Abstract
Genetic aspects of alcoholism have been modeled using rats selectively bred for extremes of alcohol preference and voluntary alcohol intake. These lines show similar alcohol drinking phenotypes but have different genetic and environmental backgrounds and may therefore display diverse behavioral traits as seen in human alcoholics. The multivariate concentric square field™ (MCSF) test is designed to provoke exploration and behaviors associated with risk assessment, risk taking and shelter seeking in a novel environment. The aim was to use the MCSF to characterize behavioral profiles in rat lines from selective breeding programs in the United States (P/NP, HAD1/LAD1, HAD2/LAD2), Italy (sP/sNP) and Finland (AA/ANA). The open field and elevated plus maze tests were used as reference tests. There were substantial differences within some of the pairs of selectively bred rat lines as well as between all alcohol-preferring rats. The most pronounced differences within the pairs of lines were between AA and ANA rats and between sP and sNP rats followed by intermediate differences between P and NP rats and minor differences comparing HAD and LAD rats. Among all preferring lines, P, HAD1 and HAD2 rats shared similar behavioral profiles, while AA and sP rats were quite different from each other and the others. No single trait appeared to form a common ‘pathway’ associated with a high alcohol drinking phenotype among all of the alcohol-preferring lines of rats. The marked behavioral differences found in the different alcohol-preferring lines may mimic the heterogeneity observed among human alcoholic subtypes.
Keywords: Alcohol non-preferring rats, alcohol-preferring rats, elevated plus maze (EPM), multivariate concentric square field™ (MCSF), open field (OF), selective breeding
INTRODUCTION
The development of animal models using selective breeding has been useful as a strategy for understanding the genetic, environmental and neurobiological underpinnings of excessive alcohol intake and dependence (Grahame 2000). To this end, several different lines of rats have been selectively bred for high and low oral alcohol preference and intake, including the University of Chile alcohol-drinking/non-drinking (UChB/UChA) rats (Mardones & Segovia-Riquelme 1983), the Alko alcohol/non-alcohol (AA/ANA) rats (Eriksson 1968), the Indiana alcohol-preferring/non-preferring (P/NP) rats and the two replicate lines of high/low alcohol-drinking (HAD1-2/LAD1-2) rats (Li et al. 1987; Li, Lumeng & Doolittle 1993), the Sardinian alcohol-preferring/non-preferring (sP/sNP) rats (Colombo et al. 2006) and the Warsaw high-/low-preferring (WHP/WLP) rats (Dyr & Kostowski 2008). These lines were bred for the same phenotypes, i.e. high or low alcohol preference and consumption under a standard, home cage, two-bottle free choice paradigm with continuous access to alcohol, water and food. One common goal of all of these selective breeding programs has been to compare the high and low alcohol-drinking lines to determine behavioral characteristics associated with selection for extremes of alcohol preference in alcohol naive animals.
The multivariate concentric square field™ (MCSF) test (Meyerson et al. 2006) has an ethological foundation and is designed to provoke exploration and behaviors associated with risk assessment, risk taking and shelter seeking by rodents in a novel environment. The arena involves a variety of zones, including sheltered, open and elevated areas, exploratory incentives, areas with different illumination and corridors enclosed by walls. The purpose of this multivariate design is to gather information that, taken together, enables a behavioral profiling of the animal in one and the same test situation (Meyerson et al. 2006).
The MCSF test has been useful for behavioral profiling of lines selectively bred for high and low alcohol intake, i.e. the AA and ANA rats (Roman et al. 2007) and the sP and sNP rats (Roman & Colombo 2009). The present investigation replicates and extends this work by comparing five different pairs of selectively bred lines of rats: the AA/ANA, sP/sNP, P/NP and the two replicate HAD1-2/LAD1-2 lines. The AA/ANA, sP/sNP and P/NP rats were generated from outbred Wistar rats, while the AA/ANA rats were raised from a mixed background including Wistar rats (Hilakivi et al. 1984; Murphy et al. 2002; Colombo et al. 2006). The HAD1-2 and LAD1-2 rats were bred from N/Nih rats, a heterogeneous foundation stock generated by crossing eight different inbred rat strains (Li et al. 1993). Therefore, Wistar rats and N/Nih rats were also evaluated in the present investigation along with the selectively bred lines.
A notable feature of this study is that all of the lines were tested concurrently, at the same age, by the same experimenters and in the same laboratory. This allowed direct comparison not just within each pair of lines but also across all of the lines selectively bred for differential alcohol drinking. The main goal was to use the MCSF test as well as the elevated plus maze (EPM) and open field (OF) tests to (i) compare each pair of lines; (ii) replicate previous results obtained from testing AA/ANA and sP/sNP rats; and (iii) investigate possible conformity in behavioral patterns linked to the alcohol-preferring and alcohol non-preferring phenotypes.
MATERIALS AND METHODS
Animals and housing
Adult male alcohol-naive AA and ANA rats [generation S97; National Institute for Health and Welfare (THL), Helsinki, Finland; n = 12/group], sP and sNP rats (generation S71; Charles River Laboratories, Calco, Italy; n = 16/group), P and NP rats (generation S64-65), HAD1 and LAD1 rats (generation S54) and HAD2 and LAD2 rats (generation S52; Indiana University School of Medicine, Indianapolis, IN; n = 12/group) were used. Age-matched, male Wistar rats (Hsd:WI; Harlan, Indianapolis, IN) and N/Nih rats (Indiana University School of Medicine, Indianapolis, IN) were also included (n = 12/group). The rats (12–14 weeks old when tested) were housed two per cage in acrylic cages (45 × 23 × 20 cm) with wood-chip bedding in a temperature-controlled and humidity-controlled animal room under a reversed 12-hour light/dark cycle. All rats were maintained for at least 3 weeks prior to behavioral testing. Research protocols were approved by the School of Science Institutional Animal Care and Use Committee and are in accordance with the guidelines of the National Institutes of Health, the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council of the National Academies 2003) and the European Communities Council Directive (86/609/EEC).
Procedure
During the week before testing, rats were handled, weighed and adapted to the bucket used to transport them from the home cage to the testing rooms. The MCSF was the main test for this investigation, with the OF and EPM tests serving as reference tests. Each animal was tested only once in each of the three tests over three consecutive days with the MCSF test completed on the first day, the OF test on the second and finally the EPM test on the third day. The sequential order of the three tests was based on a pilot study indicating that the MCSF test was most sensitive to previous experience and should be performed first to avoid carry-over effects (Augustsson 2004). The pairs of lines were tested in the following order: AA/ANA, P/NP, HAD1-2/LAD1-2 and N/Nih, and sP/sNP and Wistar. Testing was performed in separate rooms during the dark period of the light/dark cycle. Between rats, the apparatus was wiped with 10% alcohol solution and allowed to dry.
The MCSF test
The MCSF apparatus and its defined zones are shown in Fig. 1a, and the parameters measured are listed in Table 1 and Appendix S1. Each animal was released in the CENTER facing the wall between the CENTER and BRIDGE, and the test session lasted 20 minutes. The approximate light conditions (lx) in the MCSF arena were as follows: DCR: < 1; CENTER: < 20; CORRIDORs and HURDLE: < 10; SLOPE: < 30; BRIDGE: 600–650.
Figure 1.
