Abstract
Several arylpiperazines capable of reversing multidrug resistance (MDR) in Escherichia coli overexpressing acrAB and acrEF but not in pump-deficient mutant strains were identified. 1-(1-Naphthylmethyl)-piperazine, one of the more active compounds, enhanced susceptibility to fluoroquinolones and other agents and increased the intracellular concentration of levofloxacin and ethidium bromide, suggesting efflux pump inhibition as the mechanism of MDR reversal.
Bacterial resistance to chemically unrelated antimicrobial agents (multidrug resistance [MDR]) may be caused by overexpression of MDR efflux pumps (3, 6). Among gram-negative bacteria, many of these MDR efflux pumps belong to the RND (resistance-nodulation-cell division) type family of tripartite efflux pumps. MDR in selected gram-negative bacteria has been shown to be reversible by using compounds like Phe-Arg-β-naphthylamide (PAβN) or other small N-heterocyclic organic compounds thought to inhibit RND type efflux pump activity through unknown mechanisms (4, 5, 7, 9). In this study, we describe another novel type of putative efflux pump inhibitor (EPI) identified through screening of an N-heterocyclic organic compound library for MDR reversal activity in Escherichia coli.
Selected arylpiperazines were synthesized at Steinbeis Transferzentrum Technische Chemie (Reutlingen, Germany) or purchased from Chess GmbH (Mannheim, Germany), ChemBridge Corporation (San Diego, Calif.), and Sigma Chemicals (St. Louis, Mo.). We initially screened the compound library through evaluating MICs of levofloxacin alone and in the presence of putative EPIs in E. coli test strains overexpressing acrAB and acrEF (see Table 1 for strain descriptions). Compounds were further tested if their minimal concentration required to reduce the levofloxacin MIC by fourfold (LVX-MRC4) was at least fourfold lower than the intrinsic MIC of the test compound and if their MDR reversal effect (defined as reducing by at least fourfold the MICs of levofloxacin plus another antimicrobial agent) was observed in efflux pump-overexpressing test strains 2-DC14PS and 3-AG100MKX but not efflux pump-deficient control strains 1-DC14PS and HS276 (Table 1). MICs were determined by a standard microdilution assay using Luria-Bertani (LB) broth and a final inoculum of 5 × 105 CFU/ml.
TABLE 1.
E. coli strains used in this study
| Strain | Description | Reference |
|---|---|---|
| 3-AG100MKXa | acrAB-overexpressing gyrA mutant derived from AG100MKX (AG100 marA::Kmr) | 2 |
| DC14 | AG100 ΔacrAB::Kmr | 1 |
| 1-DC14PS | gyrA mutant derived from DC14PS after selection with ofloxacin | 1 |
| 2-DC14PSb | acrEF-overexpressing mutant derived from 1-DC14PS after selection with ofloxacin | 1 |
| HS414 | Wild-type W3110 | 8 |
| HS276 | HS414 ΔacrEF ΔacrAB ΔyhiUV ΔacrD ΔyegMNO::Kmr | 8 |
Enhanced expression of acrAB without expression of acrEF was shown by reverse transcription-PCR and was associated with diminished intracellular accumulation of ofloxacin in the absence of CCCP (2).
Enhanced expression of acrEF was shown by reverse transcription-PCR and was associated with diminished intracellular accumulation of ofloxacin in the absence of CCCP (2).
