Abstract
The iodothyronine deiodinases are selenoenzymes that regulate the activity of thyroid hormone via specific inner- or outer-ring deiodination. In humans, type 1 deiodinase (D1) is highly expressed in the liver, but the mechanism by which its gene expression is regulated remains to be elucidated. Liver X receptor α (LXRα), a transcription factor of the nuclear receptor superfamily, is highly expressed in the liver, where it functions as a sensor for excess intracellular oxysterols. LXRα interacts with other nuclear receptors on promoters of genes that contain a binding core sequence for nuclear receptors. In addition, it is reported that the promoter of the gene encoding human D1 (hDIO1) contains the core sequence for one of nuclear receptors, thyroid hormone receptor (TR). We investigated the involvement of LXRα in the regulation of hDIO1, in the liver. We performed hDIO1 promoter–reporter assays using a synthetic LXR agonist, T0901317, and compared promoter activity between a human liver carcinoma cell line, HepG2, and a clone of human embryonic kidney cells, TSA201. We defined the region between nucleotides −131 and −114, especially nucleotides −126 and −125, of the hDIO1 promoter as critical for basal and LXRα-mediated specific transcriptional activation in HepG2 cells. An increase in hDIO1 expression was observed in LXRα-stimulated cells, but absent in cycloheximide-treated cells, indicating that new protein synthesis is required for LXRα-mediated regulation of hDIO1. On the other hand, electrophoretic mobility shift assays revealed that LXRα and RXRα bound to the hDIO1 promoter. We also demonstrated that LXRα and TRβ compete with each other on this specific region of the promoter. In conclusion, our results indicated that LXRα plays a specific and important role in activation of TH by regulating D1, and that LXRα binds to and regulates the hDIO1 promoter, competing with TRβ on specific sequences within the promoter.
Introduction
Iodothyronine deiodinases type 1, 2, and 3 (D1, D2, and D3, respectively) are selenoenzymes that regulate the activity of thyroid hormone (TH) via specific inner- or outer-ring deiodination [1]. Biologically active intracellular triiodothyronine (T3) is supplied from the serum and via activation of thyroxine (T4) by D1 and D2 [1]. In human, D1 is expressed at high levels in the liver, kidney, and thyroid, whereas D2 is expressed in the brain, pituitary, and brown adipose tissue. The local T3 concentration in hepatic cells depends on serum TH concentration, T3 generated from T4 by D1, and clearance of T3 from hepatic cells. The expression and activity of D1 are modulated by multiple factors including T3, cAMP, retinoic acid, and TSH [2–4]. In a previous report, we demonstrated that expression of the human D1 gene (hDIO1) is liver-specifically and cooperatively regulated by forkhead box A1 (FOXA1), FOXA2, and upstream stimulatory factor (USF) via specific regions of the hDIO1 promoter [5]. However, much remains unknown regarding the mechanisms of regulation of hDIO1 and its physiological role in humans.
Liver X receptors (LXRs) are ligand-activated transcription factors of the nuclear receptor superfamily that bind oxidized cholesterol and function as sensors for excess intracellular oxysterols [6]. LXRs exist in two isoforms, LXRα and LXRβ. LXRβ is expressed ubiquitously, whereas LXRα is highly expressed in the liver, and at lower levels in adipose tissue, adrenal glands, intestines, lungs, kidneys, and cells of myeloid origin [7]. LXRs form heterodimers with the retinoid X receptor (RXR) and bind to the LXR-responsive element (LXRE) in promoters of target genes that contain direct repeats (DRs) of the core sequence AGGTCA separated by four nucleotides (DR4) [8].
LXRs bind to and compete with another subgroup of nuclear receptor, the thyroid hormone receptors (TRs), at the same sites in the 5´-region of promoters, e.g., those of the genes encoding acetyl-CoA carboxylase-α, cholesterol 7-alpha-hydroxylase, and ATP-binding cassette transporter A1 [9–11]. Toyoda et al. previously reported that the hDIO1 promoter contains two functional TH response elements (TREs), each of which also consists of two DRs with the core sequence, AGGTCA [3]. Although LXRs and TRs belong to two distinct receptor subgroups with respect to ligand-binding affinity [12], the two receptor systems have similar molecular mechanisms, i.e., both form heterodimers with RXRs and to bind to DR4 with identical geometry and polarity [13,14]. To date, it remains unknown whether LXRs regulate the expression of hDIO1. Because LXRα is expressed at very high levels in the liver, and the hDIO1 promoter contains TREs, we hypothesized that LXRα is involved in regulation of hDIO1 in a liver-specific manner by unknown mechanisms.
Thus, in this study, we investigated the involvement of LXRα in the regulation of hDIO1, in the liver using a human liver carcinoma cell line HepG2.
Materials and methods
Ligand
A synthetic LXR agonist, T0901317 (TO), was purchased from Cayman Chemical (Ann Arbor, MN, USA), and a synthetic LXR antagonist, GSK2033 (GSK), was purchased from Axon Medchem BV (Groningen, The Netherlands). TO and GSK were dissolved in and diluted with dimethyl sulfoxide at various concentrations.
Cell culture
A human liver carcinoma cell line, HepG2 [5], and a clone of human embryonic kidney 293 cells, TSA201 [15], were cultured as described previously.
Plasmid construction
Deletion mutants of the 5´-flanking region of hDIO1 (−2023, −803, −187, −131, −113, −103/−4; the transcription start site was defined as +1, nucleotide 53894211, NC_000001.11) were prepared and subcloned into pGL 4.10 (Promega, Madison, WI, USA) to create a fusion with the luciferase gene (−2023, −803, −187, −131, −113, −103/−4 hDIO1-Luc) as described previously [5]. Mutations were created in the −131/−4 hDIO1-Luc construct at nucleotides −124, −119/−118, −108/−106, −126/−125, or both −126/−125 and −108/−106 of the hDIO1 promoter using −131/−4 hDIO1-Luc construct as a template and the QuikChange® Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA). Sequences of the 5´-flanking region of mutated constructs are provided in Table 1. These constructs were verified by sequencing. Each plasmid expressing cDNA for human TRβ, human RXRα, human LXRα, and human farnesoid X receptor (FXR) was subcloned into pCMX as described previously [16]. A plasmid expressing cDNA for human pregnane X receptor (pFN21A-hPXR) was generated by Kazusa DNA Research Institute (Chiba, Japan) and purchased from Promega. We also prepared a plasmid expressing cDNA for human PAR-related orphan receptor alpha (RORα). The open reading frame of RORα was generated by PCR using cDNA from HepG2 cells as a template. We used primers containing SgfI or PmeI linker: 5´-GACCGCGATCGCCATGGAGTCAGCTCCGGCAGCCC-3´ and 5´-ATTAGTTTAAACCCCATCAATTTGCATTGCATTGCTGGTCA-3´. The PCR product was digested with SgfI and PmeI and cloned into SgfI/PmeI-digested pFN21A CMV Flexi vector (Promega). The plasmid was verified by sequencing and synthesis of RORα was confirmed by western blot.
Table 1. Wild-type and mutated sequence of constructs used in mutational analysis.
| Mutated construct and control | Sequence of 5´-flanking region of the construct between the nucleotides −131 and −104 |
|---|---|
| −126/−125, −108/−106 Mut −131/−4 hDIO1-Luc | 5´-TCTGAAATGACTCCTTCCCCTGAAAAGG |
| −126/−125 Mut −131/−4 hDIO1-Luc | 5´-TCTGAAATGACTCCTTCCCCTGACCCGG |
| −124 Mut −131/−4 hDIO1-Luc | 5´-TCTGACCCGACTCCTTCCCCTGACCCGG |
| −119/−118 Mut −131/−4 hDIO1-Luc | 5´-TCTGACCTGACTTGTTCCCCTGACCCGG |
| −108/−106 Mut −131/−4 hDIO1-Luc | 5´-TCTGAAATGACTCCTTCCCCTGAAAAGG |
| −131/−4 hDIO1-Luc | 5´-TCTGACCTGACTCCTTCCCCTGACCCGG |
The mutated base pairs between nucleotides −131 and −104 of the hDIO1 promoter are indicated by bold italic letters with underlines.
