Abstract
BACKGROUND
Acute traumatic coagulopathy (ATC) afflicts 20–30% of trauma patients, but the extensive collinearity of the coagulation cascade complicates attempts to clarify global clotting factor dysfunction. This study aims to characterize phenotypes of clotting factor dysfunction and their contributions to mortality after major trauma.
METHODS
This prospective cohort study examines all adult trauma patients of the highest activation level presenting to San Francisco General Hospital between 2/2005 and 2/2015. Factors II, V, VII, VIII, IX, X, and protein C activity on admission and mortality status at 28 days were assessed. Predictors of 28-day mortality in univariate analysis were included in multiple logistic regression controlling for traumatic brain injury (TBI), acidosis, age, and mechanism of injury. Principal component analysis (PCA) was utilized to identify phenotypic coagulation.
RESULTS
Complete coagulation factor data was available for 876/1,429 (61%). In multiple logistic regression, factors V (OR: 0.86, CI95%: 0.76–0.97), VIII (OR: 0.97, CI95%: 0.95–0.99), X (OR: 0.79, CI95%: 0.68–0.92), and protein C (OR: 1.17, CI95%: 1.05–1.30) significantly predicted 28-day mortality after controlling for age, base deficit, mechanism of injury, and TBI. PCA identified 2 significant principal components (Phenotypes 1 and 2) that accounted for 66.3% of the total variance. Phenotype 1 (factors II, VII, IX, X, and protein C abnormalities) explained 49.3% and was associated with increased injury, coagulopathy, TBI, and mortality. Phenotype 2 (factors V and VIII abnormalities) explained 17.0% and was associated with increased coagulopathy, blunt injury, and mortality. Only Phenotype 2 remained significantly associated with 28-day mortality in multiple logistic regression.
CONCLUSIONS
PCA identified 2 distinct phenotypes within the entirety of global clotting factor abnormalities, and these findings substantiate the crucial association of factors V and VIII on mortality following trauma. This may be the first step toward identifying unique phenotypes after injury and personalizing hemostatic resuscitation.
LEVEL OF EVIDENCE
Prognostic study, Level III
Keywords: Clotting factors, trauma-related mortality, hemorrhage
BACKGROUND
Trauma is the primary cause of mortality under 47 years old, and hemorrhage is the second leading cause of trauma-related deaths, surpassed only by CNS injury (1, 2). A majority of deaths from hemorrhage arise within the first 24 hours. An endogenous form of coagulopathy, termed acute traumatic coagulopathy (ATC), affects 20–30% of patients immediately after injury and is believed to contribute to early hemorrhagic death. ATC occurs independently of the iatrogenic dilutional coagulopathy elicited by resuscitation efforts, and has been associated with poorer outcomes, notably larger fluid resuscitation requirements, prolonged intensive care unit (ICU) stays, and higher overall mortality rates (3–5).
Prior work has shown the pathophysiology of ATC reflects overall global clotting factor dysfunction; however, the individual clotting factor roles in the development of this coagulopathy are not well defined (6, 7). Uncovering the key individual factors in ATC could allow development of targeted therapies aimed at blunting the effect of early coagulopathy. The clotting cascade is highly inter-related and elucidating the individual factor contributions to ATC requires a statistical approach that can overcome the extensive collinearity of the clotting factors. This inter-dependence of the factors renders traditional parametric techniques problematic and insufficient to truly understand mechanistic drivers and outcome-defined phenotypes of ATC. In contrast, non-parametric techniques such as principal component analysis (PCA) allow for examination of highly collinear data and have been used by our group in small analysis and others in a proof of concept manner to examine coagulation after injury (7, 8).
Preliminary work by our group examined PCA for assessing the role of individual clotting factors in the development of ATC in 2013. Amongst a 163 person cohort studied, complete coagulation data was actually only available on 88 patients and the PCA approach identified three potential coagulopathic phenotypes as major factors for ATC (7). Only two of the three phenotypes were associated with increased mortality including one representing global clotting dysfunction (of prothrombin, Factors V, VII, VIII, IX, X, Protein C, and antithrombin III) and the other representing activation of Protein C and fibrinolysis. Although the earlier work remains promising, the sample size was small and PCA is most effective in larger sample sizes to better define the relationships between inter-related variables. Utilizing a larger, more complete cohort, we hypothesized that PCA would confirm specific coagulopathic phenotypes important in ATC.
METHODS
This study investigates the association of individual coagulation factors to mortality in adult trauma patients of the highest activation level presenting to San Francisco General Hospital between 2/2005 and 2/2015 and who were enrolled into the prospective Activation of Coagulation and Inflammation in Trauma (ACIT) on-going cohort study. Enrolled patients include those in whom demographic, clinical, and laboratory data was collected on admission and in-hospital mortality status was assessed at 6 hours, 24 hours, 28 days, and discharge. Those under 15 years old, had >20% burns, or were pregnant, incarcerated, or transferred from outside hospitals were excluded. Efforts were made to obtain time zero (Emergency Department, ED) blood samples in patients eligible for enrollment. Study design and implementation were completed with approval from the Institutional Review Board at the University of California, San Francisco School of Medicine.
