Highlights
-
•
The manuscript deals with the characterization of lepidopteran-specific cry1 and cry2 gene harbouring Bt isolates.
-
•
We were able to find certain Bt isolates containing both cry1 and cry2 genes.
-
•
Both cry1 and cry2 genes were found in isolates containing vip3A genes, hence can result in complementation of toxicity.
-
•
13.63% of the Bt isolates were found to be toxic against Helicoverpa armigera.
Keywords: Bacillus thuringiensis, Lepidopteran-specific cry genes, Helicoverpa armigera
Abstract
Bacillus thuringiensis (Bt) based biopesticides are feasible alternatives to chemical pesticides. Here, we present the distribution of lepidopteran-specific cry1 and cry2 genes in native B. thuringiensis. Forty four out of 86 colonies were found to harbour crystals by phase contrast microscopy exhibiting a Bt index of 0.51. PCR analysis resulted in the amplification of cry1 in 24 and cry2 in 14 isolates. Twelve of the isolates showed presence of both cry1 and cry2, while 18 isolates did not show presence of either of the genes. Toxicity screening using spore-crystal mixtures against 2nd instar larvae of Helicoverpa armigera revealed that the isolates (50%) were either mildly toxic or not toxic (36.36%), and only 13.63% were toxic. The results are interesting, particularly so because the same isolates were previously reported to contain lepidopteran specific vip3A genes also, hence can complement the toxicity of the isolates harbouring vip3A genes.
1. Introduction
As the world’s population is increasing geometrically, achieving global food security-making safe and nutritious food accessible to everyone, and achieving so sustainably is a challenging task. Feeding estimated 9.2 billion people in 2050 would require raising overall food production by about 70% [1]. A major bottleneck in achieving this challenge is the competition from the insect pests. Insect pests are responsible for destroying one fifth of the world’s total crop production annually, leading to heavy economic losses. The major damaging insect pests of crops belong to the order Lepidoptera [2] and Helicoverpa armigera is one of the most significant lepidopteran pests with potential to attack more than 180 species of plants [3]. It is widely distributed in Asia, Europe, Africa and Australasia causing damages worth 2 billion US dollars annually, excluding the socio-economic and environmental costs associated with the use of chemical insecticides and the introduction of GM crops [4], [5], [6]. H. armigera has over the years developed resistance to various chemical insecticides [7], [8] and of late, its resistance to genetically modified crops expressing insecticidal protein from B. thuringiensis has also been reported [9,10].
The most common method to control insect pest populations is the use of chemical insecticides. Two of their properties, long residual action and toxicity to a wide spectrum of organisms made chemical insecticides very useful against insect pests. However, extended use of certain chemical insecticides have caused many environmental problems like persistence, toxicity to non-target organisms including humans and development of insect resistance [11,12]; reviewed in [13]. One of the most promising alternatives to the man-made chemical pesticides is the use of natural insect pathogen, Bacillus thuringiensis (Bt). The entomopathogenic potential of Bt is primarily due to its ability to produce insecticidal crystalline proteins (Cry and Cyt) [14] and in certain cases due to the production of vegetative insecticidal proteins (Vips) [15]. The crystalline and vegetative insecticidal proteins are respectively produced during the sporulation and vegetative stages of Btgrowth. Upto November 2016, seventy four classes of Cry proteins (Cry1-Cry74), three classes of Cyt proteins (Cyt1-Cyt3) and four classes of Vip proteins (Vip1-Vip4) have been designated based on their amino acid sequence homology [16]. These toxins are highly specific in action, harmless to humans and other vertebrates and are biodegradable. Presently, there are more than 50,000 known strains of B. thuringiensis isolated from diverse environments around the world [17], [18]. These strains exhibit varying degree of toxicity against different pests. Despite the availability of such large collection of B. thuringiensis strains and their insecticidal genes, three events have rendered the search for novel insecticidal strains/genes more urgent. First, a significant number of pests are not controlled with the available Cry proteins. Second, at times the level of expressed toxins is not high enough to kill the host and third, after many years of successful use in the field, the first cases of resistance to B. thuringiensis have appeared [19].
Jammu and Kashmir (32°00′–36°10′ North and 73°22′–77°40′ East) falls in the great North-Western complex of the Himalayan ranges having complex geomorphology. The variations in topographical features along longitude, latitude and altitude of the region create climatic variations resulting in unique and rich biodiversity [20], thereby making this North-Western Himalayan region a critical biodiversity hotspot of the world. These distinctive features and diversity of insects in the region provide an opportunity for prospecting novel B. thuringiensis strains with novel combinations of crystalline protein coding genes having wide insecticidal spectrum. The ecological distribution of this bacterium in Jammu and Kashmir region remains largely unexplored. The aim of this study was to isolate B. thuringiensis strains from Jammu and Kashmir region and to assess their geographical diversity with respect to the presence of lepidopteran-specific (cry1 and cry2) genes content to assess their toxicity against H. armigera.
