Abstract
Human exposure to intermediate frequency (IF) fields is increasing due to new applications such as electronic article surveillance systems, wireless power transfer and induction heating cookers. However, limited data is available on effects of IF magnetic fields (MF) on male fertility function. This study was conducted to assess possible effects on fertility indicators from exposure to IF MF. Male C57BL/6J mice were exposed continuously for 5 weeks to 7.5 kHz MF at 12 and 120 μT. Sperm cells from cauda epididymis were analysed for motility, total sperm counts, and head abnormalities. Motile sperm cells were classified as progressive or non-progressive. Testicular spermatid heads were counted as well. The body weight development and reproductive tissue weights were not affected. No exposure-related differences were observed in sperm counts or sperm head abnormalities. Proportion of non-motile cells was significantly decreased in the 120 µT group, and a corresponding increase was seen in the percentage of motile cells (significant in non-progressive motile cells). In conclusion, no adverse effects on fertility indicators were observed. Increased sperm motility is an interesting finding that needs to be confirmed in further studies.
Keywords: Intermediate frequency magnetic field, 7.5 kHz, Mouse, Male fertility
Highlights
-
•
Human exposure to intermediate frequency magnetic fields is increasing.
-
•
Mice were exposed to 7.5 kHz magnetic fields to evaluate possible effects on male fertility.
-
•
No adverse effects on fertility indicators were observed.
-
•
Sperm motility was increased in the highest exposure group.
1. Introduction
Exposure to electromagnetic fields is ubiquitous in the environment because of new technologies and novel applications being actively developed and commercialized. Extremely low frequency (ELF) magnetic fields (MF) associated with the distribution and use of electric power and radio-frequency (RF) electromagnetic fields (EMF) from wireless communication devices have been studied extensively for possible health effects. However, much less data are available on possible health effects of intermediate frequency (IF) fields between the ELF and RF ranges from 300 Hz to between 100 kHz and 30 MHz (upper limit depends on how RF is defined; Ahlbom et al., 2008; Litvak et al., 2002; Kurokawa et al., 2004). Intermediate frequency EMF are increasingly used at work and at home in applications such as electronic article surveillance (EAS) systems (Roivainen et al., 2014), induction heating cookers, magnetic resonance imaging machines, and inductively coupled wireless power transmission (Floderus et al., 2002; ICNIRP, 2008).
The present study was conducted to investigate possible effects on male fertility-related variables after long-term exposure to IF MFs. While several studies have evaluated developmental and reproductive effects of maternal exposure to IF MFs (Huuskonen et al., 1998, Frölén et al., 1993, Kim et al., 2004), only two studies have addressed possible effects on male fertility. These studies used 10 kHz MFs of the type used in inductively coupled power transmission (Dawson et al., 1998), or 20 kHz and 60 kHz fields relevant to induction heating cookers (Nishimura et al., 2012). In the present study, male mice were exposed to 7.5 kHz MFs similar to those emitted by an EAS technology commonly used in supermarkets and other stores to protect merchandise against theft. The magnetic flux densities used were 12 and 120 μT. The higher flux density exceeded the International Commission on Non-Ionizing Radiation Protection reference level (100 µT in the frequency range 3 kHz–10 MHz) for occupational exposure (ICNIRP, 2010) and was higher than the maximum exposure levels (up to 60 µT) found around EAS systems used in supermarkets and libraries (Roivainen et al., 2014).
Sperm count, sperm motility and sperm head abnormalities were measured, as they are considered to be important indicators for detecting the adverse effects of various factors on spermatogenesis (Blazak et al., 1985, Wyrobek and Bruce, 1975).
2. Materials and methods
2.1. Exposure system
The exposure system consisted of a wooden rack with five coils with rectangular cross section (Fig. 1). The dimensions of the coils were 40 cm × 120 cm. The top and bottom coils had 6 turns of copper wire (diameter 1.8 mm), while the three middle coils had 4 turns. The vertical distance between the coils was 25 cm. There were 4 shelves for animal cages; each shelf was 4 cm above one of the coils. The 7.5 kHz signal was generated by a Thandar TG501 function generator (Thurlby Thandar Instruments, UK) and amplified with a Behringer Europower EP 4000 amplifier (Music Group Services, USA) nominally capable of producing 1250 W of continuous power per channel. A 1.47 Ω resistor was connected in series with the coil system which itself has a wire resistance of 0.53 Ω, yielding a total resistance of 2 Ω. As the inductance of the coil system considerably resists flow of current at 7.5 kHz, a 1.5 µF capacitor was added to series resonate the coil system providing, a pure real load at 7.5 kHz.
Fig. 1.
Coil system used for exposure.
The animals were group housed in transparent polypropylene cages (3–10 animals per cage) with maximum dimensions of 39 × 28 × 14 cm at the cage top and minimum dimensions of 33 × 23 × 14 cm at the bottom. During the exposure, the cages were placed in the middle of on the second and fourth shelves (counted from the top). Control animals were sham-exposed in an identical unenergized coil system. Background MF in the sham-exposure coil system was measured with the calibrated coil described below. No MF exceeding the resolution of the measurement system (0.02 µT) was detected in the sham-exposure system when the exposure system was on.
2.2. Estimation of the field strength
The homogeneity of the field was evaluated both numerically and experimentally. The magnetic field strength was measured with a calibrated single- axis field sensing coil (diameter 2.0 cm, length 1.3 cm). The sensing coil was shielded against electric fields and was connected to a multimeter (Agilent U1241B, Agilent Technologies, Malaysia) to measure voltage induced in the coil.