Coagulation factors including factors II, V, VII, VIII, IX, X, and protein C percent activity were measured on patients at time zero having an available blood sample. An INR ≥1.3 was selected to denote coagulopathy according to previous multicenter discrimination analysis by Kutcher et al. identifying patients receiving increased packed red blood cells in the first 24 hours as well as formal laboratory definitions of coagulopathy found in the literature (9–11). Univariate logistic regression was performed to assess predictors of 28 day mortality using factor levels. Those with a significance of p≤0.20 were selected for multiple logistic regression modeling of 28 day mortality controlling for age, mechanism of injury, base excess, and traumatic brain injury (TBI). Significance was determined at the p<0.05 level for independent predictors of 28 day mortality. Model fit was assessed by area under the receiver operator characteristic curves (AUC-ROCs).
Principal component analysis (PCA) was utilized for further examination of coagulopathic phenotypes with factors II, V, VII, VIII, IX, X, and protein C selected as predictor variables. PCA is a data-driven statistical technique aimed at dimensionality reduction – simplifying large-volume, collinear data by maximizing the variance along new linear coordinate axes (12). In contrast to interaction terms which can result in a tremendous loss of information, PCA restructures the data according to the most fundamental components of the population. These axes or principal components individually represent unique patient phenotypic subgroupings and can be used to interpret data much more efficiently. PCA defines patterns in the data, and then expresses these as principal components or phenotypes, to illustrate similarities and differences between the various phenotypes driving outcome. Sampling adequacy was assessed by Kaiser-Meyer-Olkin test. Eigenvalues distinguish meaningful principal components or phenotypes, and those >1 were deemed significant in concordance with the Kaiser Rule. Loading values quantify variable contribution to each principal component, and those >0.30 were considered significant. Promax rotation, a form of oblique principal component transformation, was utilized for improved data interpretability, and results were visualized graphically by loading plots and score plots.
Continuous phenotype scores were calculated for each patient, and included in multiple logistic regression controlling for age, mechanism of injury, base excess, and TBI. Patients were stratified by mortality status at 6 hours, 24 hours, 28 days, and discharge, and phenotype scores of living and deceased cohorts were compared by the Wilcoxon Rank Sum test. Lastly, the upper and lower quartiles of phenotype scores were examined for differences in demographic, clinical, and laboratory variables by Chi Square and Wilcoxon Rank Sum tests. Outcomes evaluated included: age, body mass index (BMI), gender, injury severity score (ISS), Glasgow coma scale (GCS), temperature, base excess, systolic blood pressure (SBP), mechanism of injury, TBI, international normalized ratio (INR), partial thromboplastin time (PTT), platelets, hemoglobin, and mortality at 28 days.
RESULTS
Complete coagulation factor data was available for 876 of 1,429 patients (61%), of whom 81% were male, 34% were Caucasian, and the median age was 35 years old. Median ISS was 16, with 57% suffering blunt injury and 39% experiencing TBI. 14% were coagulopathic on ED arrival (as defined by INR ≥ 1.3), and overall mortality at 28 days was 19% (TABLE 1).
TABLE 1.
Baseline Characteristics
| Variable | % of Median (IQR) |
|---|---|
| Age (Years) | 35 (29) |
| Gender | |
| Male | 80.8% |
| Female | 19.2% |
| BMI (kg/m2) | 26.0 (6.5) |
| Race | |
| Caucasian | 32.3% |
| Latino | 25.3% |
| Black | 25.0% |
| Asian | 14.6% |
| Native American | 0.7% |
| Pacific Islander | 0.6% |
| Other | 0.8% |
| Unknown | 0.6% |
| ISS | 16 |
| Base Excess (mEq/L) | −3.8 (7.6) |
| GCS | 14 |
| TBI | 39.5% |
| Mechanism of Injury | |
| Blunt | 57.0% |
| Penetrating | 43.0% |
| Mortality | |
| 6 Hours | 5.4% |
| 24 Hours | 9.0% |
| 28 Days | 19.1% |
| Discharge | 20.0% |
BMI, Body Mass Index; ISS, Injury Severity Score; GCS, Glasgow Coma Score; TBI, Traumatic Brain Injury
In univariate analysis, dysfunction of clotting factors II, V, VIII, IX, X, and protein C on admission were significantly associated with mortality at 6 hours, 24 hours, 28 days, and discharge (TABLE 2). Dysfunction of factor VII was similarly predictive of increased mortality at 6 hours, but this effect diminished starting at 24 hours and persisted for all subsequent time points.
TABLE 2.
