Abstract
The marked increase in the incidence of infections due to antibiotic-resistant gram-negative bacilli in recent years is of great concern, as patients infected by those isolates might initially receive antibiotics that are inactive against the responsible pathogens. To evaluate the effect of inappropriate initial antimicrobial therapy on survival, a total of 286 patients with antibiotic-resistant gram-negative bacteremia, 61 patients with Escherichia coli bacteremia, 65 with Klebsiella pneumoniae bacteremia, 74 with Pseudomonas aeruginosa bacteremia, and 86 with Enterobacter bacteremia, were analyzed retrospectively. If a patient received at least one antimicrobial agent to which the causative microorganisms were susceptible within 24 h of blood culture collection, the initial antimicrobial therapy was considered to have been appropriate. High-risk sources of bacteremia were defined as the lung, peritoneum, or an unknown source. The main outcome measure was 30-day mortality. Of the 286 patients, 135 (47.2%) received appropriate initial empirical antimicrobial therapy, and the remaining 151 (52.8%) patients received inappropriate therapy. The adequately treated group had a 27.4% mortality rate, whereas the inadequately treated group had a 38.4% mortality rate (P = 0.049). Multivariate analysis showed that the significant independent risk factors of mortality were presentation with septic shock, a high-risk source of bacteremia, P. aeruginosa infection, and an increasing APACHE II score. In the subgroup of patients (n = 132) with a high-risk source of bacteremia, inappropriate initial antimicrobial therapy was independently associated with increased mortality (odds ratio, 3.64; 95% confidence interval, 1.13 to 11.72; P = 0.030). Our data suggest that inappropriate initial antimicrobial therapy is associated with adverse outcome in antibiotic-resistant gram-negative bacteremia, particularly in patients with a high-risk source of bacteremia.
Gram-negative bacilli such as Enterobacteriaceae and Pseudomonas aeruginosa are the leading causes of nosocomial bloodstream infections. Antibiotic-resistant strains have emerged among the gram-negative bacilli and are being increasingly recognized (8, 20). This marked increase in the incidence of infections due to antibiotic-resistant gram-negative bacilli in recent years is of great concern. It is presumed that infections caused by antibiotic-resistant bacteria result in greater mortality, longer hospitalization, and higher costs than infections caused by antibiotic-susceptible bacteria, although little data are available to support this intuitive concept (2, 3, 12). The assumption that infections caused by antibiotic-resistant bacteria are associated with a higher mortality rate is based on the possibility that appropriate antimicrobial therapy for such infections might be initiated later than for infections caused by antibiotic-susceptible bacteria (14).
Appropriate antimicrobial therapy has been shown to reduce mortality among patients with gram-negative bacteremia (10, 15) and, when initiated early, to have a favorable effect on outcome in critically ill patients with bacteremia or other serious infections (5-7, 10, 16-18, 23). However, this issue has not been studied in detail, and several reports have noted that appropriate antimicrobial therapy did not result in a notable difference in the outcomes of patients with severe infections (1, 21-23). In addition, although appropriate antimicrobial therapy has been shown to reduce mortality rates in gram-negative sepsis, little information exists about whether inappropriate initial empirical antimicrobial therapy given during the first 48 to 72 h, before microbiological results are available, adversely affects outcome. Thus, we have evaluated the effect of inappropriate initial antimicrobial therapy on survival in patients with antibiotic-resistant gram-negative bacteremia. The aim of the study was to identify the risk factors for mortality and to explore the overall association between increased mortality and inappropriate empirical therapy in antibiotic-resistant gram-negative bacteremia.
MATERIALS AND METHODS
Patients and bacterial strains.
The database at our clinical microbiology laboratory was reviewed in order to identify patients with gram-negative bacteremia. Of the gram-negative bacillus isolates, the most common strains isolated from the bloodstream were Escherichia coli (39.5%), Klebsiella pneumoniae (20.7%), Enterobacter species (8.0%), and P. aeruginosa (7.5%). Since these four are the medically important gram-negative bacilli and the most common organisms, we included them in this study cohort. Patients older than 16 years of age with antibiotic-resistant gram-negative bacteremia were included in the analysis. Only the first bacteremic episode for each patient was included in the analysis. We reviewed the medical records of individuals diagnosed from January 1998 to December 2002 at Seoul National University Hospital, Seoul, Korea, a 1,500-bed tertiary care university hospital and referral center.
