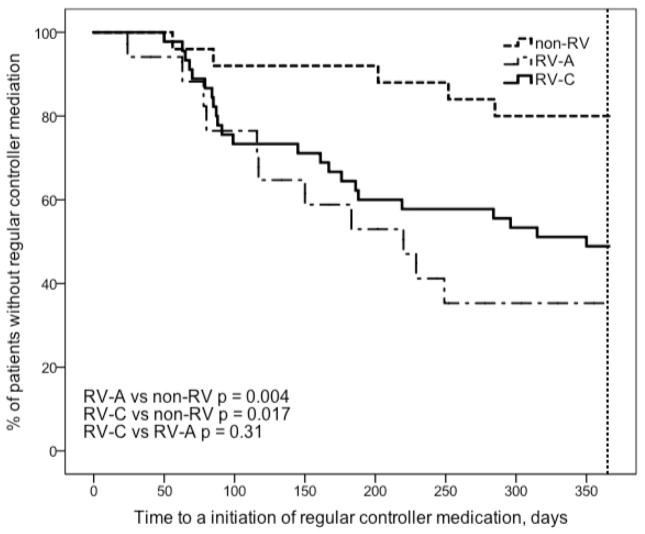
Children with RV-A (HR 3.9, p = 0.009) and RV-C (HR 3.9, p = 0.001) associated wheezing episode had a greater risk for recurrence than those with non-RV etiology (Table 2, Figure 1). RV-A (HR 4.5, p = 0.001) and RV-C (HR 5.0, p < 0.001) also had a greater risk to have a new RV-associated relapse than those with non-RV etiology (Table 2, Figure 2). A regular controller medication was initiated more promptly in children who initially wheezed with RV-A (HR 4.7, p = 0.004) or RV-C (HR 3.2, p = 0.017) when compared to children who wheezed with non-RVs (Table 2, Figure 3).
Figure 1.
Time to a new physician-confirmed wheezing episode in children after the first wheezing episode caused by RV-A, RV-C and non-RV. See Table 2 for more details.
Figure 2.
Time to a new RV induced physician-confirmed wheezing episode after the first wheezing episode caused by RV-A, RV-C and non-RV. See Table 2 for more details.
Figure 3.
Time to an initiation of regular controller medication after the first wheezing episode caused by RV-A, RV-C and non-RV. See Table 2 for more details.
Any sensitization was also a risk factor for the initiation of regular controller medication (HR 2.7 [95% CI 1.4, 5.1], p = 0.003). No other significant risk factors with non-RV group (RV-A 24%, RV-C 65%, non-RV 12%, overall p = 0.001). Furthermore, oral corticosteroid courses were observed more frequently in children with RV-A and RV-C infections than with were found. When adjusted for any sensitization, children who wheezed first with RV-A (HR 3.9 [95% CI 0.3, 11], p = 0.012) and RV-C (HR 2.8 [95% CI 1.0, 7.3], p = 0.041) associated first time wheezing had a greater risk for the initiation of regular controller medication in shorter time than children with non-RV associated wheezing. The risk for these primary outcomes was similar (all p > 0.3) for children who had wheezed first with RV-C compared to children who had wheezed first with RV-A during the 12-months of follow-up (Table 2, Figure 1–3).
Secondary clinical outcomes
Short-term outcomes differed between the three groups (Table 3). Children with RV-A and RV-C infections had more doctor visits for expiratory breathing difficulty during the 2-month follow-up than children non-RV infections (RV-A 24%, RV-C 47%, non-RV 4.2%, overall p = 0.001). No other differences were observed in secondary outcomes (Table 3).
Table 3.