Univariate Analysis & Multiple Logistic Regression of Coagulation Factors
| Univariate Analysis
|
Multiple Logistic Regression
|
||||||
|---|---|---|---|---|---|---|---|
| n | OR | CI (95%) | p | OR | CI (95%) | p | |
| Factorsa | |||||||
| II, (% activity) | 939 | 0.76 | (0.70–0.83) | <0.001 | |||
| V, (% activity) | 953 | 0.77 | (0.71–0.83) | <0.001 | 0.86 | (0.76–0.97) | 0.02 |
| VII, (% activity) | 913 | 0.99 | (0.95–1.04) | 0.77 | |||
| VIII, (% activity) | 964 | 0.98 | (0.97–0.99) | 0.001 | 0.97 | (0.95–0.99) | 0.007 |
| IX, (% activity) | 935 | 0.89 | (0.86–0.93) | <0.001 | |||
| X, (% activity) | 938 | 0.75 | (0.70–0.81) | <0.001 | 0.79 | (0.68–0.92) | 0.002 |
| Protein C a, (% activity) | 963 | 0.94 | (0.89–0.99) | 0.02 | 1.17 | (1.05–1.30) | 0.005 |
| TBI | 1,426 | 10.92 | (7.81–15.27) | <0.001 | 17.00 | (8.78–32.92) | <0.001 |
| Blunt Injury | 1,424 | 2.75 | (2.02–3.73) | <0.001 | 0.43 | (0.21–0.87) | 0.02 |
| Age (Years) | 1,427 | 1.04 | (1.03–1.04) | <0.001 | 1.04 | (1.03–1.06) | <0.001 |
| Base Excess, (mEq/L) | 1,023 | 0.94 | (0.92–0.97) | <0.001 | 0.90 | (0.86–0.94) | <0.001 |
| n = 631 | |||||||
| AUC = 0.89 | |||||||
Per 10 unit change
OR, Odds Ratio; CI (95%), Confidence Interval (95%); TBI, Traumatic Brain Injury
Coagulation factors remain linearly correlated, with factors II and VII, II and IX, II and X, VII and X, and IX and X exhibiting the highest correlation coefficients (SUPPLEMENTARY DIGITAL CONTENT TABLE 1). In multiple logistic regression, only factors V (OR: 0.86, 95% CI 0.76–0.97), VIII (OR: 0.97, 95% CI 0.95–0.99), X (OR: 0.79, 95% CI 0.68–0.92), and protein C (OR: 1.17, 95% CI 1.05–1.30) significantly predicted mortality 28 days after trauma after controlling for age, base excess, mechanism of injury, and TBI (p<0.05) (AUC: 0.89) (TABLE 1). For every 10% decrease in the activity of factors V, VIII, and X, there was a 14%, 3%, and 21% increase in the odds of mortality respectively.
To overcome the difficulties with collinearity incumbent in a sequential protease activation sequence such as the clotting cascade, we explored coagulopathic phenotypes after trauma using PCA. All coagulation factors and protein C exhibited a Kaiser-Meyer-Olkin value >0.50. Phenotypes 1 and 2 displayed eigenvalues >1.0 and collectively explained 66.3% of the data variance (TABLE 3).
TABLE 3.
Principal Component Analysis
| Phenotype 1 | Phenotype 2 | Total | |
|---|---|---|---|
| Eigenvalue | 3.45 | 1.19 | |
| Percent Variance | 49.3% | 17.0% | 66.3% |
|
| |||
| Factors | |||
| II, (% activity) | 0.47 | −0.06 | |
| V, (% activity) | 0.22 | 0.47 | |