Species identification was carried out with Vitek-GNI Card (bioMérieux, Hazelwood, Mo.) by standard methods (9), and antibiotic susceptibility testing was performed by using the disk diffusion method according to the recommendations of the National Committee for Clinical Laboratory Standards (19). Strains showing inhibition zone diameters in the intermediate range were considered resistant.
Study design and data collection.
A retrospective observational cohort study was conducted to identify the risk factors for mortality and the impact of inappropriate initial antimicrobial therapy on patient outcome in antibiotic-resistant gram-negative bacteremia. We reviewed the medical records of the patients and compared data from patients that received appropriate initial antimicrobial therapy with data from patients that received inappropriate therapy.
The data collected included age, sex, underlying disease, primary site of infection, severity of illness as calculated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score (13), and antimicrobial therapy regimen. The presence of the following comorbid conditions was also documented: neutropenia, presentation with septic shock, care in an intensive care unit (ICU), receipt of immunosuppressive agents within 30 days prior to bacteremia, corticosteroid use, the presence of a central venous catheter or an indwelling urinary catheter, and postoperative state. As this study was retrospective, the patients' physicians, not the researchers, had chosen the antimicrobial therapy regimens. The main outcome measure used was the 30-day mortality rate.
Definitions.
Gram-negative bacteremia was defined as the isolation of gram-negative bacilli in a blood culture specimen. Clinically significant bacteremia was defined as at least one positive blood culture together with clinical features compatible with systemic inflammatory response syndrome. Antibiotic resistance was defined as in vitro resistance to cefotaxime or ceftazidime. P. aeruginosa isolates were considered to be antibiotic resistant when resistance to at least one of the following antipseudomonal antibiotics was seen: piperacillin, ciprofloxacin, ceftazidime, and imipenem. The initial empirical antimicrobial therapy was considered appropriate if the initial antibiotics, which were administered within 24 h after acquisition of a blood culture samples, included at least one antibiotic that was active in vitro against the causative microorganisms and when the dosage and route of administration conformed with current medical standards (6). Inappropriate initial antimicrobial therapy referred to the administration of antimicrobial agents to which the causative microorganisms were resistant in vitro or to the lack of an antimicrobial therapy for a known causative pathogen. If the antimicrobial agent was not administered within 24 h of bacteremia onset, antimicrobial use was considered inappropriate. For P. aeruginosa bacteremia, aminoglycoside monotherapy was considered inappropriate.
The source of the bacteremia was determined on the basis of the isolation of gram-negative bacteria from the presumed portal of entry and clinical evaluation. The sources of bacteremia were divided into two categories: low risk (associated mortality, ≤30%), which included the urinary tract, intravenous catheter, and pancreaticobiliary tract, and high risk (associated mortality, >30%), which included the lung, peritoneum, and unknown sources (2).
The bacteremia was categorized as polymicrobial if additional microorganisms were recovered from the blood cultures. Nosocomial infection was defined as an infection that occurred >48 h after hospital admission, an infection that occurred <48 h after admission to the hospital in patients that had been hospitalized in the 2 weeks prior to admission, and an infection that occurred <48 h after admission to the hospital in patients that had been transferred from another hospital or nursing home. Nosocomial bloodstream infections, as well as other nosocomial infections, were defined according to the criteria proposed by the Centers for Disease Control and Prevention (4). Neutropenia was defined as an absolute neutrophil count below 500 cells/mm3. Septic shock was defined as sepsis associated with evidence of organ hypoperfusion and a systolic blood pressure <90 or >30 mm Hg less than the baseline or a requirement for the use of a vasopressor to maintain blood pressure.
Statistical analysis.
The Student's t test was used to compare continuous variables, and χ2 or Fisher's exact test was used to compare categorical variables. In identifying the independent risk factors for mortality, a backward stepwise logistic regression analysis was used to control for the effects of confounding variables. Variables with a P value of <0.05 in the univariate analysis were candidates for multivariate analysis as well as the main variable of interest (i.e., inappropriate initial antimicrobial therapy). We used backward elimination of any variable that did not contribute to the model on the grounds of the likelihood ratio test, using a significance cutoff of 0.05. The Kaplan-Meier method was used for the survival analysis. All P values were two tailed, and P values of <0.05 were considered statistically significant. The SPSS (version 10.0) software package was used for all analyses.
RESULTS
Study population.