The dose or exposure to IF magnetic fields can be expressed in several different ways, namely:
-
i)
Magnetic field level
-
ii)
Induced current densities (ICNIRP, 1998)
-
iii)
Induced electric field strengths (ICNIRP, 2010)
The dosimetry has been performed in line with best practice as described in Kuster et al. (2006), and the uncertainties in the calculated or measured quantities have been determined. The magnetic field exposure at these frequencies is equal to the incident magnetic field, in this case 12 or 120 µT, and is not discussed further.
For induced current densities and E-fields, SEMCAD v14 (SPEAG, Zurich, Switzerland) and the Magneto-Quasi Static solver was used to perform simulations. The dielectric body parameters of Gabriel et al. (1996) were used for all tissues, with the exception of the values in Supplementary Table 1, which were taken from the IT’IS LF tissue database (www.itis.ethz.ch/database). The conditions for validity of the quasi static approximation ωε < σ were always met.
Numerical dosimetry was performed to predict the induced exposure levels for male mice starting at an age of between 8 and 9 weeks for the exposure period of 5 weeks. A total of seven different mouse models – intended to represent realistic mice of different ages – were created starting from the available male voxel model having body weight of 37.6 g. This model was scaled and the resulting body masses were calculated. For a male C57BL/6J mouse in the age range of 8–14 weeks, the weights can be expected to be in the range of 22–33 g corresponding to the largest three of the seven mouse models. The mouse models were exposed in the simulation to a homogeneous vertically oriented magnetic field to determine the induced field quantities. In addition to determining the dosimetric quantities the uncertainties for the simulations and modeling were also estimated.
2.3. Field homogeneity
The coils generated a field of approximately 18.3 µT per Ampere of current flowing with the variations shown in Table 1 for the maximum and minimum cross-sections of the cages. Note that the cages reached the largest dimensions only at the very top and that the mice could only partially occupy this part of the volume when rearing up. Therefore, the variation over the inner dimension is more representative of the average area of occupancy for the mouse during normal activity. The measured fields using the single axis probe showed an overall homogeneity (SD) of 3.8%, with all measured points within ± 9% total field strength of the overall average. The average field strength was 12.03 µT for the low-dose group. The impact of using a single axis probe was investigated, and it was determined that the vertical component comprises >99.7% of the field and standard deviations are essentially the same. Thus, measurements confirmed the simulation results over the volume normally occupied by the mice.
Table 1.
Simulated field distribution statistics for a single cage.
| Shelf | Cage dimension | SD | Min | Max |
|---|---|---|---|---|
| 1st | Outer | 4.5% | −17.0% | 15.3% |
| 2nd | Inner | 2.3% | −8.2% | 3.7% |
| 3rd | Outer | 4.4% | −16.5% | 5.5% |
| 4th | Inner | 2.3% | −8.1% | 9.5% |
2.4. Mouse dosimetry results
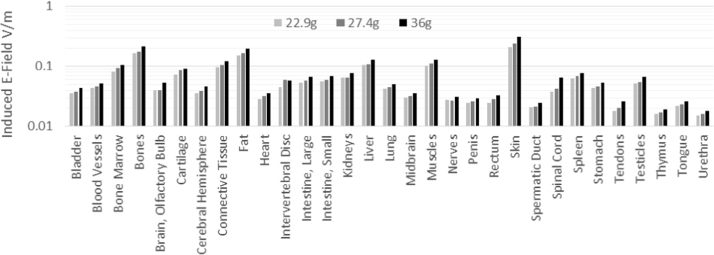
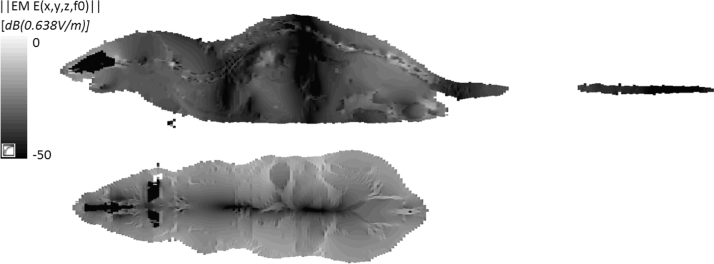
Dosimetry was performed to determine the induced E-fields and induced currents. In ICNIRP (2010) a cube of 2 mm per side is used for E-field averaging in humans, and here for the mouse, we use a cube of 0.24 mm side length, based on the ratio of human and mouse sizes. Volume averaging is performed to remove artefacts introduced by the rectilinear discretization of the models. All models were discretized with voxels of 0.12 mm side length and the average performed over 8 voxels. The peak values from the volume averages calculated for each individual tissue were determined, the most highly exposed tissues and those of interest to the study are shown in Fig. 2 for the range of weights observed for 8–14 week old mice. The E-field distribution in the center slices shown in Fig. 3. The highest exposure occurred in the skin in largest mouse size and was 0.036 and 0.36 V/m for the two exposure levels respectively, both well below the general public basic restriction of 1.06 V/m. The exposure in the testes is 0.11 V/m for the high dose group. The 50th percentile exposure can be anywhere between 1.4 and 15 times lower than the peak depending on the distribution of the tissue in question, for testes the ratio is ~5. An analysis of the uncertainties related to exposure field, and numerical dosimetry (mouse model scaling, dielectric parameters, discretization and simulation convergence) shows that the uncertainty is between 14% and 22% for the weights 33 g and 22 g respectively, hence all exposures even at k = 2 expanded uncertainty are below the basic restriction by a factor of 2.
