Abstract
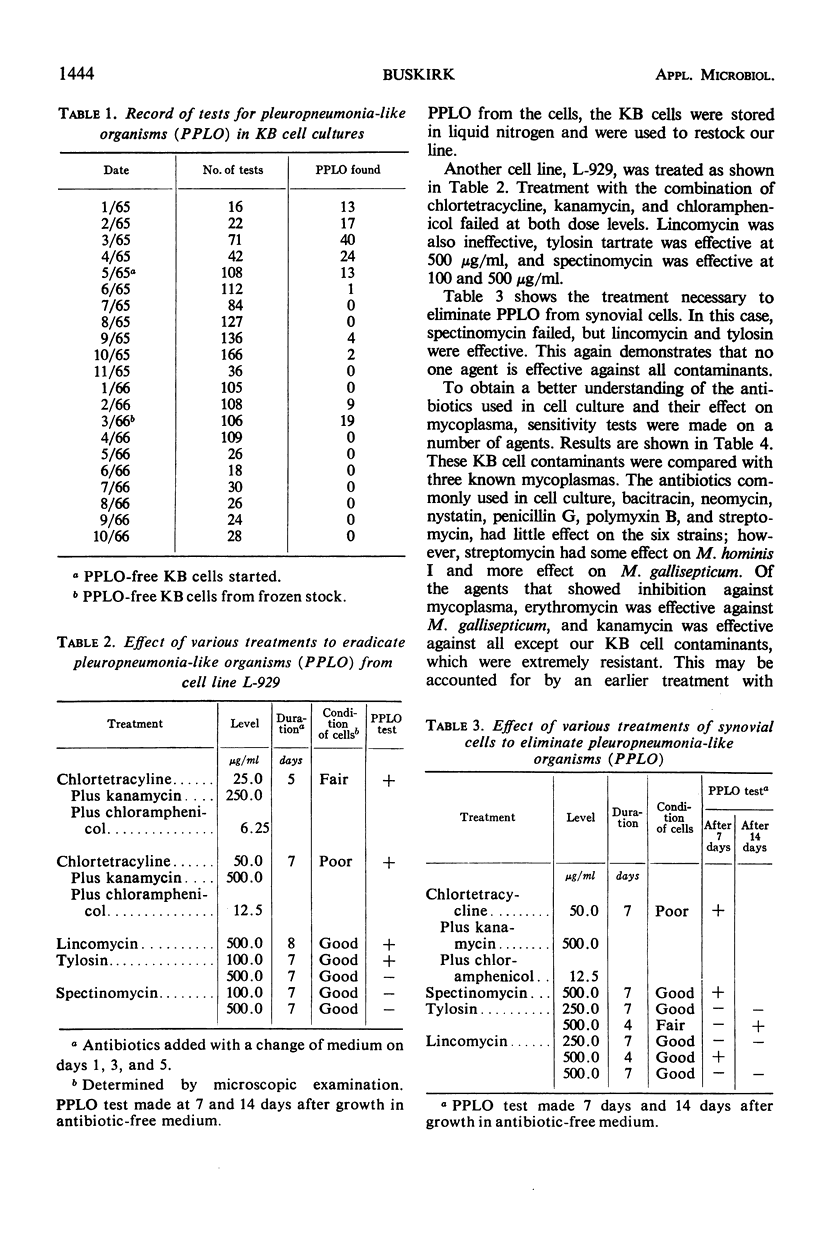
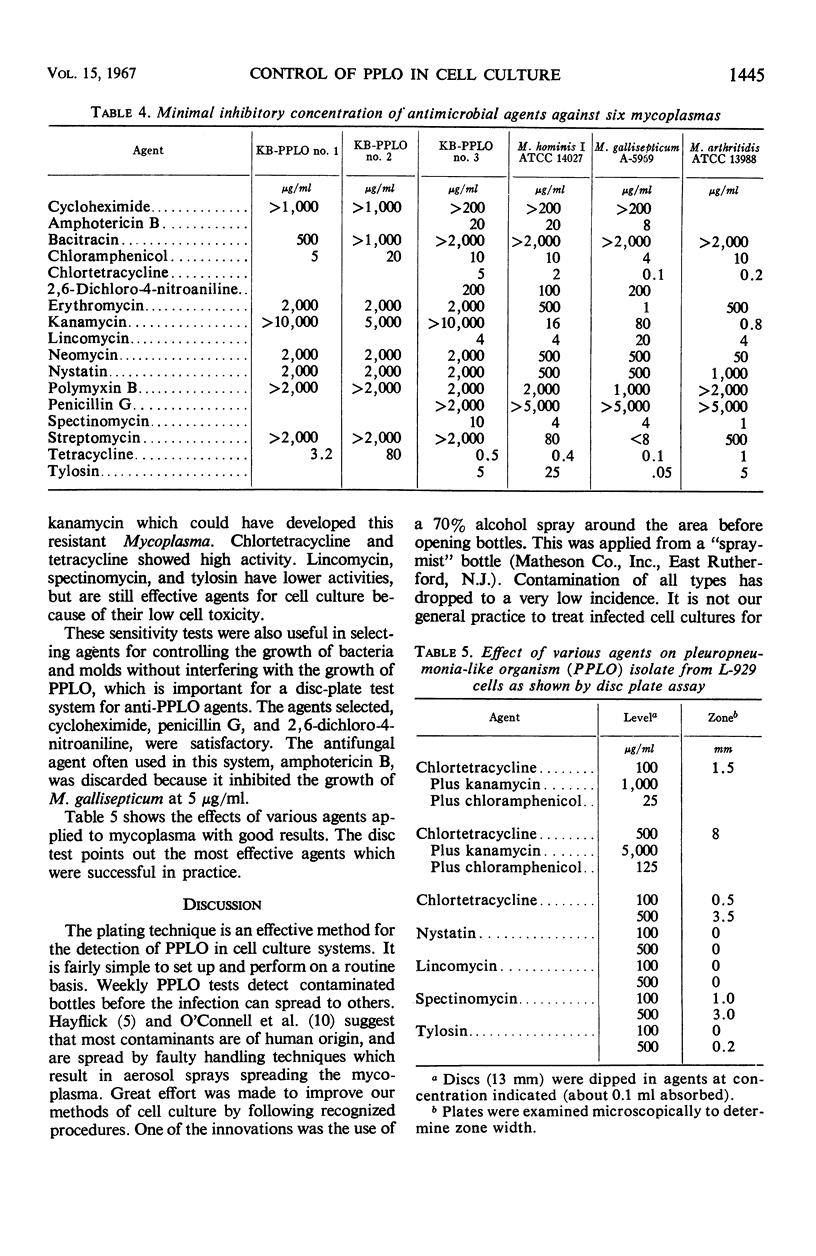
Mammalian cell culture systems were maintained free of mycoplasmas by using a 3-day agar plate test as a weekly routine to monitor the conditions of the cells. If contaminated cell cultures were found, they were discarded and replaced from a pleuropneumonia-like organism (PPLO)-free cell bank. PPLO-free lines were established by treatment with various antibiotics. The KB cell line was freed of mycoplasmas by treatment for 1 week with a mixture of chlortetracycline, kanamycin, and chloramphenicol. L-929 cells were cleared of contamination with either spectinomycin or tylosin, and a synovial cell line was cleared with lincomycin or tylosin. Each cell line, after eradication of the contaminant, was stored in liquid nitrogen. A number of agents were tested to determine minimal inhibitory concentration against three known and three unidentified mycoplasmas. Chlortetracycline and tetracycline were found to be highly active against all strains, whereas tylosin, spectinomycin, and lincomycin, though less active, were equally useful because of their low toxicity against cells. Kanamycin was highly active against three strains, but inactive at high levels against the KB cell contaminants. A disc plate test was used to check isolated cell contaminants for sensitivity to various agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARILE M. F., YAGUCHI R., EVELAND W. C. A simplified medium for the cultivation of pleuropneumonia-like organisms and the L-forms of bacteria. Am J Clin Pathol. 1958 Aug;30(2):171–176. doi: 10.1093/ajcp/30.2_ts.171. [DOI] [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- EDWARDS G. A., FOGH J. Fine structure of pleuropneumonia-like organisms in pure culture and in infected tissue culture cells. J Bacteriol. 