During the study period, a total of 1,045 patients with E. coli bacteremia, 499 patients with K. pneumoniae bacteremia, 190 patients with P. aeruginosa bacteremia, and 183 patients with Enterobacter bacteremia were identified. Among these patients, 61 (5.8%) patients with E. coli bacteremia, 65 (13.0%) patients with K. pneumoniae bacteremia, 74 (38.9%) patients with P. aeruginosa bacteremia, and 86 (47.0%) patients with Enterobacter bacteremia were infected by antibiotic-resistant strains and were included in the study cohort. Thus, 286 patients with clinically significant antibiotic-resistant gram-negative bacteremia were analyzed retrospectively.
The mean (± standard deviation) patient age was 55 ± 16 years (range, 16 to 95), and 183 (64.0%) patients were male. The most common underlying disease was a solid tumor (n = 110 [38.5%]), and the most common primary site of infection was the pancreaticobiliary tract (n = 109 [38.1%]). A total of 88.5% of the episodes were nosocomial infections, and 19.6% of the patients were neutropenic.
Of the 286 patients, 135 (47.2%) received appropriate initial empirical antibiotics and were classified as the appropriate initial antimicrobial therapy group. The remaining 151 (52.8%) patients were classified as the inappropriate initial antimicrobial therapy group. The demographic and clinical characteristics of these two groups are shown in Table 1. No significant differences between the two groups were observed in age, sex, underlying disease, primary site of infection, comorbid condition, and APACHE II score (Table 1). However, of the causative microorganisms, K. pneumoniae and Enterobacter spp. isolates were more frequent in the appropriate initial antimicrobial therapy group (31.1 versus 15.2% [P = 0.001] and 36.3 versus 24.5% [P = 0.030], respectively), whereas P. aeruginosa isolates were more frequent in the inappropriate initial antimicrobial therapy group (10.4 versus 39.7% [P < 0.001]) (Table 1).
TABLE 1.
Demographic characteristics of study populationa
| Characteristic | Value for groupb
|
P value | |
|---|---|---|---|
| Appropriate initial antibiotics (n = 135) | Inappropriate initial antibiotics (n = 151) | ||
| No. of males | 83 (61.5) | 100 (66.2) | NS |
| Age (yr) (mean ± SD, range) | 53 ± 16, 16-86 | 56 ± 16, 16-95 | NS |
| APACHE II score (mean ± SD, range) | 11.39 ± 4.85, 0-25 | 11.02 ± 5.15, 0-24 | NS |
| Underlying disease | |||
| Leukemia | 20 (14.8) | 21 (13.9) | NS |
| Malignant lymphoma | 3 (2.2) | 4 (2.6) | NS |
| Solid organ transplantation | 7 (5.2) | 7 (4.6) | NS |
| Liver cirrhosis | 13 (9.6) | 14 (9.3) | NS |
| End-stage renal disease | 6 (4.4) | 6 (4.0) | NS |
| Pancreaticobiliary tract disease | 13 (9.6) | 20 (13.2) | NS |
| Solid tumor | 51 (37.8) | 59 (39.1) | NS |
| Others | 22 (16.3) | 20 (13.2) | NS |
| Comorbid condition | |||
| Neutropenia | 27 (20) | 29 (19.2) | NS |
| Presentation with septic shock | 33 (24.4) | 42 (27.8) | NS |
| Long hospital stay (> 14 days) | 79 (58.5) | 76 (50.3) | NS |
| ICU care | 27 (20) | 26 (17.2) | NS |
| Postoperative state | 19 (14.1) | 21 (13.9) | NS |
| Polymicrobial | 18 (13.3) | 25 (16.6) | NS |
| Primary site of infection | |||
| Catheter related | 3 (2.2) | 5 (3.3) | NS |
| Pancreaticobiliary tract | 50 (37) | 59 (39.1) | NS |
| Urinary tract | 14 (10.4) | 18 (11.9) | NS |
| Lung | 4 (3) | 12 (7.9) | NS |
| Peritoneum | 18 (13.3) | 21 (13.9) | NS |
| Unknown | 45 (33.3) | 32 (21.1) | NS |
| Microorganism | |||
| E. coli | 30 (22.2) | 31 (20.5) | NS |
| K. pneumoniae | 42 (31.1) | 23 (15.2) | 0.001 |
| Enterobacter species | 49 (36.3) | 37 (24.5) | 0.030 |
| P. aeruginosa | 14 (10.4) | 60 (39.7) | <0.001 |
NS, not significant (P > 0.05); SD, standard deviation.
Number and percentage (in parentheses) of patients with each characteristic are shown.