Fig. 2.
Induced peak volume averaged electric (E) fields in individual tissues in three mouse models with body weights of 22.9 g, 27.4 g, and 36 g exposed to a 100 µT magnetic field.
Fig. 3.
Induced field distribution inside the mouse model for a 100 µT of magnetic field. EM E(x,y,z,f0) is the Electromagnetic field – Electric field magnitude expressed in dB where 0 dB is 0.638 V/m at the frequency f0 = 7.5 kHz.
In ICNIRP (1998), the standard most often enforced by legislation, exposure to low frequency (LF) magnetic fields is assessed in terms of induced currents, and, in the case of a human, the current density is averaged over an area of 1 cm2. We used an averaging area of a 1.2 mm radius circle in accordance with the relative sizes of humans and mice and discretized all models using voxels of 0.12 mm side length. Though the induced currents are less than the limit at the reference level (30.7 µT) they exceed the general public limit for most tissues at 120 µT and by a factor of 3 for the most highly exposed tissues, the overall uncertainty is less than 20%. In the testes at 120 µT the current density is estimated to be 20 mA/m2 and 10x less in the low dose group.
2.5. Animals and experimental design
C57BL/6 J male mice obtained from the Lab Animal Centre (Kuopio, Finland) were used. The animals were 2 months old in the beginning of the exposure. They were exposed continuously (except for about one hour per week that was needed for changing the cages) for 5 weeks to 7.5 kHz magnetic fields at 12 μT (n = 20), 120 μT (n = 20), or were sham exposed in the coil system without current (n= 30). The study included three cohorts. Each of the first two cohorts included 10 sham-exposed mice and 10 mice exposed to 12 μT. Because of a technical fault (malfunctioning magnetic field meter) a 120 µT group could not be successfully included in the two first cohorts, and exposure to this level was done separately using 10 sham-exposed mice and 20 mice exposed at 120 μT in the third cohort. After the exposure, the animals were ear marked (by an animal care-taker to leave the researcher blinded) and singly housed in 40 × 24 × 14 cm metal cages in a controlled environment (temperature 21 ± 1 °C, humidity 50–60%, lights on 7:00–19:00). Food and water were available ad libitum. After ear-marking the animals were tested for behavioral effects (not reported in this paper) for three weeks. The testing was identical for all exposure group and there was no exposure during this time. Sampling for fertility indicators was done after the behavioral tests. The experiments were conducted according to the guidelines of the Council of Europe (Directive 86/609) and Finland, and approved by the Animal Experiment Board in Finland (the license number ESAVI/2477/04.10.07/2013).
2.6. Reagents
The following reagents were used: pentobarbiturate-chloralhydrate cocktail, Hanks’ Balanced Salt solution (HBSS, KCl 5.3 mM, KH2PO4 0.4 mM, NaCl 137 mM, NaHCO3 4 mM, Na2HPO4 0.3 mM, d-Glucose 5.6 mM), Eosin Y (Sigma-Aldrich, Germany), Methanol (J.T. Backer, Holland), Merthiolate (Sigma-Aldrich), Ethanol (Altia Corporation, Finland).
2.7. Epididymal sperm motility and total sperm counts
The mice were deeply anesthetized with an intraperitoneal injection of pentobarbiturate-chloralhydrate cocktail (105 mg/kg pentobarbiturate and 425 mg/kg chloral hydrate). Left cauda epididymides (CE) were removed and weighed, put in 2 ml HBSS in 15 ml tubes and stored on ice. Proximal vas deferens was transected and the sperm were allowed to passively flow into a Petri dish at 37 °C in 2 ml HBSS for 10 min. Aliquots of 10 µl sperm suspension were moved into haemocytometer (Neubauer, Germany). Videos were taken through light microscope (Carl Zeiss microimaging GmbH, Germany) for 3 s for evaluation of sperm motility. Then sample were spun down at 161 g for 5 min to make cells immotile and 10 µl of suspension were loaded to haemocytometer. Photographs were taken for total sperm counts. Sperm cells were counted and cells with displacement were considered progressive, while cells having in-situ movement without displacement were counted as non-progressive, and those not moving at all were considered immotile cells (Vásquez et al., 2012).
2.8. Morphologic analysis
Morphologic analysis was done by modifying the method described by Wyrobek and Bruce (1975). Aliquots of sperm suspension used for motility assessment were used for morphology staining. Sperm suspension was stained by mixing with 2% Eosin Y (10:1) for 1 h. Two slides were made, air dried and fixed with absolute methanol for 5 min. Five hundred sperms per slide and two slides per animal were examined using light microscope to determine possible morphological abnormalities. Sperm head morphology was scored under the categories of normal, lack of usual hook, banana-like, amorphous head, triangular head, folded on themselves and two-tailed (Wyrobek and Bruce, 1975, Padmanabhan et al., 2008).
2.9. Testicular spermatid counts
Homogenization-resistant testicular sperm heads were prepared and counted by the standard method described by Blazak et al. (1993) with slight modifications. The left testis was collected from each animal, snap frozen in liquid nitrogen and stored at −70 °C until the analysis. Each sample was cut in small pieces by a razor blade and transferred into 25 ml of 0.9% saline containing 0.01% merthiolate and 0.05% Triton X-100 (SMT). The samples were blended for 2 min using a single-speed ultra turrax blender (Kika-werk GmbH and Co. KG, Staufen, Germany). Since the homogenates contained unblended material, they were filtered through a mesh (10 µm pore size, Merk Millipore Ltd. Germany). The non-filterable material was rinsed with an additional 25 ml of SMT. Then 10 µl of testis sperm homogenate was loaded onto a haemocytometer, photomicrographed using a standard light microscope with phase contrast, and counted later. The results of total cells count from testis were expressed as total numbers of spermatids per gram of testis.
