Abstract
The interaction of posaconazole and amphotericin B was evaluated in concomitant treatment of Candida albicans systemic infections in immunocompetent mice by using four strains of C. albicans with different susceptibilities to fluconazole. Posaconazole and amphotericin B were each tested at four dose levels alone and in all possible combinations against each C. albicans strain. Survival curves of mice treated with combinations of posaconazole and amphotericin B were statistically compared with those of mice treated with the component monotherapies. Of the 64 total combinations evaluated against the C. albicans strains (16 combinations per strain), 20.3% were more effective in prolonging mouse survival than both of the monotherapies, 45.3% were more effective than one of the monotherapies, and 32.8% were similar to both monotherapies. No evidence of antagonism was observed between posaconazole and amphotericin B in this mouse model, consistent with in vitro results against the same strains.
The clinical use of azoles in combination with amphotericin B (AMB) is still controversial because of the potential for antagonism between the two drugs (9, 12, 18, 22). This potential comes from their mechanisms of action; azoles block ergosterol biosynthesis, while AMB causes membrane damage by binding to ergosterol. Various experimental fungal infection models have been used to address the issue of combinational dosing, but the results have been mixed. In systemic candidiasis models in mice with Candida albicans, Louie et al. (11) observed antagonism between the triazole fluconazole (FLC) and AMB, while Sugar and Liu (23) reported antagonism between another triazole, itraconazole, and AMB. Louie et al. (10, 12) also found that FLC was antagonistic to AMB therapy against experimental C. albicans endocarditis, endophathalmitis, and pyelonephritis in rabbits. However, Sanati et al. (17) did not observe antagonism when FLC and AMB were used in combination against C. albicans in invasive candidiasis in neutropenic mice or in endocarditis in rabbits. Sugar et al. (21) reported no antagonism between FLC and AMB in invasive candidiasis with C. albicans in immunocompetent or immunocompromised mice.
Posaconazole (POS) is a broad-spectrum antifungal triazole which recently completed phase III clinical trials (7). The experiments described in this report were performed to determine the interaction between POS and AMB in concomitant combination therapy against systemic C. albicans infection in mice.
(A preliminary report of this research was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002 [A. F. Cacciapuoti, M. Gurnani, J. Halpern, F. Gheyas, R. Hare, and D. Loebenberg, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1814, p. 415, 2002].)
MATERIALS AND METHODS
Antifungal agents.
POS clinical oral suspension was used in these experiments, and dilutions were made in sterile water for injection. AMB (Fungizone) was obtained from Apothecon, Bristol-Myers Squibb, Princeton, N.J., and prepared according to the manufacturer's directions.
C. albicans strains and in vitro activity testing.
All C. albicans strains were from the Schering-Plough Research Institute fungal culture collection and included one FLC-susceptible (FLC-S) strain C43, one FLC-susceptible, dose-dependent (FLC S-DD) strain C210, and two FLC-resistant (FLC-R) strains C284 and C335. MICs were determined by the standard NCCLS method M27-A (14). MICs of FLC for strains C43, C210, C284, and C335 were 0.125, 16, 64, and 64 μg/ml, respectively. Drug interactions between POS and AMB were determined by a checkerboard microdilution method. The endpoints for POS alone and for the POS-AMB combinations were read at 80% inhibition, while that for AMB alone was read at 100% inhibition. The fractional inhibitory concentration (FIC) index (5) was defined as synergistic if the FIC was ≤0.5, indifferent if it was >0.5 but ≤4, and antagonistic if it was >4.
Mice.
Charles River Laboratories (Wilmington, Mass.) CF1 mice (white, male) were used in these studies. At the time of infection, the mice weighed 18 to 20 g. These studies were carried out in accordance with the Guide to the Care and Use of Laboratory Animals of the National Institutes of Health (15) and the Animal Welfare Act in an Association for Assessment Accreditation of Laboratory Animal Care-accredited program.
Systemic infection model and drug therapy.