| VII, (% activity) | 0.44 | −0.21 | |
| VIII, (% activity) | −0.10 | 0.85 | |
| IX, (% activity) | 0.41 | 0.01 | |
| X, (% activity) | 0.49 | −0.03 | |
| Protein C, (% activity) | 0.36 | 0.11 | |
Eigenvalues >1 were considered significant
Loading values > |0.30| were considered significant
II, factor II; V, factor V; VII; factor VII; VIII, factor VIII; IX, factor IX; X, factor X
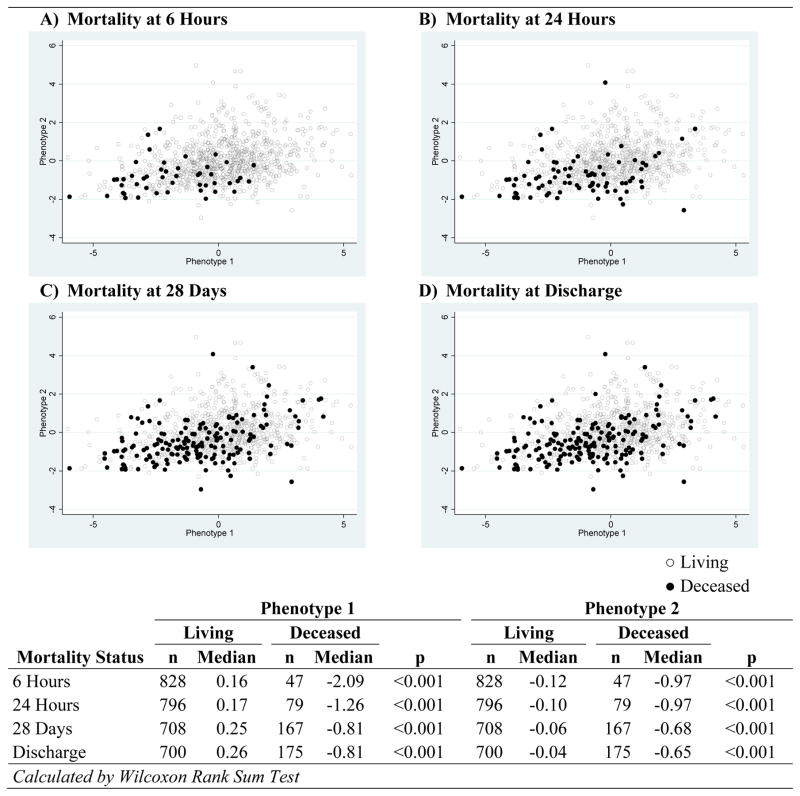
Phenotype 1 accounted for 49.3% of the variance, and factors II, VII, IX, X, and protein C were significant contributors (loading values >0.30). Phenotype 2 described 17.0% of the variance, and factors V and VIII were significant contributors (loading values >0.30). In score plots with patients identified by mortality status, deceased patients demonstrated significantly decreased Phenotype 1 and Phenotype 2 scores compared to living patients that are most notable at 6 hours and persists across all mortality time points (FIGURE 1). In multiple logistic regression, only Phenotype 2 (OR: 0.55, 95% CI 0.42–0.72) remained a significant predictor of mortality at 28 days after controlling for age, base excess, mechanism of injury, and TBI (TABLE 4).
Figure 1.
Phenotype Score Comparisons by Mortality Status
TABLE 4.
Multiple Logistic Regression of Phenotype Scores
| Variable | OR | CI (95%) | p |
|---|---|---|---|
| Phenotype 1 | 0.93 | (0.81–1.07) | 0.30 |
| Phenotype 2 | 0.55 | (0.42–0.72) | <0.001 |
| TBI | 15.40 | (8.03–29.52) | <0.001 |
| Age, (Years) | 1.05 | (1.03–1.06) | <0.001 |
| Base Excess, (mEq/L) | 0.91 | (0.87–0.94) | <0.001 |
| Blunt Injury | 0.44 | (0.22–0.88) | 0.02 |
| n = 631 | |||
| AUC = 0.88 | |||
OR, Odds Ratio; CI (95%), Confidence Interval (95%); TBI, Traumatic Brain Injury
Patients in the lowest quartile of Phenotype 1 were significantly more injured than the upper quartile (ISS 26 vs 10, p<0.001), exhibiting lower GCS (11 vs. 14, p<0.001) and higher percentage of TBI (44.0% vs 34.7%, p=0.046) (TABLE 5). Additionally this lower quartile was more acidotic (base excess −5.6 vs −2.9, p<0.001), hypothermic (36.2 vs 36.4°C, p=0.032), hypotensive (systolic blood pressure 128 vs 140 mmHg, p<0.001), and anemic (hemoglobin 12.8 vs 14.4 g/dL, p<0.001). Low Phenotype 1 patients displayed prolonged INR (INR 1.3 vs. 1.0, p<0.001), elevated PTT (30.8 vs 26.9 sec, p<0.001); and decreased platelets (238 vs 288 x103/μL, p<0.001) as well as demonstrated a higher overall mortality rate (33.9% vs. 12.3%, p<0.001). Upper and lower Phenotype 1 quartiles displayed no significant difference in age, gender, or mechanism of injury.
TABLE 5.