Transient transfection and luciferase assay
Transient transfections were performed in 24-well tissue culture plates using the LipofectamineTM LTX regent (Thermo Fisher Scientific, Waltham, MA, USA) for HepG2 cells, and the LipofectamineTM 2000 reagent (Thermo Fisher Scientific) for TSA201 cells, as described previously [5]. Briefly, cells were seeded in 24-well plates 1 day before transfection and maintained in 0.5 ml of antibiotic-free medium supplemented with 10% charcoal-stripped bovine serum (Thermo Fisher Scientific). For transfection of HepG2 cells, we used 500 ng of experimental reporter constructs, 50 ng of each expression vector, and 25 ng of pGL 4.74, which contains the cDNA encoding Renilla luciferase (Promega) as an internal control for transfection efficacy. For transfection of TSA201 cells, we used 100 ng of experimental reporter constructs, 10 ng of each expression vector, and 5 ng of pGL 4.74. In both cell types, culture media were replaced with antibiotic-free medium supplemented with 10% charcoal-stripped bovine serum with vehicle or 10−7 M TO 6–8 h after transfection. The cells were then cultured for an additional 48 h and harvested. Luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega), and luminescence was measured by a 2030 ARVOX multilabel reader (PerkinElmer, Waltham, MA, USA). Firefly luciferase activity was normalized against Renilla luciferase activity in each well to control for transfection efficacy.
RNA isolation, reverse transcription, and quantitative PCR
HepG2 cells were cultured in antibiotic-free medium supplemented with 10% charcoal-stripped bovine serum (Thermo Fisher Scientific) and treated with TO at various concentrations and 10−6 M GSK, or pre-treated with cycloheximide for 30 min before treatment with 10−7 M TO for 24 h. Then, total RNA was extracted from HepG2 cells using the RNeasy® Plus Mini Kit (QIAGEN, Valencia, CA, USA). One microgram of total RNA was reverse-transcribed with random hexamers using First-strand cDNA Synthesis Kit (GE Healthcare UK Ltd. Buckinghamshire, UK). The resultant cDNA was diluted 1:10 by addition of TE buffer.
Quantitative PCR was performed, recorded, and analyzed using TaqMan® Gene Expression Assays with the StepOnePlus™ Real-time PCR System (Thermo Fisher Scientific). The probe/primer sets were Hs00174944_m1 (hDIO1), Hs00230861_m1 (THRB), and Human PPIA (Cyclophilin A) Endogenous Control, purchased from Thermo Fisher Scientific. Diluted cDNA was amplified as described previously [5]. Expression levels of each gene were normalized against the corresponding mRNA levels of cyclophilin A to compensate for variations in input RNA.
Preparation of electrophoresis mobility shift assay (EMSA)
Nuclear extracts were prepared from HepG2 cells and TSA201 cells using the Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA). HepG2 cells were treated with vehicle or TO, with or without co-transfection of expression vectors for LXRα and RXRα (LXRα/ RXRα). One microgram of each expression vector was transfected into HepG2 cells in 60-mm dishes using the LipofectamineTM LTX regent (Thermo Fisher Scientific). Vehicle or TO was added to the culture media 24 h after transfection. The cells were then cultured for an additional 24 h and harvested. EMSAs and supershift assays were conducted using the LightShiftTM Chemiluminescent EMSA kit (Thermo Fisher Scientific) as directed by the manufacturer with slight modifications, as described previously [5]. The antibodies used in the supershift assays were as follows: anti–human LXRα mouse monoclonal antibody (PP-PPZ0412-00, Perseus Proteomics, Tokyo, Japan), anti–human RXRα mouse monoclonal antibody (PP-K8508-00, Perseus Proteomics), anti–human TRβ1 mouse monoclonal antibody (sc-738X, Santa Cruz Biotechnology, Dallas, TX, USA), and normal mouse IgG (sc-2025, Santa Cruz Biotechnology). The sequences of oligonucleotides used for EMSA are provided in Table 2.
Table 2. Sequences of double-stranded oligonucleotides used in EMSA.
| Oligonucleotides | Sequences |
|---|---|
| Wt1 | 5´-GCAAACATCTTCTGACCTGACTCCTTCCCC-3´ |
| −124 mut1 | 5´-GCAAACATCTTCTGACCCGACTCCTTCCCC-3´ |
| −126/−125 mut1 | 5´-GCAAACATCTTCTGAAATGACTCCTTCCCC-3´ |
| Wt2 | 5´-TCTGACCTGACTCCTTCCCCTGACCCGG-3´ |
| −126/−125 mut2 | 5´-TCTGAAATGACTCCTTCCCCTGACCCGG-3´ |
| LXRE | 5´-GATCTTAGTTCACTCAAGTTCAAGGATC-3´ |
Wt1, oligonucleotide containing the wild-type sequence of the region between nucleotides −141 and −112 of the hDIO1 promoter; −124 mut1, oligonucleotide containing a mutation at nucleotide −124 of the Wt1 oligonucleotide; −126/−125 mut1, oligonucleotide containing mutations at nucleotides −126 and −125 of the Wt1 oligonucleotide; Wt2, oligonucleotide containing the sequence of the region between nucleotides −131 and −104 of the hDIO1 promoter [3]; −126/−125 mut2, oligonucleotide containing mutations at nucleotides −126 and −125 of the Wt2 oligonucleotide; LXRE, oligonucleotide containing the consensus sequence of the LXR response element [17]. The oligonucleotide sequence between nucleotides −131 and −114 of the hDIO1 promoter is indicated by bold letters, and mutated base pairs are indicated by bold italic letters with underlines.
Transfection of short interfering RNA (siRNA)
An aliquot of 2.5 pmol siRNA specific for THRB (s14121, Silencer Select® siRNA, Thermo Fisher Scientific) or a negative control siRNA (Negative Control #2, Silencer Select® siRNA, Thermo Fisher Scientific) was introduced into HepG2 cells using 24-well plates and the Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific) by reverse transfection. Transfections of siRNA were performed 24 h before transfections of expression vectors for the luciferase assay. To determine the knockdown efficacy of siRNA, mRNA was extracted 72 h after siRNA transfection and analyzed by quantitative PCR as described above. Changes in levels of TRβ protein (coded by THRB) following siRNA transfections were verified by western blot. See S1 File for detailed information.
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM) obtained from at least three separate experiments. Significance of differences was evaluated using analysis of variance (ANOVA) followed by the Tukey–Kramer method, unless otherwise specified. P values < 0.05 were considered to be statistically significant.
Results
Identification of the specific region of the hDIO1 promoter required for its basal activity and LXRα-mediated in HepG2 cells
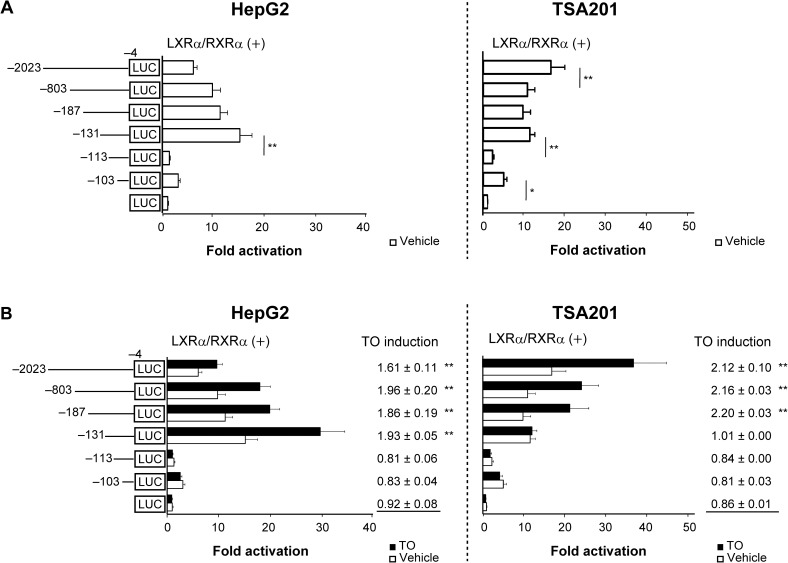
To identify the specific region of the hDIO1 promoter important for its activation by the synthetic LXR agonist, TO, we transiently transfected a series of 5´-deletion constructs into HepG2 and TSA201 cells, along with or without expression vectors for LXRα and RXRα (LXRα/RXRα). The cells were then cultured with or without TO (Fig 1 and S2 File). In both cell lines, basal luciferase activity was significantly decreased by deletion of nucleotides −131 to −114 (P < 0.01) (Fig 1A and Fig A in S2 File). Luciferase activity following addition of TO was significantly increased (1.48-fold) without expression vectors for LXRα/RXRα (Fig B in S2 File") and further increased (1.93-fold) with those vectors in HepG2 cells transfected with the −131/−4 hDIO1-Luc construct (P < 0.01) (Fig 1B). These increases in response to TO were abolished by deletion of nucleotides −131 to −114. On the other hand, in TSA201 cells transfected with the −131/−4 hDIO1-Luc construct, no significant increase in luciferase activity was observed in response to TO (Fig 1B). These results indicated the importance of the specific region between nucleotides −131 and −114 for both basal activity and LXRα-mediated activation of the hDIO1 promoter in HepG2 cells.