2.10. Statistical analysis
IBM-SPSS 23.0 for Windows (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Linear mixed model analysis was performed, with exposure group as a fixed factor and cohort as a random factor. Least significant difference test was run as the post-test with the sham group as a comparison group in case of a significant overall effect. For presentation in figures, sperm counts and percentages of cells in different motility categories were normalized to the mean of the sham-exposed group of each cohort, and data were presented as relative values in order to control for bias arising from between-cohort differences.
3. Results
3.1. Body and organ weight
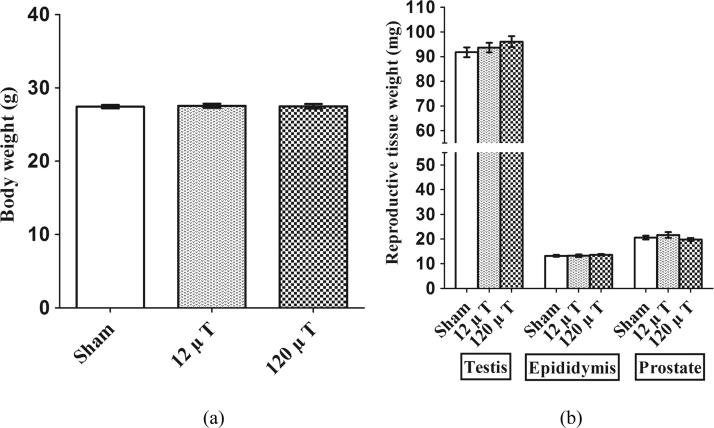
The body weights did not differ between the sham-exposed and IF MF exposed groups after the end of exposure (p = 0.95) (Fig. 4a). There were no between-group differences in the weights of testis (p = 0.34), CE (p = 0.72) or prostate (p = 0.54) after 5 weeks of IF MF exposure (Fig. 4b).
Fig. 4.
(a) Body weight in male mice exposed to 7.5 kHz magnetic fields at 12 or 120 µT. Means±SEM are shown at the age of 4 months (b) Reproductive tissue weights in mice exposed to 7.5 kHz magnetic fields at 12 or 120 µT. Means±SEM are shown at the age of 4 months N=20 (30 in the sham-exposed group). Both testes and epididymis were weighed, and the average of right and left side values was used as the tissue weight of an individual animal.
3.2. Sperm motility and sperm counts
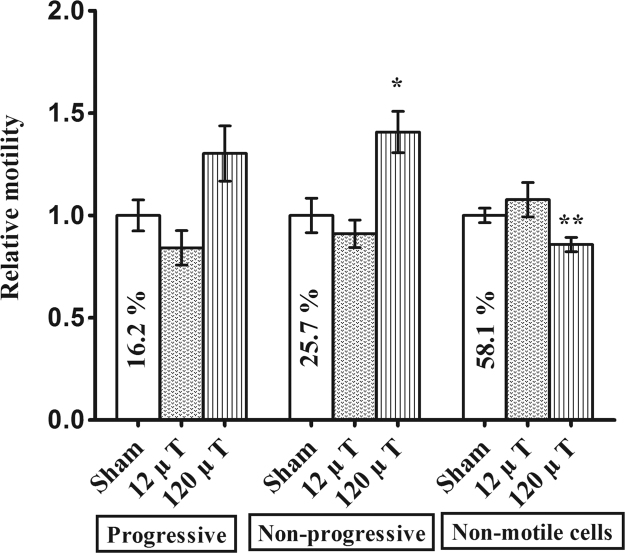
The motility of sperm cells in terms of progressive, non-progressive and non-motile cells is shown in Fig. 5. Because of a technical fault in the analysis, motility results of the first cohort are not included in the data. The exposure groups did not differ in the percentage of progressive cells (p = 0.28), but a significant exposure effect (p = 0.013) was found on the percentage of non-progressive sperm cells, such that the 120 µT group had higher counts than the sham group (p = 0.006). A corresponding main effect was observed in non-motile cells (p = 0.029), with the 120 µT group having lower counts than the sham group (p = 0.019).
Fig. 5.
Epididymal sperm motility in mice exposed to 7.5 kHz magnetic fields at 12 or 120 µT. Percentage of progressive, non-progressive and non-motile cells was calculated. To control for between-cohort differences in motility, relative values (means±SEM) are shown in relation to the percentage observed in the sham-exposed group of each cohort. Means of sham-exposed animals are given as numerical percentages. N=20 (10 in the 12 µT group). *P<0.05 and **P<0.01 in comparison to the sham-exposed group.
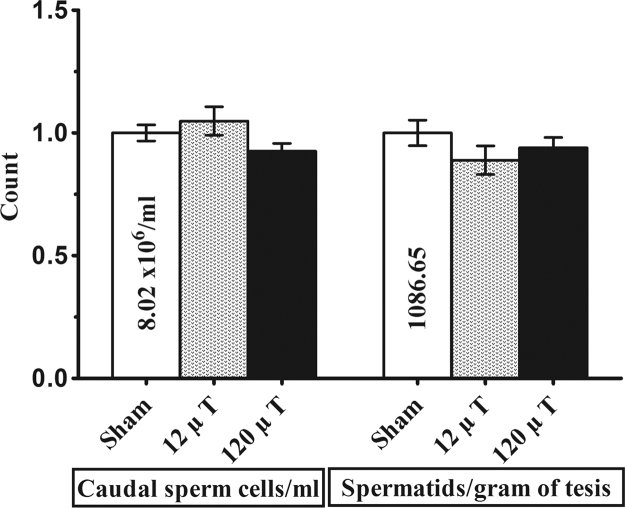
No differences between the exposure groups were observed in the epididymal (p = 0.35) or testicular (p=0.38) sperm counts (Fig. 6).