2. Materials and methods
2.1. Sample collection and isolation of Bacillus thuringiensis
A total of 86 isolates were analysed in this study which were obtained from various soil samples, collected from forests, lake sediments and agricultural fields of Kashmir valley in our previous study [21]. Sites with no history of Bt application were selected (Table 1). Isolation of Bt was done by enrichment using acetate selection as described by Travers et al. [22]. Briefly, 1 g of soil samples were incubated in 10 ml of LB broth buffered with sodium acetate solution (0.3 M, pH 6.8) at 30 °C for 4 h. In order to eliminate non-sporulated microbes that germinated during incubation, 2 ml aliquot of each sample was heated at 80 °C for 10 min. The surviving spores were diluted (10–1000 folds) in sodium acetate buffer (pH 6.8) from which 300 μl of each was spread on T3 agar plates and incubated at 30 °C to grow for 72 h. For each plated sample, well isolated colonies representing Bacillus like morphology were picked and purified on T3 agar plates containing penicillin at a concentration of 10 μgml−1.
Table 1.
Features of sampling sites, success of Bt isolation and the distribution of cry1 and cry2 genes in the native isolates.
| Soil Type | Total No. of colonies | No. of Bacillus likea isolates | No. of Bt isolates | Bt indexb |
cry genes present |
|
|---|---|---|---|---|---|---|
| cry1%/no. | cry2%/no. | |||||
| Forest | 513 | 45 | 27 | 0.60 | 55.55/15 | 37.03/10 |
| Lake sediment | 18 | 8 | 6 | 0.75 | 66.66/4 | 00.00/0 |
| Agricultural (Maize field) | 609 | 33 | 11 | 0.33 | 45.45/5 | 36.36/4 |
| Total | 1140 | 86 | 44 | 0.51 | 54.54/24 | 31.81/14 |
Off-white, opaque, slightly raised, and regular outlined.
Bt Index: Bacillus thuringiensis isolation index was calculated by dividing the number of Bt isolates by the total number of Bacillus like colonies obtained.
2.2. Scanning electron microscopy
Following acetate selection, isolates that tested positive for growth on T3 agar medium amended with penicillin at a concentration of 10 μgml−1 were examined for the presence of parasporal crystals [23,24]. The different stages of bacterial growth as well as the crystal production was analysed by Scanning Electron Microscopy (SEM) as described previously [21]. Bt index was calculated as described by Baig et al. [25].
2.3. 16S rRNA gene sequencing
Total cell DNA was extracted from the native and reference strains using GenElute Bacterial Genomic DNA Kit (Sigma-Aldrich, USA) as per the manufacturer’s instructions. PCR amplification of 16S rRNA gene from the selected Bacillus isolates was performed using the universal primers: forward (27f) 5′-AGAGTTTGATCCTGGCTCAG-3′, reverse (5210r) 5′-AAGGAGCTGATCCAGCCGCA-3′. The purified amplicons of 16S rRNA gene were sequenced using primers 27f and 5210r with fluorescent terminators (Big Dye, Applied Bio systems). The identity of the sequences obtained were determined by NCBI BLAST [26].
2.4. PCR amplification of cry1 and cry2 genes
Using total DNA as template, the cry genes were amplified using primers (+)5′-MDATYTCTAKRTCTTGACTA-3’ and (−)5′-TRACRHTDDBDGTATTAGAT-3′ [27] for the detection of cry1 and (+)5′-GTTATTCTTAATGCAGATG-AATGGG-3′ and (−)5′-CGGATAAAATAATCTGGGAAATAGT-3′ [28] for the detection of cry2 class of genes. PCR was performed in an Eppendorf Mastercycler and amplification conditions were as follows: an initial denaturation at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 60 s, annealing at 47 °C and 59 °C for cry1 and cry2 primers, respectively for 60 s, and extension at 72 °C for 60 to 90 s (depending on the expected size of amplicon), and a final extension step at 72 °C for 10 min. An aliquot of the samples were mixed with 6X DNA loading buffer so that the final concentration of the mixture is 1X, electrophoresis was carried out at 70–80 mA current in 1X TAE, for 45–90 min. An appropriate DNA marker was run alongside in one of the lanes. To visualize the DNA bands gels were viewed under UV light (λ = 254 nm) and images captured using AlphaImager HP (ProteinSimple, USA) gel documentation system.
2.5. Insect bioassays
The insecticidal activity of cry gene harbouring Bt isolates was evaluated against 2nd instar larvae of H. armigera by “quick screen” method [29]. The spore crystal mixtures were obtained as described for microscopy analysis. Higher doses of spore crystal mixtures (1 mgml−1) were smeared on young chick pea leaves in triplicates. The larvae were initially left for 10 h with the leaves smeared with spore crystal mixtures and then allowed to feed on fresh leaves for 12 h. Based on their ability to subsequently feed on the fresh diet, the treated larvae were scored as: not eaten was assigned a score of 3; partially eaten a score of 2 and completely eaten a score of 1. Based on the scores assigned, the toxicity potential of the isolates was classified as, non-toxic for isolates displaying score of 1.0, uncertain toxicity for isolates with score > 1.0 but < 2.0 and toxic for isolates exhibiting score of ≥2.0. B. thuringiensis subsp. kurstaki HD-1 was used as positive control and solubilisation buffer as negative control. The conditions for bioassay were as; 25 °C, 50 ± 10% RH and a 14:10 (light/dark [h]) photoperiod.