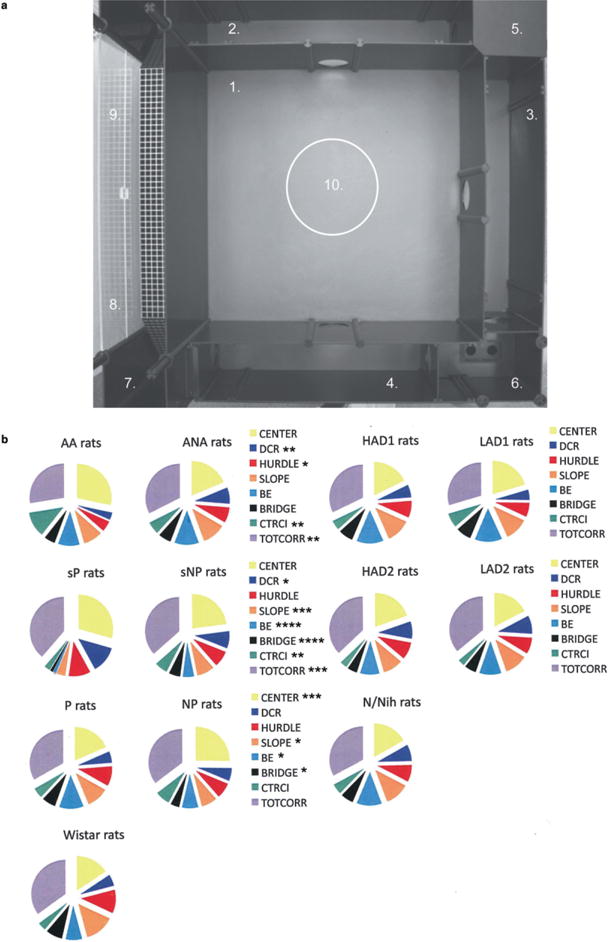
(a) The MCSF arena (100 × 100 cm) and the defined zones (Roman & Colombo 2009) numbered as follows: 1, CENTER, 70 × 70 cm, open area; 2–4, CORRIDORs, transit areas; 5, dark corner room (DCR), area for shelter seeking; 6, HURDLE, high passage to hole board with photocell to count head dips, exploratory incentive; 7, SLOPE, leading up to BRIDGE, risk assessment area; 8, BRIDGE ENTRANCE, risk assessment area; 9, BRIDGE, elevated and illuminated, risk area; 10, CENTRAL CIRCLE, 25 cm diameter, risk area. (b) The percentage number of visits to the CENTER, dark corner room (DCR), HURDLE, SLOPE, BRIDGE ENTRANCE (BE), BRIDGE, CENTRAL CIRCLE (CTRCI) and the CORRIDORs (TOTCORR) in the MCSF test in AA and ANA rats, sP and sNP rats, P and NP rats, all originally related to outbred Wistar rats, and HAD1 and LAD1 rats and HAD2 and LAD2 rats, derived from N/Nih rats. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 comparing the respective alcohol-preferring and non-preferring lines (Mann–Whitney U-test)
Table 1.
A summary table over MCSF parameters for which there were significant differences between high and low drinking lines.
| Functional categories | Parameters | P versus NP | AA versus ANA | sP versus sNP | H1 versus L1 | H2 versus L2 |
|---|---|---|---|---|---|---|
| General activity | TOTACT | P < NP* | AA < ANA* | sP < sNP**** | ||
| FRQ TOTCORR | P < NP* | AA < ANA* | sP < sNP** | H2 >L2 * | ||
| FRQ CENTER | P < NP* | sP < sNP*** | ||||
| DUR CENTER | AA > ANA*** | |||||
| DUR/FRQ CENTER | P > NP* | AA > ANA* | sP > sNP ** | |||
| Exploratory activity | LAT LEAVE | P > NP** | AA > ANA**** | sP > sNP*** | ||
| DUR TOTCORR | P > NP* | sP > sNP*** | ||||
| DUR/FRQ TOTCORR | P > NP** | AA > ANA* | sP > sNP**** | |||
| LAT HURDLE | AA > ANA** | sP > sNP** | H2 < L2* | |||
| FRQ HURDLE | AA < ANA** | |||||
| DUR HURDLE | sP < sNP* | |||||
| DUR/FRQ HURDLE | AA > ANA** | sP < sNP** | H1 < L1*** | H2 < L2** | ||
| FRQ HEAD DIP | AA < ANA* | |||||
| # ZONES VISITED | sP < sNP** | |||||
| OCC VISIT ALL ZONES | sP < sNP### | |||||
| REARING | sP < sNP**** | |||||
| Risk assessment | LAT SLOPE | AA > ANA* | sP > sNP*** | H2 < L2* | ||
| FRQ SLOPE | AA < ANA** | sP < sNP**** | ||||
| DUR SLOPE | sP < sNP**** | |||||
| DUR/FRQ SLOPE | AA > ANA*** | |||||
| OCC SLOPE | sP < sNP## | |||||
| LAT BRIDGE ENTRANCE | AA > ANA* | sP > sNP*** | H2 < L2** | |||
| FRQ BRIDGE ENTRANCE | AA < ANA** | sP < sNP**** | ||||
| DUR BRIDGE ENTRANCE | sP < sNP** | |||||
| DUR/FRQ BRIDGE ENTRANCE | AA > ANA* | |||||
| OCC BRIDGE ENTRANCE | sP < sNP## | |||||
| SAP TO CENTER | sP > sNP** | |||||
| OCC SAP TO CENTER | sP > sNP## | |||||
| SAP TO SLOPE | sP > sNP**** | H2 < L2* | ||||
| Risk taking | LAT BRIDGE | AA > ANA* | sP > sNP** | H2 < L2** | ||
| FRQ BRIDGE | AA < ANA** | sP < sNP**** | ||||
| DUR BRIDGE | sP < sNP**** | |||||
| DUR/FRQ BRIDGE | AA > ANA* | |||||
| OCC BRIDGE | sP < sNP### | |||||
| FRQ CTRCI | AA > ANA* | sP < sNP* | H1 < L1* | |||
| DUR CTRCI | AA > ANA** | sP < sNP* | H1 < L1** | |||
| DUR/FRQ CTRCI | AA > ANA** | H1 < L1** | ||||
| OCC CTRCI | sP < sNP## | |||||
| Shelter seeking | LAT DCR | AA > ANA*** | ||||
| FRQ DCR | AA < ANA*** | |||||
| DUR DCR | AA < ANA* | |||||
| Anxiety-like behavior | DUR SHELTER/RISK INDEX | sP > sNP*** | ||||
| Impulsive-like behavior | SLOPE/BRIDGE INTERVAL | sP > sNP** |
Behavioral parameters, recorded during the 20-minute trial of the MCSF test, for which there were significant differences within the respective high and low drinking pair of selectively bred rats. Occurrence (OCC) indicates the zones that were not visited by all animals in each group or the behaviors that were not performed by all animals in each group (n = 12–16/group). The sum of frequencies to the three CORRIDORs (FRQ TOTCORR) and to all zones (TOTACT) was used for assessment of general locomotor activity. The total time spent in the three CORRIDORs was given the denomination DUR TOTCORR. The shelter/risk index is calculated from the difference in time spent in the dark corner room (DCR) and on the BRIDGE, relative to the total time spent in the two zones and is used as one way of interpreting anxiety-like behavior. A positive value indicates that the animals spent more time in the DCR than on the BRIDGE and is interpreted as higher anxiety-like behavior. The SLOPE/BRIDGE interval reveals how long time it takes the animals to enter the BRIDGE in relation to first entering the SLOPE and is here used as one way of interpreting impulsive-like behavior. Thus, a value close to zero indicates less risk assessment and a fast risk-taking response.