Fig. 6.
Epididymal spermatozoon and testicular spermatid counts in mice exposed to 7.5 kHz magnetic fields at 12 or 120 µT. To control for between-cohort differences in sperm counts, relative values (means±SEM) are shown in relation to the value observed in the sham-exposed group of each cohort. Means of sham-exposed animals are given as numerical values. N=20 (30 in sham-exposed group).
3.3. Morphologic analysis
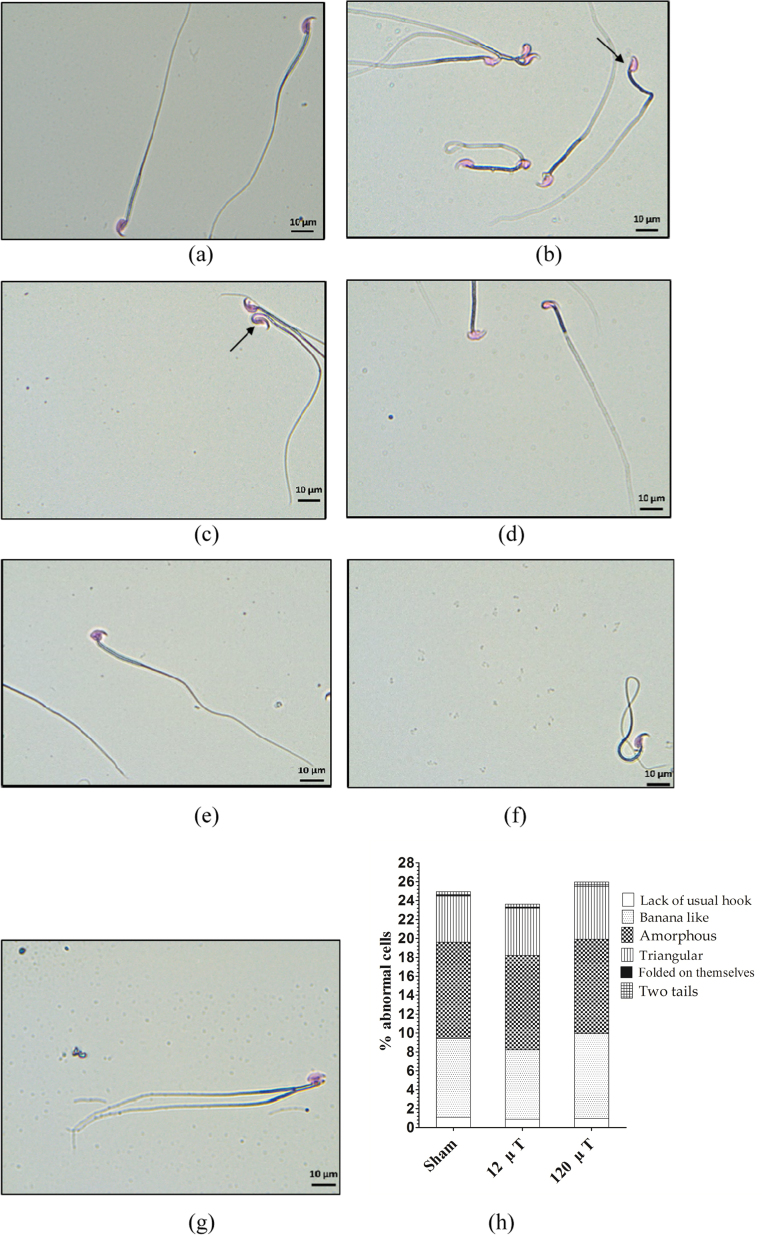
Photographs of typical examples of normal and abnormal sperm cells are shown in Fig. 7. Proportion of sperm head abnormalities in the classified abnormality categories is also shown. The proportion of abnormalities was similar in all groups, with no group differences in the categories “lack of usual hook” (p = 0.24), “banana-like” (p = 0.53), “amorphous head” (p = 0.96), “triangular head” (p = 0.64), “folded on themselves” (p = 0.64) and “two tailed” (p = 0.73).
Fig. 7.
Sperm head abnormalities. Photomicrographs of normal sperm cells (a) and various types of sperm head abnormalities: lack of usual hook (b) banana like (c) amorphous (d) triangular (e) folded on themselves (f) two tailed (g). Proportion of abnormal sperm cells in IF MF exposed mice (h). N=20 (30 in sham-exposed group).
4. Discussion
The present study was conducted to investigate possible effects of long-term exposure to IF MF on indicators of male fertility in mice. There were no adverse effects on fertility indicators. The body weight development or reproductive tissue weights were not affected by the used IF MFs, and no exposure-related differences were observed in sperm counts or sperm head abnormalities. Increased sperm motility was observed in the 120 µT group.