3. Results
3.1. Identification of B. thuringiensis
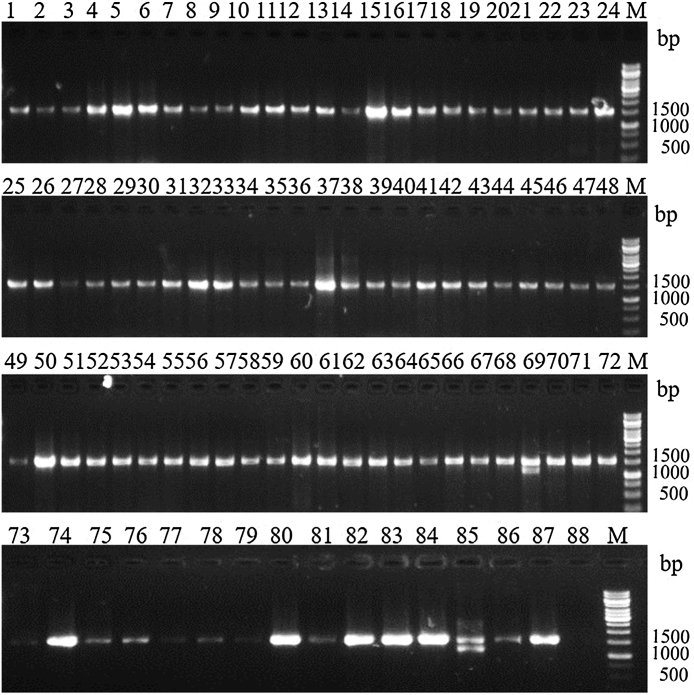
Eighty six Bacillus isolates were recovered by applying acetate-penicillin selection methodology to 30 soil samples collected from three types of soils (Table 1). Forty four isolates were found to synthesize crystal protein during sporulation by phase contrast microscopy. The cell shape of B. thuringiensis was recorded at different stages of growth (Fig. 1). B. thuringiensis was successfully isolated from all types of samples, recovery was highest from lake sediments (0.75) followed by forest soils (0.60) and the least from agricultural soils (0.33) (Table 1). The average Bt index of the study was 0.51 (Table 1). A 1500 bp amplicon corresponding to 16S rRNA was successfully amplified in all the isolates using total cell DNA as template (Fig. 2). The amplicons were sequenced and the partial sequences were aligned with the 16S rRNA gene sequences of Bt available from GenBank. It was observed that the obtained sequences showed 95–100% identity with the reported sequences on BLAST analysis (Table 2). The partial 16S rRNA gene sequences were submitted to GenBank and accession numbers were obtained [21].
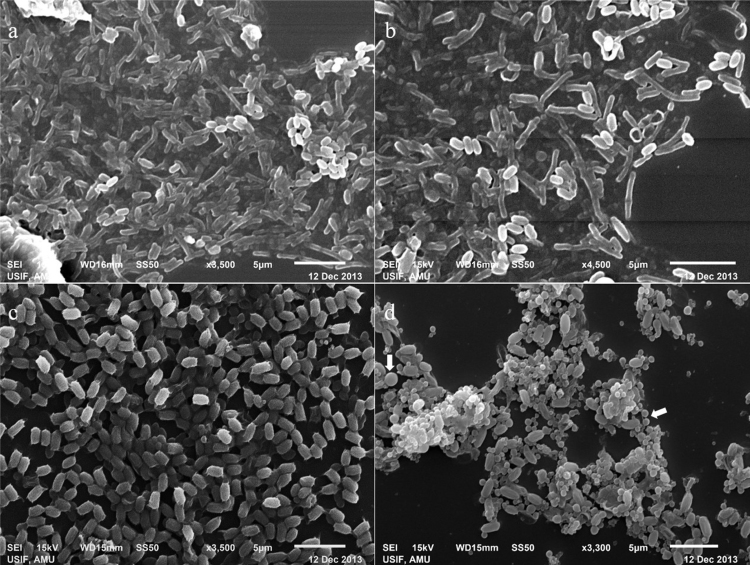
Fig. 1.
Scanning electron micrographs of B. thuringiensis cells showing different stages of growth. (a) log phase (16 h), (b) late log phase (20 h), (c) sporulation phase (48 h), (d) late sporulation/crystal production (72 h), white arrows indicate the spherical crystals.
Fig. 2.
PCR amplification of 16S rRNA gene of the native Bacillus isolates (Lanes 1–86 native isolates, 87, positive control (Bt subsp. kurstaki HD1 template), 88, negative control (without template). Lane M: 1 kb DNA size marker (Fermentas), 1.0% agarose/EtBr gel.
Table 2.
