Abstract
Background and aims
Interferon (IFN)- free direct antiviral agents (DAAs) with rapid HCV eradication might evoke immunological reconstitutions, and some early recurrences of HCC after IFN-free DAAs have been reported. This study aimed to investigate whether natural killer group 2, member D (NKG2D) predicts early emergence of HCC after IFN-free DAAs.
Methods
We conducted a clinical practice-based observational study of 101 patients infected with genotype 1 HCV who received IFN-free (DAAs), and stratified them into those who did or did not develop early (i.e., during the 6-month surveillance period following treatment.) recurrence or occurrence of clinically evident HCC. We also analyzed the peripheral blood mononuclear cells, both before treatment and at end of treatment (EOT), of 24 of the patients who received IFN-free DAAs, and 16 who received IFN-combined protease inhibitor.
Results
We found early emergence of clinically evident HCC after IFN-free DAAs in 12 (12%) patients. Higher pre-treatment NKG2D expression, higher FIB-4 score, previous HCC history and failure to achieve sustained viral response were significant factors correlating to early HCC emergence. After IFN-free DAAs, a rapid decrease of NKG2D at EOT correlated with early HCC emergence in the IFN-free DAA-treated patients, but not in patients treated with the IFN-combined regimen. The decrease of NKG2D until EOT was predictive of early HCC emergence at a cut-off of -52% (AUC = 0.92).
Conclusions
On-treatment decrease of NKG2D may be a useful predictor of early emerging HCC in patients treated with IFN-free DAAs.
Introduction
Persistent hepatitis C virus (HCV) infection, which affects about 160 million people worldwide, is the major cause of liver cirrhosis and hepatocellular carcinoma (HCC). HCV-associated HCC, like some cancers in organs such as stomach or uterine cervix, reflects a model of infection-related carcinogenesis, in which chronic inflammation (i.e., the accumulation of immune cells) promotes tumor initiation and progression[1]. Recently, interferon (IFN)-free direct antiviral agents (DAAs) have been replacing pegylated-interferon (PEG-IFN)/ ribavirin (RBV) as a first-line treatment option recommended by international guidelines[2, 3], because of their high effectiveness and limited toxicity. Many studies have shown that sustained viral responses (SVRs) through IFN-combined therapies reduce liver-related complications[4, 5]. However, although IFN-free DAAs have been shown to eradicate HCV and improve liver residual function[6, 7], whether IFN-free DAAs can effectively prevent primary or secondary HCC is still unknown.
As a practical matter, patients who receive IFN-free DAAs are usually intolerant of IFN because of hematological toxicities that may be associated with advanced liver cirrhosis, old age, or decompensated liver functions. These are also risk factors for higher HCC incidence rates. Recently, several retrospective clinical studies of early recurrence of HCC after IFN-free DAAs suggested that robust immune re-constitution may be related to earlier HCC recurrence[8–10]; conversely, another study suggested this was not the case[11].
Several studies showed that IFN-free DAAs might evoke immune reconstitution of intrahepatic interferon-stimulated genes (ISGs)[12] and early responses in natural killer (NK) cells[13, 14]. However, these studies were based on pilot studies of clinical trials, and they were not tested clinically. How these immunological changes influence immunosurveillance of HCC is still not clear.
Here, we focused on natural killer group 2, member D (NKG2D), an activating receptor for MHC class I chain-related protein A/B (MICA/B) and other ligands. NKG2D is a widely studied immunoreceptor due to its major relevance in activating immune responses against both infected and transformed cells. NKG2D has been studied in the persistence of HCV infection[15] and HCV-associated HCC[16]. Peripheral blood NKG2D and its ligands have also been found to be dysregulated during the progression of various cancers[17, 18], and may be useful biomarkers[19, 20].
To find out how HCV eradication will influence NKG2D expression, and how this difference will correlate to early post-treatment HCC emergence, we conducted a real-world practice-based observational study of patients infected with genotype-1 HCV who were treated with IFN-free DAAs, and compared to those treated with IFN-combined regimen. We analyzed the correlation of NKG2D expression to early emergence of clinically evident HCC after treatment.