Secondary clinical outcomes.
| 2 weeks, duration of symptoms | RV-A | RV-C | non-RV | p-value |
|---|---|---|---|---|
| Cough, days | 10 (5, 12) | 8 (5, 11) | 5 (4, 7) | 0.15 |
| Expiratory breathing difficulty, days | 2 (1, 6) | 2 (0, 5) | 2 (0, 4) | 0.58 |
| Noisy breathing, days | 7 (3, 9) | 6 (3, 9) | 5 (2, 9) | 0.90 |
| Rhinitis, days | 9 (4, 11) | 8 (4, 10) | 6 (4, 10) | 0.50 |
| Nocturnal symptoms, days | 1 (0, 4) | 1 (0, 4) | 2 (0, 3) | 0.89 |
| Use of bronchodilator, days | 7 (1, 11) | 5 (2, 7) | 3 (0, 7) | 0.28 |
|
| ||||
| 2 months, recurrent wheezing | ||||
|
| ||||
| Doctor visit, %* | 24% | 56% | 12% | 0.001 |
| Hospitalization, %* | 0% | 16% | 4.2% | 0.14 |
| Oral corticosteroid, %* | 24% | 47% | 4.2% | 0.001 |
| Inhaled corticosteroid, %* | 5.9% | 11% | 4.0% | 0.68 |
Values are shown as medians (interquartile range) and OR (95% CI). Data were analyzed by Kruskall-Wallis test for 2 weeks secondary outcomes and by Pearson’s chi-square test for 2 months secondary outcome.
For expiratory breathing difficulty. Statistically significant results are marked with bold.
Exploratory outcome
At study entry, any sensitization was similar for the prednisolone treatment group vs. placebo group (27% vs 28%, p = 0.91). Furthermore, the prednisolone and placebo groups had similar percentages of first wheezing illnesses associated with RV-A vs. RV-C infections (33% vs. 51%, p = 0.46). RV-A or RV-C induced first wheezing episode did not modify the effect of randomly assigned prednisolone treatment on the primary outcomes. Children with RV-C infection (HR 0.66 [95% CI 0.16, 2.7], p = 0.57) did not experience a new physician confirmed wheezing episode in longer time when compared to children with RV-A infection between prednisolone and placebo groups. No association was either found with the time to a new RV-induced wheezing episode (HR 0.39 [95% CI 0.072, 2.1], p = 0.28), or with the time to the initiation of regular controller medication (HR 1.2 [95% CI 0.26, 6.0], p = 0.79) in children with RV-C compared to those with RV-A infection. Between prednisolone vs placebo, children with RV-C induced first wheezing episode were not associated with the initiation of regular controller medication in shorter time (HR 1.2 [95% CI 0.26, 6.0], p = 0.79) when compared to children with RV-A induced wheezing episode.
Discussion
In this study we show that RV is an important virus causing repetitive infections with different virus strains in young children after the first wheezing episode during 12-month follow-up. Children with RV-A or RV-C associated first wheezing episode developed a new wheezing episode sooner than children who wheezed with non-RVs. Furthermore, the risk was also increased for a new RV associated wheezing episode, and the initiation of regular controller medication occurred in shorter time in children with RV-A and RV-C associated first wheezing than non-RV associated first wheezing. The efficacy of prednisolone to reduce recurrent wheezing episodes did not differ between RV-A and RV-C associated first wheezing episodes.
To our knowledge, this is the first study to investigate viral infections and illnesses after an initial wheezing episode. Our results emphasize the importance of RV which is detected in 29% – 59% of children with respiratory symptoms after the first wheezing episode. RV infections are reported to be most common after 9–12 months of age (30), which is consistent with our findings. The second most common virus was HBoV, a recently discovered pathogen that is detected in 5.7% – 20% of hospitalized children with respiratory illnesses and episodes of wheezing (24, 31). The third most common pathogen during the one-year follow-up was RSV, which was detected in 2% – 15% of children with mean age 12 months at the study entry. The low percentage for RSV positive children may be explained by the fact that infants aged less than 3 months were not included and the median age of our subjects (12 months) was rather high (30).
