Summary
Cisplatin holds an illustrious position in the history of chemistry most notably for its role in the virtual cure of testicular cancer. Here we describe a role for this small molecule in cyanide detoxification in vivo. Cyanide kills organisms as diverse as insects, fish and humans within seconds to hours. Current antidotes exhibit limited efficacy and are not amenable to mass distribution requiring the development of new classes of antidotes. The binding affinity of the cyanide anion for the positively charged metal platinum is known to create an extremely stable complex in vitro. We therefore screened a panel of diverse cisplatin analogs and identified compounds that conferred protection from cyanide poisoning in zebrafish, mice and rabbits. Cumulatively, this discovery pipeline begins to establish the characteristics of platinum ligands that influence their solubility, toxicity, and efficacy and provides proof of concept that platinum-based complexes are effective antidotes for cyanide poisoning.
Graphical abstract
Introduction
Historically, cyanide has been an agent of murder, war and terrorism; however unintentional exposures are equally possible and lethal. Smoke inhalation is the most common cause of cyanide poisoning in western countries (Barillo et al., 1994). Cyanide reversibly binds to cytochrome c oxidase within the mitochondria (Kellin, 1929). Consequently, electron transport and oxidative phosphorylation are halted. If not reversed, the cessation of aerobic metabolism causes a fatal deficit in oxygen consumption. Manufacturers in the United States produce 300,000 tons of hydrogen cyanide annually which is used in the extraction of gold during mining and in the synthesis of dyes, synthetic fibers, and plastics, as well as in warehouses as a pesticide. The thermal breakdown of materials such as wool, plastic, and synthetic polymers produce cyanide gas in addition to isocyanates (potent respiratory irritants), leading to smoke inhalation fatalities of approximately 5,000-10,000 and injuries of 23,000 per year in the United States (Alcorta, 2004).
Industrial accidents are another major source of cyanide morbidity and mortality. The Bhopal disaster, considered the world's worst industrial accident, occurred when 45 tons of methyl isocyanate and hydrogen cyanide escaped from reservoirs killing nearly 4,000 people immediately, followed by another 15,000-20,000 individuals over the next few weeks, and leaving a half a million survivors with debilitating injuries such as chronic respiratory illnesses and blindness (Broughton, 2005). Though there are two available antidotes for cyanide poisoning, their formulation and mode of action require them to be administered intravenously in hospital settings, therefore they would not be amenable to a mass causality scenario such as the Bhopal disaster (Hall et al., 2009). The large global supply of cyanide, the morbidity and mortality from smoke inhalation and industrial accidents, and the threat to soldiers fighting nonconventional conflicts requires the development of novel classes of antidotes that are amenable to mass distribution in the field. To meet this need, chemical biology and medicinal chemistry approaches are required to discover lead compounds for development into new classes of antidotes to cyanide poisoning.
To identify novel classes of cyanide antidotes, we developed a zebrafish model of cyanide toxicity that is amenable to high through-put chemical screening (Nath et al., 2013). Upon exposure to cyanide, zebrafish larvae develop stereotypic dose-dependent cyanide pathologies including slow heart rate, deficits in standard neurobehavioral responses, and ultimately death. In addition to its usefulness for chemical screening, the zebrafish is an organism that is uniquely positioned to extend the information gained from classical in vitro structure activity relationship (SAR) studies by providing insight into some in vivo attributes of compounds, including absorbance, metabolism and toxicity (Hao et al., 2010). Therefore, this approach represents a powerful tool in the drug discovery arena.
In this study, we used in vivo SAR studies in zebrafish to examine a panel of structurally diverse cisplatin analogs. We reasoned that cisplatin analogs contain a platinum cation, which is known to interact with the cyanide anion; however it is actually the ligands that are coordinated to the platinum core that regulate the reactivity of cisplatin analogs and hence modulate their antidote efficacy in vivo. Among the analogs tested, we discovered 22 analogs with effectiveness against cyanide poisoning. To identify high value candidates to move into mammalian studies, these 22 candidates were then funneled through a series of filters: efficacy in zebrafish, in vivo toxicity in zebrafish, in vitro toxicity in human cells, aqueous solubility, and binding kinetics to cyanide. Based on these selection criteria, 3 cisplatin analogs were tested in two mammalian models of cyanide poisoning. Finally, detailed temporal measurements of oxidative metabolism were obtained in a rabbit model of cyanide exposure to ascertain the effects of these compounds on known cyanide targets. Cumulatively, this discovery pipeline provides proof of concept that platinum based complexes have antidote activity against cyanide poisoning in zebrafish, mice and rabbits, and begin to establish the characteristics of platinum ligands that influence their solubility, toxicity, and efficacy.
Results
Platinum complexes act as antidotes to cyanide poisoning by binding the cyanide anion
In a previous chemical screen in zebrafish, we discovered 4 cyanide antidotes within a library of 3,120 FDA approved drugs (Nath et al., 2013). In that chemical screen, zebrafish were exposed to a dose of cyanide that induces lethality over several hours. In the present study, in order to increase the stringency of the assay, a dose of 100 μM was used which kills zebrafish is less than 1 hour. Zebrafish larvae were treated with cisplatin, carboplatin or the known cyanide antidote hydroxocobalamin dissolved in dimethylsulfoxide (DMSO) in a five point dose response curve (Figure 1A-C). Under these conditions, a dose of 100 μM hydroxocobalamin completely blocked cyanide-induced lethality (Figure 1D). Carboplatin was not a cyanide antidote while cisplatin displayed similar efficacy as hydroxocobalamin. Survival of 100% of zebrafish was observed at 125 μM cisplatin which is approximately equimolar to the cyanide concentration (Figure 1D).
Figure 1. Platinum complexes act as antidotes to cyanide poisoning by binding the cyanide anion.
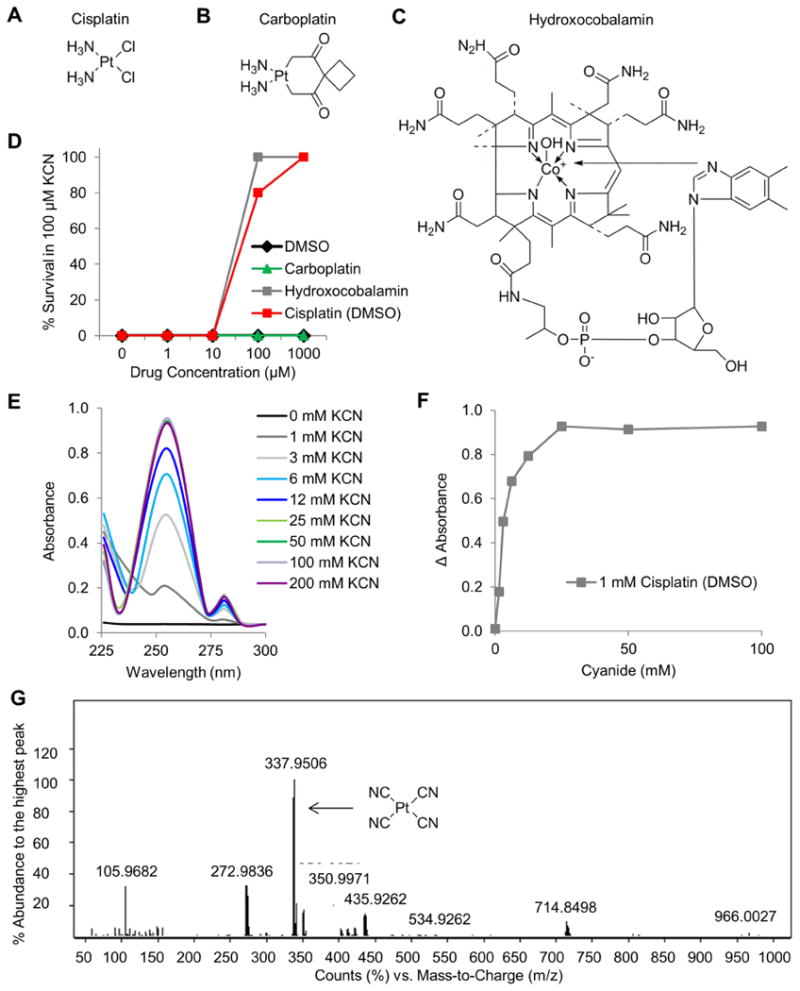
Chemical structure of A) cisplatin, B) carboplatin, and C) hydroxocobalamin. Compounds were dissolved in DMSO for the assays in this figure. D) The effects of compounds on the survival of zebrafish exposed to 100 μM KCN. E) UV-VIS spectral shift data demonstrating the binding of the cisplatin to cyanide. F) Plot of the change in absorbance of 1 mM cisplatin over increasing concentrations of cyanide. G) Analysis by mass spectrometry demonstrates that cisplatin binds 4 cyanide anions. Also see Table S1.