Fig 1. HepG2-specific regulation of the hDIO1 promoter by T0901317 (TO).
A series of 5´-deletion constructs of the hDIO1 promoter were transiently transfected into HepG2 and TSA201 cells along with expression vectors for human LXRα and human RXRα (LXRα/RXRα) with and without 10−7 M TO. Promoter activity was normalized against Renilla luciferase activity, and the normalized value is expressed relative to that of promoterless pGL 4.10 in the absence of TO. Results are expressed as means ± SEM. *, P < 0.05; **, P < 0.01. A. Basal luciferase activity of each construct. Statistical analysis was performed on pairwise comparisons of constructs, and significant pairs are presented. B. Luciferase activities of each construct with and without 10−7 M TO. TO induction indicates ratio of promoter activity with TO to the activity without TO. Statistical analysis was performed to compare TO induction of each construct with that of promoterless pGL 4.10, and significant differences are presented.
Regulation of hDIO1 expression by LXRα
To confirm the change in hDIO1 mRNA levels, we performed quantitative PCR analysis. The hDIO1 mRNA level was increased by TO in a dose-dependent manner (Fig 2A). Furthermore, the increase in hDIO1 mRNA following addition of TO was diminished by co-treatment with a LXR antagonist GSK (Fig 2B).
Fig 2. Analysis of relative hDIO1 mRNA levels in HepG2 cells.
hDIO1 mRNA levels were normalized against the corresponding levels of cyclophilin A mRNA, and the value in HepG2/vehicle cells was defined as 1. Values are expressed as means ± SEM. *, P < 0.05; **, P < 0.01; N.S., not significant. A. HepG2 cells were treated at different concentrations of TO for 24 h. B. HepG2 cells were treated with vehicle, 10−6 M TO, or 10−6 M TO and 10−6 M GSK2033 (GSK) for 24 h. C. HepG2 cells were exposed to cycloheximide for 30 min before being treated with TO (10−7 M) for 24 h.
TO is a ligand for FXR, PXR, and RORα as well as LXRα [18,19]. To verify that the effect of TO on the hDIO1 promoter depends on LXRα, we performed luciferase assays using expression vectors for these receptors in HepG2 cells (S3 File). Exclusively in cells co-transfected with expression vectors for LXRα/RXRα, luciferase activity in response to TO was significantly higher than in cells transfected with empty vectors. Overexpression of FXR and RORα did not alter luciferase activity, and PXR instead decreased, in comparison with the results of each empty vector. These results indicated that LXRα specifically activates the hDIO1 promoter.
To determine whether LXRα needs de novo protein synthesis to stimulate hDIO1 transcription, we performed the TO stimulation experiment using HepG2 cells pre-treated with cycloheximide. In this case, the mRNA level of hDIO1 was not increased by TO (Fig 2C).
Investigation of the key nucleotides within the region between nucleotides −131 and −114 of the hDIO1 promoter
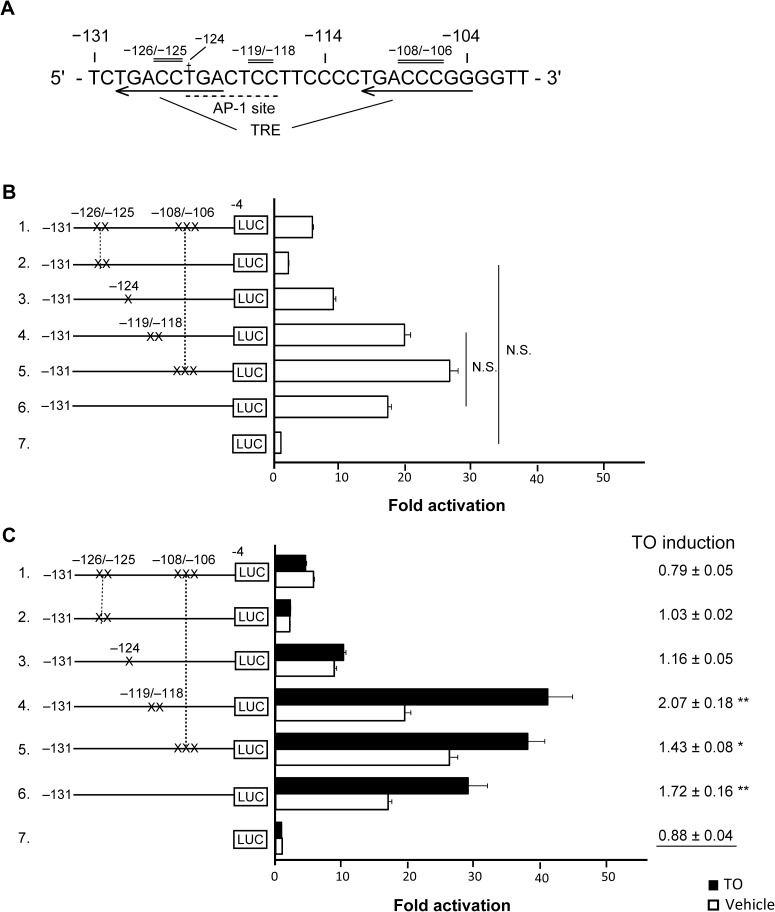
To narrow down the specific site required for the basal activity and LXRα-mediated activation of the hDIO1 promoter, we performed luciferase assays using HepG2 cells transfected with wild-type or mutant hDIO1 promoter–reporter constructs. From the previous reports of Toyoda et al. [3] and a computational analysis [20], the region between nucleotides −131 and −114 of the hDIO1 promoter contains a half-site of TRE, whose binding site consists of two octamer half-site motifs (YYRGGTCA) separated by 10 bp [3], as well as an activator protein 1 (AP-1) site, whose consensus sequence contains TGA(C/G) TCA [21–23], as shown in Fig 3A. Thus, mutations were introduced to disrupt the sequences constituting the TRE [3] and putative AP-1 site (Fig 3A). Basal luciferase activity was significantly reduced when nucleotides −126/−125 or nucleotide −124 was mutated, in comparison to the construct lacking any mutations (Fig 3B); in the former case, the reduction was larger, reaching the baseline level (i.e., the level in cells transfected with promoterless pGL 4.10). On the other hand, basal luciferase activity increased when nucleotides −108/−106 were mutated, but did not change when nucleotides −119/−118 were mutated (Fig 3B). The increase in luciferase activity of the hDIO1 promoter by TO was abolished when nucleotides −126/−125 or a nucleotide −124 was mutated, but not when nucleotides −108/−106 or −119/−118 were mutated (Fig 3C).
Fig 3. Mutational analysis of activation of the hDIO1 promoter by T0901317 (TO).