C. albicans strains were grown for 48 h on Sabouraud dextrose agar, and inocula were prepared as saline suspensions as described previously (6). Initiation of systemic infection occurred on day 0 by intravenous injection (tail vein). Inocula ranged from ca. 5 × 106 (strains C43 and C210) to ca. 1 × 107 (strains C284 and C335) CFU/mouse. Drug therapy to groups of 10 mice began at 4 h postinfection on day 0 and continued once daily through day 3. POS and AMB were each tested at 4 dose levels alone and in all possible combinations in a checkerboard fashion against each C. albicans strain (see Table 2). The 4 dose levels for each drug were selected from preliminary dose-response experiments (data not shown) to include the full range of survival efficacy, from maximum to intermediate to minimal, in the survival curves. This was done to ensure that POS and AMB would be tested in combinations involving high-, intermediate-, and low-dose levels of each drug (similar to doing a checkerboard in vitro MIC test). For each strain, there were 16 drug combination groups and 8 monotherapy groups of mice. Concomitant combination therapy was achieved by administering POS orally followed immediately by AMB intraperitoneally. Control animals were administered sterile water for injection. Survival was monitored for 10 days. Mice were not cultured for organ fungal burdens.
TABLE 2.
Effect of POS-AMB combinations on mouse survival compared to POS or AMB alone against C. albicans
| Strain | Combination
|
POS alonea
|
AMB alonea
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose levels (mg/kg)b | % Survival on dayc:
|
% Survival on dayd:
|
P valuee | % Survival on dayd:
|
P valuee | ||||
| 4 | 10 | 4 | 10 | 4 | 10 | ||||
| C43 | 1, 1 | 100 | 100 | 100 | 20 | <0.0001 | 85 | 70 | 0.0088 |
| 1, 0.5 | 100 | 100 | 100 | 20 | <0.0001 | 70 | 55 | <0.0001 | |
| 1, 0.1 | 95 | 60 | 100 | 20 | 0.0037 | 50 | 45 | 0.0370 | |
| 1, 0.02 | 100 | 40 | 100 | 20 | 0.0410 | 35 | 0 | <0.0001 | |
| 0.5, 1 | 80 | 60 | 55 | 5 | 0.0065 | 85 | 70 | 0.5500 | |
| 0.5, 0.5 | 100 | 70 | 55 | 5 | <0.0001 | 70 | 55 | 0.1900 | |
| 0.5, 0.1 | 95 | 40 | 55 | 5 | 0.0067 | 50 | 45 | 0.1900 | |
| 0.5, 0.02 | 95 | 15 | 55 | 5 | 0.0054 | 35 | 0 | <0.0001 | |
| 0.2, 1 | 60 | 60 | 15 | 0 | 0.0150 | 85 | 70 | 0.3500 | |
| 0.2, 0.5 | 75 | 65 | 15 | 0 | 0.0006 | 70 | 55 | 0.4800 | |
| 0.2, 0.1 | 60 | 55 | 15 | 0 | 0.0230 | 50 | 45 | 0.6300 | |
| 0.2, 0.02 | 45 | 10 | 15 | 0 | 0.2900 | 35 | 0 | 0.1300 | |
| 0.05, 1 | 90 | 85 | 20 | 0 | <0.0001 | 85 | 70 | 0.2500 | |
| 0.05, 0.5 | 70 | 50 | 20 | 0 | 0.0010 | 70 | 55 | 0.9500 | |
| 0.05, 0.1 | 55 | 55 | 20 | 0 | 0.0280 | 50 | 45 | 0.6700 | |
| 0.05, 0.02 | 25 | 10 | 20 | 0 | 0.4200 | 35 | 0 | 0.3000 | |
| C210 | 25, 5 | 100 | 80 | 75 | 40 | 0.0150 | 75 | 65 | 0.2100 |
| 25, 1 | 100 | 70 | 75 | 40 | 0.0820 | 85 | 70 | 0.7300 | |