PCA Interquartile Range Comparisons
| Phenotype 1 (n=437)
|
Phenotype 2 (n=438)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low (p25) (n=218)
|
High (p75) (n=219)
|
Low (p25) (n=219)
|
High (p75) (n=219)
|
|||||||
| n | Median | n | Median | p | n | Median | n | Median | p | |
| Age, (Years) | 218 | 34.5 | 219 | 37.0 | 0.15 | 218 | 36.0 | 219 | 34.0 | 0.46 |
| BMI, (kg/m2) | 174 | 25.3 | 153 | 27.2 | 0.003 | 157 | 25.2 | 169 | 26.2 | 0.19 |
| Gender | ||||||||||
| Female | 46 | 21.1% | 49 | 22.4% | 0.75 | 44 | 20.1% | 33 | 15.1% | 0.17 |
| Male | 172 | 78.9% | 170 | 77.6% | 175 | 79.9% | 186 | 84.9% | ||
| ISS | 212 | 26.0 | 218 | 10.0 | <0.001 | 212 | 17.5 | 216 | 17.0 | 0.31 |
| GCS | 217 | 11.0 | 218 | 14.0 | 0.001 | 218 | 8.5 | 217 | 14.0 | <0.001 |
| Temp, (°C) | 133 | 36.2 | 174 | 36.4 | 0.03 | 138 | 36.4 | 158 | 36.2 | 0.59 |
| Base Excess, (mEq/L) | 164 | −5.6 | 141 | −2.9 | <0.001 | 160 | −4.1 | 170 | −4.4 | 0.67 |
| SBP, (mmHg) | 213 | 128.0 | 217 | 140.0 | <0.001 | 216 | 131.0 | 216 | 136.0 | 0.054 |
| Mechanism of Injury | ||||||||||
| Penetrating | 94 | 43.1% | 94 | 42.9% | 0.97 | 79 | 36.1% | 140 | 45.9% | 0.04 |
| Blunt | 124 | 56.9% | 125 | 57.1% | 100 | 63.9% | 118 | 54.1% | ||
| TBI | 44.0% | 34.7% | 0.046 | 42.9% | 37.9% | 0.28 | ||||
| INR | 192 | 1.3 | 214 | 1.0 | <0.001 | 194 | 1.1 | 208 | 1.1 | <0.001 |
| PTT, (sec) | 197 | 30.8 | 214 | 26.9 | <0.001 | 198 | 30.1 | 209 | 26.5 | <0.001 |
| Platelets, (×103/μL) | 212 | 238.0 | 217 | 288.0 | <0.001 | 214 | 248.5 | 216 | 287.0 | <0.001 |
| Hemoglobin, (g/dL) | 212 | 12.8 | 217 | 14.4 | <0.001 | 214 | 13.7 | 216 | 13.9 | 0.38 |
| Mortality at 28 Days | 74 | 33.9% | 27 | 12.3% | <0.001 | 75 | 34.3% | 26 | 11.9% | <0.001 |
Calculated by Chi Square or Wilcoxon Rank Sum Tests as appropriate
PCA, Principal Component Analysis; ISS, Injury Severity Score; GCS, Glasgow Coma Scale; Temp, Temperature; SBP, Systolic Blood Pressure; TBI, Traumatic Brain Injury; INR, International Normalized Ratio; PTT, Partial Thromboplastin Time
Regarding Phenotype 2, the lowest quartile also exhibited elevated PTT (30.1 vs 26.5 sec, p<0.001) and decreased platelets (248.5 vs 287.0 x103/μL, p<0.001) (TABLE 5). Low Phenotype 2 patients had a significantly lower GCS (8.5 vs. 14.0, p=0.001), greater percentage of blunt injury (63.9% vs. 54.1%, p=0.037), and higher overall mortality (34.3% vs 11.9%, p<0.001). However, quartiles exhibited no statistical difference in ISS, base excess, temperature, systolic blood pressure, TBI status, or hemoglobin.
DISCUSSION
The precise mechanism of ATC is unknown, but one leading theory postulates it is mediated by anticoagulant and anti-inflammatory arms of the activated protein C pathway (4, 13, 14). Following tissue damage, thrombin binds thrombomodulin, subsequently activating protein C bound to the endothelial protein C receptor (EPCR). Activated protein C works as an anticoagulant by both proteolytically cleaving coagulation factors Va and VIIIa as well as de-repressing fibrinolysis through inhibition of plasminogen activator inhibitor 1 (PAI-1), triggering dysregulated fibrinolysis by tissue-plasminogen activator (t-PA) (4, 15). Along with aPC, other likely mechanisms for ATC include: factor dysfunction, fibrinolysis, and activation of other anticoagulant pathways such as antithrombin III (ATIII) (16–18). Consistent with our previous work, our data here confirmed that abnormalities of coagulation factors II, V, VIII, IX, X, and protein C were significantly associated with mortality after major trauma in univariate analysis, but only factors V, VIII, X, and protein C remained significantly predictive after controlling for age, base excess, TBI, and mechanism of injury. This is consistent with the theory that protein C activation is the driving factor V and VIII dysfunction after injury. These results provide evidence supporting the crucial roles of factor V, VIII, and X to impaired hemostasis after trauma.
Additionally, factor VII is the only coagulation factor not significantly associated with mortality at 28 days. While it was predictive of mortality at 6 hours, this effect was absent at all subsequent time points, thereby suggesting factor VII may only be a useful component for early mortality. Akin to events found in the CONTROL randomized clinical trial of recombinant factor VII in trauma, patients receiving recombinant factor VII demonstrated no change in mortality compared to placebo(19). Further work to identify phenotypes associated with relative factor VII dysfunction would potentially identify patients who may benefit from rVIIa.