Nine patients were classified as being in the inappropriate therapy group because antimicrobial therapy was not started within 24 h after onset of bacteremia. The remaining 277 patients received any antibiotics as initial empirical therapy, and of these, 90 patients received monotherapy and 187 patients received combination therapy. There was no significant difference in 30-day mortality between monotherapy and combination therapy groups (33 out of 90 [36.7%] versus 59 out of 187 [31.6%] [P = 0.397]). The patients who received combination therapy were more common in the appropriate therapy group than in the inappropriate therapy group (109 out of 135 [80.7%] versus 78 out of 142 [54.9%] [P < 0.001]). The reasons why the patients received inappropriate initial antimicrobial therapy are shown in Table 2.
TABLE 2.
Reasons for inappropriate initial antimicrobial therapy
| Reasons for inappropriate therapy | No. of patients
|
|||
|---|---|---|---|---|
| E. coli (n = 31) | K. pneumoniae (n = 23) | Enterobacter (n = 37) | P. aeruginosa (n = 60) | |
| Cephalosporin use and resistance to druga | 11 | 12 | 21 | |
| Combination therapy and resistance to multiple drugs | 17 | 10 | 11 | 5 |
| Monotherapy and resistance to drugb | 3 | 1 | 1 | 17 |
| Monotherapy with aminoglycoside in Pseudomonasc | 33 | |||
| No therapy | 0 | 0 | 4 | 5 |
Applied to bloodstream infections caused by E. coli, K. pneumoniae, and Enterobacter spp.
Patients who received cephalosporin monotherapy for bloodstream infections caused by E. coli, K. pneumoniae, or Enterobacter spp. were not included. Those were classified under cephalosporin use and resistance to drug.
Applied to P. aeruginosa bacteremia.
Thirty-day mortality and predictors of mortality.
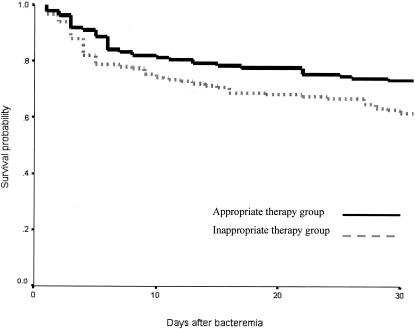
The overall 30-day mortality rate of all patients was 33.2% (95 out of 286): 21.3% (13 out of 61) for E. coli bacteremia, 32.3% (21 out of 65) for K. pneumoniae bacteremia, 33.7% (29 out of 86) for Enterobacter bacteremia, and 43.2% (32 out of 74) for P. aeruginosa bacteremia. The appropriate initial antimicrobial therapy group had a 27.4% mortality rate, whereas the inappropriate therapy group had a 38.4% mortality rate (P = 0.049). The survival curve also shows that the inappropriate initial antimicrobial therapy group had a lower probability of survival than the appropriate therapy group (Fig. 1).
FIG. 1.
Survival curve using the Kaplan-Meier method for patients with antibiotic-resistant gram-negative bacteremia who received appropriate initial antimicrobial therapy compared to those who received inappropriate therapy. The inappropriate therapy group has the lower probability of survival than the appropriate therapy group. The 30-day mortality rate of the appropriate initial antimicrobial therapy group was 27.4%, whereas that of the inappropriate therapy group was 38.4% (P = 0.049).
By univariate analysis, the factors associated with 30-day mortality in antibiotic-resistant gram-negative bacteremia were inappropriate initial antimicrobial therapy, a long hospital stay, immunosuppressive therapy, the prior receipt of corticosteroids, ICU care, a high-risk source of bacteremia, neutropenia, polymicrobial, presentation with septic shock, an increasing APACHE II score, and P. aeruginosa infection (all P values, <0.05) (Table 3). Bacteremia due to E. coli was associated with a lower mortality (odds ratio [OR], 0.47; 95% confidence interval [CI], 0.24 to 0.92).
TABLE 3.