Cisplatin contains a positively charged platinum core, which indicates that it may act as an antidote by binding the cyanide anion. To test this hypothesis, we conducted an ultraviolet-visible (UV-VIS) spectrophotometry experiment. Increasing amounts of potassium cyanide were added to a solution of 1 mM cisplatin in 0.1% DMSO/H2O and the absorbance was measured across the UV-VIS spectrum. At concentrations ranging from 1-200 mM cyanide a spectral shift was observed at 255 nm (Fig 1E). At approximately 25 mM cyanide, the reaction between cyanide and cisplatin (1 mM) reached saturation (Fig 1F). These results indicate that cyanide binds cisplatin and suggests that the mechanism of action of platinum complexes may involve binding of the cyanide anion to the metal core of cisplatin.
To confirm this hypothesis, we next performed ESI-MS to identify the composition of the reaction product created by the addition of cyanide to cisplatin. The spectra contained a major peak with an m/z of 337.9 and minor peaks with m/z's of 272.9 and 350.9 (Figure 1G). Using MS/MS and isotope distribution comparison, we identified the major peak as platinum bound to 4 cyanide anions and the minor peaks as platinum bound to 3 cyanide anions (Table S1). This finding suggests that platinum complexes may act as antidotes to cyanide poisoning by chelating cyanide anions via the platinum atom.
Cyano-platinum complexes are considered strong metal cyanide complexes because they do not dissociate easily at physiological pH (Smith and Martel, 1976). To determine if cyanide dissociates from cyano-platinum complexes in vivo and if the released cyanide induces toxicity, zebrafish were treated with 1-1000 μM potassium cyanide (KCN) or potassium tetracyanoplatinate(II) [K2Pt(CN)4] for 24 hours. KCN treatment at a dose of 20 μM resulted in 100% lethality. In contrast, zebrafish treated with K2Pt(CN)4 were alive and active with no gross morphological defects at all doses of K2Pt(CN)4 tested. These results suggest that the cyano-platinum species produced by administration of a platinum based cyanide antidote are relatively non-toxic.
Structure activity relationship analysis identifies novel antidotes to cyanide poisoning
The finding that carboplatin and cisplatin exhibit differential efficacy as cyanide antidotes suggested that the ligands bound to platinum affect antidote potency (Figure 1D). To explore the potential use of platinum complexes as cyanide antidotes and to mitigate the known insolubility and toxicity associated with cisplatin, an in vivo SAR study was performed.
Cisplatin analogs are coordination complexes containing three components: a positively charged metallic atom that is the coordination center, ligands that are leaving groups and ligands that remain conjugated to the platinum atom (typically cis ammine groups). In the case of cisplatin, upon exposure to the intracellular aqueous environment, an associative substitution reaction occurs in which the chloride leaving groups are replaced by water molecules (Siddik, 2003). This complex enters the nucleus and a second associative substitution reaction occurs. Purine bases displace the water leaving groups, generating two DNA adducts per cisplatin molecule (Fichtinger-Schepman et al., 1985). In the case of cyanide exposure, the carbon of the cyanide anion is the nucleophile that forms a bond with the platinum atom and displaces a ligand of the platinum complex.
A panel of 35 structurally diverse cisplatin analogs was assembled and doses of 1-1000 μM were tested (10 point dose response curve). The doses that rescued 100% of zebrafish (EC100) from a challenge with 100 μM KCN were determined. In a separate assay, the doses that caused 100% lethality (LD100) in the absence of KCN were determined. The EC100 was determined in both DMSO and PBS solvents (Figure 2, blue and red, respectively). The LD100 was determined for complexes dissolved in DMSO (Figure 2, black). NA indicates instances in which the complex did not induce any toxicity or did not rescue cyanide lethality at any of the doses tested. The cisplatin analogs spanned the following classes: platinum (IV) (1-6), square planar (7-13), FDA approved drugs (14-19), pyridine (20-24), triphenylphosphine (25-28), alkene (29-32), and sulfur-containing complexes (33-35). The SARs of complexes solvation in DMSO are discussed below (Figure 2, blue), subsequently the SAR results after solvation in PBS are discussed (Figure 2, red).
Figure 2. Structure activity relationships of cisplatin analogs.
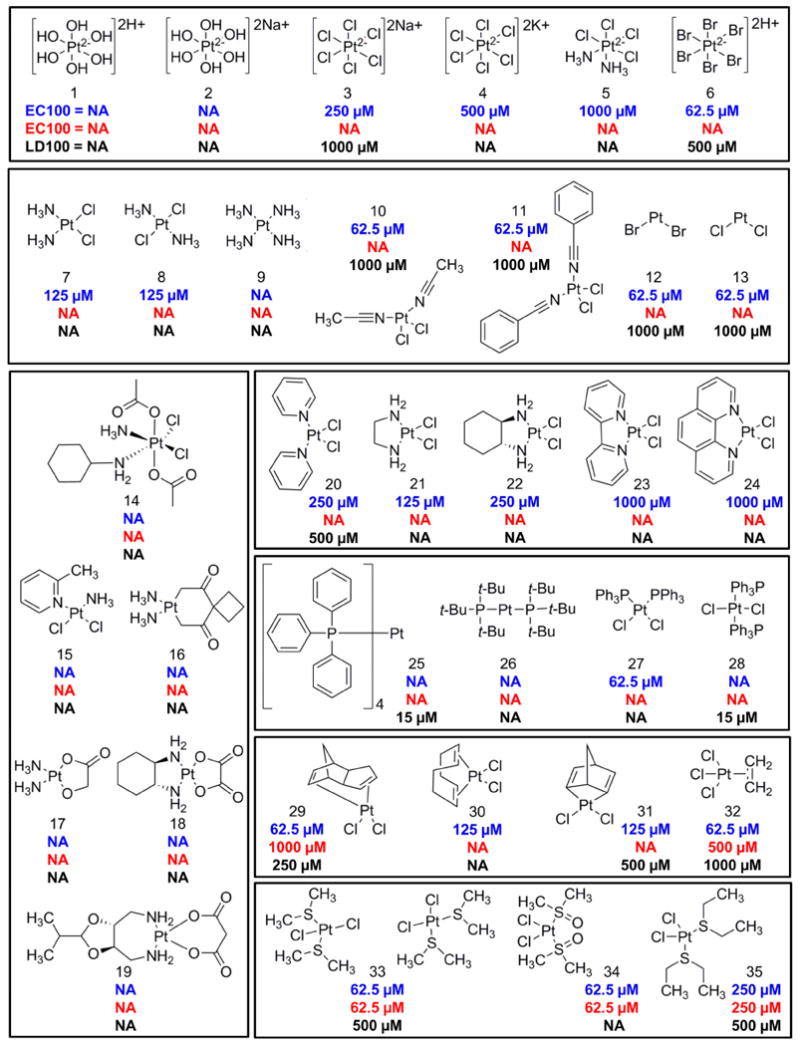
A panel of 35 cisplatin analogs was grouped into the following classes: platinum (IV) (1-6), square planar (7-13), FDA approved (14-19), pyridine (20-24), triphenylphosphine (25-28), alkene (29-32), and sulfur-containing complexes (33-35). A ten point dose curve ranging from 1-1000 μM was tested. The doses that rescued 100% of zebrafish (EC100) from a challenge with 100 μM KCN were determined. The EC100 was determined in both DMSO and PBS solvents (blue and red, respectively). In a separate assay, the doses that caused 100% lethality (LD100) in the absence of KCN were determined for complexes dissolved in DMSO (black). NA indicates instances in which the complex did not induce any toxicity or did not rescue cyanide lethality at any of the doses tested.
Platinum (IV) complexes
Tetravalent platinum complexes have an octahedral geometry and a coordination number of 6. Complexes with hydroxyl groups (1-2) displayed no toxicity in zebrafish and were not cyanide antidotes at any of the doses tested. Increasing the number of chloride ligands from 2 (7) to 4-6 (3-5) reduced the efficacy of the antidote from 125 μM to 250-1000 μM. The increased efficacy and toxicity of the sodium salt (3) compared to the potassium salt (4) may be due to its greater aqueous solubility. The most effective complex (6) had bromide leaving groups (EC100 = 62.5 μM). Platinum IV complexes with ligands that are good leaving groups were more effective cyanide antidotes than those with poor leaving groups (-Br > -Cl > -OH > -NH3). These results suggest that the ease with which the leaving groups are lost is a critical determinant of efficacy of platinum based cyanide antidotes.
Square planar complexes
These complexes have a square planar geometry with a coordination number of 4. Both cisplatin (7) and its trans stereoisomer transplatin (8) were equipotent antidotes. In contrast, the cisplatin analog with 4 ammonia ligands (9) was completely ineffective as an antidote, likely due to the fact that –NH3 is a poor leaving group and typically is considered to be a stable ligand in platinum complexes. The 4 other compounds in this class (10-13) were more potent than cisplatin with minimal toxicity in zebrafish. The two complexes with nitrile ligands (10-11) were effective antidotes at 62 μM. The two least sterically hindered platinum complexes tested in this study had a coordination number of 2, a bent geometry, and either 2 bromide or 2 chloride ligands. Both were effective antidotes at 62 μM (12-13). As a group, these complexes were a potent class of antidotes suggesting that square planar or bent complexes can be effective cyanide chelators.