Principle Component Analysis
While the univariate analysis was compelling, we are acutely aware of issues with collinear data when examining a sequential coagulation activation cascade. Importantly, PCA provides a means of combatting the specific complications arising from predictor variable interdependence. Re-examining the data using effective linear axes can help uncover noteworthy characteristics and overarching trends that may otherwise remain hidden within statistical noise from traditional regression. Furthermore, PCA has been previously utilized in the setting of ATC to examine the biological mechanisms of coagulopathy as well as corresponding functional phenotypes via rapid thromboelastography (TEG). Both Chin et al. and Kutcher et al. identified global clotting factor abnormalities and hyperfibrinolysis to be unique processes contributing to the development of ATC(7, 8). This PCA study expands the preliminary work of our group completed by Kutcher et al. but with a specific focus on clotting factor abnormalities and implemented in a much larger patient cohort.
In this analysis, we found global clotting factor dysfunction is broken into two subgroups – 1) factors II, VII, IX, X, and protein C (represented by Phenotype 1); 2) factors V and VIII (represented by Phenotype 2). Together, Phenotype 1 and Phenotype 2 account for 66.3% of the variability, and 49.3% and 17.0%, respectively. Phenotypes exhibit positive loadings of all contributing components, and accordingly, low phenotype scores denote clotting factor abnormalities (defined by decreased clotting factor or protein c percent activity).
In score plots stratified by mortality status, the deceased cohort expressed significantly lower Phenotype 1 and Phenotype 2 scores compared to their living counterparts. This effect was most prominent at 6 hours, but remained significant until hospital discharge. A majority of deaths from trauma hemorrhage occur within the first 24 hours, during which the hypocoagulable early phase of ATC is most prominent(1, 16). These findings suggest 1) global clotting factor dysfunction is most influential in the hours immediately following injury, and 2) these aftereffects are ultimately longstanding and continue to associate with mortality up to a month beyond the initial insult.
In multiple logistic regression, only Phenotype 2 remained a significant predictor of mortality at 28 days after controlling for TBI, base excess, age, and mechanism of injury. Even when accounting for the issues of collinearity, factors V and VIII continue to identify themselves as significant components to trauma outcomes. Unfortunately, no prior studies exist specifically delving into the roles of factors V and VIII in the setting of trauma. However, previous analyses of the broader ATC revealed levels of factor V associate with hypoperfusion, and dysfunction of coagulation factors V and VIII correlate with activation of protein C(4, 20). Factor V has been more extensively studied in non-trauma situations, including liver transplantation and shock. Zulian et al. found factor V serves as an early predictor of allograft failure and death in liver transplant patients(21). Additionally, the PROWESS trial found heterozygous factor V leiden carriers displayed reduced mortality in the setting of sepsis(22). In subsequent mouse studies, Liang et al identified protein C cleaved factor V as a crucial cofactor inhibiting a novel inflammatory tissue factor signaling pathway mediated by activated protein C(23). Discovery of factor V’s role in a new murine protein C mediated inflammatory pathway is exciting and insinuates factor V may play an even larger function in dictating the balance of hypocoagulability, hypercoagulability, and inflammation after trauma. Of course, substantiation of this pathway and its interacting components must first occur in humans. Together, these works evidence the impact of factors V and VIII and their possible roles in the coordination of trauma outcomes and the inflammatory response post-trauma. While preliminary, our work suggests that individualized phenotypes of post trauma coagulopathy can be identified which in the era of personalized medicine can be uniquely targeted. In the future, we speculate that perhaps targeted replacement of factors V and VIII may more effectively guide resuscitation than comprehensive non-targeted replacement via plasma. Further studies assessing clotting factor repletion after trauma would could be particularly promising.
In interquartile comparisons of phenotype groups, dysfunction of clotting factors II, VII, IX, X, and protein C (low Phenotype 1) was associated with more severe injury, denoted by significantly worse ISS, GCS, base excess, temperature, and systolic blood pressure. Intuitively, coagulation factor dysfunction should be apparent on laboratory testing, and this association was evident by elevated INR, prolonged PTT, and decreased platelets and hemoglobin. Patients also displayed a higher percentage of TBI and overall mortality. Components of Phenotype 1 encompass both intrinsic and extrinsic arms of the coagulation cascade, thus paralleling identification of global clotting factor dysfunction in prior PCAs from Chin et al. and Kutcher et al. (7, 8).
In contrast, abnormalities of factors V and VIII (low Phenotype 2) was not associated with an increased level of injury, as low Phenotype 2 patients displayed no statistical difference in ISS, base excess, temperature, or systolic blood pressure. Interestingly despite a similar level of injury, these patients demonstrated prolonged PTT, decreased platelets, and higher overall mortality. Furthermore, low Phenotype 2 individuals displayed an increased proportion of blunt injury. Opposed to penetrating injury, in blunt injury kinetic energy is dispersed over a greater surface area, thus yielding more pronounced global tissue damage. With tissue factor extensively expressed by the cellular components of the vascular system and released during trauma, we surmise blunt injury may result in a greater efflux of tissue factor (24–27). The extrinsic coagulation pathway, initiated by tissue factor release, has long been considered the primary driver of hemostasis post-trauma. However, the role of tissue factor in coagulopathy is poorly understood. Moreover, the increase in thrombin generating potential has been a puzzling aspect of protein C-mediated coagulopathy. In previous work by our group in mouse models of trauma, ATC is facilitated by thrombin activated by the tissue factor pathway (28). Though correlate interactions have yet to be validated in humans, this putative mechanism explains the association of factor V and VIII dysfunction with blunt injury as well as the increased thrombin generation of protein C-mediated ATC.