Risk factors associated with 30-day mortality in bloodstream infections caused by antimicrobial-resistant gram-negative bacilli based on univariate analysis
| Risk factor | No. of survivors (n = 191) (%) | No. of nonsurvivors (n = 95) (%) | OR (95% CI) | P value |
|---|---|---|---|---|
| Inappropriate initial empirical antimicrobial therapy | 93 (48.7) | 58 (61.1) | 1.65 (1.001-2.73) | 0.049 |
| Old age (>65 yrs) | 49 (25.7) | 28 (24.2) | 0.93 (0.52-1.64) | 0.791 |
| Long hospital stay (>14 days)a | 87 (45.5) | 68 (71.6) | 3.01 (1.77-5.11) | <0.001 |
| Immunosuppressive therapy | 14 (7.3) | 16 (16.8) | 2.56 (1.19-5.50) | 0.013 |
| Corticosteroid use | 30 (15.7) | 32 (33.7) | 2.73 (1.53-4.85) | 0.001 |
| Care in ICU | 20 (10.5) | 33 (34.7) | 4.55 (2.43-8.52) | <0.001 |
| High-risk source of bacteremia | 62 (32.5) | 70 (73.7) | 5.83 (3.37-10.08) | <0.001 |
| Neutropenic state | 30 (15.7) | 26 (27.4) | 2.02 (1.11-3.67) | 0.019 |
| Postoperative state | 24 (12.6) | 16 (16.8) | 1.41 (0.71-2.00) | 0.326 |
| Polymicrobial | 23 (12) | 20 (21.1) | 1.95 (1.01-3.76) | 0.045 |
| Presentation with septic shock | 10 (5.2) | 65 (68.4) | 39.22 (18.16-84.68) | <0.001 |
| APACHE II score | ||||
| ≤7 | 66 (34.6) | 6 (6.3) | ||
| 8-15 | 109 (57.1) | 44 (46.3) | <0.001 | |
| ≥16 | 16 (8.4) | 45 (47.4) | ||
| Microorganism | ||||
| E. coli | 48 (25.1) | 13 (13.7) | 0.47 (0.24-0.92) | 0.026 |
| K. pneumoniae | 44 (23) | 21 (22.1) | 0.95 (0.53-1.71) | 0.859 |
| Enterobacter species | 57 (29.8) | 29 (30.5) | 1.03 (0.61-1.77) | 0.906 |
| P. aeruginosa | 42 (22) | 32 (33.7) | 1.80 (1.04-3.11) | 0.033 |
Hospital stay prior to onset of bacteremia.
Multivariate analysis using a logistic regression model including the variables associated with mortality by univariate analysis (P < 0.05) showed that the significant independent risk factors for mortality were presentation with septic shock, a high-risk source of bacteremia, P. aeruginosa infection, and an increasing APACHE II score (Table 3). Inappropriate initial antimicrobial therapy did not reach statistical significance as a risk factor for mortality (OR, 2.08; 95% CI, 0.86 to 5.02; P = 0.102). However, a subgroup multivariate analysis including patients with a high-risk source of bacteremia (n = 132) compared to patients with a low-risk source of bacteremia (n = 154) showed that inappropriate initial antimicrobial therapy was independently associated with increased mortality (OR, 3.64; 95% CI, 1.13 to 11.72; P = 0.030) in the high-risk group but not in the low-risk group (OR, 1.08; 95% CI, 0.28 to 4.18; P = 0.908) (Table 4).
TABLE 4.
Independent risk factors for mortality in bloodstream infections caused by antimicrobial-resistant gram-negative bacilli based on multivariate analysisa
| Risk factor | Adjusted OR (95% CI) | P value |
|---|---|---|
| All patients of study population (n = 286) | ||
| High-risk source of bacteremia | 3.95 (1.66-9.41) | 0.002 |
| Presentation with septic shock | 40.99 (15.30-109.82) | <0.001 |
| APACHE II score (per 1-point increment) | 1.29 (1.16-1.44) | <0.001 |
| P. aeruginosa bacteremia | 3.07 (1.21-7.79) | 0.018 |
| Patients with low-risk source of bacteremia (n = 154)b | ||
| Presentation with septic shock | 23.01 (6.42-82.49) | <0.001 |
| APACHE II score (per 1-point increment) | 1.25 (1.09-1.43) | 0.001 |
| P. aeruginosa bacteremia | 4.27 (1.33-13.67) | 0.015 |
| Patients with high-risk source of bacteremia (n = 132)c | ||
| Presentation with septic shock | 100.87 (17.98-565.89) | <0.001 |
| APACHE II score (per 1-point increment) | 1.348 (1.13-1.61) | 0.001 |
| Inappropriate initial antimicrobial therapy | 3.64 (1.13-11.72) | 0.030 |
Variables with a P value of <0.05 in the univariate analysis were candidates for multivariate analysis as well as the main variable of interest (i.e., inappropriate initial antimicrobial therapy).