FDA approved drugs and compounds in clinical evaluation
Many of the compounds in this class (14-19) have a bidentate carboxyl ligand which is a moderate leaving group (16-19). Except for satraplatin (14), they all have a coordination number of 4, and are divalent. The lipophilic Pt (IV) complex satraplatin (14) is an orally available prodrug which to be active must be converted to a Pt (II) complex by loss of the two axial acetate groups. In our assay, it displayed no toxicity however it was not a cyanide antidote. Complex 15 (picoplatin) is a square planar complex that contains a methyl group on the pyridine ring that is perpendicular to the square plane which reduces the substitution kinetics. It also was not a cyanide antidote. Similarly, carboplatin (16), nedaplatin (17), PHM (JM-74) (18) and oxaliplatin (19) were not antidotes when challenged with 100 μM KCN. As a group, this class of complexes displayed no toxicity in zebrafish however none display antidote activity, indicating that compounds with bidentate carboxyl ligands are ineffective as antidotes to cyanide poisoning in zebrafish.
Pyridine complexes
Pyridine complexes are divalent cisplatin analogs with two chloride leaving groups and either a pyridine or bidentate pyridine ligand. Whereas complex 15 was developed to contain a methyl group on the pyridine ring that is perpendicular to the square plane thereby reducing the substitution kinetics, complex 20 does not contain a methyl group on either of the pyridine rings. The removal of the methyl group in complex 20 converts the complex into a cyanide antidote although not as effective as cisplatin (250 versus 125 μM). Complexes with bidentate ligands (21-24) displayed decreased efficacy as the size of rings increased suggesting that these rigid ligands may cause steric hindrance that reduces reactivity (EC100 = 1000 μM). Accordingly the most compact complex (21) in this class was the most effective (EC100 = 125 μM). It contains an ethylenediamine group in place of the two ammine groups of cisplatin and displayed no toxicity in zebrafish.
Triphenylphosphine complexes
These complexes contain lipophilic triphenylphosphine ligands (25-28). One of the four complexes was an effective antidote at 62.5 μM (27). Its trans stereoisomer (28) was not an antidote potentially due to the bulky triphenylphosphine ligands reducing access to the platinum atom whereas in the cis position the compact nature of the 2 cis chlorides allows access to the platinum atom. A caveat to this class of complexes is that they are poorly soluble.
Alkene complexes
Alkene complexes are tetrahedral complexes with chloride and alkene ligands (29-32). Complex 32, also known as Zeise's salt, contains a η2-ethylene ligand while the other complexes in the group contain cyclodiene ligands. In this class of compounds the platinum is coordinated to one (32) or two alkenes (29-31). They were equipotent or more effective than cisplatin (EC100 ≤125 μM), however there was some toxicity in this class of complexes as noted by the LD100. Of note, these complexes contain no ammine ligands unlike the majority of the platinum complexes in clinical development. Additionally, 1,5 cyclodiene ligands such as in dichloro(1,5-cyclooctadiene)platinum(II) (30) are often used in organometallic chemistry as these ligands are easily displaced (Elschenbroich, 2006). Though there is some toxicity in this class of complexes, the substitution kinetics of the 1,5 cyclodiene ligand may be a favorable feature for the development of a platinum based cyanide antidote.
Sulfur-containing complexes
Cisplatin analogs containing two sulfur-based ligands and two chloride ligands (33-35) were tested. The three complexes were effective antidotes at 62.5-250 μM. The change from diethylsulfide ligands in complex 35 to sulfoxide ligands in complex 34 increased efficacy from 250 to 62.5 μM and decreased toxicity. The cis/trans racemic mixture of complex 33 which contains dimethylsulfide ligands compared to complex 35 which contains diethylsulfide ligands was a more potent antidote (62.5 vs 250 μM). It is unclear if the cis or trans stereoisomer was conferring the dominant effect.
SAR summary of DMSO solvated complexes
In a panel of 35 platinum complexes, we identified 22 novel cyanide antidotes with an EC100 of 62.5-1000 μM. The two most effective and least toxic antidotes in zebrafish were the triphenylphoshine complex 27 and the sulfur-containing complex 34 (EC100 = 62.5 μM and no observed toxicity in zebrafish). However, complex 27 is poorly soluble in water (0.079 g/L, logP = 8.2) whereas complex 34 is aqueous soluble (84 g/L; logP = -1.35). This is a ∼33 fold improvement in solubility over cisplatin (2.5 g/L with a logP = -2.19). Additionally, the reported dose of complex 34 that causes acute toxicity in mice is ∼20 fold higher than cisplatin (LD50 = 133 versus 6.6 mg/kg IP) (Braddock et al., 1975).
SAR summary of PBS solvated complexes
Surprisingly, when cisplatin analogs were dissolved in PBS, the majority were not cyanide antidotes at any dose tested (Figure 2). However, 2 of the 4 alkene complexes (29 and 32) were antidotes, albeit requiring doses 16 and 8 fold higher, respectively, than when solvated in DMSO. All three sulfur-containing complexes (33-35) were equipotent antidotes when dissolved in DMSO and PBS. These findings motivated us to explore the effects of solvation on the efficacy of cisplatin analogs as cyanide antidotes.
cis-diamminechloro(dimethylsulfoxide)platinum(II) (complex 36) binds cyanide faster than cis-diamminedichloroplatinum(II) (cisplatin)
Cisplatin's mechanism of action requires aquation, the replacement of the chloride ligands with water molecules. This generates the active form of cisplatin (37) which is more reactive than the chloride complex (7). Due to the high concentration of chloride ions in the blood (∼100 mM) versus inside the cell (∼4 mM), aquation is favored once cisplatin enters the cell. When cisplatin is dissolved in dimethylsulfoxide (DMSO), the sulfur in DMSO undergoes nucleophilic attack of platinum. This results in the substitution of a chloride ligand with a DMSO ligand, changing its structure and creating a new chemical species (36). Due to the influence of the leaving ligands on the kinetics of associative substitution and given the SAR data in PBS versus DMSO, we hypothesized that the DMSO leaving group influences the kinetics of the reaction with cyanide.
To determine if dissolving cisplatin in PBS versus DMSO affected cyanide binding, we conducted UV-VIS experiments. Cisplatin was dissolved in water generating the aquated form of cisplatin (37), in PBS preventing the chloride ligands from being displaced by water molecules (7), or in DMSO creating the DMSO-adduct species (36) (Figure 3A, C, E). In complex 37, cyanide would be predicted to displace a water ligand first (Figure 3A). In complex 7, the cyanide anion would be predicted to displace a chloride ligand first (Figure 3C). In complex 36, based on previous associative substitution studies with platinum complexes, cyanide would be predicted to displace the ammine ligand (Banerjea et al., 1957) (Figure 3E). To determine the binding affinity of cyanide for these three complexes, increasing concentrations of cyanide were added while the absorbance across the UV-VIS spectrum was measured (Fig 3B, D, F). Both cisplatin (7) and the aquated form of cisplatin (37) did not induce a significant spectral shift when cyanide was added (Figure 3B, 3D). In contrast, complex 36 induced a significant spectral shift in the presence of increasing concentrations of cyanide (Figure 3F). Performing this experiment in a polar, aprotic environment did not affect the binding of cyanide to complex 7. These findings suggest that during nucleophilic attack, the cyanide anion cannot easily displace the chloride or water ligands in platinum; however, when a DMSO ligand is conjugated to the platinum complex associative substitution is highly favorable.
Figure 3. Identification of cis-diamminechloro(dimethylsulfoxide) platinum(II) as a potent cyanide antidote in zebrafish.