Patients and methods
Study subjects
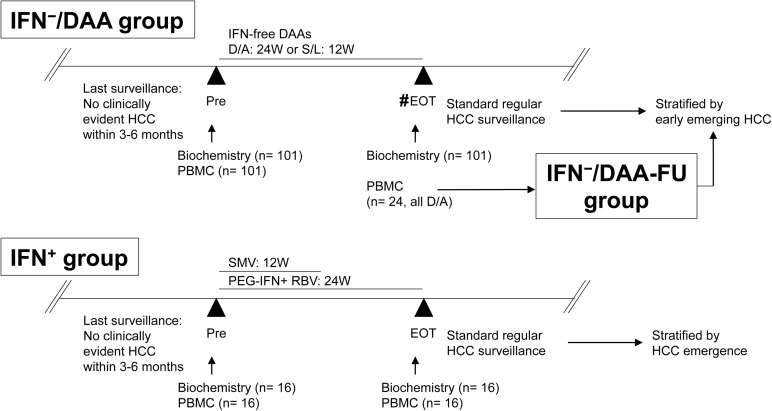
This observational case-control study was specifically approved before we started, by the institutional review board of Keio University School of Medicine (No. 20140177), according to the guidelines of the 1975 Declaration of Helsinki (2008 revision). Recruited study subjects provided prior written informed consents, which included blood sampling, study participation and analysis of clinical data. All of the study subjects received standard of care and treatment, according to their clinical presentation. Measurement of viral kinetics was performed as previously described[15]. Negative plasma HCV-RNA at week 4 is defined as early viral response (EVR). Negative plasma HCV-RNA at 12 weeks after the end of treatment is considered to have a sustained virological response (SVR). We use RECICL2009[21], or its minor revision RECICL2015[22] accordingly during the study period, for evaluation of HCC treatment effect and response. Schema of the study groups is summarized in Fig 1.
Fig 1. Scheme of this study.
#: Samples were drawn at end of treatment (EOT) for most of the cases other than two cases that suffered from viral breakthrough (VBT). Abbreviations: IFN, interferon; DAAs, direct-acting anti-viral agents; D/A; daclatasvir and asuneprevir; S/L: sofosbuvir and ledipasvir; HCC, hepatocellular carcinoma; Pre, pre-treatment; EOT, end of treatment, PBMC, peripheral blood mononuclear cells; SMV, simeprevir; PEG, pegylated, RBV, ribavirin;.
IFN−/DAA group (the group that received IFN-free DAAs) included 101 IFN-free treatment-naïve patients with chronic HCV genotype-1 infections who had no evident HCC and were undergoing standard HCC surveillance by international recommendations[2] every 3–6 months. No patient of decompensated liver cirrhosis was included in this study. They were treated with daclatasvir (60mg once daily, DCV) and asunaprevir (100mg twice daily, ASV; both Bristol-Myers Squibb, New York, NY, USA) for 24 weeks, or sofosbuvir (400mg once daily, SOF) and ledipasvir (90mg once daily, LDV; both Gilead, Foster City, CA, USA) for 12 weeks. Their treatment started from November 2014 to June 2016. Biochemical studies were performed on sera from these patients retrieved on day 0 (pre-treatment; Pre) and on the end of treatment (EOT; 24 weeks for DAC/ASV and 12 weeks for SOF/LDV) for most cases who achieved SVR or for one case whose HCV-RNA relapsed, or at the time of viral breakthrough (VBT). All 101 patients had PBMCs retrieved on day 0 as part of their pre-treatment evaluations.