Phylogenetic identification and cry gene profiling of native isolates obtained from various locations of Kashmir Valley.
| Isolates | Isolation site | Accession No. | Nearest Phylogenetic Neighbor | Identity (%) | cry gene profiled |
|---|---|---|---|---|---|
| JK7 | Brarinar (Forest soil) | KJ125312 | KF631232 Bacillus thuringiensis strain MU9 | 100 | I, II |
| JK9 | Brarinar (Forest soil) | KJ125313 | KF512665 Bacillus thuringiensis strain BFB35 | 99 | I, II |
| JK10 | Brarinar (Forest soil) | KJ125314 | KF550441 Bacillus thuringiensis strain WBD10A | 100 | NA |
| JK11 | Brarinar (Forest soil) | KJ125315 | KF555624 Bacillus thuringiensis strain Dr45 | 99 | NA |
| JK12 | Brarinar (Forest soil) | KJ125316 | FR877760 Bacillus thuringiensis strain BD12 | 100 | I, II |
| JK13 | Brarinar (Forest soil) | KJ125317 | KF444375 Bacillus thuringiensis strain WG47(1) | 100 | NA |
| JK14 | Brarinar (Forest soil) | KJ125318 | JX500188 Bacillus thuringiensis strain EAPL17 | 100 | I |
| JK15 | Brarinar (Forest soil) | KJ125319 | KF550441 Bacillus thuringiensis strain WBD10A | 99 | I, II |
| JK16 | Brarinar (Forest soil) | KJ125320 | KF550441 Bacillus thuringiensis strain WBD10A | 99 | I, II |
| JK17 | Brarinar (Forest soil) | KJ125321 | KF500576 Bacillus thuringiensis strain Bt100 | 99 | NA |
| JK18 | Brarinar (Forest soil) | KJ125322 | KC778385 Bacillus thuringiensis strain BGB20 | 100 | I |
| JK19 | Brarinar (Forest soil) | KJ125323 | KF010790 Bacillus thuringiensis strain B45V | 100 | I, II |
| JK20 | Pangkong (Lake Sediment) | KJ125324 | JQ004442 Bacillus thuringiensis strain GTG-47 | 99 | NA |
| JK21 | Pangkong (Lake Sediment) | KJ125325 | JQ004442 Bacillus thuringiensis strain GTG 47 | 99 | I |
| JK22 | Pangkong (Lake Sediment) | KJ125326 | EU240956 Bacillus thuringiensis strain DW-1T | 98 | I |
| JK23 | Pangkong (Lake Sediment) | KJ125327 | KF017270 Bacillus thuringiensis strain VITGS | 99 | NA |
| JK26 | Pangkong (Lake Sediment) | KJ125330 | KF010790 Bacillus thuringiensis strain B45V | 100 | I |
| JK27 | Pangkong (Lake Sediment) | KJ125331 | KF631232 Bacillus thuringiensis strain MU9 | 99 | I |
| JK33 | Kalgi (Maize Field) | KJ125337 | KF550441 Bacillus thuringiensis strain WBD10A | 100 | I, II |
| JK35 | Kalgi (Maize Field) | KJ125338 | JQ004425 Bacillus thuringiensis strain GTG-21 | 99 | NA |
| JK36 | Kalgi (Maize Field) | KJ125339 | KF017270 Bacillus thuringiensis strain VITGS | 99 | II |
| JK37 | Kalgi (Maize Field) | KJ125340 | JQ004442 Bacillus thuringiensis strain GTG-47 | 98 | I |
| JK38 | Kalgi (Maize Field) | KJ125341 | KF017270 Bacillus thuringiensis strain VITGS | 99 | I |
| JK39 | Kalgi (Maize Field) | KJ125342 | KF631232 Bacillus thuringiensis strain MU9 | 99 | NA |
| JK40 | Kokarnag (Forest soil) | KJ125343 | EF495116 Bacillus thuringiensis strain 19198 | 95 | I |
| JK41 | Kokarnag (Forest soil) | KJ125344 | JQ004425 Bacillus thuringiensis strain GTG-21 | 100 | NA |
| JK42 | Kokarnag (Forest soil) | KJ125345 | EF113653 Bacillus thuringiensis strain GDFT2 | 99 | NA |
| JK52 | Mairan (Maize field) | KJ125355 | EU429663 Bacillus thuringiensis serovar kurstaki | 96 | NA |
| JK57 | Gulmarg (Forest soil) | KJ125358 | KF555624 Bacillus thuringiensis strain Dr45 | 98 | NA |