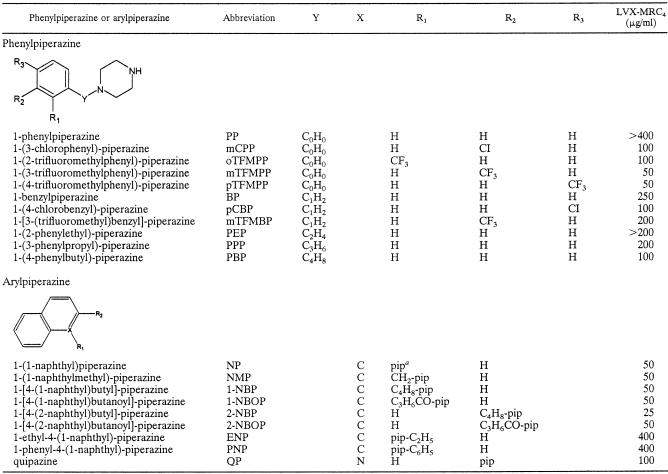
Analysis of the relationship between the structure of phenylpiperazines and MDR reversal activity suggested that elongation of the spacer between the benzene ring and the piperazine ring would enhance potency (Table 2). A fivefold increase in potency was observed for 1-(4-phenylbutyl)-piperazine (LVX-MRC4, 100 μg/ml; spacer length, 4) compared to the weakest compound, 1-phenylpiperazine (PP) (LVX-MRC4, >400 μg/ml; spacer length, 0). Halogenic substitutions at the benzene ring independently caused a significant increase in potency, with the most successful being the introduction of a trifluoromethyl group at the meta position of the benzene ring of PP. This compound, called mTFMPP (LVX-MRC4, 50 μg/ml), became the most effective phenylpiperazine tested in our assay, whereas the naphthylpiperazines 1-(1-naphthyl)piperazine, 1-(1-naphthylmethyl)-piperazine (NMP), and 1-[4-(2-naphthyl)butyl]-piperazine were among the most potent unsubstituted arylpiperazines. Interestingly, the simple addition of an ethyl or phenyl group to the piperazine ring, as in 1-ethyl-4-(1-naphthyl)-piperazine or 1-phenyl-4-(1-naphthyl)-piperazine, led to a dramatic loss in potency.
TABLE 2.
Structure and activity as MDR reversal agents of selected phenyl- and arylpiperazines tested with acrAB-overexpressing E. coli 3-AG100MKX cells
pip, piperazine.
NMP and mTFMPP had similar intrinsic MICs (NMP, 400 μg/ml; mTFMPP, 800 μg/ml) in efflux pump-overexpressing as well as efflux pump-deficient test strains and MDR reversal activities at the LVX-MRC4 were limited to efflux pump-overexpressing test strains. Both compounds were compared with PAβN in their ability to increase the intracellular accumulation of levofloxacin, measured as described previously (2). Briefly, levofloxacin (final concentration of 10 μg/ml) was added to E. coli 3-AG100MKX cells suspended to an optical density at 600 nm (OD600) of 1 in 7 ml of 50 mM sodium phosphate buffer (pH 7.0) containing 0.2% glucose. After incubation at 37°C for 10 min, NMP or mTFMPP (each at a final concentration of 100 μg/ml), PAβN (final concentration of 25 μg/ml), or carbonyl cyanide m-chlorophenyl-hydrazone (CCCP) (200 μM) were added. PAβN and CCCP were from Sigma Chemicals. The concentration of PAβN chosen represented the LVX-MRC4 of PAβN for strain 3-AG100MKX. At timed intervals, 1-ml samples were removed and centrifuged through silicone oil, and the pellet was resuspended in 300 μl of 0.1 M glycine hydrochloride (pH 3.0). After overnight incubation at room temperature, samples were again centrifuged, and the amount of released levofloxacin was determined spectrofluorometrically (excitation, 292 nm; emission, 496 nm) in the supernatant. Experiments were done in triplicate.
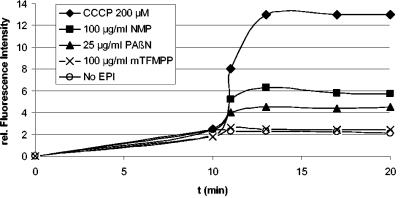
As shown in Fig. 1, addition of both NMP and PAβN but not of mTFMPP resulted in increased intracellular levofloxacin accumulation, consistent with an EPI effect. To further confirm this finding, we measured intracellular ethidium bromide (EtBr). 3-AG100MKX cells were grown overnight on LB agar plates and suspended in phosphate-buffered saline plus 0.4% glucose (pH 7.4) to an OD600 of 1, and NMP was added at increasing concentrations. Samples were placed into a 96-well plate, EtBr was added (final concentration, 1.0 μg/ml), and fluorescence was read in a Safire (Tecan, Crailsheim, Germany) fluorescence plate reader (excitation, 518 nm; emission, 605 nm). Figure 2 shows that addition of NMP resulted in a dose-dependent increase in relative fluorescence intensity. Similar results were obtained with 2-DC14PS and HS414 but not with 1-DC14PS and HS276, in which the effects were much smaller (data not shown). These observations suggested that NMP may exert its MDR reversal activity primarily through inhibition of the AcrAB and AcrEF efflux pumps.