P ≤ 0.05,
P < 0.01,
P < 0.001,
P < 0.0001 comparing each pair of selectively bred rats (Mann–-Whitney U-test);
P 0.05,
P < 0.01,
P < 0.001 comparing each pair of selectively bred rats (Pearson Chi-square test). Raw data values are shown in Appendix S1. CTRCI = central circle; DCR = dark corner room; DUR = duration (s); DUR/FRQ = duration per visit (s); FRQ = frequency; LAT = latency (s); OCC = occurrence; SAP = stretched attend posture; TOTACT = total activity, i.e. the sum of all frequencies; TOTCORR = total corridor, i.e. the sum of all corridors.
The OF test
The OF arena was a square field (90 × 90 cm). Lines divided the arena into a 6 × 6 array of small squares (15 × 15 cm) used to score number of crossings. Note, the center portions of the OF and MCSF were of different shapes and relative sizes. The parameters tested are described in Table 2A and Appendix S1. Each rat was released in the peripheral zone, and the test session lasted 10 minutes. The approximate light intensity in the OF arena was 35 lx.
Table 2.
A summary table of OF parameters (A) and EPM parameters (B) for which there were significant differences between high and low drinking lines.
| Functional categories | Parameters | P versus NP | AA versus ANA | sP versus sNP | H1 versus L1 | H2 versus L2 |
|---|---|---|---|---|---|---|
| A. Open field | ||||||
| General activity | TOTACT | sP < sNP**** | H1 < L1*** | H2 > L2* | ||
| CROSS | P > NP*** | sP < sNP**** | H1 < L1*** | H2 > L2*** | ||
| Risk taking | FRQ CENTER | sP < sNP*** | H1 < L1*** | H2 > L2* | ||
| DUR CENTER | sP < sNP*** | H1 < L1**** | ||||
| DUR/FRQ CENTER | AA > ANA* | H1 < L1* | ||||
| OCC CENTER | sP < sNP## | |||||
| %DUR CENTER | sP < sNP**** | H1 < L1**** | ||||
| Thigmotaxis | FRQ PERIPHERY | sP < sNP**** | H1 < L1*** | H2 > L2* | ||
| DUR PERIPHERY | sP > sNP**** | H1 > L1**** | ||||
| DUR/FRQ PERIPHERY | sP > sNP**** | H1 > L1*** | H2 < L2* | |||
| %DUR PERIPHERY | sP > sNP**** | H1 > L1**** | ||||
| B. Elevated plus maze | ||||||
| General activity | TOTACT | AA < ANA**** | sP < sNP**** | |||
| Exploratory activity | LAT LEAVE | P > NP* | ||||
| Risk assessment | FRQ CENTER | AA < ANA**** | sP < sNP*** | |||
| DUR CENTER | P > NP* | AA > ANA* | H1 < L1** | |||
| DUR/FRQ CENTER | AA > ANA**** | sP > sNP** | ||||
| %DUR CENTER | P > NP* | AA > ANA* | H1 < L1** | |||
| Risk taking | LAT OPEN | H2 > L2* | ||||
| FRQ OPEN | sP < sNP* | |||||
| DUR OPEN | AA > ANA* | sP < sNP* | H2 < L2* | |||
| DUR/FRQ OPEN | AA > ANA** | H2 < L2* | ||||
| OCC OPEN | sP < sNP### | |||||
| %DUR OPEN | AA > ANA* | sP < sNP**** | ||||
| %FRQ OPEN | sP < sNP**** | |||||
| “Shelter” seeking | FRQ CLOSED | AA < ANA**** | sP < sNP* | |||
| DUR CLOSED | AA < ANA* | sP > sNP** | H1 > L1* | |||
| DUR/FRQ CLOSED | sP > sNP*** | H1 > L1** | ||||
| %DUR CLOSED | AA < ANA* | sP > sNP** | H1 > L1* |
Behavioral parameters, recorded during the 10-minute trial of the OF test (A) and the 5-minute trial of the EPM test (B), for which there were significant differences within the respective high and low drinking pair of selectively bred rats. Occurrence (OCC) indicates the zones that were not visited by all animals in each group or the behaviors that were not performed by all animals in each group (n = 11–16/group). In the OF test, duration and frequency of visits into a peripheral zone, 15 cm from each of the four walls of the arena was used for assessment of thigmotaxis, whereas duration and frequency of visits into the central part (60 × 60 cm) revealed center activity. The total number of lines crossed (CROSSINGS) and the sum of visits to the periphery and center (TOTACT) were used as measures of general locomotor activity. In the EPM test, the sum of visits to the open and closed arms (TOTACT) was used for assessment of general locomotor activity.
P ≤ 0.05,
P < 0.01,
P < 0.001,
P < 0.0001 comparing each pair of selectively bred rats (Mann–Whitney U-test);
P 0.05,
P < 0.01,
P < 0.001 comparing each pair of selectively bred rats (Pearson Chi-square test). Raw data values are shown in Appendix S1 Tables. CROSS = number of lines crossed in the OF; DUR = duration (s); DUR/FRQ = duration per visit (s); FRQ = frequency; LAT = latency (s); LAT LEAVE = time to leave the periphery area of the OF or center area of the EPM at the beginning of the session; OCC = occurrence; TOTACT = total activity, i.e. the sum of entries into the center and periphery of the OF or into the open and closed arms of the EPM.
The EPM test
The EPM apparatus (AccuScan, Columbus, OH) was white acrylic plastic with two open arms (50 × 10 cm) at right angles to two wall-enclosed and covered arms (50 × 10 × 50 cm), raised 90 cm off the floor. The parameters tested are listed in Table 2B and Appendix S1. Each rat was released in the center facing an open arm, and the test session lasted 5 minutes. The light conditions on the open and closed arms were approximately 45 lx and 10 lx, respectively.
Behavioral recordings
The animals were monitored by video cameras, while the observer watched remotely from an adjacent room. In the MCSF test, the numbers of stretched attend postures (SAPs; from the CORRIDORs into the CENTER and from the CORRIDOR into the SLOPE) and rearings were recorded by direct observation, and the number of head dips into the hurdle hole board was noted. Scoring was performed using the Observer XT 8.0 (Noldus Information Technology, Wageningen, the Netherlands). Visits to the defined zones were only scored as such if both hind legs had crossed over into that section.
Statistical analysis
The majority of the data was not normally distributed, and analysis was done using non-parametric statistics. The Mann–Whitney U-test or Kruskal–Wallis test was used where appropriate. The Pearson Chi-square test was used for analysis of occurrence, i.e. the number of animals entering a zone or performing a behavior. Differences were considered statistically significant at P ≤ 0.05. Statistica 8.0 (StatSoft Inc., Tulsa, OK) was used for the statistical analyses.
A trend analysis, conceptually similar to a multivariate analysis of variance, was used for the primary analyses of the MCSF data. For each of five functional behavioral categories, related variables were combined into single composite dependent variables. Individual rank values were summarized within the functional categories general activity (TOTAL ACTIVITY, FRQ TOTCORR and CENTER, and DUR/FRQ TOTCORR), exploratory activity (DUR TOTCORR, CENTER and HURDLE, REARING, and number of PHOTOCELL COUNTS in the hole board), risk assessment (SAP to CENTER and SLOPE, and DUR/FRQ SLOPE and BRIDGE ENTRANCE), risk-taking behavior (FRQ BRIDGE and CENTRAL CIRCLE, DUR BRIDGE and CENTRAL CIRCLE, and DUR/FRQ BRIDGE and CENTRAL CIRCLE) and shelter-seeking behavior (FRQ, DUR, and DUR/FRQ DCR). The individuals were ranked against each other, either within each selectively bred pair of rats or within the group of preferring rats and the group of non-preferring rats. The rank values were then summed into a sum rank for each functional category and analyzed.