IFN−/DAA-FU (follow-up) group was a sub-group of IFN−/DAA group. Twenty-four patients who had been treated by DAC/ASV had additional PBMCs retrieved at the EOT. Cases of both IFN−/DAA group and IFN−/DAA-FU group were thereafter followed-up for HCC surveillance within 6 months after EOT, according to the guidelines[2]. They were stratified by emergence of HCC, including both recurrences and first occurrences. Any HCC recognized during this period was considered to be an early-emerging HCC. Clinical characteristics of IFN−/DAA group and IFN−/DAA-FU group are shown in Table 1 and Table 2, respectively. Liver function and tumor-related variables at 3 points in the study of the 12 patients who developed early-emerging HCC are summarized in S1 Table. Chronological flows of the 8 patients with recurrences of HCC, including tumor numbers, maximal sizes, BCLC stages, treatment, and follow-up image studies, are shown in S1 Fig. Comparison between IFN−/DAA group and IFN−/DAA-FU group is shown in S2 Table. Non-invasive fibrosis scores including aspartate aminotransferase (AST) to platelet ratio index (APRI, cut-off for METAVIR F4 cirrhosis as > 2.0), FIB-4 (cut-off for METAVIR F3-F4 advanced fibrosis as > 3.25), and Forns index (cut-off for METAVIR F0-F1 as < 4.2) were calculated as described before[23–25].
Table 1. Clinical characteristics of IFN−/DAA group, and comparison of cases stratified by early-emerging HCC.
| Variables | Total | Post-treatment Early-emerging HCC | ||
|---|---|---|---|---|
| No | Yes | P (uni) | ||
| n (%) | 101 | 89(88) | 12(12) | – |
| Age, years | 67 [24–85] | 67 [24–85] | 79 [50–82] | 0.19 |
| ≧65 yrs, n (%) | 62(62) | 53(60) | 9(75) | 0.36 |
| Sex (M: F), n (%) | 38(38): 63(62) | 35(37): 54(53) | 3(25): 9(75) | 0.53 |
| Pre-treatment parameters | ||||
| ALT, IU/L | 44[13–284] | 44[13–284] | 45[23–83] | 0.97 |
| AST, IU/L | 49[19–246] | 48[19–246] | 73[20–129] | 0.22 |
| G-GTP, IU/L | 31[11–268] | 32[11–268] | 30[18–79] | 0.92 |
| T-Bil, mg/dl | 0.8[0.3–2.7] | 0.8[0.3–2.7] | 1.1[0.4–2.0] | 0.0171* |
| Albumin, g/dl | 4.3[3.3–4.9] | 4.4[3–4.9] | 3.8[3.3–4.3] | 0.0002** |
| Platelet, 103/μl | 136[33–457] | 145[33–457] | 93[37–172] | 0.007** |
| PT-INR | 1.00[0.86–1.30] | 1.00[0.86–1.30] | 1.07[1.00–1.29] | 0.0003** |
| 4COL7s, ng/ml | 6.7[3.4–17] | 6.4[3.4–15] | 9[5.1–17] | 0.001** |
| AFP, ng/ml | 6[1–685] | 5[1–685] | 17.5[3–171] | 0.002* |
| HCV-RNA, LogIU/ml | 6.3[3.5–7.5] | 6.3[3.5–7.5] | 6.0 [5.3–7.1] | 0.25 |
| Total cholesterol, mg/dl | 159[107–241] | 161[107–241] | 133[110–184] | 0.013* |