1960 Feb;79:267–276. doi: 10.1128/jb.79.2.267-276.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORI G. B., LEE D. Y. A METHOD FOR ERADICATION OF MYCOPLASMA INFECTIONS IN CELL CULTURES. Proc Soc Exp Biol Med. 1964 Dec;117:918–921. doi: 10.3181/00379727-117-29735. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., CHANOCK R. M. MYCOPLASMA SPECIES OF MAN. Bacteriol Rev. 1965 Jun;29:185–221. doi: 10.1128/br.29.2.185-221.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L., STINEBRING W. R. Intracellular growth of pleuropneumonialike organisms (PPLO) in tissue culture and in ovo. Ann N Y Acad Sci. 1960 Jan 15;79:433–449. doi: 10.1111/j.1749-6632.1960.tb42709.x. [DOI] [PubMed] [Google Scholar]
- MASON D. J., DIETZ A., SMITH R. M. Actinospectacin, a new antibiotic. I. Discovery and biological properties. Antibiot Chemother (Northfield) 1961 Feb;11:118–122. [PubMed] [Google Scholar]
- O'CONNELL R. C., WITTLER R. G., FABER J. E. AEROSOLS AS A SOURCE OF WIDESPREAD MYCOPLASMA CONTAMINATION OF TISSUE CULTURES. Appl Microbiol. 1964 Jul;12:337–342. doi: 10.1128/am.12.4.337-342.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. E., TREADWELL P. E., KENNY G. E. MAMMALIAN CELL CULTURES CONTAMINATED WITH PLEUROPNEUMONIA-LIKE ORGANISMS. II. EFFECT OF PPLO ON CELL MORPHOLOGY IN ESTABLISHED MONOLAYER CULTURES. Exp Cell Res. 1963 Aug;31:321–328. doi: 10.1016/0014-4827(63)90009-0. [DOI] [PubMed] [Google Scholar]
- ROBINSON L. B., WICHELHAUSEN R. H., BROWN T. M. Sensitivity studies on human pleuropneumonia-like organisms. J Lab Clin Med. 1952 Feb;39(2):290–302. [PubMed] [Google Scholar]
- ROBINSON L. B., WICHELHAUSEN R. H. Contamination of human cell cultures by pleuropneumonialike organisms. Science. 1956 Dec 7;124(3232):1147–1148. doi: 10.1126/science.124.3232.1147. [DOI] [PubMed] [Google Scholar]