A. The nucleotide sequence of the 5´-flanking region of the hDIO1 promoter used in the analysis, along with the positional relationship among mutated oligonucleotides, thyroid hormone response element (TRE), and the putative activator protein 1 (AP-1) site. † and black horizontal bars represent the site-specific mutation. B and C. A series of mutated hDIO1 promoter constructs was transiently transfected into HepG2 cells along with expression vectors for LXRα/RXRα, with or without 10−7 M TO. Schematic diagram to the left of the figure representing mutant (No. 1–5) and wild-type (No. 6) hDIO1 promoters, which were introduced upstream of the luciferase gene. No. 7 represents the promoterless pGL 4.10 construct. Promoter activity was normalized against Renilla luciferase activity, and is expressed relative to that of promoterless pGL 4.10 in the absence of TO. Values are expressed as means ± SEM. *, P < 0.05; **, P < 0.01; N.S., not significant. B. Basal luciferase activity of each construct. Statistical analysis was performed on comparisons between all constructs. Because most pairs exhibited significance, only non-significant pairs are presented. C. Luciferase activities of each construct with and without 10−7 M TO. TO induction indicates ratios of promoter activity with TO to the activity without TO. Statistical analysis was performed on comparisons of TO induction of each construct with that of promoterless pGL 4.10, and significant differences are presented.
Identification of nuclear proteins that bind to nucleotides −131 to −114 of the hDIO1 promoter in HepG2 and TSA201 cells
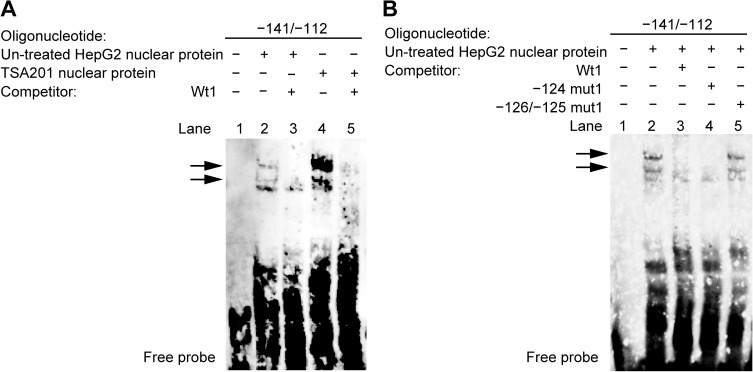
To confirm the interaction between DNA and proteins within nucleotides −131 to −114 of the hDIO1 promoter, we performed EMSA using oligonucleotides containing the wild-type sequence of the region between nucleotides −141 and −112 of the hDIO1 promoter (Wt1) (Fig 4A). Incubation of nuclear proteins from HepG2 cells or TSA201 cells with biotin-labeled Wt1 oligonucleotides led to the formation of several DNA/protein complexes, as shown in lanes 2 and 4, respectively. In both cell lines, specific formation of these complexes was inhibited by incubation with excess unlabeled Wt1 oligonucleotides (lanes 3 and 5).
Fig 4. Specific binding of transcription factors to the region between nucleotides −131 and −114 of the hDIO1 promoter.
A. EMSA with oligonucleotide containing the wild-type sequence of the region between nucleotides −141 and −112 of the hDIO1 promoter (Wt1) and nuclear extracts from un-treated HepG2 cells and TSA201 cells. In lane 1, as a control, only the biotin-labeled Wt1 oligonucleotide was present. Biotin-labeled Wt1 oligonucleotide was incubated with nuclear extracts from HepG2 or TSA201 cells without competitors in lanes 2 and 4, respectively, and with 25-fold molar excesses of unlabeled Wt1 oligonucleotides in lanes 3 and 5, respectively. The specific DNA/protein complexes formed are indicated by arrows. B. EMSA with mutant oligonucleotides and nuclear extracts from HepG2 cells. Biotin-labeled Wt1 oligonucleotide was incubated with nuclear extracts from HepG2 cells without competitors in lane 2, and with 25-fold molar excesses of unlabeled Wt1, −124mut1, and −126/−125mut1 oligonucleotides in lanes 3, 4, and 5, respectively. The specific DNA/protein complexes formed are indicated by arrows.
Furthermore, we performed EMSA using nuclear extracts from HepG2 cells and mutated unlabeled nucleotides (−124 mut1 or −126/−125 mut1) (Fig 4B). The same DNA/protein complexes observed in previous experiments (lane 2) were abolished when the nuclear proteins were incubated with excess unlabeled Wt1 oligonucleotides (lane 3), but not with excess unlabeled −126/−125 mut1 oligonucleotides (lane 5), supporting the idea that the specific DNA/protein complexes formed within the region between nucleotides −131 and −114 of the hDIO1 promoter. However, both DNA/protein complexes were abolished when nuclear extracts were incubated with excess unlabeled −124 mut1 oligonucleotides (lane 4).
Binding of LXRα and RXRα on the hDIO1 promoter
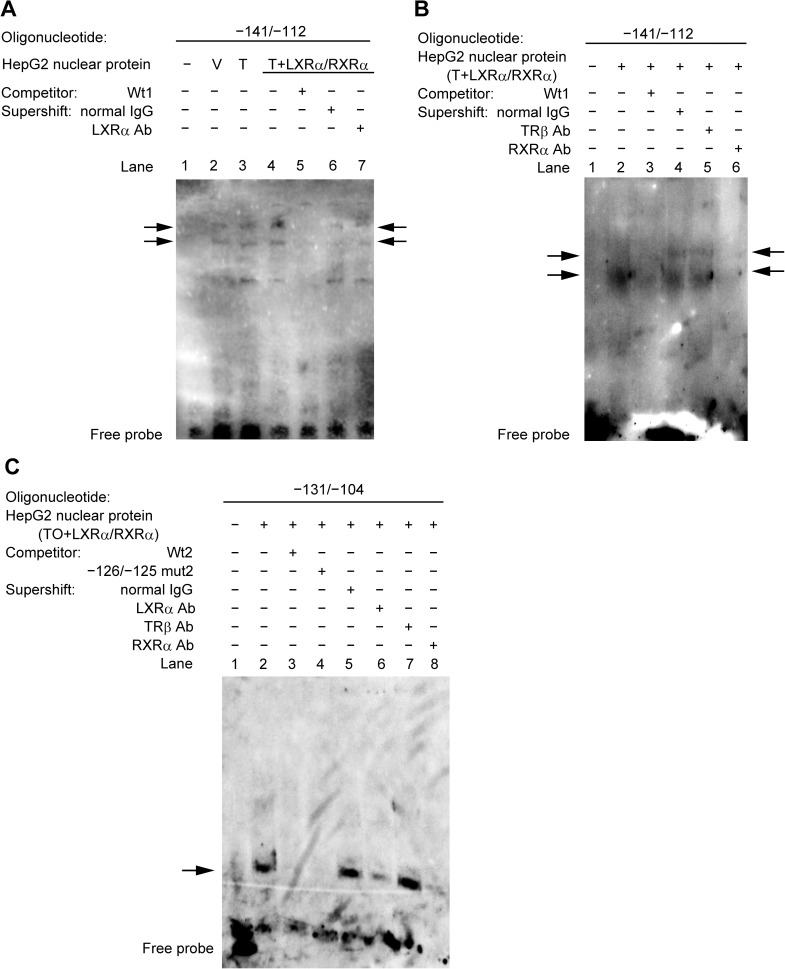
The results of EMSA using nuclear extracts from vehicle-treated, TO-treated, and TO-treated and LXRα/RXRα-overexpressing HepG2 cells were shown in Fig 5A. The DNA/protein complexes were observed at the same positions and appeared slightly stronger using nuclear extracts from TO-treated cells (lane 3) and much stronger using nuclear extracts from TO-treated and LXRα/RXRα-overexpressing cells (lane 4), in comparison with those using nuclear extracts from vehicle-treated cells (lane 2).
Fig 5. Binding of LXRα on the hDIO1 promoter.