| Forns index | 7.6[2.5–13] | 7.5[2.5–13] | 9.2[6.8–13] | 0.001** |
| <4.2, n (%) | 6(6) | 6(7) | 0(0) | 1.00 |
| FIB-4 | 3.87 [0.62–107] | 3.58 [0.62–107] | 10.14 [1.41–16.56] | 0.002** |
| >3.25, n (%) | 61(60) | 50(56) | 11(92) | 0.03* |
| APRI | 1.11[0.13–52] | 1.00[0.13–52] | 3.18[0.33–5.4] | 0.007** |
| >2.0, n (%) | 26(26) | 18(20) | 8(67) | 0.002** |
| Previous HCC, n (%) | 16(16) | 8(9) | 8(67) | <0.0001** |
| Previous IFN-combined, n (%) | 43(43) | 40(45) | 3(25) | 0.23 |
| DAA type, n (%) | 0.75 | |||
| DCV/ASV | 62(61) | 55(62) | 7(58) | |
| SOF/LDV | 39(38) | 34(38) | 5(42) | |
| NKG2D, % | 23.4 [1–71] | 22 [1–53] | 33[19–71] | 0.006** |
| Post-treatment parameters | ||||
| SVR, n (%) | 98(97) | 88(99) | 10(83) | 0.04* |
*, P< 0.05
**, P< 0.01.
Data are shown as median with the range within brackets. Abbreviations: 4COL7s: type 4 collagen 7s domain; AFP: alpha-fetoprotein; ASV: asunaprevir; CI: confidence interval; DAA: direct-acting agent; DCV: daclatasvir; F: female; G-GTP, gamma- glutamyl transpeptidase; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; IFN: interferon; LDV: ledipasvir; M: male; SOF: sofosbuvir; SVR: sustained viral response; T-Bil, total bilirubin; uni: univariate.
Table 2. Clinical characteristics of IFN−/DAA-FU group, and comparison of cases stratified by early-emerging HCC.
| Variables | Total | Early-emerging HCC | ||
|---|---|---|---|---|
| No | Yes | P | ||
| N | 24 | 19 | 5 | – |
| Age, years | 67.5 [45–79] | 67 [45–79] | 72 [50–79] | 0.39 |
| Sex (M/F), n | 10 /14 | 9/ 10 | 1/ 4 | 0.36 |
| Pre-treatment parameters | ||||
| ALT, IU/L | 58 [26–284] | 59 [27–284] | 57 [26–83] | 0.89 |
| AST, IU/L | 67 [27–246] | 61 [27–246] | 76 [32–92] | 0.72 |
| G-GTP, IU/L | 43 [19–268] | 47 [24–268] | 30 [19–79] | 0.39 |
| T-Bil, mg/dl | 0.9 [0.4–2.7] | 0.8 [0.4–2.7] | 1.2 [0.7–1.8] | 0.24 |
| Albumin, g/dl | 3.9 [3.3–4.8] | 4.0 [3.3–4.8] | 3.9 [3.3–4.1] | 0.23 |
| Platelet, 103/μl | 108 [47–221] | 111 [47–221] | 65 [49–94] | 0.01* |
| 4COL7s, ng/ml | 7.9 [3.9–15] | 7.5 [3.9–15] | 8.1 [7.2–15] | 0.26 |
| AFP, ng/ml | 12 [3–171] | 9 [3–109] | 23 [9–171] | 0.14 |
| HCV-RNA, LogIU/ml | 6.3 [5.8–7.5] | 6.4 [5.9–7.5] | 5.9 [5.8–6.4] | 0.02* |
| Total cholesterol, mg/dl | 148 [107–230] | 152 [107–230] | 127 [120–173] | 0.08 |
| FIB-4 | 4.49 [1.21–25.80] | 4.40 [1.21–25.80] | 12.73 [4.34–16.56] | 0.12 |
| APRI | 1.80[0.35–14.95] | 1.49[0.35–14.95] | 3.52 [0.97–4.43] | 0.10 |
| Previous HCC, n (%) | 5 (21) | 2 (11) | 3 (60) | 0.04* |
| Post-treatment parameters | ||||
| EVR, n (%) | 20 (83) | 16 (84) | 4 (80) | 1.00 |
| SVR, n (%) | 21 (88) | 18 (95) | 3 (60) | 0.10 |
| ALT, IU/L | 21 [11–235] | 17 [11–235] | 29 [21–30] | 0.24 |
| AST, IU/L | 28 [17–102] | 25 [19–102] | 33 [28–46] | 0.07 |
| G-GTP, IU/L | 23 [12–150] | 22 [12–150] | 23 [15–28] | 0.91 |
| T-Bil, mg/dl | 1.0 [0.5–2.0] | 0.9 [0.5–1.4] | 1.1 [1.0–2.0] | 0.05 |
| Albumin, g/dl | 4.3 [3.7–4.9] | 4.4 [3.7–4.9] | 4.2 [4.1–4.5] | 0.24 |
| Platelet, 103/μl | 115 [54–202] | 120 [54–202] | 120 [59–125] | 0.10 |
| 4COL7s, ng/ml | 6.9 [4.3–12] | 6.3 [4.3–12] | 8.1 [7.5–9.2] | 0.08 |
| AFP, ng/ml | 5 [3–591] | 4 [3–9] | 10 [4–591] | 0.04* |
| Total cholesterol, mg/dl | 166 [121–233] | 167 [127–233] | 143 [121–193] | 0.08 |
| FIB-4 | 3.06 [0.99–10.25] | 2.73 [0.99–9.66] | 5.92 [2.27–10.25] | 0.08 |
| APRI | 0.64 [0.24–3.07] | 0.61 [0.30–3.07] | 1.15 [0.69–2.08] | 0.04* |
| ΔNKG2D (NK cells), % | −42 [−80–+21] | −34 [−73–+21] | −57 [−80–−51] | 0.005** |