| 25, 0.1 | 90 | 45 | 75 | 40 | 0.6500 | 50 | 50 | 0.2900 | |
| 25, 0.02 | 65 | 5 | 75 | 40 | 0.0280 | 35 | 15 | 0.3300 | |
| 10, 5 | 75 | 70 | 35 | 0 | 0.0002 | 75 | 65 | 0.6700 | |
| 10, 1 | 90 | 55 | 35 | 0 | 0.0004 | 85 | 70 | 0.4300 | |
| 10, 0.1 | 55 | 50 | 35 | 0 | 0.1300 | 50 | 50 | 0.9000 | |
| 10, 0.02 | 55 | 20 | 35 | 0 | 0.0720 | 35 | 15 | 0.2100 | |
| 5, 5 | 100 | 95 | 50 | 0 | <0.0001 | 75 | 65 | 0.1700 | |
| 5, 1 | 85 | 70 | 50 | 0 | <0.0001 | 85 | 70 | 0.9700 | |
| 5, 0.1 | 60 | 50 | 50 | 0 | 0.0790 | 50 | 50 | 0.8600 | |
| 5, 0.02 | 85 | 10 | 50 | 0 | 0.0021 | 35 | 15 | 0.0210 | |
| 1, 5 | 100 | 95 | 15 | 0 | <0.0001 | 75 | 65 | 0.0170 | |
| 1, 1 | 85 | 70 | 15 | 0 | <0.0001 | 85 | 70 | 0.9300 | |
| 1, 0.1 | 50 | 35 | 15 | 0 | 0.1300 | 50 | 50 | 0.4600 | |
| 1, 0.02 | 20 | 5 | 15 | 0 | 0.4200 | 35 | 15 | 0.4800 | |
| C284 | 25, 5 | 90 | 90 | 70 | 55 | 0.0160 | 75 | 60 | 0.0420 |
| 25, 1 | 75 | 65 | 70 | 55 | 0.4800 | 55 | 50 | 0.2200 | |
| 25, 0.2 | 80 | 55 | 70 | 55 | 0.8900 | 30 | 15 | 0.0042 | |
| 25, 0.05 | 80 | 55 | 70 | 55 | 0.6900 | 15 | 10 | 0.0005 | |
| 10, 5 | 75 | 70 | 80 | 55 | 0.5200 | 75 | 60 | 0.8100 | |
| 10, 1 | 75 | 70 | 80 | 55 | 0.5600 | 55 | 50 | 0.2700 | |
| 10, 0.2 | 70 | 70 | 80 | 55 | 0.6600 | 30 | 15 | 0.0210 | |
| 10, 0.05 | 85 | 80 | 80 | 55 | 0.1400 | 15 | 10 | 0.0001 | |
| 5, 5 | 80 | 65 | 55 | 35 | 0.0370 | 75 | 60 | 0.7800 | |
| 5, 1 | 55 | 55 | 55 | 35 | 0.3000 | 55 | 50 | 0.9500 | |
| 5, 0.2 | 60 | 60 | 55 | 35 | 0.2000 | 30 | 15 | 0.1300 | |
| 5, 0.05 | 50 | 40 | 55 | 35 | 0.9800 | 15 | 10 | 0.5100 | |
| 1, 5 | 60 | 55 | 20 | 10 | 0.0380 | 75 | 60 | 0.3200 | |
| 1, 1 | 65 | 65 | 20 | 10 | 0.0110 | 55 | 50 | 0.6200 | |
| 1, 0.2 | 55 | 35 | 20 | 10 | 0.2000 | 30 | 15 | 0.6000 | |
| 1, 0.05 | 45 | 15 | 20 | 10 | 0.5700 | 15 | 10 | 0.8300 | |
| C335 | 100, 5 | 100 | 100 | 100 | 65 | 0.0040 | 75 | 70 | 0.0088 |
| 100, 1 | 95 | 95 | 100 | 65 | 0.0280 | 70 | 70 | 0.0300 | |
| 100, 0.25 | 95 | 90 | 100 | 65 | 0.1100 | 45 | 40 | 0.0005 | |
| 100, 0.1 | 95 | 55 | 100 | 65 | 0.3500 | 20 | 10 | <0.0001 | |
| 25, 5 | 95 | 95 | 100 | 30 | 0.0001 | 75 | 70 | 0.0370 | |
| 25, 1 | 100 | 100 | 100 | 30 | <0.0001 | 70 | 70 | 0.0087 | |
| 25, 0.25 | 100 | 75 | 100 | 30 | 0.0130 | 45 | 40 | 0.0020 | |
| 25, 0.1 | 90 | 35 | 100 | 30 | 0.9700 | 20 | 10 | <0.0001 | |
| 5, 5 | 90 | 90 | 70 | 40 | 0.0030 | 75 | 70 | 0.1200 | |
| 5, 1 | 85 | 65 | 70 | 40 | 0.1300 | 70 | 70 | 0.9000 | |
| 5, 0.25 | 75 | 55 | 70 | 40 | 0.7500 | 45 | 40 | 0.1900 | |
| 5, 0.1 | 70 | 40 | 70 | 40 | 0.8900 | 20 | 10 | 0.0014 | |
| 1, 5 | 80 | 75 | 30 | 5 | <0.0001 | 75 | 70 | 0.6400 | |
| 1, 1 | 55 | 55 | 30 | 5 | 0.0490 | 70 | 70 | 0.5500 | |
| 1, 0.25 | 45 | 40 | 30 | 5 | 0.3200 | 45 | 40 | 0.9400 | |
| 1, 0.1 | 35 | 15 | 30 | 5 | 0.8500 | 20 | 10 | 0.7300 | |
POS or AMB alone at dose levels used in the combination.