| JK58 | Gulmarg (Forest soil) | KJ125359 | KF444375 Bacillus thuringiensis strain WG47(1) | 99 | I, II |
| JK59 | Gulmarg (Forest soil) | KJ125360 | KF550441 Bacillus thuringiensis strain WBD10A | 99 | NA |
| JK60 | Gulmarg (Forest soil) | KJ125361 | KF631232 Bacillus thuringiensis strain MU9 | 99 | NA |
| JK65 | Gulmarg (Forest soil) | KJ125362 | KF017270 Bacillus thuringiensis strain VITGS | 99 | I, II |
| JK66 | Gulmarg (Forest soil) | KJ125363 | JQ004425 Bacillus thuringiensis strain GTG-21 | 99 | I, II |
| JK67 | Gulmarg (Forest soil) | KJ125364 | KF017270 Bacillus thuringiensis strain VITGS | 99 | I |
| JK70 | Venkara (Forest soil) | KJ125366 | KF512665 Bacillus thuringiensis strain BFB35 | 100 | NA |
| JK71 | Venkara (Forest soil) | KJ125367 | JX051373 Bacillus thuringiensis strain T106 | 98 | NA |
| JK72 | Venkara (Forest soil) | KJ125368 | JX437002 Bacillus thuringiensis strain RX-MKV2 | 99 | I, II |
| JK73 | Venkara (Forest soil) | KJ125369 | GQ497139 Bacillus thuringiensis strain KKK 2 | 98 | I |
| JK74 | Venkara (Forest soil) | KJ125370 | KF631232 Bacillus thuringiensis strain MU9 | 99 | NA |
| JK75 | Saripara (Maize Field) | KJ125371 | JQ004425 Bacillus thuringiensis strain GTG-21 | 99 | II |
| JK76 | Saripara (Maize Field) | KJ125372 | KF631232 Bacillus thuringiensis strain MU9 | 99 | NA |
| JK88 | Udusa (Maize Field) | KJ125383 | KF631232 Bacillus thuringiensis strain MU9 | 99 | I |
| JK92 | Udusa (Maize Field) | KJ125387 | JQ004442 Bacillus thuringiensis strain GTG-47 | 99 | I, II |
3.2. Amplification of cry genes
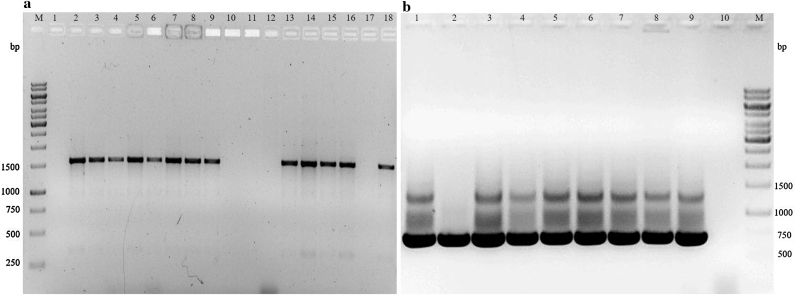
PCR screening using two pairs of universal primers was performed to detect cry1 and cry2 gene families in our collection. Primers for the detection of both cry1 and cr2 showed successful amplification as indicated by their specific product sizes of 1500 and 700 bp, respectively on agarose gel electrophoresis (Fig. 3). Twenty six isolates of the collection were found to contain either or both the cry genes tested while as 18 isolates did not show the presence of either of the genes. Compared to cry2, cry1 genes were found to be more abundant and in most of the isolates (12) they were found together.
Fig. 3.
Amplification of (a) cry1 and (b) cry2 genes in representative B. thuringiensis isolates indicating amplification of 1500 bp and 700 bp amplicons, respectively. Lane M: 1 kb DNA size marker (Fermentas). 1.0% agarose/EtBr gel.
3.3. “Quick screen” and toxicity assay against Helicoverpa
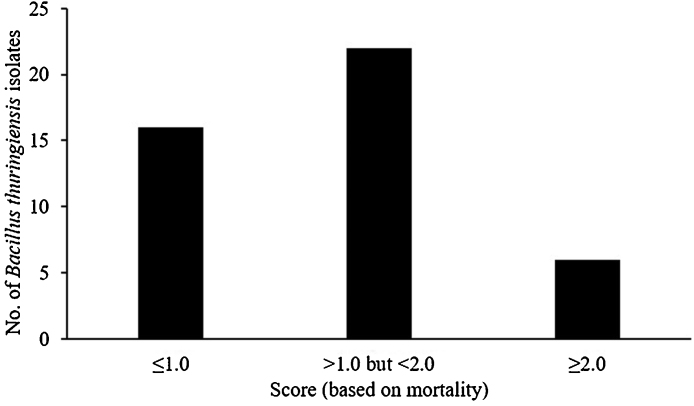
All the 44 native B. thuringiensis isolates and the reference strain HD1 were subject to toxicity screening using concentrated spore crystal suspensions (1 mgml−1). Twenty two out of 44 isolates were assigned a score of 1.0-2.0 as the leaves were partially eaten, 16 isolates were assigned a score of 1.0 because the leaves were completely eaten in this case while as only six isolates and the reference strain were assigned a score of 2 as the leaves were not eaten at all by the larvae. The scores indicate the mild toxicity, absence of toxicity and high toxicity of the isolates, respectively (Fig. 4).
Fig. 4.
Toxicity distribution of B. thuringiensis isolates against H. armigera larvae. The values indicate ≤1.0, non-toxic; >1.0 but <2.0, mildly toxic; ≥2.0, toxic.