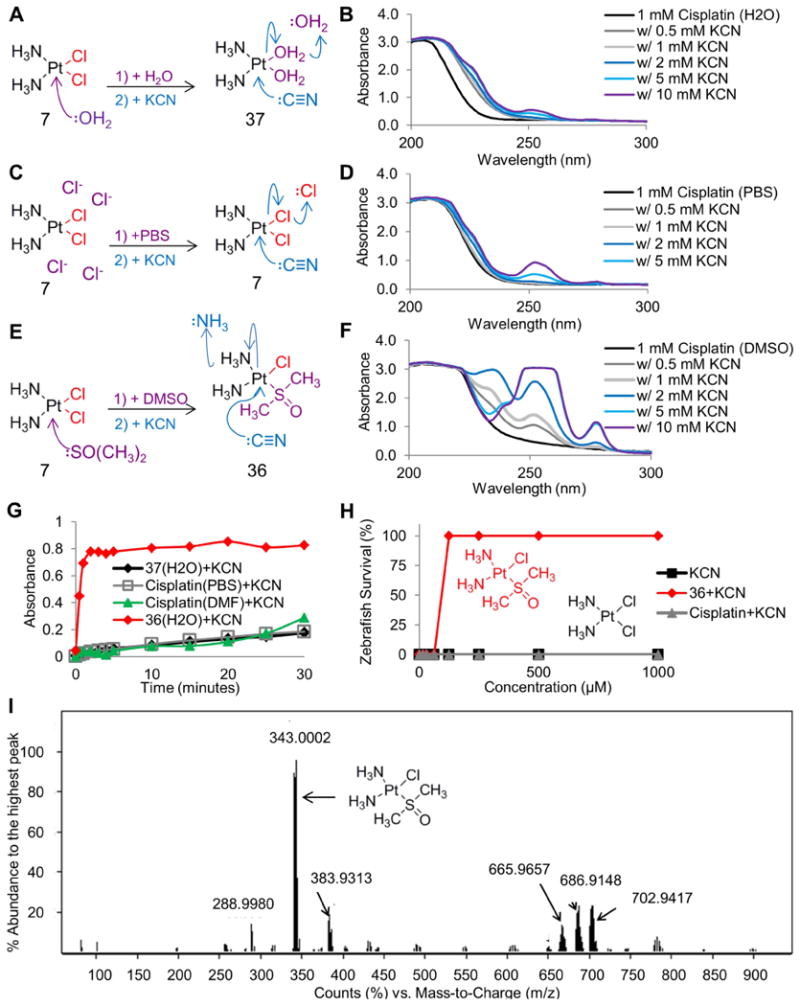
A) Depiction of the associative substitution reaction of water with cisplatin (7), and cyanide with the aquated form of cisplatin (37). B) Spectral shift data demonstrating minimal binding of the aquated form of cisplatin to increasing concentrations of cyanide. C) Depiction of the solvation effect of PBS on cisplatin, and the associative substitution reaction of cyanide with cisplatin (7). D) Spectral shift data demonstrating minimal binding of cisplatin to increasing concentrations of cyanide. E) Depiction of the associative substitution reaction of DMSO with cisplatin generating complex 36, and cyanide with complex 36. F) Spectral shift data demonstrating binding of complex 36 to increasing concentrations of cyanide. G) Graph of the binding of 1 mM complex 37 (black), complex 7 (gray), complex 36 (red), and cisplatin dissolved in DMF (green) to 5 mM cyanide over time, demonstrating the rapid and increased binding rate of complex 36 to cyanide. H) Survival assay in zebrafish demonstrating that complex 36 (red) is a cyanide antidote while cisplatin (gray) is not an antidote. I) Mass spectrometry identification of cis-diamminechloro(dimethylsulfoxide)platinum(II) (complex 36) as the species created by the associative substitution reaction between DMSO and cisplatin. See also Table S2.
Next, we performed a time-course experiment to evaluate the reaction rate of cyanide with these complexes. To 1 mM of complex, 5 mM of cyanide was added and the absorbance was measured every 1 minute for 5 minutes followed by every 5 minutes for 30 minutes (Figure 3G). Cisplatin (7) dissolved in PBS and assayed in PBS had a reaction rate of 0.021 AU/min. The aquated form of cisplatin (cisplatin dissolved in water and assayed in water, 37) had a reaction rate of 0.013 AU/min. Complex 36 (cisplatin dissolved in DMSO and assayed in water), had a reaction rate of 0.367 AU/min. To create a polar aprotic environment DMF was used as the solvent and assay buffer. Cisplatin dissolved in and assayed in DMF had a reaction rate of 0.019 AU/min. These results demonstrate that the reaction between cyanide and complex 36 occurred at a rate 17 fold faster than cisplatin (7) and 28 fold faster than the aquated form of cisplatin (37).
Identification of complex 36 as a cyanide antidote in vivo
We hypothesized that the reaction rate of complex 36 with cyanide, compared to that with cisplatin, drives the different efficacies of these two complexes in vivo. Zebrafish treated with cisplatin dissolved in PBS (cisplatin) displayed no activity as a cyanide antidote while cisplatin dissolved in DMSO (complex 36) was an effective antidote (Fig 3H). Theoretically DMSO could undergo nucleophilic attack of the platinum atom, generating multiple reaction products. To decipher the exact chemical species created when we dissolved cisplatin in DMSO, we used ESI-MS. The most abundant ion signal detected was at m/z = 343 corresponding to the molecular weight of [Pt(NH3)2(Cl)(DMSO)] (Figure 3I). In this complex one chloride ligand was displaced by a DMSO ligand generating cis-diamminechloro(dimethylsulfoxide)platinum(II) (36). We observed near complete conversion of cisplatin to complex 36 within a few hours, consistent with the literature (Fischer et al., 2008). Other minor species were detected consistent with published studies (Table S2). Collectively these results demonstrate that the cisplatin analog, cis-diamminechloro(dimethylsulfoxide)platinum(II), is a cyanide antidote. Concomitantly, the SAR dataset revealed that complexes dissolved in DMSO were cyanide antidotes, whereas those same complexes dissolved in PBS were not cyanide antidotes suggesting that sulfur based ligands are a key feature of platinum based cyanide antidotes.
Cisplatin analogs solvated in DMSO display decreased cytotoxicity in H1975 cells
The toxic side effects of platinum based drugs are thought to be due to their mechanism of action, DNA damage leading to cell death. DMSO is known to inactivate chemotherapeutic drugs (cisplatin, carboplatin and oxaliplatin) by inserting into the complex, disrupting its ability to interact with DNA and hence induce cell death (Fischer et al., 2008). To test the cytotoxicity of the compounds under study, cisplatin responsive non-small cell lung cancer cells (H1975) were used. Cells were treated with 0-300 μM of a platinum complex for 72 hours and cell viability was assessed by measuring ATP levels. Cells treated with 50 μM cisplatin appeared rounded, shrunken and fragmented, while those treated with complexes 36 or 34 displayed similar morphology to control cells (Figure 4A). At 72 hours, dose dependent cell killing was observed in cisplatin treated cells, however not in cells treated with complex 34 or 36 (Figure 4B). The IC50 for cisplatin was 62 μM. In an expanded dose response curve, the IC50 of complexes 34 and 36 were 702 and 689 μM, a ∼10 fold decrease in cytotoxicity.
Figure 4. A subset of cisplatin analogues solvated in DMSO exhibit decreased cytotoxicity in human cells.
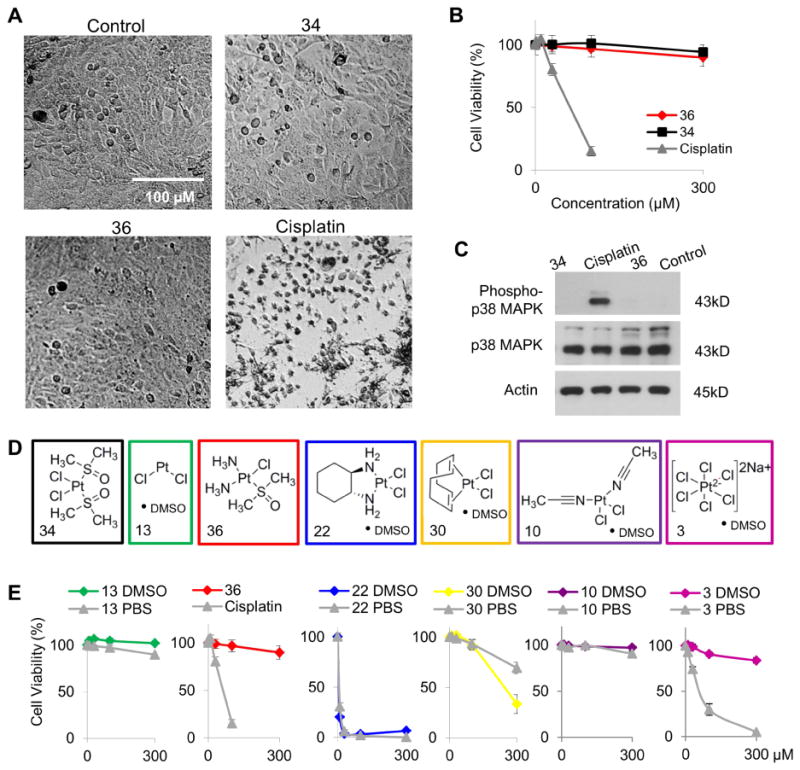
A) Images of H1975 cells treated with vehicle, 34, 36, or cisplatin. B) Cell viability over increasing concentrations of 36 (red), cisplatin (gray) and 34 (black). Data represented as the mean ± SD. C) Western blots for phospho-p38 MAPK on lysates from cells treated with vehicle, 34, 36, or cisplatin. Complexes from each structural class (D) were dissolved in PBS (gray) or DMSO (color) and cell viability over increasing concentrations was determined (E). Also see Figure S1.
Activation of p38 MAPK in response to cisplatin induced DNA damage is a requisite step in the mechanism of action of cisplatin (Galan-Moya et al., 2008). We measured the phosphorylation state of the kinase p38 MAPK in lysates from cells treated with 50 μM of the indicated complexes for 24 hours. As expected, cisplatin activated p38 by inducing phosphorylation (Figure 4C). However, complexes 34 and 36 did not increase activated p38 levels, indicating that these complexes are not initiating the stress-associated signaling pathway that is triggered by cisplatin. These findings are consistent with previous in vitro and in vivo studies demonstrating the detoxifying effect of DMSO formulations of cisplatin (Hall et al., 2014).