Doses of POS are given first, followed by doses of AMB.
Percent survival of combination-treated mice on day 4 (day after final dose) or day 10 (end of experiment) postinfection (average of the results from two experiments per strain, 10 mice per group per experiment).
Percent survival of POS alone- or AMB alone-treated mice on day 4 (day after final dose) or day 10 (end of experiment) postinfection (average of the results from two experiments per strain, 10 mice per group per experiment).
P values from Wilcoxon tests comparing survival curves (from day 0 to day 10) of combination versus POS or AMB alone at the dose level used in the combination. A significant difference between survival curves was observed if the P value was <0.05.
Statistical analysis.
Each C. albicans strain was tested twice, and the results were combined for statistical analysis. Wilcoxon tests were performed to compare the survival curves (Kaplan Meier). A P value of <0.05 indicated that the overall survival curves were statistically significantly different. No multiplicity adjustments were made.
RESULTS
In vitro interaction between POS and AMB.
POS and AMB were tested in vitro alone and in combination against the four C. albicans strains which exhibited a range of susceptibilities to FLC (Table 1). The MICs of POS ranged from 0.03 to 1, while the MICs of AMB ranged from 1 to 4. The drug interaction results were mixed but were primarily indifferent. The POS-AMB interactions with strains C43 and C284 were synergistic and indifferent in different experiments, while those with strains C284 and C335 were indifferent. No antagonism was observed.
TABLE 1.
In vitro interaction between POS and AMB against four C. albicans strains with different susceptibilities to FLC
| Strain | MICa (μg/ml) of:
|
POS-AMB FIC | Interpretation | ||
|---|---|---|---|---|---|
| POS | AMB | POS-AMB | |||
| C43 (FLC-S) | 0.03 | 2 | 0.008, 0.5 | 0.50 | Synergy |
| C43 (FLC-S) | 0.03 | 2 | 0.016, 0.5 | 0.78 | Indifferent |
| C210 (FLC S-DD) | 1 | 2 | 0.5, 0.5 | 0.75 | Indifferent |
| C284 (FLC-R) | 0.25 | 1 | 0.125, 0.125 | 0.62 | Indifferent |
| C284 (FLC-R) | 0.25 | 4 | 0.06, 1 | 0.49 | Synergy |
| C335 (FLC-R) | 0.125 | 2 | 0.016, 1 | 0.63 | Indifferent |
MICs were determined using NCCLS method M27-A (14).
Effect of combination dosing against systemic candidiasis in mice.
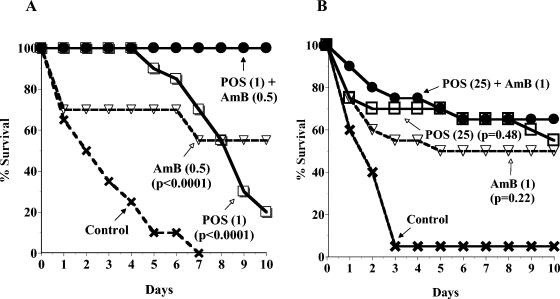
The four C. albicans strains were tested in a systemic infection model with immunocompetent mice. Table 2 shows the 16 POS and AMB combinations used for each strain and the averaged percent survival data from two experiments per strain on day 4 (the day after the last dose) and day 10 (end of experiment) for the combinations and their component monotherapies. Survival curves from day 0 to day 10 for each concomitant combination of POS and AMB were statistically compared to those for the POS and AMB monotherapies, and the resulting P values are also shown in Table 2. Survival curves are shown as examples of the data in Fig. 1A (strain C43) and 1B (strain C284). Both graphs show the combination of the highest dose level of POS and the second-highest dose level of AMB used against that strain, compared to the component monotherapies and controls. The emphasis in the graphs on the second-highest dose level of AMB is to show the drug interaction with a lower, but still efficacious, level of AMB, where potential antagonism could still be observed, as opposed to the highest level of AMB, where antagonism may be masked by the maximum efficacy of AMB. In Fig. 1A, for the FLC-S strain C43, mice treated with the POS and AMB combination survived significantly longer than those treated with the component monotherapies (P < 0.05) or the controls. In Fig. 1B, for the FLC-R strain C284, mice treated with the POS and AMB combination survived similarly to those treated with the component monotherapies (P > 0.05) but longer than the controls.