The 5-week IF MF exposure was started at the age of 2 months, and the fertility indicators were evaluated 3 weeks after the termination of exposure. As the duration of mouse spermatogenesis is 35 days (Attia et al., 2015), many of the evaluated sperm cells had been exposed to the IF field during the whole spermatogenesis and then stored in the cauda epididymis for 3 weeks. The shortest possible exposure was experienced by those cells that had just reached maturity at the time of analysis; such cells were exposed for the two first weeks of spermatogenesis.
Rodents are considered a suitable model for studying environmental effects on reproduction because they have high fertility and short generation times (Okano et al., 2016). Spermatogenesis is a very complex process and represents one of the most dramatic examples of cellular proliferation and differentiation in any mammalian organ system (Evenson et al., 1985). In normal mice, the incidence of spontaneous changes in sperm morphology is low and the percentage of normal sperm ranges from 78% to 85%. An increase in the frequency of sperm abnormalities can consequently be considered as evidence of damage to germ cells (Wyrobek and Bruce, 1975). In our experimental conditions the proportion of normal sperm cells were 74–76%. However, there were no differences between the groups, which suggests that the MF used does not interfere with spermatogenesis in such a way that it would increase sperm head abnormalities. Sufficient sperm count is obviously also important for successful fertilization (Nikolettos et al., 1999). The present study did not provide evidence of adverse effects of IF MFs on sperm counts in cauda epididymis or testis.
Sperm cell motility is an important indicator of fertility (Nikolettos et al., 1999). The percentage of motile cells in the sham-exposed control groups of each cohort of mice varied from 41.9% to 46.2%, which is comparable to the percentages (38–98%) reported in other studies (Kamiya et al., 2003, Montoto et al., 2011). The increased sperm motility observed in the 120 µT group is of interest. There is no previous evidence of stimulation of sperm motility by IF MFs. However, Iorio et al. (2007) reported that exposure of human semen samples to 50 Hz MFs at 5 mT increased sperm motility after 2 h of exposure, and the increased motility persisted 21 h after the end of the 3-h exposure. This effect was observed in cells exposed to a MF with square waveform, whereas a sinusoidal MF with the same amplitude and frequency did not affect sperm motility. The difference between the two waveforms may be relevant with respect to the effects observed in the present study, as the spectrum of a square waveform (as revealed by Fourier transform) contains a high number of multiples of the fundamental frequency (kHz frequencies most likely being present in a 50 Hz square wave). These higher frequencies might be responsible for the motility-stimulating effect of the square wave. Understanding of the mechanisms of the regulation of sperm motility is limited, but motion naturally needs energy, and reported evidence indicates that mitochondrial oxidative phosphorylation has a key role in mediating the effect of 50-Hz square wave MF on sperm motility (Turner, 2006, Iorio et al., 2011).
Two previous studies have addressed possible adverse effects of IF MF on fertility of rats. Nishimura et al. (2012) exposed rats to 20 kHz, 0.2 mT and 60 kHz, 0.1 mT MF for 22 h/d for 14 days. No exposure-related changes were observed in body weight, reproductive tissue weights (testes and CE weight), epididymal sperm count, motility or sperm morphology. Dawson et al. (1998) exposed male rats to 10 kHz IF MF at 0.095, 0.24, and 0.95 mT for 20–23.5 h/d for 45 days. Maternal rats were also exposed during gestation or 1 month prior to mating. There were no exposure-related effects on spermatogenesis or any other signs of reproductive toxicity. The results of the present study are in agreement with these two studies with respect to lack of adverse effects on indicators of male fertility. Together, these studies have covered four different IF MF frequencies (7.5, 10, 20 and 60 kHz) and magnetic flux densities from 12 to 950 µT.
There is still uncertainty concerning the mechanisms of the biological effects of alternating MFs, and understanding of the frequency dependence of the effects is therefore limited. It is thus of interest to compare effects of IF MFs to those of ELF MFs. Although many studies have reported no effects on spermatogenesis or male fertility in rodents exposed to 50 or 60 Hz MFs (Akdag et al., 2006, Akdag et al., 2013, Duan et al., 2014, Elbetieha et al., 2002; Heredia‐Rojas et al., 2004), the evidence is inconsistent as there are also positive studies reporting adverse effects of ELF MFs on rat (Al-Akhras et al., 2006), boar (Bernabo et al., 2007) and human (Li et al., 2010) sperm cells, as well as fertility of rats (Al-Akhras et al., 2001) and swine (Bernabo et al., 2010). The negative findings of the present study (and previous studies on IF MFs) indicate that if fertility is affected by MFs, such effects may not occur at frequencies ≥7.5 kHz. However, further studies that directly address fertility in animals and/or humans exposed to IF MFs would be useful to reduce the remaining uncertainties.
5. Conclusion
In conclusion, no adverse effects on fertility indicators were observed in mice exposed to IF MFs. The body weight development or reproductive tissue weights were not affected by the IF MFs used, and no exposure-related differences were observed in sperm counts or sperm head abnormalities. Increased sperm motility is an interesting finding that needs to be confirmed in further studies.
Acknowledgements
The research leading to these results has received funding from the European Union Seventh Framework Programme under Grant agreement no: 603794, and from the University of Eastern Finland. Authors wish to thank Ms. Hanne Säppi for her skillful technical assistance.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2017.05.014.