*, P< 0.05
**, P< 0.01.
Data are shown as median with the range within brackets. Abbreviations: 4COL7s: type 4 collagen 7s domain; AFP: alpha-fetoprotein; EVR: early viral response; F: female; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; M: male; SVR: sustained viral response.
IFN+ group (the group received SMV/PEG-IFN/RBV triple therapy), a control group whose treatment started from June to October, 2014, included 16 patients with chronic HCV genotype-1 infection but no evident HCC, who underwent standard HCC surveillance by international recommendations[2] every 3–6 months. They were treated with a combination of weekly PEG-IFN-α2b, ribavirin (daily dose based on body weight; Schering-Plough KK, Tokyo, Japan) for 24 weeks, and simeprevir (150 mg once daily, SMV; Janssen KK, Tokyo, Japan) for the first 12 weeks. Serum biochemical studies and PBMCs were retrieved on day 0 (Pre) and on EOT (24 weeks). After EOT, these patients were followed-up for HCC surveillance every 3–6 months regularly, according to the guidelines[2]. Clinical characteristics that compare IFN−/DAA-FU group and IFN+ group are shown in S3 Table.
Flow cytometry analysis
Patients’ PBMCs were isolated freshly without cryopreservation on the day of sampling, and were analyzed by flow cytometry as previously described[15]. Anti-human monoclonal antibodies, including those for CD3 (FITC, clone HIT3a, Becton-Dickinson [BD], Franklin Lake, NJ, USA), CD56 (PE-Cy7, clone B159, BD), CD4 (APC, clone Leu3a, BD), CD8 (APC-Cy7, clone SK1, BD), 7-amino-actinomycin D (7-AAD), NKG2D (PE, clone 1D11, BioLegend, San Diego, CA, USA), CD25 (APC-Cy7, clone M-A251, BD), and CD127 (PE, clone HIL-7R-M21, BD) were used. Dead cells were excluded from assessment by 7-AAD. Irrelevant anti-rat isotype antibodies (BD) were used to assess background fluorescence. Stained cells were analyzed using FACS Canto II (BD). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA). Gating strategies are shown in S2 Fig.
Statistical analysis
Data were analyzed using JMP12 (SAS Institute, Inc. Cary, NC, USA), and are expressed as median with range or average± standard deviation (SD), as appropriate. Non-parametric Kruskal–Wallis tests were used to assess differences between groups. Categorical data were analyzed using Fisher’s exact test. To investigate the independent determining factors for early-emerging HCC, logistic regression models adjusted for covariates were generated. Receiver operating characteristic (ROC) analysis was performed to confirm the usefulness of various parameters to predict early HCC emergence. P < 0.05 was considered significant.