FIG. 1.
Concomitant combination treatment with POS and AMB of systemic infection in mice with FLC-S C. albicans C43 (A) or FLC-R C. albicans C284 (B). The combination of POS and AMB, POS alone, or AMB alone was administered once daily for 4 days starting 4 h postinfection on day 0. Dose levels (in milligrams/kilogram of body weight) are indicated in parentheses. Controls were administered sterile water for injection. The survival data were averaged from two experiments (10 mice per group in each experiment). The P values were determined by comparing the survival curve for the combination to each of those for the drugs alone.
Table 3 shows a summary of all of the interactions between POS and AMB listed in Table 2. In these studies, an antagonistic combination was defined as providing less survival efficacy than one or both of the component drugs alone. Against all four C. albicans strains, 20.3% of the POS and AMB combinations tested were more effective than either POS or AMB alone (P < 0.05) in prolonging the survival of mice. In addition, 45.3% of the combinations were more effective than one of the drugs alone (P < 0.05) and similar to the other drug alone (P > 0.05), while 32.8% of the combinations were similar to both drugs alone (P > 0.05). Only one combination of POS and AMB was less effective than one of the drugs alone (POS in this case), and no combinations were less effective than both POS and AMB alone. Overall, 98.4% of the 64 combinations tested against the four C. albicans strains showed no evidence of antagonism between POS and AMB in this model of systemic candidiasis in immunocompetent mice.
TABLE 3.
Summary of interactions of POS-AMB combinations compared to one or both of POS or AMB alone against the four C. albicans strainsa
| Strain | No. of combinations tested | No. of combinations:
|
||||
|---|---|---|---|---|---|---|
| More effectiveb than both alone | More effectiveb than one alone | Similarb to both alone | Less effectiveb than one alone | Less effectiveb than both alone | ||
| C43 (FLC-S) | 16 | 5 | 9c | 2 | 0 | 0 |
| C210 (FLC S-DD) | 16 | 2 | 6c | 7 | 1c | 0 |
| C284 (FLC-R) | 16 | 1 | 7c | 8 | 0 | 0 |
| C335 (FLC-R) | 16 | 5 | 7c | 4 | 0 | 0 |
| Total (%) | 64 | 13 (20.3) | 29 (45.3) | 21 (32.8) | 1 (1.6) | 0 |
Based on statistical analysis of survival curves through day 10 postinfection with 4 dose levels of each drug alone and in all possible combinations (data from 2 experiments per strain were pooled).
More effective or less effective means the survival curves for the combinations were significantly different from those for the drug(s) alone (P < 0.05). Similar means the survival curves for the combinations and the drug(s) alone were not significantly different (P > 0.05).
These combinations were also similar to the other drug alone.
DISCUSSION
Our studies demonstrate that concomitant combination treatment of systemic candidiasis in immunocompetent mice with POS and AMB was not antagonistic, as determined by using survival as the efficacy endpoint. Instead, 20.3, 45.3, and 32.8% of the total combinations tested against four C. albicans strains resulted in survival of mice, which was more effective than both monotherapies, more effective than one of the monotherapies, and similar to both monotherapies, respectively. In addition, the combination of POS and AMB was effective and not antagonistic even against strains with reduced susceptibility to FLC, where much higher doses of POS were used in the combinations. The finding of no antagonism in the in vivo experiments was consistent with the in vitro results against the same strains.