FIG. 1.
Intracellular accumulation of levofloxacin with or without addition of NMP, mTFMPP, PAβN, and CCCP in the acrAB-overexpressing E. coli strain 3-AG100MKX. Levofloxacin (10 μg/ml) was added at time point 0, and NMP, mTFMPP, PAβN, or CCCP were added after 10 min. Samples removed at timed intervals were centrifuged through silicone oil, and the pellet was resuspended in 0.1 M glycine hydrochloride (pH 3.0). After overnight incubation at room temperature, samples were again centrifuged, and the amount of released levofloxacin in the supernatant was determined spectrofluorometrically. Shown are representative results from one out of three experiments.
FIG. 2.
Effect of the addition of increasing concentrations of NMP on EtBr accumulation in acrAB-overexpressing E. coli strain 3-AG100MKX. Cells grown in LB agar overnight were suspended in phosphate-buffered saline containing 0.4% glucose, and NMP was added at increasing concentrations. EtBr was added (1 μg/ml) at time point 0, and fluorescence changes over time were measured by fluorescence spectroscopy.
The spectrum of antimicrobial agents affected by addition of NMP (at a concentration of 100 μg/ml) and, for comparison, of PAβN (at a concentration of 25 μg/ml, corresponding to one-fourth the MIC for pump-deficient strain 1-DC14PS) was determined by evaluating the reduction of MICs of several agents. As shown in Table 3, NMP reduced the MIC of levofloxacin by 8- to 16-fold in E. coli strains overexpressing acrAB or acrEF. Effects on other agents were similar or slightly smaller, with 4- to 8-fold reductions of the MICs of oxacillin, rifampin, chloramphenicol, and clarithromycin. The MIC of linezolid was differently affected in the acrEF-overexpressing strain 2-DC14PS. The reduction was 32-fold for this strain versus 8-fold for the acrAB-overexpressing strain 3-AG100MKX (Table 3). The results obtained with E. coli strain HS414 and its pump-deficient derivative HS276 were similar (data not shown) to those obtained with 1-DC14PS and 2-DC14PS (Table 3). Generally, the MICs for pump-deficient strains 1-DC14PS and HS276 were not affected by addition of NMP, with the exception of rifampin, while PAβN addition to the pump-deficient mutant strains led to decreased MICs of agents other than chloramphenicol. Addition of PAβN at 25 μg/ml in efflux pump-overexpressing strains appeared to be more effective than addition of NMP, in particular regarding clarithromycin but not levofloxacin, linezolid, and tetracycline susceptibilities (Table 3). This finding did not change after increasing the concentration of PAβN to 100 μg/ml (data not shown).
TABLE 3.
Effect of the putative EPI NMP and of PAβN on MICs of different drugs in efflux pump-overexpressing E. coli strains 2-DC14PS and 3-AG100MKX and ΔacrAB control strain 1-DC14PS
| Strain | Drug | MIC (μg/ml) with:
|
||
|---|---|---|---|---|
| No EPI | NMP (100 μg/ml) | PAβN (25 μg/ml) | ||
| 1-DC14PS | Levofloxacin | 0.125 | 0.125 | 0.06 |
| Linezolid | 16 | 16 | 8 | |
| Clarithromycin | 2 | 2 | 1 | |
| Oxacillin | ≤0.5 | ≤0.5 | ≤0.5 | |
| Rifampin | 8 | 2 | ≤0.06 | |
| Chloramphenicol | 1 | 1 | 1 | |
| Tetracycline | 1 | 0.5 | 0.25 | |
| 2-DC14PS | Levofloxacin | 4 | 0.25 | 1 |
| Linezolid | 512 | 16 | 64 | |
| Clarithromycin | 256 | 32 | 8 | |
| Oxacillin | 64 | 16 | 32 | |
| Rifampin | 16 | 4 | 0.25 | |
| Chloramphenicol | 8 | 1 | 1 | |
| Tetracycline | 2 | 0.5 | 2 | |
| 3-AG100MKX | Levofloxacin | 4 | 0.5 | 0.5 |
| Linezolid | 256 | 16 | 64 | |
| Clarithromycin | 256 | 32 | 2 | |
| Oxacillin | 64 | 16 | 32 | |
| Rifampin | 16 | 4 | 0.125 | |
| Chloramphenicol | 8 | 1 | 2 | |
| Tetracycline | 2 | 0.5 | 2 | |
Additional representatives of diverse classes of agents were tested with 3-AG100MKX. A MIC reduction (fourfold or greater) after addition of NMP was observed for other fluoroquinolones (ciprofloxacin, norfloxacin, enoxacin, and pefloxacin), erythromycin, azithromycin, clindamycin, doxycycline, nitrofurantoin, and EtBr but not for the ketolides telithromycin and ABT-773, glycopeptides, aminoglycosides, trimethoprim-sulfamethoxazole, and fosfomycin (data not shown).