Multivariate analysis using a logistic regression model included the following variables: a long hospital stay, ICU care, neutropenia, polymicrobial, septic shock, APACHE II score, Pseudomonas infection, and inappropriate initial antimicrobial therapy.
Multivariate analysis included ICU care, septic shock, APACHE II score, Pseudomonas infection, old age, and inappropriate initial antimicrobial therapy.
Of the 132 patients with a high-risk source of bacteremia, 67 (50.8%) received appropriate initial antimicrobial therapy against the causative microorganisms, and 65 (49.2%) received inappropriate initial antimicrobial therapy. Of the 67 patients who received appropriate antimicrobial therapy, 29 (43.3%) died, and of the 65 patients who received inappropriate antimicrobial therapy, 41 (63.1%) died (P = 0.023).
On the other hand, of the 154 patients with a low-risk source of bacteremia, 68 (44.2%) received appropriate initial antimicrobial therapy, and 86 (55.8%) received inappropriate initial antimicrobial therapy. Of the 68 patients who received appropriate antimicrobial therapy, 8 (11.8%) died, and of the 86 patients who received inappropriate antimicrobial therapy, 17 (19.8%) died (P = 0.181).
DISCUSSION
This study was undertaken to evaluate the risk factors for mortality and the effect of inappropriate initial antimicrobial therapy on the outcome of patients with antibiotic-resistant gram-negative bacteremia. We found that severity of illness, septic shock, a high-risk source of bacteremia, and P. aeruginosa infection were strong prognostic factors of mortality. Inadequately treated patients had significantly higher mortality than adequately treated patients, although statistical significance was not reached by multivariate analysis after adjusting for confounding variables. The overall mortality rate was 33.2%, with rates of 27.4 and 38.4% for patients that received appropriate and inappropriate antimicrobial therapy, respectively. Thus, an 11% reduction in the overall crude mortality rate was associated with adequate early empirical antimicrobial therapy. Furthermore, for patients with a high-risk source of bacteremia, such as lung, peritoneum, or an unknown source, inappropriate initial antimicrobial therapy was identified as an independent risk factor for mortality (OR, 3.64; 95% CI, 1.13 to 110.72) after adjusting for confounding variables. Thus, the adequacy of initial antimicrobial therapy was an important determinant of survival, especially in patients with a high-risk source of bacteremia.
These results are in line with those of others who found that inappropriate empirical antimicrobial therapy of bacteremia was independently associated with higher mortality in different groups of patients (5, 16). Our study findings and the published literature strongly support the concept that inappropriate initial antimicrobial therapy has an adverse effect on survival in patients with gram-negative sepsis (10, 15).
In our study, it was noted that inappropriate initial antimicrobial therapy was independently associated with increased mortality in patients with a high-risk source of bacteremia, whereas inappropriate initial antimicrobial therapy was not found to be associated with increased mortality in patients with a low-risk source of bacteremia. Our study did not find a significant association between inappropriate initial antimicrobial therapy and the outcome of patients with low-risk sources of bacteremia including pancreaticobiliary tract, urinary tract, or a catheter-related source, although the 95% CI was relatively wide (OR, 1.08; 95% CI, 0.28 to 4.18) and thus likely contributed to the lack of ability to detect a significant difference. In these patients, the independent risk factors for mortality were septic shock, an increasing APACHE II score, and P. aeruginosa infection. The lack of significant association between inappropriate initial antimicrobial therapy and the outcome of patients with a low-risk source of bacteremia may have been due to a high proportion of catheter removal or early intervention for decompression of biliary or urinary obstruction in the majority of patients. It might indicate that nonmedical interventions such as decompression of obstruction or the removal of infection foci are also important aspects of the treatment of infection. Our results suggest that when gram-negative bacteremia is suspected, the most significant prognostic variables are the primary site of infection (i.e., high-risk or low-risk site) and the severity of the underlying illness (i.e., higher APACHE II score or septic shock). Also, our data suggest that a delay in appropriate antimicrobial therapy might not lead to an adverse outcome in patients with a low-risk source of bacteremia if appropriate definitive antimicrobial therapy is administered and appropriate decompression procedures are conducted. Similarly, in our previous study of Staphylococcus aureus bacteremia, we suggested that the effect of inappropriate antimicrobial therapy and methicillin resistance on outcome depended on the primary foci of infection (11). The outcomes of bloodstream infections may depend on underlying conditions, the severity of illness, the adequacy of antimicrobial agents, and the primary site of infection. Also, the effect of inappropriate initial antimicrobial therapy may also depend on the severity of the underlying illness, the duration of delay in appropriate therapy, the removal of infection foci, the virulence of the microorganism, and the primary site of infection.