An additional multivariate data analysis was used to examine MCSF performance. A partial least squares to latent structures (PLS) analysis (Eriksson et al. 2006) was used to investigate the similarity between all the alcohol-preferring lines. SIMCA-P+ 12.0 (Umetrics AB, Umeå, Sweden) was used.
RESULTS
Descriptive statistics
Tables with a complete listing of the means ± standard errors of the mean (SEM) for each line are provided in Appendix S1. Tables 1 and 2 show the MCSF, OF and EPM parameters for which there were significant differences between the high and low drinking lines. There were many more differences between the sP and sNP lines and between the AA and ANA lines compared with the P/NP, HAD1/LAD1 or HAD2/LAD2 pairs of lines. This pattern was confirmed by the trend analysis and PLS analysis.
Within pair comparisons
AA and ANA rats
MCSF test
Figure 1b illustrates the number of visits to the different zones in relation to the activity of each individual. AA rats made significantly more percentage visits to the CENTER and CENTRAL CIRCLE than ANA rats. Furthermore, AA rats made significantly fewer visits to the DCR, HURDLE and CORRIDORs than ANA rats. The trend analysis for AA and ANA rats is shown in Fig. 2a. AA rats were characterized by significantly lower general activity, exploration and shelter-seeking behavior than ANA rats. Furthermore, AA rats had significantly higher risk assessment and risk-taking behavior compared with ANA rats.
Figure 2.
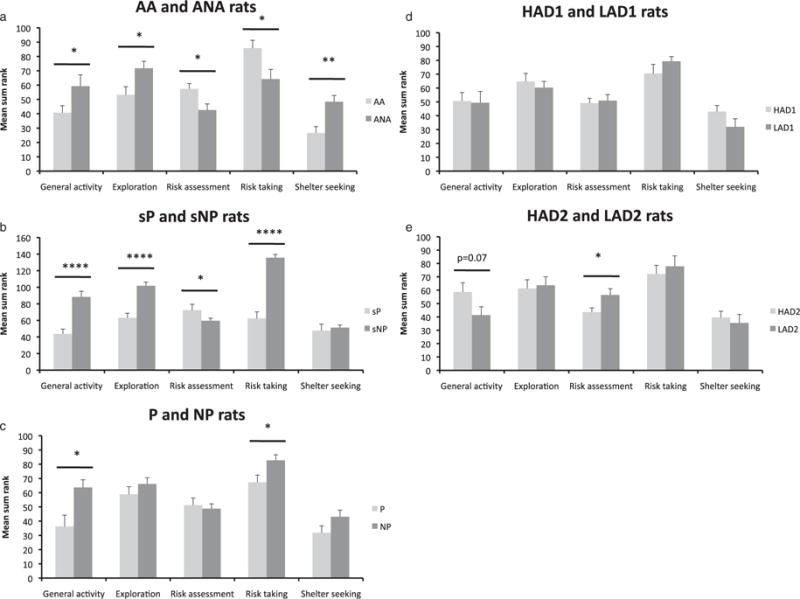
The trend analysis for AA and ANA rats (a), sP and sNP rats (b), P and NP rats (c), HAD1 and LAD1 rats (d), and HAD2 and LAD2 rats (e). Values represent mean ± SEM. *P ≤ 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 when comparing the respective alcohol-preferring and alcohol non-preferring rats (Mann–Whitney U-test)
OF test
A summary of the significant differences between AA and ANA rats in the OF test is shown in Table 2A and reveals that AA rats spent more time per visit in the center than ANA rats.
EPM test
A summary of the significant differences between AA and ANA rats in the EPM test is shown in Table 2B. The AA rats had higher open arm and center activity and lower closed arm activity than the ANA rats.
sP and sNP rats
MCSF test
sP rats made significantly more percentage visits to the CORRIDORs and DCR than sNP rats. Furthermore, sP rats made significantly fewer visits to the risk areas SLOPE, BRIDGE ENTRANCE, BRIDGE and CENTRAL CIRCLE than sNP rats (Fig. 1b). The trend analysis (Fig. 2b) indicated that general activity, exploration and risk-taking behavior were significantly lower in sP than in sNP rats. Furthermore, risk assessment was significantly higher in sP rats compared with sNP rats.
OF test
Fewer sP than sNP rats visited the center, and sP rats had lower center activity than sNP rats. Moreover, sP rats were less active compared with sNP rats (Table 2A).
EPM test
Fewer sP than sNP rats visited the open arms of the EPM. The sP rats had lower open arm activity and higher closed arm activity than the sNP rats. Furthermore, sP rats were less active than sNP rats (Table 2B).
P and NP rats
MCSF test
P rats made significantly fewer visits to the CENTER compared with NP rats. Moreover, P rats made significantly more visits to the risk areas SLOPE, BRIDGE ENTRANCE and BRIDGE compared with NP rats (Fig. 1b). The trend analysis (Fig. 2c) indicated that P rats were characterized by significantly lower general activity and risk-taking behavior compared with NP rats, while exploration, risk assessment and shelter-seeking behavior did not differ between the two groups.
OF test
P rats made more crossings than NP rats (Table 2A).
EPM test
P rats spent longer time in the center compared with NP rats (Table 2B).
HAD1 and LAD1 rats
MCSF test
HAD1 and LAD1 rats did not differ in number of visits to the different zones (Fig. 1b). The trend analysis (Fig. 2d) detected no significant differences in general activity, exploration, risk assessment, risk taking and shelter-seeking behavior.
OF test
HAD1 rats had lower activity in the center of the OF compared with LAD1 rats. Furthermore, HAD1 rats were characterized by lower general activity compared with LAD1 rats (Table 2A).
EPM test
HAD1 rats spent more time on the closed arms and less time in the center compared with the LAD1 rats (Table 2B).
HAD2 and LAD2 rats
MCSF test
HAD2 and LAD2 rats did not differ in number of visits to the different zones (Fig. 1b). The trend analysis (Fig. 2e) revealed significantly lower risk assessment in HAD2 rats than LAD2 rats and a tendency for a higher general activity in HAD2 rats compared with LAD2 rats (P = 0.07). No line differences were found for exploration, risk taking and shelter-seeking behavior.
OF test
HAD2 rats had higher activity and spent less time per visit in the periphery than LAD2 rats (Table 2A).
EPM test
HAD2 rats spent less time in total and shorter time per visit on the open arms compared with the LAD2 rats (Table 2B).
Wistar and N/Nih rats
The results from the MCSF, OF and EPM tests in Wistar and N/Nih rats are shown in Appendix S1 (Tables S9 and S10). Figure 1b illustrates the number of visits to the different zones in relation to the activity of each individual in Wistar and N/Nih rats, respectively.