A. EMSA with oligonucleotide containing the wild-type sequence of the region between nucleotides −141 and −112 of the hDIO1 promoter (Wt1) with nuclear extracts from vehicle-treated (V), T0901317 (TO)-treated (T), or TO-treated and LXRα/RXRα-overexpressing (T+LXRα/RXRα) HepG2 cells; also shown is a supershift assay with an antibody against LXRα. Specific DNA/protein complexes are indicated by arrows. In lane 1, as a control, only biotin-labeled Wt1 oligonucleotide was present. In lanes 2, 3, and 4, biotin-labeled Wt1 oligonucleotide was incubated with nuclear extracts from V, T, and T+LXRα/RXRα HepG2 cells without competitor, respectively. In lane 5, biotin-labeled Wt1 oligonucleotide was incubated with nuclear extracts from T+LXRα/RXRα HepG2 cells with excess unlabeled Wt1 oligonucleotides as competitor. The results of the supershift assay are shown with normal mouse IgG as a control in lane 6 and with an antibody against LXRα in lane 7. B. EMSA with Wt1 oligonucleotides with nuclear extracts from T+LXRα/RXRα HepG2 cells and a supershift assay with antibodies against TRβ and RXRα. Specific DNA/protein complexes are indicated by arrows. In lane 1, as a control, only biotin-labeled Wt1 oligonucleotide was present. Biotin-labeled Wt1 oligonucleotide was incubated with the nuclear extracts in lane 2 and with the nuclear extracts and excess unlabeled Wt1 oligonucleotides as competitor in lane 3. The results of the supershift assay are shown with normal mouse IgG as a control in lane 4, with an antibody against TRβ in lane 5, and with an antibody against RXRα in lane 6. C. EMSA was performed with oligonucleotides containing the wild-type sequence of the region between nucleotides −131 and −104 of the hDIO1 promoter (Wt2) with nuclear extracts from T+LXRα/RXRα HepG2 cells; also shown is a supershift assay with antibodies against LXRα, TRβ, and RXRα. A specific DNA/protein complex is indicated by an arrow. In lane 1, as a control, only biotin-labeled Wt2 was present. Biotin-labeled Wt2 was incubated with nuclear extracts in lane 2, with nuclear extracts and excess unlabeled Wt2 oligonucleotides as competitor in lane 3, and with excess unlabeled −126/−125 mut2 as competitor in lane 4. The results of the supershift assay are shown with normal mouse IgG as a control in lane 5, with an antibody against LXRα in lane 6, with an antibody against TRβ in lane 7, and with an antibody against RXRα in lane 8.
To identify the protein that binds this region, we performed supershift assays using antibodies against LXRα, TRβ, and RXRα with nuclear extracts from TO-treated and LXRα/RXRα-overexpressing HepG2 cells (Fig 5A and 5B). The results of validation of the antibodies used in supershift assays are shown in S4 File. The supershift of the complexes was not observed with antibodies against LXRα (Fig 5A, lane 7) or TRβ (Fig 5B, lane 5), but was observed with an antibody against RXRα (Fig 5B, lane 6). These results indicated that the DNA/protein complexes formed within nucleotides −131 to −114 of the hDIO1 promoter contained RXRα.
Considering that RXRα forms a heterodimer with other nuclear receptors on the 3´ side of RXRα binding position, such as LXRα and TRβ [24], and that nucleotides −131 to −114 contain a half-site of TRE while nucleotides −113 to −104 contain another half-site, we performed EMSA using an oligonucleotide containing the sequence of the region between nucleotides −131 and −104 (Wt2) [3] with nuclear extracts from TO-treated and LXRα/RXRα-overexpressing HepG2 cells (Fig 5C). As a result, a DNA/protein complex was formed (lane 2), but this complex abolished when the nuclear extracts were incubated with excess unlabeled Wt2 oligonucleotides (lane 3) or excess unlabeled −126/−125 mut2 oligonucleotides (lane 4). In a supershift assay, the formed complex was supershifted with antibodies against both LXRα and RXRα (lanes 6 and 8), but not TRβ (lane 7). These results indicated that LXRα, as well as RXRα, can bind to the region between nucleotides −131 and −104 of the hDIO1 promoter. In addition, unlike RXRα binding, LXRα binding also required the region between nucleotides −113 and −104.
Furthermore, to determine whether LXRα binds to the hDIO1 promoter under physiological conditions, we performed ChIP assays using an antibody against LXRα. We described materials and methods in S1 File. Using both primer sets, approximately 6-fold to 8-fold enrichment of ChIP-DNA following LXRα immunoprecipitation was observed in comparison with the negative control (S5 File), indicating that LXRα could physiologically bind to the hDIO1 promoter.
Interaction of LXRα/RXRα and TRβ on a specific region of the hDIO1 promoter
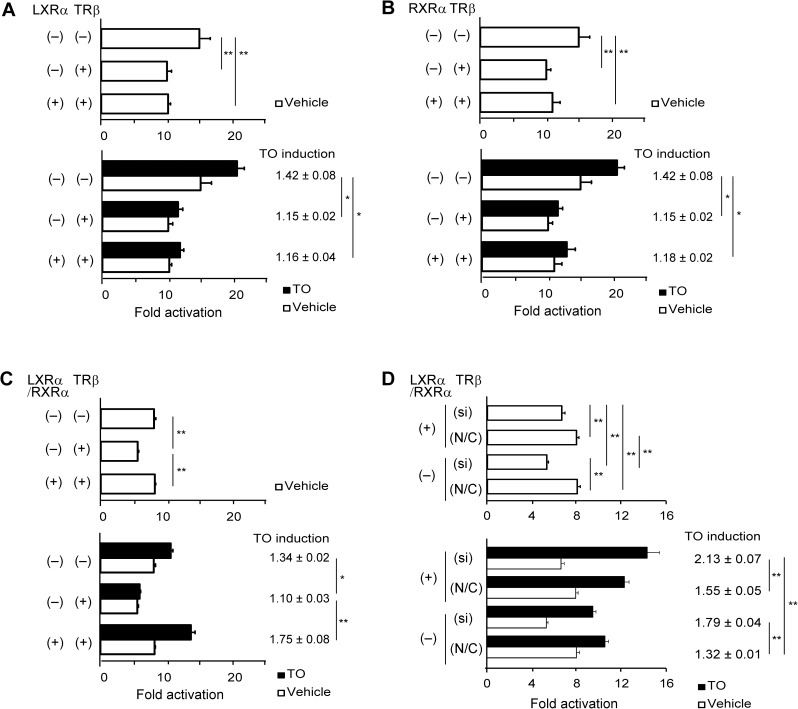
TRs bind to or compete with LXRs on the same regions in the promoters of some genes [9–11], and basal suppression of gene promoters by un-liganded TRs has been reported [25,26]. Therefore, we examined the interaction of LXRα, RXRα, and TRβ on a specific region of the hDIO1 promoter using the −131/−4 hDIO1-Luc construct (Fig 6). Basal luciferase activity, which was significantly decreased by overexpression of TRβ, was not abolished when expression vector for either LXRα or RXRα was transfected alone (Fig 6A and 6B), but was abolished when expression vectors for LXRα/RXRα were co-transfected (Fig 6C). Similarly, overexpression of TRβ decreased TO induction, except when expression vectors for LXRα/RXRα were co-transfected (Fig 6C).
Fig 6. Interaction of LXRα/RXRα and TRβ on the activity of the hDIO1 promoter.
HepG2 cells were transfected with or without expression vectors for LXRα, RXRα, or LXRα/RXRα, with or without TRβ or siRNA specific for THRB. We performed luciferase assay using a −131/−4 hDIO1-Luc construct. All upper figures show only basal luciferase activities, whereas lower figures show both basal activities and activities with or without 10−7 M TO. TO induction indicates ratios of promoter activity with TO to the activity without TO. Promoter activity was normalized against Renilla luciferase activity, and the normalized value is expressed relative to that of promoterless pGL 4.10 in the absence of TO. Results are expressed as means ± SEM. Statistical analysis was performed on comparisons among all conditions; the results of basal activity are shown in the upper figure, and those of TO induction are shown in the lower figure; *, P < 0.05; **, P < 0.01. A. Comparison with and without overexpression of LXRα or TRβ. B. Comparison with and without overexpression of RXRα or TRβ. C. Comparison with and without overexpression of LXRα/RXRα or TRβ. D. Comparison with and without overexpression of LXRα/RXRα and TRβ knockdown using siRNA. si, siRNA specific for THRB; N/C, negative control siRNA.