In the present study, no significant differences in severity of illness (as measured by the APACHE II score) or incidence of septic shock hampered the outcome comparison between the appropriate initial antimicrobial therapy group and the inappropriate therapy group. However, one possible weakness of our study is that the outcome comparison between the two patient groups may have been confounded by differences in the clinical virulence of the types of gram-negative bacteria involved. For example, P. aeruginosa isolates, which are known to be extremely virulent, were more common in the inappropriate antimicrobial therapy group. Nevertheless, by multivariate analysis including patients with a high-risk source of bacteremia, inappropriate initial antimicrobial therapy was found to be independently associated with a higher mortality.
Kollef suggested that several factors were associated with a greater likelihood of inappropriate antimicrobial therapy, namely, the presence of multiple infecting organisms, fungal infection, P. aeruginosa infection, and antibiotic-resistant bacteria (14). However, in the present study, cases with polymicrobial infection were distributed evenly among those treated with appropriate and inappropriate initial antibiotics, and thus, in contrast to Kollef's findings, there wasn't a higher proportion of inappropriate antimicrobial therapy in the group of patients with polymicrobial infections. Also, it may be presumed that serious infections caused by antibiotic-resistant bacteria have a worse prognosis because of the delay in initiation of appropriate antibiotic therapy (14). In our study of antibiotic-resistant gram-negative bacteremia, we found that inappropriate initial antimicrobial therapy might adversely affect outcome. These findings suggest that continuing efforts should be directed at reducing inappropriate empirical antimicrobial therapy to septic patients, especially in cases of suspected gram-negative sepsis. Therefore, broad-spectrum cephalosporin monotherapy might not provide an optimal therapeutic option as an empirical antimicrobial regimen for the treatment of gram-negative sepsis if antibiotic-resistant gram-negative bacilli are prevalent, especially in patients with risk factors of antibiotic-resistant bacterial infection. However, empirical antibiotics for serious infections should be recommended on the basis of the distribution of pathogens and their susceptibility patterns in the institution where the regimen is administered. Furthermore, the effect of inappropriate antimicrobial therapy might depend on the severity of the underlying illness and comorbid conditions of the patients.
The main limitation of the present investigation is that it was observational and not randomized, and thus, an unknown risk factor for mortality might have been unequally distributed between the two groups. Also, we did not identify the factors that influenced the attending physician's delay in administrating appropriate antimicrobial therapy; therefore, we can't exclude the possibility of unmeasured confounding factors. However, the association between early empirical antimicrobial therapy and mortality in patients with sepsis should be investigated in an observational manner, because ethical considerations preclude a prospective randomized trial. In addition, it is unlikely that randomized controlled trials on therapy for infections due to antibiotic-resistant gram-negative bacteremia will be performed in the near future. Although the evidence of a statistical association between initial inappropriate antimicrobial therapy and mortality does not prove causality, our data suggest that mortality in inadequately treated patients can't be attributed to comorbid conditions, the severity of illness, baseline organ dysfunction, or the causative microorganisms alone.
In the present study, the analysis data set did not include very high APACHE II scores (above 25), and only 19% of patients required ICU care. Thus, the results of our study may not be applicable to the critically ill patients who require ICU care. Indeed, there is controversy regarding the impact of inappropriate initial antimicrobial therapy on the outcome of critically ill patients in the ICU setting (2, 7, 17, 23, 24). Further investigations in a larger data set are warranted.
In conclusion, this investigation shows that administration of inappropriate initial antimicrobial therapy might be associated with an adverse outcome in those patients with antibiotic-resistant gram-negative bacteremia. In particular, in patients with a high-risk source of bacteremia such as lung, peritoneum, or an unknown source, inappropriate initial antimicrobial therapy had an independent adverse effect on the outcome of patients after adjusting for a large number of potential confounders.