Comparisons within all of the alcohol-preferring and within all of the alcohol non-preferring lines
Trend analysis
Among the alcohol-preferring lines (Fig. 3a), overall differences were detected for general activity, exploratory activity, risk taking and shelter-seeking behavior. sP rats had significantly lower general activity than all other preferring lines. HAD1 rats were characterized by significantly higher exploratory activity compared with the other preferring rat lines. AA rats displayed significantly higher risk-taking behavior than all other lines, and sP rats were found to have significantly lower risk-taking behavior compared with all other preferring rat lines. Finally, AA rats displayed significantly lower shelter-seeking behavior than all other preferring lines, and P rats seek less shelter than HAD2 rats.
Figure 3.
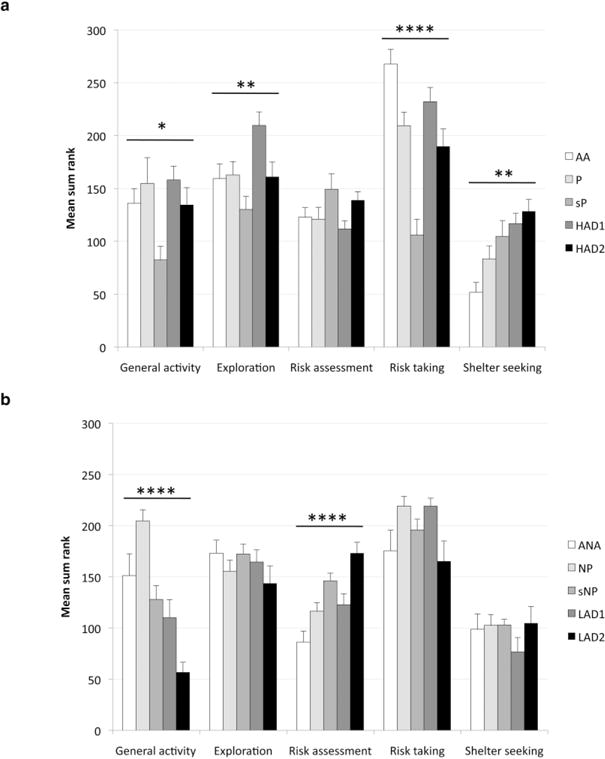
The trend analysis for alcohol-preferring (a) and alcohol non-preferring (b) lines. Values represent mean ± SEM. *P ≤ 0.05, **P < 0.01, ****P < 0.0001 comparing all alcohol-preferring and non-preferring lines, respectively (Kruskal–Wallis test). For specific differences between the preferring and non-preferring lines, respectively, please see the Results section
Among the non-preferring lines (Fig. 3b), overall differences were detected for general activity and risk-assessment behavior. NP rats were significantly more active than all other non-preferring lines, while LAD2 rats were significantly less active compared with the other non-preferring lines. ANA rats showed lower risk-assessment behavior compared with the other non-preferring lines, while LAD 2 rats displayed significantly higher risk-assessment behavior compared with the other non-preferring lines. Finally, NP rats showed less risk assessment than sNP rats.
PLS analysis
The PLS analysis revealed similarities between the groups of alcohol-preferring lines (Fig. 4). Groups of animals that load closer together on the PLS plot show greater similarity than groups that are located farther away. The greatest similarity in MCSF performance was found between P, HAD1 and HAD2 rats. The sP rats, loading alone in the upper left quadrant, and the AA rats, loading in the lower right quadrant, were different. The loading of sP rats was associated with latency measures and duration in the CORRIDORs (generally longer in sP rats), and parameters for shelter-seeking behavior. The loading of AA rats was associated with parameters for risk-taking behavior (generally more pronounced). Parameters for general activity loaded in the same quadrant as P, HAD1 and HAD2 rats. The picture from the PLS analysis supports the results from the trend analysis (Fig. 3a).
Figure 4.
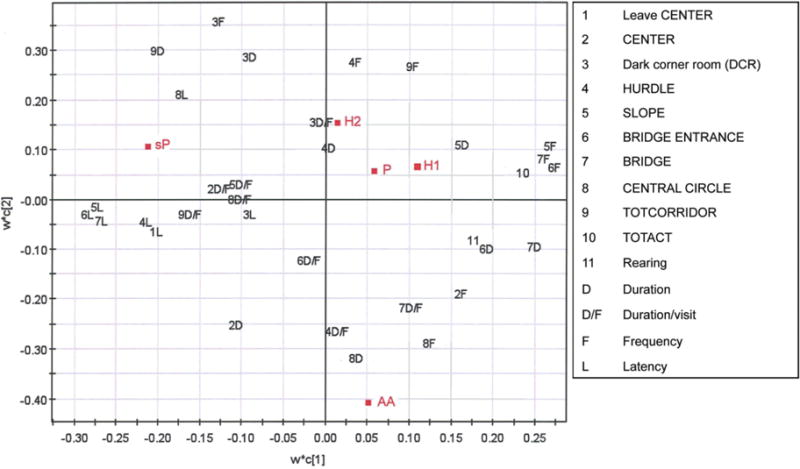
The partial least squares to latent structures (PLS) analysis showing the relationship between the alcohol-preferring lines, i.e. AA, sP, P, HAD1 (H1) and HAD2 (H2) and the MCSF parameters important for the loading of the respective group. Parameters located further away from the origin are of greater importance. TOTACT, sum of visits to all zones in the arena; TOTCORR, sum of performance in the CORRIDORs
Impulsive-like behavior and anxiety-like behavior
The latency to first enter the BRIDGE in relation to first enter the SLOPE (the SLOPE/BRIDGE interval; see Table 1) can be viewed as a measure of impulsive-like behavior. After entering the SLOPE, the animal has to assess the risk of entering the BRIDGE. Animals displaying impulsive-like behavior will have a shorter interval in entering the risk area. A significant difference was detected when comparing sP and sNP rats, implying higher impulsive-like behavior in sP than sNP rats. The relationship between time spent in the sheltered area (DCR) and the risk area (BRIDGE) in relation to the time spent in both zones (the shelter/risk index, see Table 1) can be viewed as one measure of anxiety-like behavior. The sP rats had a significantly larger index than the sNP rats, thus implying higher anxiety-like behavior in sP rats. No other significant line differences were found.
DISCUSSION
In the present investigation, the majority of the alcohol-preferring and non-preferring pairs of selectively bred rats available worldwide were tested. Great differences were found within some of the pairs of selectively bred rat lines as well as between all alcohol-preferring and all alcohol non-preferring rats, despite their evident phenotypes regarding voluntary alcohol intake. The most pronounced differences within the pairs of selectively bred lines were found when comparing AA and ANA rats, and sP and sNP rats. Minor differences were revealed when comparing HAD1 and LAD1, and HAD2 and LAD2 rats. Differences between P and NP rats were intermediate. Thus, there was marked heterogeneity in behavioral traits associated with high alcohol drinking.
The different pairs of selectively bred preferring and non-preferring lines originate from different foundation stocks. The outbred Wistar colony used to raise the P/NP lines was the WrmWRC(WI)BR stock, a closed colony housed at the Walter Reed Army Institute of Research and is now extinct (Murphy et al. 2002). The Wistar stock used to breed the sP/sNP lines was purchased from Morini (San Polo d’Enza, RE, Italy) (Colombo et al. 2006). It is likely that these Wistar stocks were genetically and phenotypically different from each other. The AA/ANA lines were originally derived from a foundation stock that included Wistar and Sprague–Dawley rats and were later crossed with F1 hybrids from a Lewis and Norwegian Brown cross (Sommer, Hyytiä & Kiianmaa 2006). The HAD1-2/LAD1-2 rats were derived from the heterogeneous N/Nih stock (Murphy et al. 2002). Because the gene pools are different among the various foundation stocks, different genes of varied behavioral traits likely cosegregated with the genes for alcohol preference. This is the most cogent reason for the highly varied behavioral dispositions that we now observe in the five different lines of alcohol-preferring rats.