Appendix A. Supplementary material
Supplementary material
References
- Ahlbom A., Bridges J., De Seze R., Hillert L., Juutilainen J., Mattsson M.O., Neubauer G., Schüz J., Simko M., Bromen K. Possible effects of electromagnetic fields (EMF) on human health – opinion of the scientific committee on emerging and newly identified health risks (SCENIHR) Toxicology. 2008;246:248–250. doi: 10.1016/j.tox.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Akdag M.Z., Dasdag S., Aksen F., Isik B., Yilmaz F. Effect of ELF magnetic fields on lipid peroxidation, sperm count, p53, and trace elements. Med. Sci. Monit. 2006;12:366–371. [PubMed] [Google Scholar]
- Akdag M.Z., Dasdag S., Uzunlar A.K., Ulukaya E., Oral A.Y., Çelik N., Akşen F. Can safe and long-term exposure to extremely low frequency (50 Hz) magnetic fields affect apoptosis, reproduction, and oxidative stress? Int. J. Rad. Biol. 2013;89:1053–1060. doi: 10.3109/09553002.2013.817705. [DOI] [PubMed] [Google Scholar]
- Al‐Akhras M.D.A., Darmani H., Elbetieha A. Influence of 50 Hz magnetic field on sex hormones and other fertility parameters of adult male rats. Bioelectromagnetics. 2006;27:127–131. doi: 10.1002/bem.20186. [DOI] [PubMed] [Google Scholar]
- Al‐Akhras M.D.A., Elbetieha A., Hasan M.K., Al‐Omari I., Darmani H., Albiss B. Effects of extremely low frequency magnetic field on fertility of adult male and female rats. Bioelectromagnetics. 2001;22:340–344. doi: 10.1002/bem.59. [DOI] [PubMed] [Google Scholar]
- Attia S.M., Ahmad S.F., Okash R.M., Bakheet S.A. Dominant lethal effects of nocodazole in germ cells of male mice. Food Chem. Toxicol. 2015;77:101–104. doi: 10.1016/j.fct.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Bernabo N., Tettamanti E., Pistilli M.G., Nardinocchi D., Berardinelli P., Mattioli M., Barboni B. Effects of 50 Hz extremely low frequency magnetic field on the morphology and function of boar spermatozoa capacitated in vitro. Theriogenology. 2007;67:801–815. doi: 10.1016/j.theriogenology.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Bernabo N., Tettamanti E., Russo V., Martelli A., Turriani M., Mattoli M., Barboni B. Extremely low frequency electromagnetic field exposure affects fertilization outcome in swine animal model. Theriogenology. 2010;73:1293–1305. doi: 10.1016/j.theriogenology.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Blazak W.F., Ernst T.L., Stewart B.E. Potential indicators of reproductive toxicity: testicular sperm production and epididymal sperm number, transit time, and motility in Fischer 344 rats. Fund. Appl. Toxicol. 1985;5:1097–1103. doi: 10.1016/0272-0590(85)90145-9. [DOI] [PubMed] [Google Scholar]
- Blazak W.F., Treinen K.A., Juniewicz P.E. Application of testicular sperm head counts in the assessment of male reproductive toxicity. Methods Toxicol. 1993;3:86–94. [Google Scholar]
- Dawson B.V., Robertson I.G.C., Wilson W.R., Zwi L.J., Boys J.T., Green A.W. Evaluation of potential health effects of 10 kHz magnetic fields: a rodent reproductive study. Bioelectromagnetics. 1998;19:162–171. doi: 10.1002/(sici)1521-186x(1998)19:3<162::aid-bem4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Duan W., Liu C., Wu H., Chen C., Zhang T., Gao P., Zhou Z. Effects of exposure to extremely low frequency magnetic fields on spermatogenesis in adult rats. Bioelectromagnetics. 2014;35:58–69. doi: 10.1002/bem.21816. [DOI] [PubMed] [Google Scholar]
- Elbetieha A., AL‐Akhras M.D.A., Darmani H. Long‐term exposure of male and female mice to 50 Hz magnetic field: effects on fertility. Bioelectromagnetics. 2002;23:168–172. doi: 10.1002/bem.109. [DOI] [PubMed] [Google Scholar]
- Evenson D.P., Higgins P.J., Grueneberg D., Ballachey B.E. Flow cytometric analysis of mouse spermatogenic function following exposure to ethylnitrosourea. Cytometry. 1985;6:238–253. doi: 10.1002/cyto.990060311. [DOI] [PubMed] [Google Scholar]
- Floderus B., Stenlund C., Carlgren F. Occupational exposures to high frequency electromagnetic fields in the intermediate range (> 300 Hz–10 MHz) Bioelectromagnetics. 2002;23:568–577. doi: 10.1002/bem.10050. [DOI] [PubMed] [Google Scholar]
- Frölén H., Svedenstal B.M., Paulsson L.E. Effects of pulsed magnetic fields on the developing mouse embryo. Bioelectromagnetics. 1993;14:197–204. doi: 10.1002/bem.2250140303. [DOI] [PubMed] [Google Scholar]
- Gabriel S., Lau R.W., Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996;41:2271. doi: 10.1088/0031-9155/41/11/003. [DOI] [PubMed] [Google Scholar]
- Heredia‐Rojas J.A., Caballero‐Hernandez D.E., Rodriguez‐de la Fuente A.O., Ramos‐Alfano G., Rodriguez‐Flores L.E. Lack of alterations on meiotic chromosomes and morphological characteristics of male germ cells in mice exposed to a 60 Hz and 2.0 mT magnetic field. Bioelectromagnetics. 2004;25:63–68. doi: 10.1002/bem.10184. [DOI] [PubMed] [Google Scholar]
- Huuskonen H., Juutilainen J., Julkunen A., Maeki-Paakkanen J., Komulainen H. Effects of gestational exposure to a video display terminal-like magnetic field (20-kHz) on CBA/S mice. Teratology. 1998;58:190–196. doi: 10.1002/(SICI)1096-9926(199811)58:5<190::AID-TERA5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz) Health Phys. 1998;74:494–522. [PubMed] [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection ICNIRP statement on EMF-emitting new technologies. Health Phys. 2008;94:376–392. doi: 10.1097/01.HP.0000304155.41715.73. [DOI] [PubMed] [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz–100 kHz) Health Phys. 2010;99:818–836. doi: 10.1097/HP.0b013e3181f06c86. [DOI] [PubMed] [Google Scholar]
- Iorio R., Scrimaglio R., Rantucci E., Monache S.D., Di Gaetano A., Finetti N., Francavilla F., Santucci R., Tettamanti E., Colonna R.A. Preliminary study of oscillating electromagnetic field effects on human spermatozoon motility. Bioelectromagnetics. 2007;28:72–75. doi: 10.1002/bem.20278. [DOI] [PubMed] [Google Scholar]
- Iorio R., Delle Monache S., Bennato F., Di Bartolomeo C., Scrimaglio R., Cinque B., Colonna R.C. Involvement of mitochondrial activity in mediating ELF‐EMF stimulatory effect on human sperm motility. Bioelectromagnetics. 2011;32:15–27. doi: 10.1002/bem.20602. [DOI] [PubMed] [Google Scholar]
- IT’IS LF tissue database. 〈www.itis.ethz.ch/virtual-population/tissueproperties/database/〉). (26/04/2017).