Study Limitations
This study has several potential limitations. First, complete coagulation data was only available for 876/1,429 patients (61%) as not all patients had a time zero blood sample drawn. Those patients who were transported immediately to the operating room due to being in extremis were more likely to have a time zero sample obtained in the emergency department. Our clinical practice is to attempt to obtain rapidly at least one intravenous access point to get a type and cross blood sample prior to transport to the operating room. Our research samples are drawn by protocol from this same intravenous line prior to use of the line for blood products or intravenous fluids. Given this reflection of clinical practice it is not surprising that we found individuals with non-missing data to bemore injured than their missing-data counterparts (ISS 16 vs. 10, p<0.001; Base excess −3.8 vs. −2.6, p=0.0045). Though our data are missing in a non-random fashion, we succeeded in targeting the most severely injured patients and subsequently believe our results to be robust to elucidate trauma pathophysiology in the highest risk patient group.
Additionally, PCA as a technique possesses limited generalizability. Phenotypes are not directly clinically interpretable, and phenotypes scores cannot be calculated for patients outside the original sample population. Further, the need for an a priori inclusion of predictor variables opens the possibility of predictor selection bias as coagulopathy after trauma is governed by numerous proteins beyond those included in this analysis. However, we intended this study to be an exploratory investigation focused on clotting factor dysfunction immediately after injury. Identifying patterns of coagulation factors is a valuable step toward unmasking the complexities of extrinsic and intrinsic arms of the coagulation cascade post-trauma. Expanding these findings with inclusion of inflammatory mediators and thromboelastography (TEG) results would be a crucial next step.
CONCLUSION
In summary, these findings substantiate the crucial association of factors V and VIII on mortality following severe trauma. The complex collinearity of coagulation factors can prove difficult for standard regression techniques, but we found PCA identifies 2 distinct patterns within the entirety of global clotting factor abnormalities forming a theoretical basis for identification of unique phenotypes after injury which may potentially benefit from personalized targeted treatment rather than one-size-fits-all plasma based hemostatic resuscitation. Both exhibit clear correlation with clinical outcomes and further evidence the impact of factors V and VIII on early and late trauma mortality.
Supplementary Material
Acknowledgments
FUNDING/SUPPORT
Ryan Kunitake is supported by the National Center for Advancing Translational Sciences, National Institutes of Health via the University of California San Francisco Clinical and Translational Institute (TL1 TR000144). Rachael Callcut is supported with a career development award from the National Institute of Environmental Health Sciences, National Institute of Health Big Data to Knowledge Initiative (K01ES026834). Additional funding was provided by the Department of Defense (W81XWH-10-1-0509) and NIH Tactic Grant (1UM1HL120877). The contents of this paper are solely the responsibility of the authors and do not represent the official views of the NIH.
Footnotes
MEETINGS: This work was presented in part at the Pacific Coast Surgical Association Annual Meeting, February 2016, in Kona, Hawaii.
CONFLICTS OF INTEREST: None
AUTHOR CONTRIBUTIONS:
Literature search – Kunitake, Callcut
Study design – Kunitake, Christie, Conroy, Cohen, Callcut
Data collection – Howard, Kornblith, Christie, Conroy
Data analysis – Kunitake, Callcut
Data interpretation – Kunitake, Howard, Kornblith, Christie, Cohen, Callcut
Writing – Kunitake, Cohen, Callcut
Critical Revision - Howard, Kornblith, Christie, Cohen, Callcut
FINANCIAL DISCLOSURES: Ryan Kunitake is supported by the National Center for Advancing Translational Sciences, National Institutes of Health via the University of California San Francisco Clinical and Translational Institute (TL1 TR000144). Rachael Callcut is supported with a career development award from the National Institute of Environmental Health Sciences, National Institute of Health Big Data to Knowledge Initiative (K01ES026834).