Another distinct possibility is that many of the differences observed between the lines are because of chance changes in frequency and fixation of alleles resulting from inbreeding. Inbreeding, omnipresent in closed animal colonies and roughly proportional to the number of generations without interbreeding with other lines or strains, would alter genes in a random fashion. Inbreeding would tend to yield behavioral differences between lines that are unrelated to the selection pressure originally applied from drinking differences (Crabbe et al. 1990). Suggestive that inbreeding plays a role here is that the older (in terms of number of generations of selective breeding), and therefore presumably more inbred lines (sP/sNP and AA/ANA) showed more behavioral differences than the newer (HAD/LAD) lines.
Finally, animal handling and other routines vary in different breeding laboratories (Wahlsten et al. 2003), and early environmental and social influences have been suggested to be as important as genetics in determining later life behavior (Lathe 2004). These factors may induce variation and individual differences produced in various ways including by epigenetic mechanisms. Differences in spontaneous behaviors such as exploration, risk assessment and risk taking are probably conserved during the process of selective breeding. The exception should be if a specific behavioral trait(s) is truly associated with preference for alcohol in which case both the behavior and alcohol intake will differ between preferring and non-preferring rats. The underlying neural mechanisms involved in alcohol intake are likely more limited compared to those factors responsible for behaviors of importance for the species overall behavioral repertoire, which often is necessary for survival. It may well be that only a small part of the functions regulating spontaneous behavior is associated with alcohol preference and intake. The following sections are summaries of the within and between line comparisons found in the present investigation.
AA and ANA rats
The results from the MCSF test reveal that AA rats generally are less active, explorative and shelter seeking and more risk assessing and risk taking compared with ANA rats. These results replicate a previously published comparison of the AA and ANA rats using the MCSF test, in spite of the use of a smaller arena in the previous study (Roman et al. 2007). The profiles are here interpreted as higher risk taking and lower anxiety-like behavior in AA compared with ANA rats. In agreement with the findings in the MCSF, higher open arm activity was observed in the EPM test and minor indications for higher center activity were noted in the OF test in AA rats relative to ANA rats. Taken together, these findings are in agreement with previous studies that compared the AA and ANA rats using a variety of tests (Knapp et al. 1997; Möller et al. 1997; Sandbak et al. 1998; Roman et al. 2005).
sP and sNP rats
The results from the MCSF test reveal lower general activity, exploratory drive and risk-taking behavior and higher risk-assessment behavior in sP rats relative to sNP rats. The profile of the sP rats is interpreted as higher vigilance and anxiety-related behaviors compared with the sNP rats. The lower activity and risk-taking behavior in sP rats is also a consistent finding in the OF and EPM tests. Lower activity and exploratory drive have previously been demonstrated in sP rats compared with sNP rats (Colombo et al. 1998; Agabio et al. 2001). The profiles obtained using the MCSF test replicate a previous study in which sP and sNP rats were investigated (Roman & Colombo 2009). sP rats have also been characterized by higher anxiety-related behaviors in various behavioral tests (Colombo et al. 1995; Richter et al. 2000; Leggio et al. 2003).
P and NP rats
The results from the MCSF test reveal lower general activity and to some extent also lower exploration in P rats than in NP rats. When their lower general activity was taken into account, P rats made more percentage visits to the risk areas compared with NP rats, which suggested higher risk-taking behavior. However, when differences in activity were not taken into account (as in the trend analysis), lower risk-taking behavior was observed in P than in NP rats. Observations with the OF and EPM tests did not support any line differences in risk-taking behavior and offered little support for higher anxiety-like behavior in P rats. These findings contrast significantly with previous and recent reports demonstrating higher anxiety-like behavior in P rats compared with NP rats (Stewart et al. 1993; Hwang et al. 2004; Pandey et al. 2005; Zhang et al. 2010), but see also Badishtov et al. (1995) and Viglinskaya et al. (1995) who found no differences between P and NP rats. The factors that underlie the discrepancy between the present results and previous reports of higher anxiety-like behavior in P rats are unclear at this time and could include undefined differences in procedural variables. Herein, rats were tested in the EPM and OF after testing in the MCSF, and it cannot be excluded that this sequence of testing somehow affected the results. It is of interest to note that considerable instability in genetic differences in EPM behavior in mice has been observed, even when investigators have gone to great lengths to equalize testing procedures (Wahlsten et al. 2003). Genetic drift may also play a role.
HAD and LAD rats
The results from the MCSF test reveal only minor differences between HAD and LAD rats. A consistent finding from the MCSF and OF tests is that HAD1 rats are less risk taking in an open area (MCSF CENTRAL CIRCLE and OF center) than LAD1 rats. Furthermore, HAD2 rats are found to be less risk assessing than LAD2 rats, while no such difference was found when comparing HAD1 and LAD1 rats. The general lack of differences is in agreement with previous studies in which, for instance, no differences in anxiety-related behaviors between HAD and LAD rats have been found (Viglinskaya et al. 1995; Overstreet et al. 1997; Hwang et al. 2004). More recently, HAD1 and HAD2 rats were found to discount delayed and probabilistic rewards more steeply than LAD1 and LAD2 rats, which was interpreted as higher impulsive-like behavior in the HAD rats (Wilhelm & Mitchell 2008). The lower risk assessment behavior and shorter SLOPE/BRIDGE interval (higher impulsive-like behavior) in HAD2 rats relative to LAD2 rats demonstrated herein is in agreement with this finding.
Comparisons within all of the alcohol-preferring lines
General activity was similar in all lines except the sP rats, which were characterized by the lowest activity. Explorative drive was highest in the HAD1 rats and more similar in the other lines. No differences in risk assessment behavior were revealed among the different lines. With regard to risk-taking behavior, AA rats showed the highest, while sP rats showed the lowest. Shelter-seeking behavior was lowest in AA rats and highest in HAD2 rats. The PLS analysis revealed similar behavioral strategies in P, HAD1 and HAD2 rats, while the behavioral strategies of AA and sP rats were quite different from each other and the others. Thus, AA rats constitute one subgroup with more pronounced risk-taking behavior, and sP rats exert another subgroup characterized by lower activity, explorative drive and lower risk-taking behavior. From this pattern, it can be concluded that the different alcohol-preferring lines share no common behavioral profile.
This is one of the few studies in which behavioral traits in addition to alcohol drinking/self-administration have been concurrently investigated in so many pairs of selectively bred alcohol-preferring and non-preferring rodent lines. Perhaps the most consistent behavioral finding among different alcohol-preferring lines is elevated consumption of sweet-tasting solutions compared with the non-preferring lines, previously observed in all of the pairs of selectively bred rats except the sP/sNP lines (Murphy et al. 2002; Colombo et al. 2006; Sommer et al. 2006). With regard to neurochemical factors underlying opposite alcohol consumption behavior in preferring and non-preferring lines, a number of differences have been demonstrated. For example, P, sP and HAD rats seem to have a dysfunction in the serotonin system not shared by the AA rats. Furthermore, the mesolimbic dopamine pathway appears to be of less importance for the positive reinforcing properties of alcohol in AA rats than in P, HAD and sP rats (Murphy et al. 2002). Thus, differences in neurochemical and neurobiological factors have been found, which may underlie divergent expressions of explorative strategies and behavioral profiles in the different pairs of selected lines.