In these studies we did not investigate the effect of POS and AMB on fungal burdens in organs or the effect of sequential dosing of POS relative to AMB. However, Najvar et al. (13) reported that concomitant POS and AMB or sequential dosing (initial dosing with POS followed a day later by AMB) in a pulmonary Aspergillus flavus infection model in immunocompromised mice were not antagonistic, as determined by both survival and lung burden results.
Although pharmacokinetic (PK) data and PK-pharmacodynamic (PD) analyses were not obtained or performed in our combination dosing studies, the PK data for POS and the PK-PD data for POS monotherapy and AMB monotherapy were reported previously. Following oral administration of POS to mice, Nomeir et al. (16) observed a dose-related increase in the maximum concentration in serum (up to 80 mg/kg) and area under the concentration-time curve (AUC, up to 120 mg/kg). Andes et al. (4) studied PK-PD data for POS against C. albicans in neutropenic mice and reported that the 24-h AUC/MIC ratio was the PK-PD parameter associated with POS efficacy in this model. The mean free drug AUC/MIC ratio of 16.9 for POS was similar to the ratio of 25 observed with other triazoles. In addition, these authors also indicated that POS exhibited prolonged (20 to 30 h) postantifungal effect (PAFE) for free drug, potentially due to sub-MIC effects. Andes et al. (3) also reported that the PK-PD parameter predictive of efficacy for AMB was the peak serum level/MIC ratio and that AMB also had a prolonged (23 to 30 h) PAFE. The prolonged PAFEs of POS and AMB may have contributed to the antifungal efficacy of combination treatment with these drugs observed in our studies.
Antagonism between azoles and AMB has been observed in some, but not other, literature reports involving animal models of fungal infections. Louie et al. (10, 11) showed antagonism between FLC and AMB, and Sugar and Liu (23) showed the same result with itraconazole and AMB with C. albicans infection models. Lewis et al. (9) also reported that preexposure to itraconazole reduced the efficacy of subsequent treatment with AMB in murine pulmonary aspergillosis. The absence of FLC-AMB antagonism was observed in murine candidiasis studies (17, 21) and by George et al. (8) in an immunosuppressed rabbit model of aspergillosis, by Anaissie et al. (2) against Trichosporon beigelii infection in mice, and by Barchiesi et al. (5) against murine systemic cryptococcosis. Other triazole-AMB combinations were also not antagonistic, including saperconazole against murine systemic candidiasis (20) and SCH 39304 against murine systemic candidiasis (19) and cryptococcal meningitis (1).
We anticipate that POS will be used in combination with other antifungals, potentially including AMB, for treatment of serious fungal infections in patients. The efficacy observed in mice with the combination of POS and AMB suggests this combination could be effective in clinical fungal infections. However, the lack of antagonism in our studies indicates that the combination could potentially be tried clinically with less concern for antagonism.
Acknowledgments
We thank Ferdous Gheyas for statistical analysis and Paul McNicholas for critical review of the manuscript.
REFERENCES
- 1.Albert, M. M., J. R. Graybill, and M. G. Rinaldi. 1991. Treatment of murine cryptococcal meningitis with an SCH39304-amphotericin B combination. Antimicrob. Agents Chemother. 35:1721-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaissie, E. J., R. Hachem, N. C. Karyotakis, A. Gokaslan, M. C. Dignani, L. C. Stephens, and C. K. Tin-U. 1994. Comparative efficacies of amphotericin B, triazoles, and combination of both as experimental therapy for murine trichosporonosis. Antimicrob. Agents Chemother. 38:2541-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., T. Stamsted, and R. Conklin. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., K. Marchillo, R. Conklin, G. Krishna, F. Ezzet, A. Cacciapuoti, and D. Loebenberg. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barchiesi, F., A. M. Schimizzi, F. Caselli, A. Novelli, S. Fallani, D. Giannini, D. Arzeni, S. Di Cesare, L. Falconi Di Francesco, M. Fortuna, A. Giacometti, F. Carle, T. Mazzei, and G. Scalise. 2000. Interactions between triazoles and amphotericin B against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacciapuoti, A., D. Loebenberg, R. Parmegiani, B. Antonacci, C. Norris, E. L. Moss, Jr., F. Menzel, Jr., T. Yarosh-Tomaine, R. S. Hare, and G. H. Miller. 