Next, we examined TO induction in TRβ-knockdown conditions (Fig 6D). The siRNA specific for TRβ efficiently knocked down the THRB mRNA and the encoded protein (S6 File). Basal luciferase activity was significantly increased in TRβ-knockdown cells co-transfected with expression vectors for LXRα/RXRα. Furthermore, in TRβ-knockdown cells, TO induction was increased (from 1.32-fold to 1.79-fold) in the absence of co-transfection of expression vectors for LXRα/RXRα, and further increased (from 1.55-fold to 2.13-fold) in the presence of LXRα/RXRα. These results indicated that LXRα/RXRα compete with TRβ in TO induction, as well as basal activity, in this specific region of the hDIO1 promoter.
Discussion
In this study, we investigated the involvement and mechanism of regulation of hDIO1 mediated by LXRα using the synthetic LXR agonist TO. We identified a specific region between nucleotides −131 and −114 of the hDIO1 promoter that was important for basal activity and LXRα-mediated activation of hDIO1 specifically in HepG2 cells. The results of mutational analysis showed that basal and TO-induced luciferase activities of the hDIO1 promoter were abolished when nucleotides −126/−125 or a nucleotide −124 was mutated. EMSA using nuclear extracts from HepG2 and TSA201 cells revealed that some DNA/protein complexes were formed on oligonucleotides containing the sequence of the region between nucleotides −131 and −114 of the hDIO1 promoter. Supershifts of DNA/protein complexes were observed with an antibody against RXRα using oligonucleotides containing the sequence of the region between nucleotides −131 and −114, as well as with antibodies against LXRα and RXRα using the oligonucleotides containing the sequence of the region between nucleotides −131 and −104. In addition, luciferase assays revealed that LXRα/RXRα and TRβ compete with each other on this specific region of the hDIO1 promoter when TRβ is either overexpressed or knocked down.
The specific region we identified between nucleotides −131 and −114 of the hDIO1 promoter contains a TRE, as reported by Toyoda et al. [3]. Within this region, nucleotides −126/−125 were critical for both basal and LXRα-mediated activation and protein binding on the hDIO1 promoter. This result is consistent with reports that the CC residues within TRE are the most important determinants for TRβ binding [27,28]. Luciferase assays revealed that basal and LXRα-mediated activation was also abolished when a nucleotide −124 was mutated. However, based on the results of EMSA using excess mutated unlabeled oligonucleotide, a nucleotide −124 (unlike nucleotides −126/−125) did not seem to be essential for formation of DNA/protein complexes.
We used a synthetic LXR ligand, TO, in this study. Some synthetic ligands for LXRs, such as TO and GW3965, were developed because oxysterols, the natural ligands for LXRs, have relatively low affinities for LXRs [29,30]. We selected TO as a ligand for LXRs for our in vitro study because it is a stronger activator of LXRs than GW3965 in HepG2 cells [31]. TO is a potential activator of other nuclear receptors including FXR, PXR, and RORα [18,19]. To confirm that TO specifically stimulates LXRα to regulate hDIO1, we used two strategies. First, we confirmed that the activation of luciferase activity in response to TO was not augmented by overexpression of FXR, PXR, or RORα, but was augmented by LXRα/RXRα. Second, the response to TO in hDIO1 mRNA was antagonized by a LXR antagonist GSK.
The results of EMSA and supershift assay suggested that DNA/protein complexes, including LXRα and RXRα were formed with oligonucleotides containing the sequences of the region between the nucleotides −131 and −104 of the hDIO1 promoter. The supershift with an antibody against RXRα was observed not only with an oligonucleotide containing the sequences of the region between nucleotides −141 and −112 of the hDIO1 promoter (containing the sequences of the 5´ single half-site of TRE), but also with an oligonucleotide containing the sequences of the region between nucleotides −131 and −104 (containing the sequences of complete TRE). On the other hand, the supershift with an antibody against LXRα was observed only with the oligonucleotide containing the sequences of the region between nucleotides −131 and −104. These results suggest that LXRα has effects on the region between nucleotides −111 and −104 of the hDIO1 promoter, where the 3´ half-site of TRE is located.
RXR forms heterodimers with numerous other nuclear receptors, including retinoic acid receptor, vitamin D receptor, TRs, and LXRs [8,32,33]. The consensus sequence of RXR, 5´-AGGTCA-3´ [34], is located between nucleotides −129 and −124 of the hDIO1 promoter, supporting our EMSA results showing that supershift with an antibody against RXRα was observed with oligonucleotides containing the sequences of the regions between nucleotides −131 and −114 and nucleotides −131 and −104. In addition, interactions mediated by dimerization of RXRα with other receptors occur only when RXR binds to the 5´ half-site of their binding positions [35]. The importance of the polarity of RXRα was consistent with the results of the supershift assay with an antibody against LXRα. We observed that the DNA/protein complexes were more prominent when nuclear extracts from TO-treated (and especially TO-treated and LXRα/RXRα-overexpressing) HepG2 cells were used. This suggests that the DNA/protein complexes might contain some other proteins induced by TO or overexpression of LXRα/RXRα. Cycloheximide treatment abolished the increase in mRNA levels of hDIO1 by TO, suggesting that some newly synthesized proteins are involved in stimulation of hDIO1 transcription by LXRα.
Interactions between TR and LXR have been reported on promoters of some genes [9–11,36]. We confirmed that TR and LXR interact on the hDIO1 promoter; TO induction was significantly decreased by overexpression of TRβ, but this effect was abolished by co-transfection of expression vectors for LXRα/RXRα. Interestingly, the change in TO induction by TRβ was not affected by transfection of expression vector for either LXRα or RXRα alone. This highlights the relevance of the EMSA/supershift results showing that complexes including both LXRα and RXRα bound to the specific region between nucleotides −131 and −104 of the hDIO1 promoter. By contrast, in TRβ-knockdown cells, TO induction was significantly increased with or without overexpression of LXRα/RXRα, indicating that deterioration of TRβ function augments the effects of LXRα/RXRα on the hDIO1 promoter.
Our observation that stimulation of LXRα by TO increased the expression of hDIO1 in HepG2 cells was inconsistent with previous in vivo observations that TO decreases hepatic expression of Dio1 in rat and mouse models [37,38]. Importantly, there is a large difference in the sequences of this gene’s promoter regions between human and rodent; TREs have not been identified in the promoter regions of rat or mouse Dio1, whereas two TREs are present in the promoter region of human DIO1 [3]. Therefore, we performed in vitro examinations using human cell lines, and obtained meaningful data on human DIO1 gene.
In conclusion, we demonstrated that a specific region of the hDIO1 promoter, which contains a TRE, was important for the basal activity and LXRα-mediated activation of this gene. Our results provide the additional insights that LXRα plays a specific and important role in activation of TH by regulating D1. Furthermore, LXRα binds to and regulates the hDIO1 promoter, and competes with TRβ on a specific region of the hDIO1 promoter.
Supporting information
(DOCX)
A. A series of 5'-deletion constructs of the hDIO1 promoter were transiently transfected into HepG2 and TSA201 cells in the absence of expression vectors for human LXRα and human RXRα (LXRα/RXRα), with or without 10−7 M TO. Promoter activity was normalized against Renilla luciferase activity, and the normalized value is expressed relative to that of promoterless pGL 4.10 in the absence of TO. Results are expressed as means ± SEM. **, P < 0.01. A. Basal luciferase activity of each construct. Statistical analysis was performed for pairwise combinations of constructs, and significant pairs are presented. B. Luciferase activities of each construct with and without 10−7 M TO. TO induction indicates ratios of promoter activity with TO vs. without TO. Statistical analysis was performed between TO induction of each construct and that of promoterless pGL 4.10, and significant differences are presented.
(TIF)
HepG2 cells were treated with and without 10−7 M TO and transfected using expression vectors as follows. pCMX-LXRα, co-transfection with pCMX-LXRα and pCMX-RXRα; pCMX-FXR, co-transfection with pCMX-FXR and pCMX-RXRα; pCMX-empty, transfection of pCMX-empty vector alone; pFN21A-RORα, co-transfection of pFN21A-RORα and pCMX-empty vector; pFN21A-PXR, co-transfection of pFN21A-PXR and pCMX-RXRα; and pFN21A-empty, co-transfection of pCMX-empty vector and pFN21A-empty vector. Promoter activity was normalized against Renilla luciferase activity, and the normalized value is expressed relative to that of promoterless pGL 4.10 with pCMX-empty or pFN21A-empty in the absence of TO. TO induction indicates ratios of promoter activity with TO vs. without TO. Statistical analysis was performed to compare each group with the respective empty vector group. Results are expressed as means ± SEM. *, P < 0.05; **, P < 0.01; N.S., not significant.