4. Discussion
Although, distribution of cry genes of B. thuringiensis in some geographical regions of India has been investigated earlier [30,31], no researcher has systematically analysed the distribution of cry genes in soils of Jammu and Kashmir region. So, it is of interest to determine the distribution and diversity of cry genes and identify the type of cry genes. Since lepidopteran pests are the most predominant among all other insect pests and cause heavy economic loss, this study was carried to characterize and study the distribution of Bt isolates containing lepidopteran-specific cry1 and cry2 genes. Further, the toxicity of the isolates was evaluated against an economically important lepidopteran pest H. armigera.
The presence of a parasporal inclusion body is a diagnostic feature to discriminate B. thuringiensis species form its close relatives in the Bacillus cereus group [32]. Based on the presence of parasporal crystals, 44 Bacilli out of 86 were classified as B. thuringiensis. B. thuringiensis was found to be widely distributed in the soils of Jammu and Kashmir region with no history of Bt application. The estimation of the success of Bt isolation (Bt index) varied among the soil samples and the average Bt index observed in the study was 0.51. The average Bt index varies between soil samples all over the world as reported previously [25,21,33,34]. The likely reason for difference in Bt index is hard to access, however, the difference in geomorphology and the interaction of the bacterium with their insect hosts could influence their numbers in different environments.
16S rRNA gene sequencing is a routine method used for the taxonomic identification of bacteria [35]. However, the method has not been successful in clearly distinguishing the members of the B. cereus group of which B. thuringiensis is one of the member [35]. So, the partial 16S rRNA gene sequences were considered B. thuringiensis for the statistical counting if they showed maximum similarity to either B. thuringiensis or B. cereus.
PCR-based approach has been extensively utilized for the identification of known and novel cry genes in B. thuringiensis since its introduction by Carozzi et al. [36]. The rapidity and reproducibility of PCR-based detection of cry genes makes it a very useful tool even today. Both cry1 and cry2 genes were detected in the isolates, cry1 was found to be more prevalent than cry2 and most of the cry1 harbouring isolates also contained cry2 gene. The predominance of cry1 gene compared to all other cry genes in the native B. thuringiensis has been reported extensively [37,38]. However, predominance of cry genes other than cry1 has also been reported [25,28,39,40]. Twenty six out of 44 B. thuringiensis isolates showed the presence of either cry1 or cry2 genes among which 12 isolates showed the presence of cry1 and cry2 in combination (Table 2). The possible reason for both cry1 and cry2 genes being found together is that both the genes may be closely located on the genome and have evolved together [41]. Isolates containing both the cry genes are ideal for development of biocontrol agent as both the proteins encoded by these genes are specific against lepidopteran pests. Eighteen of the 44 isolates did not show presence of either cry1 or cry2, suggesting that they may contain other types of cry genes not tested in this study. A large number of isolates harbouring no cry genes has been reported in various B. thuringiensis collections previously [42,43].
All the putative B. thuringiensis isolates were subjected to toxicity screening against 2nd instar larvae of H. armigera using higher doses of spore crystal mixtures. Only 13.63% of the isolates exhibited toxicity as evidenced by the treated larvae not being able to feed on fresh leaves, 50% of the isolates were mildly toxic whereas 36.36% were found to be non-toxic (Fig. 4). The difference in toxicity is multifactorial including the absence of toxin proteins and due the poor expression of the Cry proteins.
In the present study, B. thuringiensis isolates from various soil samples were found to harbour either or both of the cry1 and cry2 genes and some of them were found to be toxic against H. armigera. More precise bioassays using different doses of spore crystal mixtures against more number of larvae shall lead to the identification of potentially toxic isolates for the control of H. armigera.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The first author is grateful to the, University grants commission (UGC), New Delhi, India for providing fellowship under Maulana Azad National Fellowship (MANF) scheme. Project Director, National Research Centre on Plant Biotechnology (NRCPB) is thanked for providing necessary facilities to carry out this research. National Agricultural Innovation Project (NAIP) of Indian Council of Agricultural Research, New Delhi is acknowledged for providing financial support. Dr. Daniel R. Zeigler, the Bacillus Genetic Stock Center (BGSC) Ohio State University, USA is thanked for providing the reference strain.