In our study, RV was the most common pathogen detected in 29% – 62% of symptomatic infections. Our results are in line with earlier studies of RV prevalence (23 – 41%) in hospitalized and outpatient children (19, 32–34) with respiratory illness. Interestingly, RV and especially RV-C, was most commonly detected in recurrent wheezing episodes which supports the previous finding that children with RV-C have an increased risk for severe wheezing leading to hospital admission (23). Asymptomatic RV infections have been reported in 8% – 36% hospitalized and outpatient children, whereas in our study RV was found in up to 40% of asymptomatic infections (18–20, 35, 36). This might be explained by deficient immune responses in the studied children with wheezing and RV infection (6, 7, 44 The second most common pathogen in asymptomatic infections was HBoV. This is consistent with earlier studies when HBoV has been found in up to 44% of asymptomatic infections with prolonged presence in immunocompetent children and suggests that many asymptomatic HBoV infections are truly asymptomatic (37, 38). Moreover, MPV, EV and CV showed similar prevalence in asymptomatic and symptomatic infections (Online Supplementary, Table S1). These findings demonstrate that young children have several virus infections that can be transient or incipient, either symptomatic or asymptomatic depending on virus species and host immune factors.
Our results show that RV reinfections were nearly always caused by different RV strains indicating new infections and, that the time interval for a new RV infection in children is relatively short. This supports earlier findings that repeated RV infections are due to reinfections and not due to persistent infections in this group of otherwise healthy children (15, 16, 20, 25, 39, 40). Different RV strains were also found in the recurrent wheezing episodes when compared to the first RV associated wheezing episodes, which is consistent with a previous study (17). Consistent with our study, most of the RVs are no longer detectable after 2 weeks regardless of RV types (20, 35).
Interestingly, our results add to previous findings that RV-A and RV-C wheezing illnesses contribute to the risk for recurrent wheezing (3, 5, 41, 42). In addition, Linsuwanon et al have earlier shown that RV-A and RV-C detected together with RSV were associated with recurrent wheezing (41, 43). However, the risk was not further increased when atopic characteristics were present which emphasizes the role of RV infection alone as a risk factor (4, 5). Whether RV is a marker of children who are at risk for wheezing or a causative agent for wheezing remains unclear. Interestingly, 62% of children with an initial RV wheezing episode developed recurrent wheezing. Also, a first wheezing episode caused by RV-A and RV-C was associated with an increased risk for subsequent RV-associated recurrent wheezing. Children with atopy or asthma can have deficient interferon responses of airway epithelial cells, which could confer susceptibility to recurrent RV illnesses (9). Asthma and atopy are also associated with reduced interferon responses of airway mononuclear cells ex vivo (44). The results of our study may be explained by the differences in immune responses beginning in early childhood.
In a recently published study, children with RV positive bronchiolitis used 4.1-fold more asthma controller medication than children with RSV positive bronchiolitis during 12-month follow-up (45). Our results add that RVA- and RVC-associated first wheezing episodes are of particular risk for the initiation of regular controller medication for asthma symptoms when compared to non-RV associated wheezing. Furthermore, we have previously shown that in a subgroup of RV infected children with high viral load having a first wheezing episode, prednisolone might be beneficial suggesting the presence of (probably pre-existing) inflammation in the lower airways (13). In a post hoc study, prednisolone decreased the recurrent wheezing episodes in children with RV infection (5). However, results from the current study do not support the hypothesis that the efficacy of prednisolone would be different between patients with RV-A or RV-C infection.
The strengths of the study include the focus on the first wheezing episode, consecutive patients, prospective design and detailed clinical data. Furthermore, the subject retention rate was high, with only 17% being lost to follow-up. For RV molecular typing, we used specific primers from both partial 5′ NCR and VP4/VP2 region of the viral genome. Our typing was mainly based on 5′ NCR, because more sequences were obtained using primers from that region. Results of the VP4/VP2 sequencing, which is the gold standard for RV typing, were used as additional data to ensure 5′ NCR based sequencing results and to verify RV-C species and type assignments. Of the type assignments which included sequences obtained from both 5′NCR and VP4/VP2 region, 93% (28/30) were congruent. Limitations of this study include a small sample size which included mostly represented hospitalized children. Therefore, our results cannot be generalized to outpatients.
In conclusion, this study demonstrated that repetitive RV infections are caused by different virus strains in children with first-time wheezing episodes. It also emphasizes the importance of RV-A and RV-C infections in the risk of recurrent wheezing episode and specifically for a new RV associated wheezing episode. Moreover, the initiation of regular controller medication was associated with RV-A and RV-C first-time wheezing episodes, which calls attention to these two RV species as identifying children who may benefit from secondary prevention strategies for asthma. Further studies are also warranted regarding the role of immune responses in children in determining the risk for subsequent recurrent wheezing episodes.