Differences in the above-cited factors may also underlie heterogeneity in alcohol-seeking behaviors among the preferring lines despite their similarities in meeting the criteria for their selective breeding—high voluntary alcohol intake and preference in the continuous-access, two-bottle choice test. For instance, when alcohol availability is contingent on lever pressing, the alcohol-preferring lines differ, with HAD1>P > sP > HAD2 > AA in magnitude of operant responding for alcohol (Files et al. 1997; Samson et al. 1998; Vacca et al. 2002). Furthermore, line differences have been observed in the tendencies to display and magnitudes of the alcohol deprivation effect (ADE), a temporary increase in voluntary alcohol intake following a period of alcohol abstinence (Sinclair & Li 1989; Agabio et al. 2000; Serra et al. 2003; Vengeliene et al. 2003; Rodd et al. 2009). Thus, selective breeding for alcohol preference has resulted in diverse behavioral outcomes among the different selectively bred lines in aspects of alcohol-seeking behavior in addition to exploratory strategies and behavioral profiles as highlighted here with the MCSF test.
General discussion
Since alcohol dependence involves several different components, e.g. gene × environment and gene × gene interactions, heterogeneity among humans as well as experimental animals is to be expected. In a recent study that attempted to classify different subtypes of alcoholics (Moss, Chen &Yi 2007), five different clusters or subtypes among alcohol-dependent individuals were identified and differed according to time of onset, genetic load, presence of psychiatric comordidity including other drug use disorders and levels of psychosocial functioning. These different subtypes illustrate the heterogeneity among alcohol-dependent individuals, which cannot be captured in one single animal model. The need for developing animal models that reflect this heterogeneity has recently been emphasized (Crabbe 2010). Among the alcoholism-related phenotypes and endophenotypes considered to be important targets for genetic studies using rodent models was behavioral under control—encompassing the constructs of impulsivity, novelty seeking and risk taking (Crabbe 2010), which are reflected in how rats explore the MCSF.
The results presented herein clearly indicate that although the five preferring lines were selected for a similar high alcohol-drinking phenotype, the different foundation stocks used together with different breeding environments have produced lines with different exploratory strategies and behavioral profiles. An analogy could be drawn between the heterogeneity observed among human alcoholic subtypes and that seen among the different alcohol-preferring lines. From a translational perspective, it is tempting to speculate about the different alcohol-preferring rats somehow mimicking the alcoholic subtypes. In analogy with the human population sample (Moss et al. 2007), different profiles exist in the different preferring rat lines, with AA and sP rats, respectively, exerting the opposing extremes in several aspects. Our results suggest that behavioral traits such as high anxiety or risk taking/impulsivity are not required for high alcohol drinking or preference. However, the possibility that either high anxiety or high risk taking/impulsivity, when present, can contribute quantitatively to high alcohol preference cannot be excluded.
Overall, the current findings argue against a single behavioral ‘pathway’ to high alcohol intake, detectable with the MCSF, shared among all the selectively bred lines of rats. In the realm of translational research, the behavioral profiles of the five alcohol-preferring lines are suggested to emulate the attributes of different alcoholic subtypes. Thus, the different alcohol-preferring lines represent valuable models for the heterogeneity of alcohol dependence in humans.
Supplementary Material
Appendix S1 Expanded material and methods, and discussion.
Table S1 Results from the multivariate concentric square field™ (MCSF) test in adult male Alko, alcohol (AA) and Alko, non-alcohol (ANA) rats.
Table S2 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Alko, alcohol (AA) and Alko, non-alcohol (ANA) rats.
Table S3 Results from the multivariate concentric square field™ (MCSF) test in adult male Sardinian alcohol-preferring (sP) and non-preferring (sNP) rats.
Table S4 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Sardinian alcohol-preferring (sP) and non-preferring (sNP) rats.
Table S5 Results from the multivariate concentric square field™ (MCSF) test in adult male Indiana preferring (P) and Indiana non-preferring (NP) rats.
Table S6 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Indiana preferring (P) and Indiana non-preferring (NP) rats.
Table S7 Results from the multivariate concentric square field™ (MCSF) test in adult male Indiana high alcohol drinking 1-2 (HAD1-2) and Indiana low alcohol drinking 1-2 (LAD1-2) rats.
Table S8 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Indiana high alcohol drinking 1-2 (HAD1-2) and Indiana low alcohol drinking 1-2 (LAD1-2) rats.
Table S9 Results from the multivariate concentric square field™ (MCSF) test in adult male Wistar (Hsd:WI) and N/Nih rats.
Table S10 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Wistar (Hsd:WI) and N/Nih rats.
Acknowledgments
The authors acknowledge technical assistance from Ms. Megan Litsheim, IUPUI and valuable comments on data analysis, in particular the introduction of the trend analysis, and on the preparation of the manuscript from Professor Bengt J Meyerson, Uppsala University.
Financial support from NIH grants AA07462, supplement to AA015512 (L.L.) and the Swedish Society for Medical Research (SSMF), the Facias Foundation and the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly (07-21:2; E.R.) is gratefully acknowledged.
Footnotes
SUPPORTING INFORMATION
Additional information may be found in the Appendix S1 in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Authors Contribution
ER, RBS, TKL and LL were responsible for the study concept and design. ER, RBS, MLB and MLJ conducted the behavioral testing and acquisition of animal data. GC, PH and LL provided experimental animals and contributed to the acquisition of data. ER, RBS and NEBE performed the data analysis. ER, RBS, NJG, TKL and LL contributed in interpretation of results. ER and RBS drafted the manuscript. ER, RBS, GC, PH, NEBE, NJG, TKL and LL provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
References
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G. Development of short-lasting alcohol deprivation effect in sardinian alcohol-preferring rats. Alcohol. 2000;21:59–62. doi: 10.1016/s0741-8329(00)00072-0. [DOI] [PubMed] [Google Scholar]
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23:123–126. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Augustsson H. Ethoexperimental studies of behaviour in wild and laboratory mice. Risk assessment, emotional reactivity and animal welfare. Acta Universitatis Agriculturae Sueciae. Veterinaria. 2004;174:7–62. [Google Scholar]
- Badishtov BA, Overstreet DH, Kashevskaya OP, Viglinskaya IV, Kampov-Polevoy AB, Seredenin SB, Halikas JA. To drink or not to drink: open field behavior in alcohol-preferring and- nonpreferring rat strains. Physiol Behav. 1995;57:585–589. doi: 10.1016/0031-9384(94)00299-k. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Vacca G, Gessa GL. Stimulation of locomotor activity by voluntarily consumed ethanol in Sardinian alcohol-preferring rats. Eur J Pharmacol. 1998;357:109–113. doi: 10.1016/s0014-2999(98)00560-3. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, Gessa GL. Sardinian alcohol-preferring rats: a genetic animal model of anxiety. Physiol Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Consilience of rodent and human phenotypes relevant for alcohol dependence. Addict Biol. 2010;15:103–108. doi: 10.1111/j.1369-1600.2009.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Dyr W, Kostowski W. Warsaw high-preferring (WHP) and Warsaw low-preferring (WLP) lines of rats selectively bred for high and low voluntary ethanol intake: preliminary phenotypic characterization. Alcohol. 2008;42:161–170. doi: 10.1016/j.alcohol.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Eriksson K. Genetic selection for voluntary alcohol consumption in the albino rat. Science. 1968;159:739–741. doi: 10.1126/science.159.3816.739. [DOI] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikström C, Wold S. Multi- and Megavariate Data Analysis. Part I: Basic Principles and Applications. Second Revised and Enlarged Edition. Umeå, Sweden: Umetrics AB; 2006. [Google Scholar]