References
- 1.FAO Global agriculture towards 2050. Proceedings of High Level Expert Forum − How to Feed the World in 2050; October 2009, Rome, Italy; 2009. pp. 12–13. [Google Scholar]
- 2.Pimentel D. Environmental and economic costs of the application of pesticides primarily in the United States. In: Rajinder P., Dhawan A., editors. Integrated Pest Management: Innovation-Development Process. Springer; Netherlands: 2009. pp. 88–91. [Google Scholar]
- 3.Smith-Pardo A.H. The old world bollworm Helicoverpa armigera (Hübner) (Lepidoptera: noctuidae: Heliothinae) its biology, economic importance and its recent introduction into the Western Hemisphere. Bol. Mus. Entomol. Univ. Valle. 2014;6(1):18–28. [Google Scholar]
- 4.CABI EPPO . EPPO quarantine pest; 1996. Helicoverpa Armigera. Data Sheets on Quarantine Pests. pp. 6. [Google Scholar]
- 5.Tay W.T., Soria M., Walsh T., Thomazoni D., Silvie P., Behere G. A brave new world for an old world pest: helicoverpa armigera (Lepidoptera: noctuidae) in Brazil. PLoS One. 2013;8(11):1–7. doi: 10.1371/journal.pone.0080134. (e80134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren A. CSIRO Blog at World Press; 2013. Mega-pest Worms Its Way into Brazil. [Google Scholar]
- 7.Fitt G.P., Wilson L.J. Genetic engineering in IPM: bt cotton. In: Kennedy G.G., Sutton T.B., editors. Emerging Technologies in Integrated Pest Management: Concepts, Research and Implementation. APS Press; St Paul,MN, USA: 2000. pp. 108–125. [Google Scholar]
- 8.Yang Y., Li Y., Wu Y. Current status of insecticide resistance in Helicoverpa armigera after 15 years of Bt cotton planting in China. J. Econ. Entomol. 2013;106:375–381. doi: 10.1603/ec12286. [DOI] [PubMed] [Google Scholar]
- 9.Downes S., Mahon R.J., Rossiter L., Kauter G., Leven T., Fitt G. Adaptive management of pest resistance by Helicoverpa species (Noctuidae) in Australia to the Cry2Ab Bt toxin in Bollgard II cotton. Evol. Appl. 2010;3(5–6):574–584. doi: 10.1111/j.1752-4571.2010.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabashnik B.E., Gould F. Delaying corn rootworm resistance to Bt corn. J. Econ. Entomol. 2012;105:767–776. doi: 10.1603/ec12080. [DOI] [PubMed] [Google Scholar]
- 11.Aktar M.W., Gupta D.S., Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2009;2:1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glazer A.N., Nikaido H. Microbial insecticides. In: Company Freeman W.H., editor. Microbial Biotechnology. Fundamentals of Applied Microbiology; New York: 1995. pp. 209–229. [Google Scholar]
- 13.Shishir A., Roy A., Islam N., Rahman A., Khan S.N., Hoq M.M. Abundance and diversity of Bacillus thuringiensis in Bangladesh and their cry genes profile. Front. Environ. Sci. 2014;20(2):1–10. [Google Scholar]
- 14.Schnepf E., Crickmore N., Van R.J., Lereclus D., Baum J., Feitelson J. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estruch J.J., Warren G.W., Mullins M.A., Nye G.J., Craig J.A., Koziel M.G. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5389–5394. doi: 10.1073/pnas.93.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.N. Crickmore , J. Baum , A. Bravo , D. Lereclus , K. Narva , K. Sampson , et al. Bacillus Thuringiensis Toxin Nomenclature 2016; (Accessed 16 November 2016) http://www.btnomenclature.info/
- 17.Ozturk E., Acik L., Ayvaz A., Bozdogan B., Suludere Z. Isolation and characterization of native Bacillus thuringiensis strains from soil and testing the bioactivity of isolates against Ephestia kuehniella zeller (Lepidoptera: pyralidae) larvae. Turk. J. Biochem. 2008;33:202–208. [Google Scholar]
- 18.Sadder M.T., Horani H.K., Al-Banna L. Application of RAPD technique to study polymorphism among Bacillus thuringiensis isolates from Jordan. World J. Microbiol. Biotechnol. 2006;22:1307–1312. [Google Scholar]
- 19.Noguera P.A., Ibarra J.E. Detection of new cry genes of Bacillus thuringiensis by use of a novel PCR primer system. Appl. Environ. Microbiol. 2010;76:6150–6155. doi: 10.1128/AEM.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray M., Doshi N., Alag N., Sreedhar R. Indian Network on Ethics and Climate Change (INECC); Visakhapatnam, India: 2011. Climate Vulnerability in North-Western Himalayas: A Contribution to the Ongoing Nation-wide Climate Studies, Vulnerability in Various Ecoregions of India. [Google Scholar]
- 21.Lone S.A., Yadav R., Malik A., Padaria J.C. Molecular and insecticidal characterization of Vip3A protein producing Bacillus thuringiensis strains toxic against Helicoverpa armigera (Lepidoptera: noctuidae) Can. J. Microbiol. 2016;62(2):179–190. doi: 10.1139/cjm-2015-0328. [DOI] [PubMed] [Google Scholar]
- 22.Travers R.S., Martin P.A.W., Reichelderfer C.F. Selective process for efficient isolation of soil Bacillus sp. Appl. Environ. Microbiol. 1987;53:1263–1266. doi: 10.1128/aem.53.6.1263-1266.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohba M., Aizaw A.K. Insect toxicity of Bacillus thuringiensis isolated from soils of Japan. Invertebr. Pathol. 1986;47:12–20. [Google Scholar]