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 3.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255(2):379–85. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peltan ID, Vande Vusse LK, Maier RV, Watkins TR. An International Normalized Ratio-Based Definition of Acute Traumatic Coagulopathy Is Associated With Mortality, Venous Thromboembolism, and Multiple Organ Failure After Injury. Crit Care Med. 2015;43(7):1429–38. doi: 10.1097/CCM.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S40–7. doi: 10.1097/TA.0b013e31828fa43d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74(5):1223–9. doi: 10.1097/TA.0b013e31828b7fa1. discussion 9–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin TL, Moore EE, Moore HB, Gonzalez E, Chapman MP, Stringham JR, Ramos CR, Banerjee A, Sauaia A. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014;156(3):570–7. doi: 10.1016/j.surg.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutcher ME, Howard BM, Sperry JL, Hubbard AE, Decker AL, Cuschieri J, Minei JP, Moore EE, Brownstein BH, Maier RV, et al. Evolving beyond the vicious triad: Differential mediation of traumatic coagulopathy by injury, shock, and resuscitation. J Trauma Acute Care Surg. 2015;78(3):516–23. doi: 10.1097/TA.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 10.Lu RP, Ni A, Lin FC, Ortiz-Pujols SM, Adams SD, Monroe DM, 3rd, Whinna HC, Cairns BA, Key NS. Major burn injury is not associated with acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;74(6):1474–9. doi: 10.1097/TA.0b013e3182923193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allard CB, Scarpelini S, Rhind SG, Baker AJ, Shek PN, Tien H, Fernando M, Tremblay L, Morrison LJ, Pinto R, et al. Abnormal coagulation tests are associated with progression of traumatic intracranial hemorrhage. J Trauma. 2009;67(5):959–67. doi: 10.1097/TA.0b013e3181ad5d37. [DOI] [PubMed] [Google Scholar]
- 12.Dunteman GH. Principal components analysis. Sage; 1989. [Google Scholar]
- 13.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiaans SC, Wagener BM, Esmon CT, Pittet JF. Protein C and acute inflammation: a clinical and biological perspective. Am J Physiol Lung Cell Mol Physiol. 2013;305(7):L455–66. doi: 10.1152/ajplung.00093.2013. [DOI] [PubMed] [Google Scholar]
- 15.Esmon CT. Protein C anticoagulant system--anti-inflammatory effects. Semin Immunopathol. 2012;34(1):127–32. doi: 10.1007/s00281-011-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson GP, Letson HL, Sharma R, Sheppard FR, Cap AP. Mechanisms of early trauma-induced coagulopathy: The clot thickens or not? J Trauma Acute Care Surg. 2015;79(2):301–9. doi: 10.1097/TA.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 17.Oshiro A, Yanagida Y, Gando S, Henzan N, Takahashi I, Makise H. Hemostasis during the early stages of trauma: comparison with disseminated intravascular coagulation. Crit Care. 2014;18(2):R61. doi: 10.1186/cc13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawamura A, Hayakawa M, Gando S, Kubota N, Sugano M, Wada T, Katabami K. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb Res. 2009;124(5):608–13. doi: 10.1016/j.thromres.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent JL, Tortella BJ, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 20.Jansen JO, Scarpelini S, Pinto R, Tien HC, Callum J, Rizoli SB. Hypoperfusion in severely injured trauma patients is associated with reduced coagulation factor activity. J Trauma. 2011;71(5 Suppl 1):S435–40. doi: 10.1097/TA.0b013e318232e5cb. [DOI] [PubMed] [Google Scholar]
- 21.Zulian MC, Chedid MF, Chedid AD, Grezzana Filho TJ, Leipnitz I, de Araujo A, Alvares-da-Silva MR, Cardoni MG, Guimaraes LS, Kruel CD, et al. Low serum factor V level: early predictor of allograft failure and death following liver transplantation. Langenbecks Arch Surg. 2015;400(5):589–97. doi: 10.1007/s00423-015-1290-2. [DOI] [PubMed] [Google Scholar]
- 22.Kerlin BA, Yan SB, Isermann BH, Brandt JT, Sood R, Basson BR, Joyce DE, Weiler H, Dhainaut JF. Survival advantage associated with heterozygous factor V Leiden mutation in patients with severe sepsis and in mouse endotoxemia. Blood. 2003;102(9):3085–92. doi: 10.1182/blood-2003-06-1789. [DOI] [PubMed] [Google Scholar]
- 23.Liang HP, Kerschen EJ, Basu S, Hernandez I, Zogg M, Jia S, Hessner MJ, Toso R, Rezaie AR, Fernandez JA, et al. Coagulation factor V mediates inhibition of tissue factor signaling by activated protein C in mice. Blood. 2015;126(21):2415–23. doi: 10.1182/blood-2015-05-644401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Utter GH, Owings JT, Jacoby RC, Gosselin RC, Paglieroni TG. Injury induces increased monocyte expression of tissue factor: factors associated with head injury attenuate the injury-related monocyte expression of tissue factor. J Trauma. 2002;52(6):1071–7. doi: 10.1097/00005373-200206000-00008. discussion 7. [DOI] [PubMed] [Google Scholar]
- 25.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59(2):421–37. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 26.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–97. [PMC free article] [PubMed] [Google Scholar]
- 27.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108(5):1447–52. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard BM, Miyazawa BY, Dong W, Cedron WJ, Vilardi RF, Ruf W, Cohen MJ. The tissue factor pathway mediates both activation of coagulation and coagulopathy after injury. J Trauma Acute Care Surg. 2015;79(6):1009–14. doi: 10.1097/TA.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.