Although the DMSO-bound form of cisplatin undermines the drug's utility as a chemotherapeutic drug, the decreased toxicity is a beneficial aspect for its use as a cyanide antidote. Therefore, antidotes identified in the SAR study were solvated in DMSO or PBS and assessed for cytotoxicity in H1975 cells (Figure 4E and S1A-D). Several complexes (4, 5, 22 and 31), in both PBS and DMSO formulations, were more cytotoxic than cisplatin. Other complexes (20, 29, 30, 33 and 35) displayed increased cytotoxicity when solvated in DMSO compared to PBS. These two groups of complexes represent compounds with chemotherapeutic potential that may not encounter resistance due to binding with thiol containing proteins. Several complexes (6, 10, 12, 13, 27 and 32) displayed minimal ability to induce cell death at doses up to 300 μM in both DMSO and PBS formulations. Finally, DMSO inactivated the cytotoxic activity of complex 3, 11 and 21. These findings indicate that the DMSO adduct form of a few structurally distinct platinum complexes exhibit reduced cytotoxicity while maintaining or improving their efficacy as cyanide antidotes.
Cisplatin Analogs Protected Mice Exposed to a Lethal Dose of Cyanide
The successful application of cisplatin analogs as antidotes to cyanide poisoning in zebrafish led us to investigate their effects in a mouse model of cyanide poisoning. In this model, a mouse is placed in a gas tight chamber and exposed to cyanide gas for 15 minutes. Subsequently, the mouse is injected intraperitoneally with vehicle or platinum complex and then re-exposed to cyanide gas for 25 minutes. Thus, total exposure time to cyanide gas is 40 minutes. All surviving animals are observed for several hours and then euthanized. In this model mice that receive saline consistently died within a 5 minute window 30-35 minutes after the onset of cyanide exposure (n=6).
Pt(II) and Pt(IV) cisplatin analogs (36, 34 and 3) were chosen based on efficacy/toxicity in zebrafish, toxicity in human cells, binding kinetics, and solubility. Several other complexes had favorable efficacy and toxicity profiles however due to low solubility they will require future formulation studies prior to mammalian testing. Of the mice receiving complex 36 (20 μmol), 83% survived the full exposure period (n=6) while of those receiving 10 μmol 33% survived (n=6). Of the mice receiving 20 μmol complex 34, 100% survived while of those receiving 10 μmol, 33% survived (n=6). For complex 3, 100% of mice receiving 5 μmol and 50% of mice receiving 2.5 μmol survived (n=6). The four-fold increased potency of complex 3 compared to complex 36 may be because complex 3 binds up to 5 cyanide anions while complex 36 binds 3-4 cyanide anions (Figure S2). Collectively, these data demonstrate that the effect of cisplatin analogs as countermeasures to cyanide poisoning is conserved in mammals.
To test the hypothesis that formulation in DMSO improves the efficacy of platinum based cyanide antidotes, we tested complex 3 with or without DMSO. Treatment with 2.5 μmol of complex 3 formulated with DMSO resulted in 50% survival (n=6). To achieve 66% survival in mice treated with complex 3 formulated without DMSO, a dose of 10 μmol was required (n=6). These results indicate that DMSO formulation improves the efficacy of complex 3 by ∼4-fold and suggests that the improvement in antidote activity is a result of the chemical reaction between DMSO and platinum complexes.
Cisplatin Analogs Reversed Cyanide-Induced Effects on Oxidative Metabolism in Rabbits
Rabbits (n=5) were infused intravenously (IV) with a sub-lethal dose of sodium cyanide (10 mg) while tissue oxygenation in the central nervous system (CNS) was monitored in real time using continuous wave near infrared spectroscopy (CWNIRS). During the 60 minute cyanide infusion, CWNIRS of the CNS detected an increase in the concentration of oxyhemoglobin (red) and decrease in deoxyhemoglobin (blue) (Figure 6A). This occurs as cyanide prevents oxygen offloading from hemoglobin in erythrocytes thus leading to an increasing fraction of hemoglobin in the oxygenated state. However when the cyanide infusion stops, both of these curves gradually reverse, indicating oxygen offloading from hemoglobin and an increase in circulating hemoglobin in the deoxygenated state (blue). Thus, the pathophysiological changes associated with sub-lethal cyanide exposure are reversed 30 minutes following the cessation of cyanide infusion.
Figure 6. Cisplatin analogs reversed cyanide induced changes in oxidative metabolism in rabbits.

A representative rabbit injected with cyanide demonstrating A) increased concentration of hemoglobin in the oxygenated state (red) compared to the deoxygenated state (blue) in the CNS and B) decreased cytochrome oxidase c redox ratio in the muscle (black). Injection of 36 (C-D) or 3 (E-F) after the cyanide infusion results in rapid reversal of cyanide induced pathophysiologic changes. n=5.
To determine if cisplatin analogs alter the kinetics of oxygen offloading from hemoglobin and ameliorate cyanide toxicity in the CNS, rabbits were treated with 15 mg/kg of 36 or 7.5 mg/kg of 3 IV after the cyanide infusion (n=5). There is no rabbit toxicity data on 34, 36 or the complex 3 DMSO adduct species in the literature, but the doses of 3 that were used are well above the LDLo in rabbits (7.5 mg/kg vs 180 mg/kg) (TOXNET). As expected, during cyanide infusion, an increase in the concentration of oxyhemoglobin (red) and decrease in deoxyhemoglobin (blue) was detected, indicating cyanide toxicity. However, immediately following the administration of 36 or 3 (Inj), the oxy- and deoxy-hemoglobin concentrations rapidly returned to baseline levels (Figure 6B, E). Restoration to baseline occurs in less than 10 minutes compared to vehicle controls in which restoration to near baseline occurs in 30 minutes (intersection of oxy- and deoxy-hemoglobin curves). Further, the time constant (Tau) which represents the decay of the oxy-hemoglobin was significantly different compared to controls (257 ± 143 min; p<0.01) for both complex 3 and complex 36 (6.61 ± 4.41 and 12.15 ± 4.42 min, respectively). Collectively, these changes indicate a reversal of the pathophysiological events induced by cyanide.
We hypothesized that the elimination pathway for cyano-platinum complexes in mammals occurs via the kidneys as both thiocyanate and cisplatin are excreted in the urine. Urine was collected 90 minutes post antidote injection and cyano-platinum complexes were measured by mass spectrometry. We detected 1.3 ± 0.7 μg/mL of Pt(CN)3 and 0.6 ± 0.3 μg/mL of Pt(CN)4 in the urine of rabbits (n=3) treated with complex 36. In rabbits (n=3) treated with complex 3 we detected 30.5 ± 17.6 μg/mL of Pt(CN)3 and 12.9 ± 7.5 μg/mL of Pt(CN)4 in the urine 90 minutes post injection. These data demonstrate that cyano-platinum species produced by the administration of platinum based antidotes are excreted into the urine.
Cisplatin Analogs Corrected Cytochrome C Oxidase Redox State in Rabbits Exposed to Cyanide
Next, we used diffuse optical spectroscopy (DOS) to monitor cytochrome c oxidase redox state in the muscle of rabbits. During the 60 minute cyanide infusion, the cytochrome c oxidase redox ratio (black) decreased due to the binding of cyanide anion to iron in cytochrome c oxidase and did not return to baseline levels after cessation of the cyanide infusion (Figure 6B). However, when 13 mg/kg of 36 or 7.5 mg/kg of 3 (n=5) was injected IV, cytochrome c oxidase redox ratio returned to baseline in 10-20 minutes (Figure 6D, F). These findings indicate that cisplatin analogs restore muscle cytochrome c oxidase redox state to baseline, indicating that cisplatin analogs are effective antidotes in mammals.
Discussion
Herein we report the structure activity relationships of cisplatin analogs and describe their efficacy as cyanide antidotes in zebrafish, mice and rabbits. Through efficacy, toxicity and binding studies, we identified the features of cisplatin analogs that confer protection against cyanide poisoning. Ultimately, we identified a Pt(IV) complex (3) as a promising antidote to pursue for further studies.
Serendipitously, we discovered that cisplatin is converted into a cyanide antidote by solvation in DMSO, the standard solvent used to dissolve compounds for chemical screening. After solvation in PBS we identified 5 of 35 complexes (14%) as antidotes whereas after DMSO solvation we identified 22 of 35 complexes (62%) as antidotes. The increased hit rate in DMSO solvated complexes occurs because the sulfur atom in DMSO attacks the platinum atom, displaces ligands, and changes the structure of the complex into a new molecule with different chemical properties.
We speculate that the trans-directing effect of sulfur underlies the new substitution kinetics of platinum complexes with DMSO ligands (Basolo and Pearson, 2007). A trans-directing ligand affects the lability of the ligand trans to itself and directs the positioning of an entering ligand to that position. For instance, complex 36 has two cis ammonia ligands, one chloride and one DMSO ligand, which is trans to one of the ammonia ligands. Ammonia ligands are extremely poor leaving groups. However, the potent trans-directing effect of sulfur in the DMSO ligand weakens the ammonia ligand bonded trans to its position. Thus in this configuration, the ammonia ligand (-NH3) leaves first (Ivanov et al., 1998). Our UV-VIS and MS data demonstrate that the cyanide anion rapidly displaces ligands in DMSO solvated cisplatin analogs. Therefore, the high hit rate of platinum complexes solvated in DMSO compared to PBS suggests that the presence of a sulfur-containing ligand and the trans directing effects of sulfur are key features of platinum based cyanide antidote activity.