1992. Comparison of SCH 39304, fluconazole, and ketoconazole for treatment of systemic infections in mice. Antimicrob. Agents Chemother. 36:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cacciapuoti, A., D. Loebenberg, E. Corcoran, F. Menzel, Jr., E. L. Moss, Jr., C. Norris, M. Michalski, K. Raynor, J. Halpern, C. Mendrick, B. Arnold, B. Antonacci, R. Parmegiani, T. Yarosh-Tomaine, G. H. Miller, and R. S. Hare. 2000. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent against Aspergillus and Candida. Antimicrob. Agents Chemother. 44:2017-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George, D., D. Kordick, P. Miniter, T. Patterson, and V. T. Andriole. 1993. Combination therapy in experimental invasive aspergillosis. J. Infect. Dis. 168:692-698. [DOI] [PubMed] [Google Scholar]
- 9.Lewis, R. E., R. A. Prince, J. Chi, and D. P. Kontoyiannis. 2002. Itraconazole preexposure attenuates the efficacy of subsequent amphotericin B therapy in a murine model of acute invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 46:3208-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louie, A., W. Liu, D. A. Miller, A. C. Sucke, Q-F. Liu, G. L. Drusano, M. Mayers, and M. H. Miller. 1999. Efficacies of high-dose fluconazole plus amphotericin B and high-dose fluconazole plus 5-fluorocytosine versus amphotericin B, fluconazole, and 5-fluorocytosine monotherapies in treatment of experimental endocarditis, endophthalmitis, and pyelonephritis due to Candida albicans. Antimicrob. Agents Chemother. 43:2831-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie, A., P. Banerjee, G. L. Drusano, M. Shayegani, and M. H. Miller. 1999. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob. Agents Chemother. 43:2841-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie, A., P. Kaw, P. Banerjee, W. Liu, G. Chen, and M. H. Miller. 2001. Impact of the order of initiation of fluconazole and amphotericin B in sequential or combination therapy on killing of Candida albicans in vitro and in a rabbit model of endocarditis and pyelonephritis. Antimicrob. Agents Chemother. 45:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najvar, L. K., A. Cacciapuoti, S. Hernandez, J. Halpern, R. Bocanegra, M. Gurnani, F. Menzel, D. Loebenberg, and J. R. Graybill. 2004. Activity of posaconazole combined with amphotericin B against Aspergillus flavus infection in mice: comparative studies in two laboratories. Antimicrob. Agents Chemother. 48:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.National Research Council. 1996. NIH guide to the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 16.Nomeir, A. A., P. Kumari, M. J. Hilbert, S. Gupta, D. Loebenberg, A. Cacciapuoti, R. Hare, G. H. Miller, C-C. Lin, and M. N. Cayen. 2000. Pharmacokinetics of SCH 56592, a new azole broad-spectrum antifungal agent, in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 44:727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanati, H., C. F. Ramos, A. S. Bayer, and M. A. Ghannoum. 1997. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob. Agents Chemother. 41:1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbach, W. J., D. A. Stevens, and D. W. Denning. 2003. Combination and sequential therapy for invasive aspergillosis: review of published in vitro and in vivo interactions and 6281 clinical cases from 1966 to 2001. Clin. Infect. Dis. 37:S188-224. [DOI] [PubMed] [Google Scholar]
- 19.Sugar, A. M. 1991. Interactions of amphotericin B and SCH 39304 in the treatment of experimental murine candidiasis: lack of antagonism of a polyene-azole combination. Antimicrob. Agents Chemother. 35:1669-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugar, A. M., M. Salibian, and L. Z. Goldani. 1994. Saperconazole therapy of murine disseminated candidiasis: efficacy and interactions with amphotericin B. Antimicrob. Agents Chemother. 38:371-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugar, A. M., C. A. Hitchcock, P. F. Troke, and M. Picard. 1995. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob. Agents Chemother. 39:598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugar, A. M. 1995. Use of amphotericin B with azole antifungal drugs: what are we doing? Antimicrob. Agents Chemother. 39:1907-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugar, A. M., and X-P. Liu. 1998. Interactions of itraconazole with amphotericin B in the treatment of murine invasive candidiasis. J. Clin. Infect. Dis. 177:1660-1663. [DOI] [PubMed] [Google Scholar]