Among the tested arylpiperazines, the naphthyl derivative NMP matched our predefined working criteria of an ideal EPI: it significantly reduced the MICs of two or more antibiotics in efflux pump-overexpressing strains, it did not inhibit the growth of an efflux pump-deficient strain at concentrations effective in efflux-competent strains, and finally, it increased the intracellular accumulation of otherwise expelled efflux pump substrates. NMP, as the most potent compound in E. coli, appeared to be less active than PAβN, which, unlike NMP, showed inhibitory activity in strains without expression of the AcrAB and AcrEF E. coli efflux pumps and thus may exert its effect through action on other pumps or through additional mechanisms unrelated to pump inhibition.
NMP can be added to the list of MDR reversal agents with putative EPI activity in E. coli. Expanding this list to include compounds with different spectra of activity in terms of bacterial species as well as drugs may help with investigation in more detail of the mechanisms of pump substrate recognition and mechanisms of MDR efflux pump inhibition.
Acknowledgments
This study was supported in part by research grant P559/99 from the University of Ulm, by BMBF grant 01KI9951, and by the Landesstiftung Baden-Württemberg.
We thank Petra Steinke for excellent technical assistance.
REFERENCES
- 1.Jellen-Ritter, A. S., and W. V. Kern. 2001. Enhanced expression of the multidrug efflux pumps AcrAB and AcrEF associated with insertion element transposition in Escherichia coli mutants selected with a fluoroquinolone. Antimicrob. Agents Chemother. 45:1467-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kern, W. V., M. Oethinger, A. S. Jellen-Ritter, and S. B. Levy. 2000. Non-target gene mutations in the development of fluoroquinolone resistance in Escherichia coli. Antimicrob. Agents Chemother. 44:814-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li, X. Z., and H. Nikaido. 2004. Efflux-mediated drug resistance in bacteria. Drugs 64:159-204. [DOI] [PubMed] [Google Scholar]
- 4.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallea, M., A. Mahamoud, J. Chevalier, S. Alibert-Franco, P. Brouant, J. Barbe, and J. M. Pages. 2003. Alkylaminoquinolines inhibit the bacterial antibiotic efflux pump in multidrug-resistant clinical isolates. Biochem. J. 376:801-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poole, K. 2004. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 10:12-26. [DOI] [PubMed] [Google Scholar]
- 7.Renau, T. E., R. Leger, E. M. Flamme, J. Sangalang, M. W. She, R. Yen, C. L. Gannon, D. Griffith, S. Chamberland, O. Lomovskaya, S. J. Hecker, V. J. Lee, T. Ohta, and K. Nakayama. 1999. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 42:4928-4931. [DOI] [PubMed] [Google Scholar]
- 8.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorarensen, A., A. L. Presley-Bodnar, K. R. Marotti, T. P. Boyle, C. L. Heckaman, M. J. Bohanon, P. K. Tomich, G. E. Zurenko, M. T. Sweeney, and B. H. Yagi. 2001. 3-Arylpiperidines as potentiators of existing antibacterial agents. Bioorg. Med. Chem. Lett. 11:1903-1906. [DOI] [PubMed] [Google Scholar]