In the SAR study, we identified 22 complexes with antidote activity ranging from an EC100 of 62.5 to 1000 μM. Cisplatin analogs are capable of binding up to 5 cyanide anions as demonstrated by our MS data (Figure S2 and Table S2). Their efficacy as cyanide antidotes may be based on the number of ligands displaced by cyanide and the kinetics of displacement of these ligands by associative substitution. In turn, associative substitution reactions are governed by factors including 1) the nature of the metal (in this case platinum), 2) the charge or electrons to be donated by the nucleophile (in this case the cyanide anion), and 3) the characteristics of the ligands coordinated to the metal (the leaving groups). Our SAR study spanned 7 structural classes, allowing for the evaluation of the effects of an array of ligands on efficacy.
A number of observations were gleaned from the structure activity relationship study regarding the chemical features that conferred cisplatin analogs with the most potency. Critical features included the presence of a sulfur containing ligand. Further complexes should not be developed to contain ligands that are rigid (multiple ring structures) or bulky as steric hindrance may interfere with the molecular rearrangements that occur during nucleophilic attack thereby limiting cyanide anion's ability to access the platinum atom. FDA approved drugs containing leaving groups such as bidentate carboxyl ligands are less reactive and were ineffective antidotes. Although the prototypic cisplatin analog contains 2 chloride ligands and 2 ammine ligands, in 10 of the 14 most potent cyanide antidotes, the platinum was not conjugated to any ammine ligands. Instead they were coordinated to alkene, sulfur or triphenylphosphine ligands. Bidentate alkene complexes were much more effective antidotes than bidentate ammine ligands. Typically bidentate groups are moderate leavings groups due to the two-step mechanism of ligand loss, but the resonance stabilization properties of alkenes or the trans effect of sulfur may facilitate ring opening and subsequent loss of the ligand. Within the platinum (IV) class 4 of the 7 complexes were antidotes in zebrafish and 1 was in mammals. Platinum (IV) complexes tend to be less toxic than platinum (II) complexes. Accordingly, complex 3 which was a cyanide antidote at 7.5 mg/kg in rabbits, has a LDLo of 180 mg/kg SC in rabbits compared to cisplatin which has a LDLo of <10 mg/kg in several species (TOXNET). In summary, complexes should be engineered to have fast substitution kinetics for at least some of the ligands as cyanide can be lethal within minutes however the tradeoff between high reactivity and toxicity must be evaluated carefully. Therefore, platinum (IV) complexes may possess the optimal balance of reactivity, efficacy and toxicity.
One million new patients in North America receive cisplatin annually even though cisplatin is associated with a number of side effects including nephrotoxicity, neurotoxicity and ototoxicity (Strumberg et al., 2002). These drugs act by crosslinking DNA strands, which prevents DNA replication and cell division, and ultimately leads to the induction of apoptosis (Jamieson and Lippard, 1999). The histone protein λH2A.X becomes phosphorylated in response to the DNA damage caused by platinum drugs. However, DLD-1 cells treated with cisplatin, carboplatin, transplatin or [PtCl2(en)] formulated in DMSO do not exhibit this response (Hall et al., 2014). Additionally, cleaved PARP and cleaved caspase-3 were not detected, demonstrating the loss of platinum-induced apoptotic events (Hall et al., 2014). In particular one of the most concerning side effects, nephrotoxicity, was decreased in studies with rats using the cisplatin-DMSO adduct species (Fischer et al., 2008). Although the DMSO adduct form of cisplatin undermines the drug's utility as a chemotherapeutic drug, the decreased toxicity is a beneficial aspect for its use as a cyanide antidote because it reduces toxicity while improving interaction with cyanide.
Formal toxicology studies are beyond the scope of this study however much can be learned from published efforts to temper the side effects of platinum-based chemotherapeutic drugs. For instance, the decreased toxicity of the cisplatin-DMSO species initiated studies utilizing thiols as reactive "protective agents". Pretreatment with D-methionine or glutathione ester protects against cisplatin induced toxicity in central nervous system tissue and kidney, respectively (Anderson et al., 1990; Gopal et al., 2012). Further, the ability of N-acetylcysteine to prevent cisplatin-induced ototoxicity is in Phase I clinical trials (NCT02094625). Coordination of protective agents to platinum that could be displaced by the cyanide anion during the associative substitution reaction is an intriguing approach for the development of a dual cyanide antidote and is currently underway in our lab.
Promising strategies to overcome platinum toxicity being pioneered include nanoparticles and targeted encapsulations (Wisnovsky et al., 2013). Lipoplatin is a liposomal-encapsulated formulation of cisplatin that has successfully completed Phase III trials for lung cancer and demonstrated significantly reduced toxicity compared to cisplatin, especially in the kidney (6.1% vs 40%) (Stathopoulos et al., 2010). Organelle specific drug delivery using triphenylphosphine is an effective way to deliver drugs to the mitochondria (Frantz and Wipf, 2010; Zhou et al., 2014). Directly delivering a cyanide antidote to its target organelle could be a significant advance in therapy. Here, complex 22 was shown to be a potent antidote in the SAR study (EC100 = 62.5 μM). However, due to the lipophilic triphenylphosphine group, the solubility of complex 22 was in the low micromolar range. To overcome its low solubility, it will likely require specialized formulation. In addition to formulation strategies, medicinal chemistry approaches are being used to mitigate side effects. For instance, replacing the 2 ammonia groups in cisplatin with a cyclopropanammine group in JM-11 significantly decreased nephrotoxicity in rodents, demonstrating that changes in ligands influence the unique properties of platinum complexes and their in vivo biology (Harrap et al., 1980).
Collectively, using three different vertebrate animal models, the studies reported here represent a proof of concept demonstrating the efficacy of platinum-based complexes as a class of molecules to counteract cyanide poisoning. The SAR data yielded a promising antidote and generated information to rationally design additional platinum antidotes. Future cycles of medicinal chemistry and formulation chemistry are required to bring rationally designed cisplatin analogs through the pipeline that in the future may be first-in-class treatments for cyanide poisoning.
Significance
Cisplatin is a simple but elegant small molecule that has led to the virtual cure of testicular cancer. Nath, et al. discovered that cisplatin analogs are antidotes to cyanide poisoning in three vertebrate species. Using structure activity relationships they evaluate the efficacy, toxicity and solubility of cisplatin analogs in human lung cells, zebrafish, mice, and rabbits. These findings lead to the identification of the chemical features of cisplatin analogs that confer protection against cyanide poisoning, thereby contributing to the ongoing effort to discover effective cyanide antidotes and to understand the in vivo biology of cisplatin analogs.
STAR Methods
Contact for Reagent and Resource Sharing
Please contact A.K.N. (anjali.nath@aya.yale.edu) for reagents and resources generated in this study.
Experimental Model and Subject Details
Zebrafish
Animals were maintained and embryos were obtained according to standard fish husbandry protocols in accordance with the Massachusetts General Hospital Institutional Animal Care and Use Committee. Zebrafish embryos (Ekkwill strain) were grown at 28°C in HEPES buffered Tübingen E3 medium and assayed at 6 d.p.f.
Mice
All studies were carried out according to NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Veterans Administration San Diego Healthcare System's Institutional Animal Care and Use Committee. C57/BL6J male (Jackson Laboratories) male mice weighing 20-25 g were used and were fed ad libitum Teklad #7001.
Rabbits
The protocol was reviewed and approved by the University of California Irvine (UCI) Institutional Animal Care and Use Committee (IACUC). Pathogen-free New Zealand White male rabbits (Western Oregon Rabbit Supply), weighing 3.5-4.5 kg were used in this study.
Tissue Culture Cells
H1975 non-small cell cancer cells were grown in RPMI-1640 Medium supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 IU/ml penicillin and 100 μg/ml streptomycin.
Method Details
In vivo SAR Studies
The assay was carried out on 6 d.p.f. larval zebrafish loaded in 96-well plates containing HEPES buffered Tübingen E3 medium (n=5 per well). Compounds were screened using a 12 point dose response analysis 0.4-1000 μM. Potassium cyanide was added at a dose of 100 μM which induces 100% death within 1 hour in controls animals. Following the addition of cyanide the plates were sealed with adhesive PCR plate foil and incubated at 28°C. The lowest effective dose to rescue 100% of larvae (EC100) was reported 4 hours post treatment. For assessment of compound toxicity, larvae were treated for 24 hours with compounds and viability was assessed by observing heart rate and response to touch as previously described (Nath et al., 2013). The dose that causes 100% lethality was reported (LD100). All compounds were purchased from Sigma-Aldrich or Abcam. Complex 36 was prepared as previously described and its structure was confirmed by mass spectrometry (Fischer et al., 2008). In the zebrafish assay, each dose of each drug was tested on 5 larvae and these experiments were repeated on 5 separate days.