Supplementary Material
Acknowledgments
Disclosure of funding: This work was supported by grants from the Foundation for Pediatric Research, Helsinki; Allergy Research Foundation in Southwest Finland, Turku; the Sigrid Juselius Foundation, Helsinki; the Academy of Finland (grant nos. 132595 and 114034), Helsinki; the Finnish Medical Foundation, Helsinki; Turku University Foundation, Turku; The TYKS Foundation, Turku; Tampere Tuberculosis Foundation, Tampere; Ida Montin Foundation, Espoo; the Research Foundation of the Pulmonary Diseases, Helsinki; The Finnish Cultural Foundation, Helsinki; all in Finland.
References
- 1.Hyvärinen MK, Kotaniemi-Syrjänen A, Reijonen TM, et al. Eosinophil activity in infants hospitalized for wheezing and risk of persistent childhood asthma. Pediatr Allergy Immunol. 2010;21:96–103. doi: 10.1111/j.1399-3038.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 2.Guilbert TW, Singh AM, Danov Z, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol. 2011;128:532-8.e1–10. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Midulla F, Pierangeli A, Cangiano G, et al. Rhinovirus bronchiolitis and recurrent wheezing: 1-year follow-up. Eur Respir J. 2012;39:396–402. doi: 10.1183/09031936.00188210. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–5. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukkarinen M, Lukkarinen H, Lehtinen P, et al. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24:237–43. doi: 10.1111/pai.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contoli M, Ito K, Padovani A, Poletti D, et al. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy. 2015;70:910–20. doi: 10.1111/all.12627. [DOI] [PubMed] [Google Scholar]
- 7.Jakiela B, Brockman-Schneider R, Amineva S, et al. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–23. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll KN, Gebretsadik T, Minton P, et al. Influence of maternal asthma on the cause and severity of infant acute respiratory tract infections. J Allergy Clin Immunol. 2012;129:1236–42. doi: 10.1016/j.jaci.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraldo S, Contoli M, Bazzan E, et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–14. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Calışkan M, Bochkov YA, Kreiner-Møller E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turunen R, Koistinen A, Vuorinen T, et al. The first wheezing episode: respiratory virus etiology, atopic characteristics, and illness severity. Pediatr Allergy Immunol. 2014;25:796–803. doi: 10.1111/pai.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochkov YA, Watters K, Ashraf S, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112:5485–90. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jartti T, Nieminen R, Vuorinen T, et al. Short- and long-term efficacy of prednisolone for first acute rhinovirus-induced wheezing episode. J Allergy Clin Immunol. 2015;135:691–8.e9. doi: 10.1016/j.jaci.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bochkov YA, Grindle K, Vang F, et al. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52:2461–71. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–50. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 16.Engelmann I, Mordacq C, Gosset P, et al. Rhinovirus and asthma: reinfection, not persistence. Am J Respir Crit Care Med. 2013;188:1165–7. doi: 10.1164/rccm.201303-0585LE. [DOI] [PubMed] [Google Scholar]
- 17.Jartti T, Lee WM, Pappas T, et al. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32:314–20. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Principi N, Zampiero A, Gambino M, et al. Prospective evaluation of rhinovirus infection in healthy young children. J Clin Virol. 2015;66:83–9. doi: 10.1016/j.jcv.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Fry AM, Lu X, Olsen SJ, et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One. 2011;6:e17780. doi: 10.1371/journal.pone.0017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loeffelholz MJ, Trujillo R, Pyles RB, et al. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics. 2014;134:1144–50. doi: 10.1542/peds.2014-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller EK, Khuri-Bulos N, Williams JV, et al. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Virol. 2009;46:85–9. doi: 10.1016/j.jcv.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bizzintino J, Lee WM, Laing IA, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37:1037–42. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox DW, Bizzintino J, Ferrari G, et al. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med. 2013;188:1358–64. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Söderlund-Venermo M, Lahtinen A, Jartti T, et al. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg Infect Dis. 2009;15:1423–30. doi: 10.3201/eid1509.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltola V, Waris M, Osterback R, et al. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–9. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 26.Wisdom A, Leitch EC, Gaunt E, et al. Screening respiratory samples for detection of human rhinoviruses (HRVs) and enteroviruses: comprehensive VP4-VP2 typing reveals high incidence and genetic diversity of HRV species C. J Clin Microbiol. 2009;47:3958–67. doi: 10.1128/JCM.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linsuwanon P, Payungporn S, Samransamruajkit R, et al. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect. 2009;59:115–21. doi: 10.1016/j.jinf.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turunen R, Jartti T, Bochkov Y, et al. Rhinovirus species and clinical characteristics in the first wheezing episode in children. J Med Virol. 2016 May 27; doi: 10.1002/jmv.24587. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller EK, Mackay IM. From sneeze to wheeze: what we know about rhinovirus Cs. J Clin Virol. 2013;57:291–9. doi: 10.1016/j.jcv.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Jartti T, Lehtinen P, Vuorinen T, et al. Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr Infect Dis J. 2009;28:311–7. doi: 10.1097/INF.0b013e31818ee0c1. [DOI] [PubMed] [Google Scholar]
- 31.Principi N, Piralla A, Zampiero A, et al. Bocavirus Infection in Otherwise Healthy Children with Respiratory Disease. PLoS One. 2015;10:e0135640. doi: 10.1371/journal.pone.0135640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcone DN, Culasso A, Carballal G, et al. Genetic diversity and clinical impact of human rhinoviruses in hospitalized and outpatient children with acute respiratory infection, Argentina. J Clin Virol. 2014;61:558–64. doi: 10.1016/j.jcv.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcone DN, Ellis A, Videla C, et al. Viral etiology of acute respiratory infections in hospitalized and outpatient children in Buenos Aires, Argentina. Pediatr Infect Dis J. 2013;32:e105–10. doi: 10.1097/INF.0b013e31827cd06f. [DOI] [PubMed] [Google Scholar]
- 34.van Benten I, Koopman L, Niesters B, et al. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol. 2003;14:363–70. doi: 10.1034/j.1399-3038.2003.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jartti T, Lehtinen P, Vuorinen T, et al. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–9. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 36.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–90. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 37.Martin ET, Fairchok MP, Kuypers J, Magaret A, Zerr DM, Wald A, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis. 2010;201:1625–32. doi: 10.1086/652405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blessing K, Neske F, Herre U, Kreth HW, Weissbrich B. Prolonged detection of human bocavirus DNA in nasopharyngeal aspirates of children with respiratory tract disease. Pediatr Infect Dis J. 2009;28:1018–9. doi: 10.1097/INF.0b013e3181a854ae. [DOI] [PubMed] [Google Scholar]
- 39.van der Zalm MM, Wilbrink B, van Ewijk BE, et al. Highly frequent infections with human rhinovirus in healthy young children: a longitudinal cohort study. J Clin Virol. 2011;52:317–20. doi: 10.1016/j.jcv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Wright PF, Deatly AM, Karron RA, et al. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol. 2007;45:2126–9. doi: 10.1128/JCM.02553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Midulla F, Nicolai A, Ferrara M, et al. Recurrent wheezing 36 months after bronchiolitis is associated with rhinovirus infections and blood eosinophilia. Acta Paediatr. 2014;103:1094–9. doi: 10.1111/apa.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linsuwanon P, Payungporn S, Samransamruajkit R, et al. Recurrent human rhinovirus infections in infants with refractory wheezing. Emerg Infect Dis. 2009;15:978–80. doi: 10.3201/eid1506.081558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sykes A, Edwards MR, Macintyre J, et al. Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012;129:1506–1514.e6. doi: 10.1016/j.jaci.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 45.Bergroth E, Aakula M, Korppi M, et al. Post-bronchiolitis Use of Asthma Medication: A Prospective 1-year Follow-up Study. Pediatr Infect Dis J. 2016;35:363–8. doi: 10.1097/INF.0000000000001017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.