REFERENCES
- 1.Bates, D. W., K. E. Pruess, and T. H. Lee. 1995. How bad are bacteremia and sepsis? Outcomes in a cohort with suspected bacteremia. Arch. Intern. Med. 155:593-598. [PubMed] [Google Scholar]
- 2.Blot, S., K. Vandewoude, D. De Bacquer, and F. Colardyn. 2002. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin. Infect. Dis. 34:1600-1606. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulous, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 4.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 5.Hanon, F. X., D. L. Monnet, T. L. Sorensen, K. Molbak, G. Pedersen, and H. Schonheyder. 2002. Survival of patients with bacteraemia in relation to initial empirical antimicrobial treatment. Scand. J. Infect. Dis. 34:520-528. [DOI] [PubMed] [Google Scholar]
- 6.Harbarth, S., J. Garbino, J. Pugin, J. A. Romand, D. Lew, and D. Pittet. 2003. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 115:529-535. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 8.Itokazu, G. S., J. P. Quinn, C. Bell-Dixon, F. M. Kahan, and R. A. Weinstein. 1996. Antimicrobial resistance rates among aerobic gram-negative bacilli recovered from patients in intensive care units: evaluation of a national postmarketing surveillance program. Clin. Infect. Dis. 23:779-784. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen, J. H., J. D. Turnidge, and J. A. Washington. 1999. Antibacterial susceptibility tests: dilution and disk diffusion methods, p. 1526-1543. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 10.Kang, C.-I., S.-H. Kim, H.-B. Kim, S.-W. Park, Y.-J. Choi, M.-D. Oh, E.-C. Kim, and K.-W. Choe. 2003. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 37:745-751. [DOI] [PubMed] [Google Scholar]
- 11.Kim, S.-H., W.-B. Park, K.-D. Lee, C.-I. Kang, H.-B. Kim, M.-D. Oh, E.-C. Kim, and K.-W. Choe. 2003. Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci. Clin. Infect. Dis. 37:794-799. [DOI] [PubMed] [Google Scholar]
- 12.Kim, Y.-K., H. Pai, H.-J. Lee, S.-E. Park, E.-H. Choi, J. Kim, J.-H. Kim, and E.-C. Kim. 2002. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob. Agents Chemother. 46:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knaus, W. A., E. A. Drapier, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 14.Kollef, M. H. 2000. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin. Infect. Dis. 31:S131-S138. [DOI] [PubMed] [Google Scholar]
- 15.Leibovici, L., M. Paul, O. Poznanski, M. Drucker, Z. Samra, H. Konigsberger, and S. D. Pitlik. 1997. Monotherapy versus β-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob. Agents Chemother. 41:1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leibovici, L, I. Shraga, M. Drucker, H. Konigsberger, Z. Samra, and S. D. Pitlik. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244:379-386. [DOI] [PubMed] [Google Scholar]
- 17.Leone, M., A. Bourgoin, S. Cambon, M. Dubuc, J. Albanese, and C. Martin. 2003. Empirical antimicrobial therapy of septic shock patients: adequacy and impact on the outcome. Crit. Care Med. 31:462-467. [DOI] [PubMed] [Google Scholar]
- 18.MacArthur, R. D., M. Miller, T. Albertson, E. Panacek, D. Johnson, L. Teoh, and W. Barchuk. 2004. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin. Infect. Dis. 38:284-288. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A7, 7th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.National Nosocomial Infections Surveillance System. 1999. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990-May 1999, issued June 1999. Am. J. Infect. Control 27:520-532. [DOI] [PubMed] [Google Scholar]
- 21.Pittet, D., B. Thievent, R. P. Wenzel, N. Li, R. Auckenthaler, and P. M. Suter. 1996. Bedside prediction of mortality from bacteremic sepsis. A dynamic analysis of ICU patients. Am. J. Respir. Crit. Care Med. 153:684-693. [DOI] [PubMed] [Google Scholar]
- 22.Sotto, A., J. Y. Lefrant, P. Fabbro-Peray, L. Muller, J. Tafuri, F. Navarro, M. Prudhomme, and J. E. De La Coussaye. 2002. Evaluation of antimicrobial therapy management of 120 consecutive patients with secondary peritonitis. J. Antimicrob. Chemother. 50:569-576. [DOI] [PubMed] [Google Scholar]
- 23.Valles, J., J. Rello, A. Ochagavia, J. Garnacho, and M. A. Alcala. 2003. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest 123:1615-1624. [DOI] [PubMed] [Google Scholar]
- 24.Zaragoza, R., A. Artero, J. J. Camarena, S. Sancho, R. Gonzalez, and J. M. Nogueira. 2003. The influence of inadequate empirical antimicrobial treatment on patients with bloodstream infections in an intensive care unit. Clin. Microbiol. Infect. 9:412-418. [DOI] [PubMed] [Google Scholar]