UV-VIS Spectral Assay
Cisplatin was dissolved in PBS (1 mM), DMSO (1 M), H2O (1 mM) or DMF (1 mM) and heated for several hours to generate the stock solution at the concentration indicated in parentheses. Subsequently the DMSO stock was diluted to 1 mM in PBS or H2O. For dose response experiments, from a stock solution of 1M KCN in PBS or H2O, a cyanide concentration curve from 1-200 mM was generated in PBS or H2O. The reaction was incubated for 30 minute and the absorbance read over the UV-VIS spectrum on a NanoDrop (Thermo Scientific). For time course experiments, 1 mM complex was reacted with 5 mM KCN and the absorbance was measured every 1 minute for 5 minutes followed by every 5 minutes for 30 minutes. To create a polar aprotic environment, DMF was used as the solvent and assay buffer. All reactions were blanked to a solution of complex only.
Cytotoxicity Assay
Cell viability was evaluated in H1975 cells, a cisplatin responsive non-small cell lung cancer line. Cells were plated into 96-well plates. When the plates reached 80% confluency, the cells were treated with the indicated compounds for 72 hours. The compounds were dissolved in DMSO or PBS and tested at doses at 0, 3, 10, 30 and 300 μM (eight wells per dose). Viability was assayed using the CellTiter-Glo Luminescent Cell Viability Assay (Promega) by gently removing the media prior to adding CellTiter-Glo reagent to the wells. Three biological replicates were performed.
Western Blot
Cells were treated for 24 hours then lysed in NP-40 lysis buffer [50 mM Tris/HCl, pH 8.0, 150 mM NaCl, 1% NP-40, complete protease inhibitor tablets (Sigma-Aldrich) and PhosStop phosphatase inhibitor tablets (Roche)]. Lysates were ran on NuPAGE Novex 4-12% Bis-Tris Protein Gels (Invitrogen) and transferred to PVDF membranes. The membranes were blocked with 5% BSA for 1 hour at room temperature and probed with antibodies to phospho-p38 MAPK, p38 MAPK and β-actin overnight at 4°C. Three biological replicates were performed.
Mass Spectrometry
The cisplatin-DMSO solution (100 mM) was generated by adding 15 mg cisplatin into 500 μl of DMSO (warmed to 95°C). The solution was incubated in the dark for 1 hour. The hexachloroplatinate-DMSO solution (100 mM) was generated by adding 28 mg hexachloroplatinate into 500 μl of DMSO (warmed to 95°C). The solution was incubated in the dark for 1 hour. Next, a solution of 1M KCN or K13C15N was prepared in water that was adjusted to pH 7.4 with NaOH. Equal volumes of the platinum complexes and the cyanide solution were then combined, vortexed and incubated. The resulting mixture was diluted 10-fold with HPLC-grade methanol and then infused directly into an Agilent 6550 iFunnel Q-TOF Mass Spectrometer equipped with a dual AJS-ESI source at 10 μl/min. The source parameters for acquisition were set as: drying gas temperature and flow was 250°C and 14 L/min respectively; nebulizer was set at 35 psig; sheath gas temperature and flow were 350°C and 11 L/min respectively; Vcap and nozzle voltage were at 1500V and 2000V. The MS-only full scan and/or targeted MS/MS scan in positive mode was acquired through Agilent MassHunter Workstation LC/MS Data Acquisition software (version B.05.01). The mass range of TOF spectra was 70-1700 m/z and the acquisition rate was 1 spectra/sec. The reference mass of 121.050873 and 922.009798 was selected for mass correction. The acquired Q-TOF LC/MS data was analyzed with the Agilent MassHunter Workstation Qualitative Analysis software (version B.06.00) for peak identification. To further confirm the structure of identified compounds, the isotope distribution of the observed compound spectra was compared with predicted spectra generated using an Isotope Distribution Calculator and Mass Spec Plotter (http://www.sisweb.com/mstools/isotope.htm).
Mouse Cyanide Inhalation Model
Mice were placed in an acrylic glass chamber and anesthetized by injecting isoflurane into the chamber (2% v/v). The mice become anesthetized within 1-2 min, but 5 min are allowed to be sure they are in a homeostatic state before being cyanide exposure. Hydrogen cyanide is generated by injecting 0.1 M KCN into a beaker of 1 M sulfuric acid. Mice are exposed to cyanide gas for 15 min in gas chamber, removed from the chamber, injected with antidote, and re-exposed to cyanide gas for an additional 25 min. The antidotes are prepared in DMSO, diluted 10 fold in saline and injected IP. The animals are anesthetized with isoflurane throughout the cyanide exposure period and all surviving mice are euthanized at the end of the experiment. This model has been previously described (Chan et al., 2015).
Rabbit Cyanide Infusion Model
Animals were anesthetized with an intramuscular injection of ketamine HCl 50mg/kg (Ketaject, Phoenix Pharmaceutical Inc., St. Joseph, MI) and xylazine 5mg/kg (Anased, Lloyed Laboratories, Shenandoah, IA). After the injection, a 23 gauge, 1 inch catheter was placed in the animal's marginal ear vein to administer continuous intravenous anesthesia with ketamine/xylazine. The depth of anesthesia was evaluated by monitoring the animals' physical reflexes and heart rate. Animals were intubated with a 3.0 cuffed endotracheal tube secured by a gauze tie; they were mechanically ventilated (dual phase control respirator, model 32A4BEPM-5R, Harvard Apparatus, Chicago, IL) at a rate of 20 respirations per minute, a tidal volume of 50 cc, and FiO2 of 100%. A pulse oximeter (Biox 3700 Pulse Oximeter, Ohmeda, Boulder, CO) with a probe was placed on the tongue to measure SpO2 and heart rate. Sodium cyanide, 10mg in 60cc normal saline, was infused continuously intravenously at a rate of 1cc/min. Inspired oxygen remained at 100% throughout the experiment. At the end of infusion, antidote compounds were given IV and animals were monitored for an additional 90 minutes. The effects of cyanide toxicity and reversal of toxicity with antidotes were observed in real time using optical spectroscopy. Subsequently, the animals were euthanized with an intravenous injection of Euthasol (1.0 cc, Euthasol, Virbac AH, Inc. Fort Worth, Texas). This model has been previously described (Brenner et al., 2010).
In vivo Optical Spectroscopy
The details of DOS and CWNIRS methodology have been previously described (Brenner et al., 2010). Briefly, DOS measurements were obtained through a fiber-optic probe placed on the shaved surface of the right inner thigh of the rabbit. The broadband DOS system combines multi-frequency domain photon migration with time-independent near infrared spectroscopy to accurately measure bulk tissue absorption and scattering spectra. Tissue concentrations of oxyhemoglobin, deoxyhemoglobin and cytochrome c redox state (ratio of oxidized to reduced cytochrome c) were calculated by a linear least squares fit of the wavelength-dependent extinction coefficient spectra of each chromophore. CWNIRS penetrates more deeply into tissues than DOS therefore was used to assess oxy- and deoxyhemoglobin effects of cyanide toxicity in the central nervous system. The CWNIRS system consists of a light source (HL 2000, Ocean Optics, FL), a CCD spectrometer (USB4000, Ocean Optics, FL), and customized optical fiber guides. Continuous wave near infrared light was delivered to the rabbit brain using a fiber optic probe (9mm source-detector separation), and transmitted light intensities at five wavelengths (732, 758, 805, 840, 880 nm) were measured using the CCD spectrometer every second. We quantified changes in oxy- and deoxyhemoglobin concentrations throughout the experiment using a modified Beer-Lamberts' law and those changes are displayed in real time using Labview software (Labview 7.1, National Instrument, TX).
Quantification and Statistical Analysis
Statistical parameters are reported in Figure Legends or in Method Details. In the zebrafish assay, each dose of each drug was tested on 5 larvae and these experiments were repeated on 5 separate days. Cell viability experiments were tested three separate times with error bars indicating the SD of 8 wells from one of the three replicates. The Western blot experiments were performed on 3 biological replicates. The murine cyanide inhalation model used 6 mice per treatment group. The rabbit cyanide infusion model used 5 rabbits per treatment group.
Supplementary Material
Figure 5. Cisplatin analogs protected mice exposed to a lethal dose of cyanide.