(TIF)
A. EMSA with oligonucleotide containing the wild-type sequence of the region between nucleotides −131 and −104 of the hDIO1 promoter (Wt2) with nuclear extracts from HepG2 cells overexpressing TRβ and RXRα, and supershift assays with antibodies against TRβ and RXRα. Specific DNA/protein complexes are indicated by black arrows and supershifted bands are by a gray arrow. In lane 1, as a control, only biotin-labeled Wt2 oligonucleotide was present. Biotin-labeled Wt2 oligonucleotide was incubated with nuclear extracts in lane 2 and with nuclear extracts and excess unlabeled Wt2 oligonucleotides as competitor in lane 3. The results of supershift assays are shown with normal mouse IgG as a control in lane 4, with an antibody against TRβ in lane 5, and with an antibody against RXRα in lane 6. B. EMSA with oligonucleotide containing consensus sequence of LXR response element (LXRE) with nuclear extracts from HepG2 cells overexpressing LXRα and RXRα, and supershift assays with an antibody against LXRα. Specific DNA/protein complexes are indicated by black arrows and a supershifted band is by a gray arrow. In lane 1, as a control, only biotin-labeled LXRE oligonucleotide was present. Biotin-labeled LXRE oligonucleotide was incubated with nuclear extracts in lane 2 and with nuclear extracts and excess unlabeled LXRE oligonucleotides as competitor in lane 3. The results of supershift assays are shown with normal mouse IgG as a control in lane 4 and with an antibody against LXRα in lane 5.
(TIF)
We purified chromatin fragments following immunoprecipitation from HepG2 cells using an antibody against LXRα. Normal mouse IgG was used as a negative control for an antibody against LXRα. The presence of specific DNA fragments binding to LXRα was determined by quantitative PCR using primer set 1 and 2, which covers nucleotides −128/+41 and −188/−25 of the genomic hDIO1 promoter, respectively. Enrichment of LXRα-bound DNA and that of normal mouse IgG-bound DNA were normalized against 10% of the corresponding input DNA, and the results of enrichment of LXRα-bound DNA were expressed as the fold change relative to normal mouse IgG-bound DNA. Data were compared by unpaired Student’s t test. Values are expressed as means ± SEM. **, P < 0.01.
(TIF)
A. The results of quantitative PCR to detect THRB mRNA. We compared HepG2 cells transfected with negative control siRNA (N/C) or siRNA against THRB (si-TRβ). THRB mRNA levels were normalized against the corresponding levels of cyclophilin A mRNA, and the value in N/C was defined as 1. Values are expressed as means ± SEM. **, P < 0.01. B. Protein levels of TRβ after siRNA transfection were evaluated by western blot analysis. β-actin was used as a standard.
(TIF)
Acknowledgments
This work was supported by JSPS KAKENHI (Grant Number 16H06902), MEXT KAKENHI (Grant Numbers 21229013, 20790659, 18790635, and 19591075), and the Takeda Science Foundation. We gratefully acknowledge the work of past and present members of our laboratory and our discussions with them, which have helped to shape many of the ideas presented here.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by JSPS KAKENHI (Grant Number 16H06902), MEXT KAKENHI (Grant Numbers 21229013, 20790659, 18790635, and 19591075; https://www.jsps.go.jp/j-grantsinaid/), and Takeda Science Foundation (http://www.takeda-sci.or.jp). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23: 38–89. doi: 10.1210/edrv.23.1.0455 [DOI] [PubMed] [Google Scholar]
- 2.Koenig RJ (2005) Regulation of type 1 iodothyronine deiodinase in health and disease. Thyroid 15: 835–840. doi: 10.1089/thy.2005.15.835 [DOI] [PubMed] [Google Scholar]
- 3.Toyoda N, Zavacki AM, Maia AL, Harney JW, Larsen PR (1995) A novel retinoid X receptor-independent thyroid hormone response element is present in the human type 1 deiodinase gene. Mol Cell Biol 15: 5100–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreck R, Schnieders F, Schmutzler C, Kohrle J (1994) Retinoids stimulate type I iodothyronine 5'-deiodinase activity in human follicular thyroid carcinoma cell lines. J Clin Endocrinol Metab 79: 791–798. doi: 10.1210/jcem.79.3.8077363 [DOI] [PubMed] [Google Scholar]
- 5.Kanamoto N, Tagami T, Ueda-Sakane Y, Sone M, Miura M, Yasoda A, et al. (2012) Forkhead box A1 (FOXA1) and A2 (FOXA2) oppositely regulate human type 1 iodothyronine deiodinase gene in liver. Endocrinology 153: 492–500. doi: 10.1210/en.2011-1310 [DOI] [PubMed] [Google Scholar]
- 6.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383: 728–731. doi: 10.1038/383728a0 [DOI] [PubMed] [Google Scholar]
- 7.Repa JJ, Mangelsdorf DJ (2000) The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol 16: 459–481. doi: 10.1146/annurev.cellbio.16.1.459 [DOI] [PubMed] [Google Scholar]
- 8.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ (2001) Nuclear receptors and lipid physiology: opening the X-files. Science 294: 1866–1870. doi: 10.1126/science.294.5548.1866 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Yin L, Hillgartner FB (2001) Thyroid hormone stimulates acetyl-coA carboxylase-alpha transcription in hepatocytes by modulating the composition of nuclear receptor complexes bound to a thyroid hormone response element. J Biol Chem 276: 974–983. doi: 10.1074/jbc.M005894200 [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto K, Cohen RN, Yamada M, Markan KR, Monden T, Satoh T, et al. (2006) Cross-talk between thyroid hormone receptor and liver X receptor regulatory pathways is revealed in a thyroid hormone resistance mouse model. J Biol Chem 281: 295–302. doi: 10.1074/jbc.M507877200 [DOI] [PubMed] [Google Scholar]
- 11.Huuskonen J, Vishnu M, Pullinger CR, Fielding PE, Fielding CJ (2004) Regulation of ATP-binding cassette transporter A1 transcription by thyroid hormone receptor. Biochemistry 43: 1626–1632. doi: 10.1021/bi0301643 [DOI] [PubMed] [Google Scholar]
- 12.Enmark E, Gustafsson JA (2001) Comparing nuclear receptors in worms, flies and humans. Trends Pharmacol Sci 22: 611–615. [DOI] [PubMed] [Google Scholar]
- 13.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M (1994) A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol 14: 7025–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkenstam A, Farnegardh M, Gustafsson JA (2004) Convergence of lipid homeostasis through liver X and thyroid hormone receptors. Mech Ageing Dev 125: 707–717. doi: 10.1016/j.mad.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 15.Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. (2002) Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab 87: 5185–5190. doi: 10.1210/jc.2002-020209 [DOI] [PubMed] [Google Scholar]
- 16.Umesono K, Murakami KK, Thompson CC, Evans RM (1991) Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell 65: 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao LF, Iwasaki Y, Nishiyama M, Taguchi T, Tsugita M, Okazaki M, et al. (2012) Liver X receptor alpha is involved in the transcriptional regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. Diabetes 61: 1062–1071. doi: 10.2337/db11-1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitro N, Vargas L, Romeo R, Koder A, Saez E (2007) T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett 581: 1721–1726. doi: 10.1016/j.febslet.2007.03.047 [DOI] [PubMed] [Google Scholar]
- 19.Houck KA, Borchert KM, Hepler CD, Thomas JS, Bramlett KS, Michael LF, et al. (2004) T0901317 is a dual LXR/FXR agonist. Mol Genet Metab 83: 184–187. doi: 10.1016/j.ymgme.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 20.Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, et al. (1998) Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res 26: 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed AH, Gordon RD, Sukor N, Pimenta E, Stowasser M (2011) Quality of life in patients with bilateral primary aldosteronism before and during treatment with spironolactone and/or amiloride, including a comparison with our previously published results in those with unilateral disease treated surgically. J Clin Endocrinol Metab 96: 2904–2911. doi: 10.1210/jc.2011-0138 [DOI] [PubMed] [Google Scholar]
- 22.Lee W, Mitchell P, Tjian R (1987) Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell 49: 741–752. [DOI] [PubMed] [Google Scholar]