- 24.Yang Z.W., Wu H.W., Wang K.M., Xie T.J. Method for highly efficient isolation of Bacillus thuringiensis from soil. Chin. J. Biol. Control. 2000;16:26–30. [Google Scholar]
- 25.Baig D.N., Bukhari D.A., Shakoori A.R. cry genes profiling and the toxicity of isolates of Bacillus thuringiensis from soil samples against American bollworm, Helicoverpa armigera. J. Appl. Microbiol. 2010;109(6):1967–1978. doi: 10.1111/j.1365-2672.2010.04826.x. [DOI] [PubMed] [Google Scholar]
- 26.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.Juarez-Perez V.M., Ferrandis M.D., Frutos R. PCR-Based approach for detection of novel Bacillus thuringiensis cry genes. Appl. Environ. Microbiol. 1997;63(8):2997–3002. doi: 10.1128/aem.63.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Dov E., Zaritsky A., Dahan E., Barak Z., Sýnal R., Manasherob R. Extended Screening by PCR for seven cry-group genes from field-collected Strains of Bacillus thuringiensis. Appl. Environ. Microbiol. 1997;63:4883–4890. doi: 10.1128/aem.63.12.4883-4890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rampersad J., Ammons D. A Bacillus thuringiensis isolation method utilizing a novel stain, low selection and high throughput produced atypical results. BMC Microbiol. 2005;5(1):52. doi: 10.1186/1471-2180-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattar S., Maiti M.K. Molecular characterization of a novel vegetative insecticidal protein from Bacillus thuringiensis effective against sap-sucking insect pest. J. Microbiol. Biotechnol. 2011;21:937–946. doi: 10.4014/jmb.1105.05030. [DOI] [PubMed] [Google Scholar]
- 31.Shingote P.R., Moharil M.P., Dhumale D.R., Deshmukh A.G., Jadhav P.V., Dudhare M.S. Distribution of vip genes, protein profiling and determination of entomopathogenic potential of local isolates of bacillus thuringiensis. Bt Res. 2013;4(3):14–20. [Google Scholar]
- 32.Soufiane B., Baizet M., Cote J. Multilocus sequence analysis of Bacillus thuringiensis serovars navarrensis, bolivia and vazensis and Bacillus weihenstephanensis reveals a common phylogeny. Antonie van Leeuwenhoek. 2013;103:195–205. doi: 10.1007/s10482-012-9800-5. [DOI] [PubMed] [Google Scholar]
- 33.Martin P.A.W., Travers R.S. Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl. Environ. Microbiol. 1989;55:2437–2442. doi: 10.1128/aem.55.10.2437-2442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X., Zheng A., Zhu J., Wang S., Wang L., Deng Q. Characterization of vegetative insecticidal protein vip Genes of Bacillus thuringiensis from Sichuan Basin in China. Curr. Microbiol. 2011;62(3):752–757. doi: 10.1007/s00284-010-9782-3. [DOI] [PubMed] [Google Scholar]
- 35.Punina N.V., Zotov V.S., Parkhomenko A.L., Parkhomenko T.U., Topunov A.F. Genetic diversity of Bacillus thuringiensis from Different Geo-Ecological Regions of Ukraine by analyzing the 16S rRNA and gyrB Genes and by AP-PCR and saAFLP. Acta Nat. 2013;5(1):90–100. [PMC free article] [PubMed] [Google Scholar]
- 36.Carozzi N.B., Kramer V.C., Warren G.W., Evola S., Koziel M.G. Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl. Environ. Microbiol. 1991;57:3057–3061. doi: 10.1128/aem.57.11.3057-3061.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baig D.N., Mehnaz S. Determination and distribution of cry-type genes in halophilc Bacillus thuringiensis isolates of Arabian Sea sedimentary rocks. Microbiol. Res. 2010;165(5):376–383. doi: 10.1016/j.micres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Porcar M., Juarez-Perez V. PCR-based identification of Bacillus thuringiensis pesticidal crystal genes. FEMS Microbiol. Rev. 2003;26:419–432. doi: 10.1111/j.1574-6976.2003.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 39.Mendoza G., Portillo A., Arias E., Ribas R.M., Olmos J. New combinations of cry genes from Bacillus thuringiensis strains isolated from North-Western Mexico. Int. Microbiol. 2012;15:209–216. doi: 10.2436/20.1501.01.174. [DOI] [PubMed] [Google Scholar]
- 40.Pinto L.M.N., Fiuza L.M. PCR and bioassays screening of Bacillus thuringiensis isolates from rice-fields of Rio Grande do Sul, specific to lepidopterans and coleopterans. Braz. J. Microbiol. 2003;34:305–310. [Google Scholar]
- 41.Ruan L., Crickmore N., Peng D., Sun M. Are nematodes a missing link in the confounded ecology of the entomopathogen Bacillus thuringiensis? Trends Microbiol. 2015;23(6):341–346. doi: 10.1016/j.tim.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Uribe D., Martinez W., Ceron J. Distribution and diversity of cry genes in native strains of Bacillus thuringiensis obtained from different ecosystems from Colombia. J. Invertebr. Pathol. 2003;82:119–127. doi: 10.1016/s0022-2011(02)00195-7. [DOI] [PubMed] [Google Scholar]
- 43.Vilas-Boas G.T., Lemos M.V.F. Diversity of cry genes and genetic characterization of Bacillus thuringiensis isolated from Brazil. Can. J. Microbiol. 2004;50:605–613. doi: 10.1139/w04-052. [DOI] [PubMed] [Google Scholar]