- Kamiya H., Sasaki S., Ikeuchi T., Umemoto Y., Tatsura H., Hayashi Y., Kohri K. Effect of simulated microgravity on testosterone and sperm motility in mice. J. Androl. 2003;24:885–890. doi: 10.1002/j.1939-4640.2003.tb03140.x. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Song J.E., Kim S.R., Oh H., Gimm Y.M., Yoo D.S., Lee Y.S. Teratological studies of prenatal exposure of mice to a 20 kHz sawtooth magnetic field. Bioelectromagnetics. 2004;25:114–117. doi: 10.1002/bem.10164. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y., Nitta H., Kabuto M. Evaluation of residential exposure to intermediate frequency magnetic fields. Arch. Environ. Health. 2004;59:693–699. doi: 10.1080/00039890409602955. [DOI] [PubMed] [Google Scholar]
- Kuster N., Torres V.B., Nikoloski N., Frauscher M., Kainz W. Methodology of detailed dosimetry and treatment of uncertainty and variations for in vivo studies. Bioelectromagnetics. 2006;27:378–391. doi: 10.1002/bem.20219. [DOI] [PubMed] [Google Scholar]
- Li D.K., Yan B., Li Z., Gao E., Miao M., Gong D., Yuan W. Exposure to magnetic fields and the risk of poor sperm quality. Reprod. Toxicol. 2010;29:86–92. doi: 10.1016/j.reprotox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Litvak E., Foster K.R., Repacholi M.H. Health and safety implications of exposure to electromagnetic fields in the frequency range 300 Hz to 10 MHz. Bioelectromagnetics. 2002;23:68–82. doi: 10.1002/bem.99. [DOI] [PubMed] [Google Scholar]
- Montoto L.G., Magaña C., Tourmente M., Martín-Coello J., Crespo C., Luque-Larena J.J., Roldan E.R. Sperm competition, sperm numbers and sperm quality in muroid rodents. PLoS One. 2011;6:e18173. doi: 10.1371/journal.pone.0018173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolettos N., Kupker W., Demirel C., Schopper B., Blasig C., Sturm R., Al-Hasani S. Fertilization potential of spermatozoa with abnormal morphology. Hum. Reprod. 1999;14:47–70. doi: 10.1093/humrep/14.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- Nishimura I., Oshima A., Shibuya K., Mitani T., Negishi T. Absence of reproductive and developmental toxicity in rats following exposure to a 20-kHz or 60-kHz magnetic field. Regul. Toxicol. Pharmacol. 2012;64:394–401. doi: 10.1016/j.yrtph.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Okano T., Ishiniwa H., Onuma M., Shindo J., Yokohata Y., Tamaoki M. Effects of environmental radiation on testes and spermatogenesis in wild large Japanese field mice (Apodemus speciosus) from Fukushima. Sci. Rep. 2016;6:23601. doi: 10.1038/srep23601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan S., Tripathi D.N., Vikram A., Ramarao P., Jena G.B. Cytotoxic and genotoxic effects of methotrexate in germ cells of male Swiss mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008;655:59–67. doi: 10.1016/j.mrgentox.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Roivainen P., Eskelinen T., Jokela K., Juutilainen J. Occupational exposure to intermediate frequency and extremely low frequency magnetic fields among personnel working near electronic article surveillance systems. Bioelectromagnetics. 2014;35:245–250. doi: 10.1002/bem.21850. [DOI] [PubMed] [Google Scholar]
- Turner R.M. Moving to the beat: a review of mammalian sperm motility regulation. Reprod. Fertil. Dev. 2006;18:25–38. doi: 10.1071/rd05120. [DOI] [PubMed] [Google Scholar]
- Vásquez J.H., Gonzáles J.M., Pino J.L. Decrease in spermatic parameters of mice treated with hydroalcoholic. Rev. Peru. Biol. 2012;19:089–093. [Google Scholar]
- Wyrobek A.J., Bruce W.R. Chemical induction of sperm abnormalities in mice. Proc. Natl. Acad. Sci. USA. 1975;72:4425–4429. doi: 10.1073/pnas.72.11.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material