- 23.Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, et al. (1987) Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49: 729–739. [DOI] [PubMed] [Google Scholar]
- 24.Orlov I, Rochel N, Moras D, Klaholz BP (2012) Structure of the full human RXR/VDR nuclear receptor heterodimer complex with its DR3 target DNA. EMBO J 31: 291–300. doi: 10.1038/emboj.2011.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damm K, Thompson CC, Evans RM (1989) Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature 339: 593–597. doi: 10.1038/339593a0 [DOI] [PubMed] [Google Scholar]
- 26.Graupner G, Wills KN, Tzukerman M, Zhang XK, Pfahl M (1989) Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature 340: 653–656. doi: 10.1038/340653a0 [DOI] [PubMed] [Google Scholar]
- 27.Ayers S, Switnicki MP, Angajala A, Lammel J, Arumanayagam AS, Webb P (2014) Genome-wide binding patterns of thyroid hormone receptor beta. PLoS One 9: e81186 doi: 10.1371/journal.pone.0081186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz RW, Koenig RJ (1994) Specificity and mechanism of thyroid hormone induction from an octamer response element. J Biol Chem 269: 18915–18920. [PubMed] [Google Scholar]
- 29.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, et al. (2000) Role of LXRs in control of lipogenesis. Genes Dev 14: 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, et al. (2002) Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem 45: 1963–1966. [DOI] [PubMed] [Google Scholar]
- 31.Albers M, Blume B, Schlueter T, Wright MB, Kober I, Kremoser C, et al. (2006) A novel principle for partial agonism of liver X receptor ligands. Competitive recruitment of activators and repressors. J Biol Chem 281: 4920–4930. doi: 10.1074/jbc.M510101200 [DOI] [PubMed] [Google Scholar]
- 32.Bugge TH, Pohl J, Lonnoy O, Stunnenberg HG (1992) RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J 11: 1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu VC, Delsert C, Andersen B, Holloway JM, Devary OV, Naar AM, et al. (1991) RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell 67: 1251–1266. [DOI] [PubMed] [Google Scholar]
- 34.Lee MS, Kliewer SA, Provencal J, Wright PE, Evans RM (1993) Structure of the retinoid X receptor alpha DNA binding domain: a helix required for homodimeric DNA binding. Science 260: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 35.Mader S, Chen JY, Chen Z, White J, Chambon P, Gronemeyer H (1993) The patterns of binding of RAR, RXR and TR homo- and heterodimers to direct repeats are dictated by the binding specificites of the DNA binding domains. EMBO J 12: 5029–5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishida E, Hashimoto K, Okada S, Satoh T, Yamada M, Mori M (2013) Crosstalk between thyroid hormone receptor and liver X receptor in the regulation of selective Alzheimer's disease indicator-1 gene expression. PLoS One 8: e54901 doi: 10.1371/journal.pone.0054901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies JS, Kotokorpi P, Lindahl U, Oscarsson J, Wells T, Mode A (2008) Effects of the synthetic liver X receptor agonist T0901317 on the growth hormone and thyroid hormone axes in male rats. Endocrine 33: 196–204. doi: 10.1007/s12020-008-9067-9 [DOI] [PubMed] [Google Scholar]
- 38.Stulnig TM, Steffensen KR, Gao H, Reimers M, Dahlman-Wright K, Schuster GU, et al. (2002) Novel roles of liver X receptors exposed by gene expression profiling in liver and adipose tissue. Mol Pharmacol 62: 1299–1305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
A. A series of 5'-deletion constructs of the hDIO1 promoter were transiently transfected into HepG2 and TSA201 cells in the absence of expression vectors for human LXRα and human RXRα (LXRα/RXRα), with or without 10−7 M TO. Promoter activity was normalized against Renilla luciferase activity, and the normalized value is expressed relative to that of promoterless pGL 4.10 in the absence of TO. Results are expressed as means ± SEM. **, P < 0.01. A. Basal luciferase activity of each construct. Statistical analysis was performed for pairwise combinations of constructs, and significant pairs are presented. B. Luciferase activities of each construct with and without 10−7 M TO. TO induction indicates ratios of promoter activity with TO vs. without TO. Statistical analysis was performed between TO induction of each construct and that of promoterless pGL 4.10, and significant differences are presented.
(TIF)
HepG2 cells were treated with and without 10−7 M TO and transfected using expression vectors as follows. pCMX-LXRα, co-transfection with pCMX-LXRα and pCMX-RXRα; pCMX-FXR, co-transfection with pCMX-FXR and pCMX-RXRα; pCMX-empty, transfection of pCMX-empty vector alone; pFN21A-RORα, co-transfection of pFN21A-RORα and pCMX-empty vector; pFN21A-PXR, co-transfection of pFN21A-PXR and pCMX-RXRα; and pFN21A-empty, co-transfection of pCMX-empty vector and pFN21A-empty vector. Promoter activity was normalized against Renilla luciferase activity, and the normalized value is expressed relative to that of promoterless pGL 4.10 with pCMX-empty or pFN21A-empty in the absence of TO. TO induction indicates ratios of promoter activity with TO vs. without TO. Statistical analysis was performed to compare each group with the respective empty vector group. Results are expressed as means ± SEM. *, P < 0.05; **, P < 0.01; N.S., not significant.
(TIF)
A. EMSA with oligonucleotide containing the wild-type sequence of the region between nucleotides −131 and −104 of the hDIO1 promoter (Wt2) with nuclear extracts from HepG2 cells overexpressing TRβ and RXRα, and supershift assays with antibodies against TRβ and RXRα. Specific DNA/protein complexes are indicated by black arrows and supershifted bands are by a gray arrow. In lane 1, as a control, only biotin-labeled Wt2 oligonucleotide was present. Biotin-labeled Wt2 oligonucleotide was incubated with nuclear extracts in lane 2 and with nuclear extracts and excess unlabeled Wt2 oligonucleotides as competitor in lane 3. The results of supershift assays are shown with normal mouse IgG as a control in lane 4, with an antibody against TRβ in lane 5, and with an antibody against RXRα in lane 6. B. EMSA with oligonucleotide containing consensus sequence of LXR response element (LXRE) with nuclear extracts from HepG2 cells overexpressing LXRα and RXRα, and supershift assays with an antibody against LXRα. Specific DNA/protein complexes are indicated by black arrows and a supershifted band is by a gray arrow. In lane 1, as a control, only biotin-labeled LXRE oligonucleotide was present. Biotin-labeled LXRE oligonucleotide was incubated with nuclear extracts in lane 2 and with nuclear extracts and excess unlabeled LXRE oligonucleotides as competitor in lane 3. The results of supershift assays are shown with normal mouse IgG as a control in lane 4 and with an antibody against LXRα in lane 5.
(TIF)
We purified chromatin fragments following immunoprecipitation from HepG2 cells using an antibody against LXRα. Normal mouse IgG was used as a negative control for an antibody against LXRα. The presence of specific DNA fragments binding to LXRα was determined by quantitative PCR using primer set 1 and 2, which covers nucleotides −128/+41 and −188/−25 of the genomic hDIO1 promoter, respectively. Enrichment of LXRα-bound DNA and that of normal mouse IgG-bound DNA were normalized against 10% of the corresponding input DNA, and the results of enrichment of LXRα-bound DNA were expressed as the fold change relative to normal mouse IgG-bound DNA. Data were compared by unpaired Student’s t test. Values are expressed as means ± SEM. **, P < 0.01.
(TIF)
A. The results of quantitative PCR to detect THRB mRNA. We compared HepG2 cells transfected with negative control siRNA (N/C) or siRNA against THRB (si-TRβ). THRB mRNA levels were normalized against the corresponding levels of cyclophilin A mRNA, and the value in N/C was defined as 1. Values are expressed as means ± SEM. **, P < 0.01. B. Protein levels of TRβ after siRNA transfection were evaluated by western blot analysis. β-actin was used as a standard.
(TIF)
Data Availability Statement
All relevant data are within the paper.