- Files FJ, Denning CE, Hyytiä P, Kiianmaa K, Samson HH. Ethanol-reinforced responding by AA and ANA rats following the sucrose-substitution initiation procedure. Alcohol Clin Exp Res. 1997;21:749–753. [PubMed] [Google Scholar]
- Grahame NJ. Selected lines and inbred strains. Tools in the hunt for the genes involved in alcoholism. Alcohol Res Health. 2000;24:159–163. [PMC free article] [PubMed] [Google Scholar]
- Hilakivi L, Eriksson CJP, Sarviharju M, Sinclair JD. Revitalization of the AA and ANA rat lines: effects on some line characteristics. Alcohol. 1984;1:71–75. doi: 10.1016/0741-8329(84)90040-5. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Stewart R, Zhang JK, Lumeng L, Li TK. Corticotropin-releasing factor gene expression is down-regulated in the central nucleus of the amygdala of alcohol-preferring rats which exhibit high anxiety: a comparison between rat lines selectively bred for high and low alcohol preference. Brain Res. 2004;1026:143–150. doi: 10.1016/j.brainres.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Kampov-Polevoy AB, Overstreet DH, Breese GR, Rezvani AH. Ultrasonic vocalization behavior differs between lines of ethanol-preferring and nonpreferring rats. Alcohol Clin Exp Res. 1997;21:1232–1240. [PubMed] [Google Scholar]
- Lathe R. The individuality of mice. Genes Brain Behav. 2004;3:317–327. doi: 10.1111/j.1601-183X.2004.00083.x. [DOI] [PubMed] [Google Scholar]
- Leggio B, Masi F, Grappi S, Nanni G, Gambarana C, Colombo G, de Montis MG. Sardinian alcohol-preferring and non-preferring rats show different reactivity to aversive stimuli and a similar response to a natural reward. Brain Res. 2003;973:275–284. doi: 10.1016/s0006-8993(03)02533-2. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl. 1987;1:91–96. [PubMed] [Google Scholar]
- Mardones J, Segovia-Riquelme N. Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol Teratol. 1983;5:171–178. [PubMed] [Google Scholar]
- Meyerson BJ, Augustsson H, Berg M, Roman E. The concentric square field: a multivariate test arena for analysis of explorative strategies. Behav Brain Res. 2006;168:100–113. doi: 10.1016/j.bbr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Möller C, Wiklund L, Thorsell A, Hyytiä P, Heilig M. Decreased measures of experimental anxiety in rats bred for high alcohol preference. Alcohol Clin Exp Res. 1997;21:656–660. [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guidlines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: The National Academic Press; 2003. [PubMed] [Google Scholar]
- Overstreet DH, Halikas JA, Seredenin SB, Kampov-Polevoy AB, Viglinskaya IV, Kashevskaya O, Badishtov BA, Knapp DJ, Mormede P, Kiianmaa K, Li TK, Rezvani AH. Behavioral similarities and differences among alcohol-preferring and—nonpreferring rats: confirmation by factor analysis and extension to additional groups. Alcohol Clin Exp Res. 1997;21:840–848. [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Zorrilla EP, Basso AM, Koob GF, Weiss F. Altered amygdalar CRF release and increased anxiety-like behavior in Sardinian alcohol-preferring rats: a microdialysis and behavioral study. Alcohol Clin Exp Res. 2000;24:1765–1772. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, McBride WJ. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of high-alcohol-drinking (HAD) rats. Addict Biol. 2009;14:152–164. doi: 10.1111/j.1369-1600.2008.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman E, Colombo G. Lower risk taking and exploratory behavior in alcohol-preferring sP rats than in alcohol nonpreferring sNP rats in the multivariate concentric square field™ (MCSF) test. Behav Brain Res. 2009;205:249–258. doi: 10.1016/j.bbr.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Roman E, Gustafsson L, Hyytiä P, Nylander I. Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res. 2005;29:591–601. doi: 10.1097/01.alc.0000158933.70242.fc. [DOI] [PubMed] [Google Scholar]
- Roman E, Meyerson BJ, Hyytiä P, Nylander I. The multivariate concentric square field test reveals different behavioural profiles in male AA and ANA rats with regard to risk taking and environmental reactivity. Behav Brain Res. 2007;183:195–205. doi: 10.1016/j.bbr.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning C, Marvin S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. I. Ethanol initiation and limited access operant self-administration. Alcohol Clin Exp Res. 1998;22:2133–2146. [PubMed] [Google Scholar]
- Sandbak T, Murison R, Sarviharju M, Hyytiä P. Defensive burying and stress gastric erosions in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res. 1998;22:2050–2054. [PubMed] [Google Scholar]
- Serra S, Brunetti G, Vacca G, Lobina C, Carai MA, Gessa GL, Colombo G. Stable preference for high ethanol concentrations after ethanol deprivation in Sardinian alcohol-preferring (sP) rats. Alcohol. 2003;29:101–108. doi: 10.1016/s0741-8329(03)00003-x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sommer W, Hyytiä P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309. doi: 10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Vacca G, Serra S, Brunetti G, Carai MA, Samson HH, Gessa GL, Colombo G. Operant self-administration of ethanol in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res. 2002;26:1678–1685. doi: 10.1097/01.ALC.0000036285.62071.DA. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Viglinskaya IV, Overstreet DH, Kashevskaya OP, Badishtov BA, Kampov-Polevoy AB, Seredenin SB, Halikas JA. To drink or not to drink: tests of anxiety and immobility in alcohol- preferring and alcohol-nonpreferring rat strains. Physiol Behav. 1995;57:937–941. doi: 10.1016/0031-9384(94)00368-f. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, II, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sakharkar AJ, Shi G, Ugale R, Prakash A, Pandey SC. Neuropeptide y signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol Clin Exp Res. 2010;34:451–461. doi: 10.1111/j.1530-0277.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Expanded material and methods, and discussion.
Table S1 Results from the multivariate concentric square field™ (MCSF) test in adult male Alko, alcohol (AA) and Alko, non-alcohol (ANA) rats.
Table S2 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Alko, alcohol (AA) and Alko, non-alcohol (ANA) rats.
Table S3 Results from the multivariate concentric square field™ (MCSF) test in adult male Sardinian alcohol-preferring (sP) and non-preferring (sNP) rats.
Table S4 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Sardinian alcohol-preferring (sP) and non-preferring (sNP) rats.
Table S5 Results from the multivariate concentric square field™ (MCSF) test in adult male Indiana preferring (P) and Indiana non-preferring (NP) rats.
Table S6 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Indiana preferring (P) and Indiana non-preferring (NP) rats.
Table S7 Results from the multivariate concentric square field™ (MCSF) test in adult male Indiana high alcohol drinking 1-2 (HAD1-2) and Indiana low alcohol drinking 1-2 (LAD1-2) rats.
Table S8 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Indiana high alcohol drinking 1-2 (HAD1-2) and Indiana low alcohol drinking 1-2 (LAD1-2) rats.
Table S9 Results from the multivariate concentric square field™ (MCSF) test in adult male Wistar (Hsd:WI) and N/Nih rats.
Table S10 Results from the open field (OF) and elevated plus maze (EPM) tests in adult male Wistar (Hsd:WI) and N/Nih rats.