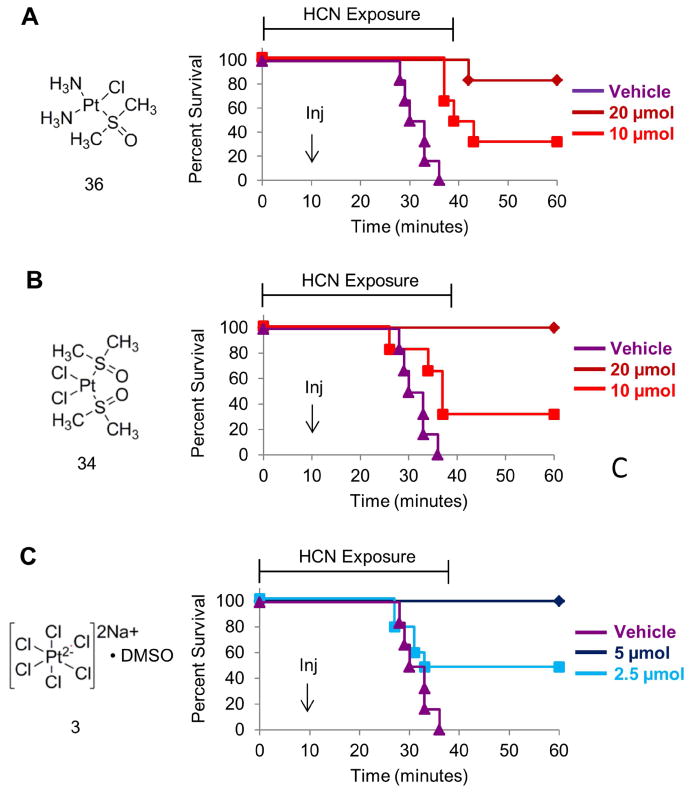
Mice were exposed to cyanide gas for 15 min, injected with the indicated complex (Inj) and placed back in the gas chamber for another 25 min. The data are shown as percent survival versus time. The animals injected with vehicle (purple) consistently died between 30-35 minutes however A) 83% of mice treated with 20 μmol of 36, B) 100% of mice treated with 20 μmol of 34, and C) 100% of mice treated with 5 μmol of 3 survived exposure to a lethal dose of cyanide. n=6. Also see Figure S2.
Highlights.
Cyanide antidotes discovered by structure activity relationships of cisplatin
Sulfur containing ligands are a key feature of platinum based cyanide antidotes
Cisplatin analogues act as antidotes by binding up to 5 cyanide anions
Antidote activity is conserved in zebrafish, mice and rabbits
Acknowledgments
This work was supported by NIH U54NS079201.
Footnotes
Author contributions: Conceptualization, A.K.N. and R.T.P; Investigation, A.K.N., J.L., S.M., D.H., A.C., J.M., P.S. and X.S; Writing—original draft, A.K.N; Writing—review and editing, all; Supervision, A.K.N. R.T.P., R.E.G. C.A.M., M.B, S.M. and G.R.B; Resources and Funding acquisition, R.T.P., R.E.G. C.A.M., M.B and G.R.B.
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcorta R. Smoke inhalation & acute cyanide poisoning. Hydrogen cyanide poisoning proves increasingly common in smoke-inhalation victims. JEMS. 2004;29(6-15) quiz suppl 16-17. [PubMed] [Google Scholar]
- Anderson ME, Naganuma A, Meister A. Protection against cisplatin toxicity by administration of glutathione ester. FASEB J. 1990;4:3251–3255. doi: 10.1096/fasebj.4.14.2227215. [DOI] [PubMed] [Google Scholar]
- Banerjea D, Basolo F, Pearson RG. Mechanism of Substitution Reactions of Complex Ions. XII. Reactions of Some Platinum(II) Complexes with Various Reactants. Journal of the American Chemical Society. 1957;79:4055–4062. [Google Scholar]
- Barillo DJ, Goode R, Esch V. Cyanide poisoning in victims of fire: analysis of 364 cases and review of the literature. J Burn Care Rehabil. 1994;15:46–57. doi: 10.1097/00004630-199401000-00010. [DOI] [PubMed] [Google Scholar]
- Basolo F, Pearson RG. Progress in Inorganic Chemistry. John Wiley & Sons, Inc.; 2007. The Trans Effect in Metal Complexes; pp. 381–453. [Google Scholar]
- Braddock PD, Connors TA, Jones M, Khokhar AR, Melzack DH, Tobe ML. Structure and activity relationships of platinum complexes with anti-tumour activity. Chem Biol Interact. 1975;11:145–161. doi: 10.1016/0009-2797(75)90095-2. [DOI] [PubMed] [Google Scholar]
- Brenner M, Kim JG, Mahon SB, Lee J, Kreuter KA, Blackledge W, Mukai D, Patterson S, Mohammad O, Sharma VS, et al. Intramuscular cobinamide sulfite in a rabbit model of sublethal cyanide toxicity. Ann Emerg Med. 2010;55:352–363. doi: 10.1016/j.annemergmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton E. The Bhopal disaster and its aftermath: a review. Environ Health. 2005;4:6. doi: 10.1186/1476-069X-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Jiang J, Fridman A, Guo LT, Shelton GD, Liu MT, Green C, Haushalter KJ, Patel HH, Lee J, et al. Nitrocobinamide, a new cyanide antidote that can be administered by intramuscular injection. J Med Chem. 2015;58:1750–1759. doi: 10.1021/jm501565k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elschenbroich C. Organometallics. Weinheim: Wiley-VCH; 2006. [Google Scholar]
- Fichtinger-Schepman AM, van der Veer JL, den Hartog JH, Lohman PH, Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985;24:707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Fischer SJ, Benson LM, Fauq A, Naylor S, Windebank AJ. Cisplatin and dimethyl sulfoxide react to form an adducted compound with reduced cytotoxicity and neurotoxicity. Neurotoxicology. 2008;29:444–452. doi: 10.1016/j.neuro.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Frantz MC, Wipf P. Mitochondria as a target in treatment. Environ Mol Mutagen. 2010;51:462–475. doi: 10.1002/em.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Moya EM, Hernandez-Losa J, Aceves Luquero CI, de la Cruz-Morcillo MA, Ramirez-Castillejo C, Callejas-Valera JL, Arriaga A, Aranburo AF, Ramon y Cajal S, Silvio Gutkind J, et al. c-Abl activates p38 MAPK independently of its tyrosine kinase activity: Implications in cisplatin-based therapy. Int J Cancer. 2008;122:289–297. doi: 10.1002/ijc.23063. [DOI] [PubMed] [Google Scholar]
- Gopal KV, Wu C, Shrestha B, Campbell KC, Moore EJ, Gross GW. d-Methionine protects against cisplatin-induced neurotoxicity in cortical networks. Neurotoxicol Teratol. 2012;34:495–504. doi: 10.1016/j.ntt.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol. 2009;39:541–552. doi: 10.1080/10408440802304944. [DOI] [PubMed] [Google Scholar]
- Hall MD, Telma KA, Chang KE, Lee TD, Madigan JP, Lloyd JR, Goldlust IS, Hoeschele JD, Gottesman MM. Say no to DMSO: dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer Res. 2014;74:3913–3922. doi: 10.1158/0008-5472.CAN-14-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrap KR, Jones M, Wilkinson CR, Clink HM, Sparrow S, Mitchley BCV, Clarke S, Veasey A. Antitumour, toxic and biochemical properties of cis-platin and eight other platinum complexes. In: Prestayko AW, Crooke ST, Carter SK, editors. cis-Platin: Current status and new developments. New York: Academic Press; 1980. pp. 193–212. [Google Scholar]
- Ivanov AI, Christodoulou J, Parkinson JA, Barnham KJ, Tucker A, Woodrow J, Sadler PJ. Cisplatin binding sites on human albumin. J Biol Chem. 1998;273:14721–14730. doi: 10.1074/jbc.273.24.14721. [DOI] [PubMed] [Google Scholar]
- Jamieson ER, Lippard SJ. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- Kellin D. Cytochrome and respiratory enzymes. Proceedings of the Royal Society B: Biological Sciences. 1929;104:206–251. [Google Scholar]
- Nath AK, Roberts LD, Liu Y, Mahon SB, Kim S, Ryu JH, Werdich A, Januzzi JL, Boss GR, Rockwood GA, et al. Chemical and metabolomic screens identify novel biomarkers and antidotes for cyanide exposure. FASEB J. 2013;27:1928–1938. doi: 10.1096/fj.12-225037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Smith RM, Martel AE. Inorganic Complexes. Plenum Press; 1976. Critical Stability Constants. [Google Scholar]
- Stathopoulos GP, Antoniou D, Dimitroulis J, Michalopoulou P, Bastas A, Marosis K, Stathopoulos J, Provata A, Yiamboudakis P, Veldekis D, et al. Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann Oncol. 2010;21:2227–2232. doi: 10.1093/annonc/mdq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumberg D, Brugge S, Korn MW, Koeppen S, Ranft J, Scheiber G, Reiners C, Mockel C, Seeber S, Scheulen ME. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. 2002;13:229–236. doi: 10.1093/annonc/mdf058. [DOI] [PubMed] [Google Scholar]
- TOXNET N. Soduim chloroplatinate. ChemIDplus [Google Scholar]
- Wisnovsky SP, Wilson JJ, Radford RJ, Pereira MP, Chan MR, Laposa RR, Lippard SJ, Kelley SO. Targeting mitochondrial DNA with a platinum-based anticancer agent. Chem Biol. 2013;20:1323–1328. doi: 10.1016/j.chembiol.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang X, Hu M, Zhu C, Guo Z. A mitochondrion-targeting copper complex exhibits potent cytotoxicity against cisplatin-resistant tumor cells through multiple mechanisms of action. Chem Sci. 2014;5:2761–2770. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