Results
Background characteristics: Significantly higher pre NKG2D correlates to early emerging of clinically evident HCC in IFN−/DAA group
In the IFN−/DAA group, 12 patients (12%) had early-emerging HCC (Table 1). Compared between patients with or without early-emerging HCC, pre-treatment parameters, including total bilirubin, serum albumin, platelet count, PT-INR, type 4 collagen domain 7s (4COL7s), FIB-4 score, APRI, Forns index, alpha-fetoprotein (AFP), total cholesterol, status of previous HCC history, SVR status, and total NKG2D expression differed significantly. Notably, 60% of the cases had advanced liver fibrosis (FIB-4 score >3.25), and 26% of them had liver cirrhosis (APRI >2.0). This reflected a fact that many patients who underwent IFN-free DAAs were ineligible for IFN-combined regimen due to advanced liver fibrosis. In multivariate analysis, the presence of pre-treatment higher total NKG2D expression, along with previous HCC history and non-SVR, was found to be significantly associated with early-emerging HCC, after adjustment for age, sex, AFP, FIB-4 score, and/or serum albumin (Table 3) in various models.
Table 3. Models of multivariate analysis in IFN-/DAA group predicting early- emerging HCC.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P |
| HCC past history | 62 | 7.4–1775 | 0.002** | 24.1 | 3.8–302 | 0.003** | 49.3 | 5.2–1700 | 0.006** |
| Non SVR | 287 | 5.6–6.1x104 | 0.01* | 127 | 2.9–2.1x104 | 0.02* | 476 | 6.1–3740 | 0.02* |
| Pre NKG2D | 285 | 3.5–1.1x108 | 0.01* | 72 | 1.9–3.4x106 | 0.01* | 840 | 5.6-x1.1x109 | 0.01* |
| Pre FIB-4 | 1.3 | 1.1–1.6 | 0.02* | - | - | - | - | - | 0.18 |
| Pre Albumin | - | - | - | 498 | 2.4–3.7x105 | 0.04* | - | - | 0.09 |
*, P< 0.05
**, P< 0.01.
Multivariate Model 1 was adjusted for “Age over 65 years,” “sex,” “pre-treatment alpha-fetoprotein,” “HCC past history,” “Non SVR,” and “pre-treatment FIB-4 scores.” Multivariate Model 2 was adjusted for all variables in Model 1 with “pre-treatment serum albumin,” exclusive of “pre-treatment FIB-4 score.”Multivariate Model 3 was adjusted for variables in Model 1 and “pre-treatment serum albumin.”“Age over 65 years,” “sex,” and “pre-treatment alpha-fetoprotein” are not significant variables in all Models. Abbreviations: HR, hazard ratio; CI, confidence interval.
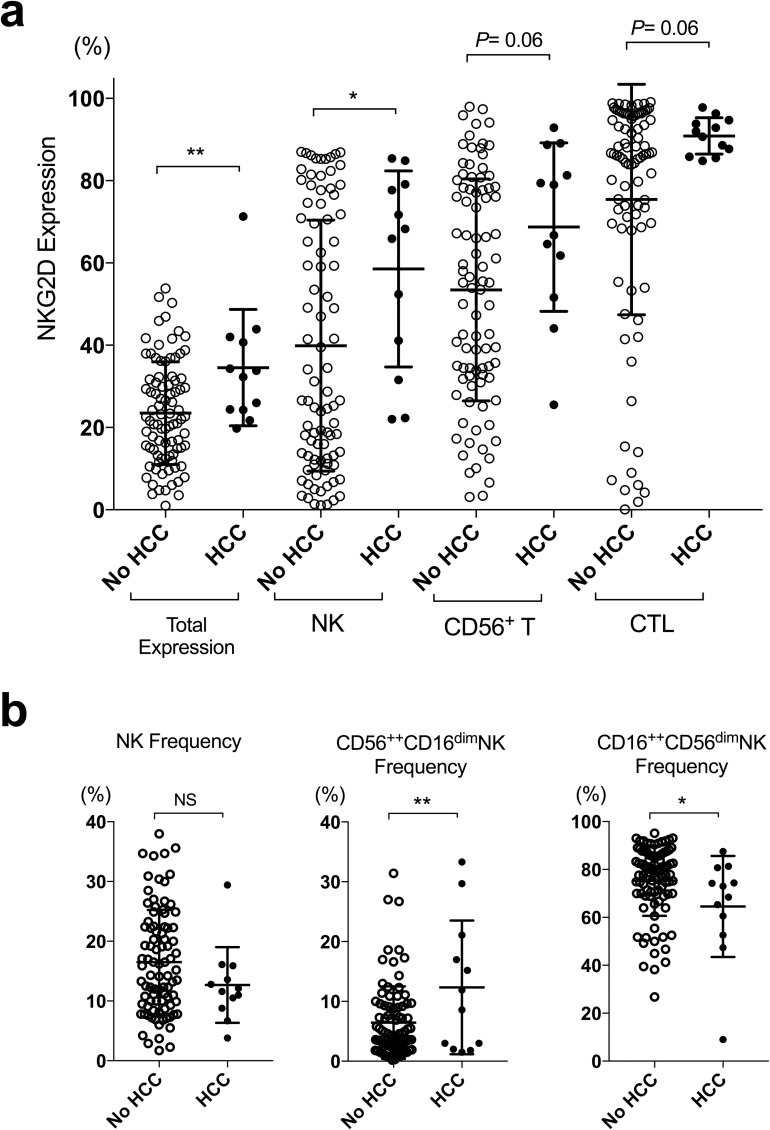
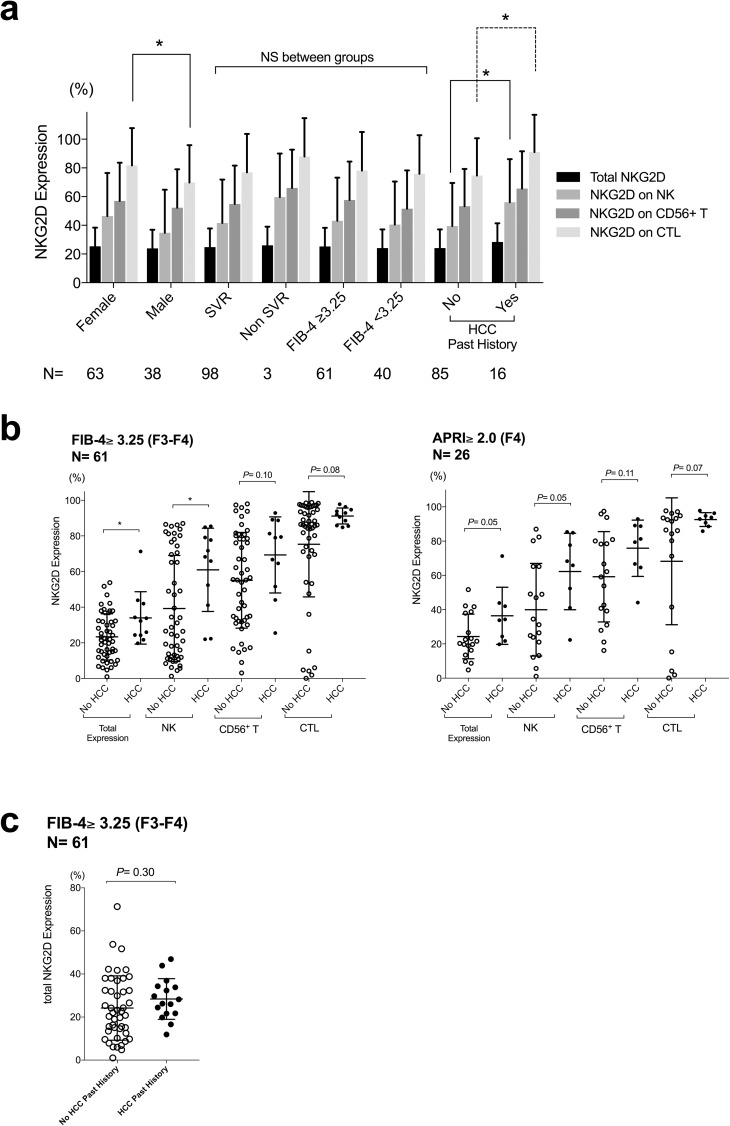
Pre-treatment peripheral blood NKG2D expression of total lymphoid cells and of individual type of immune cells, including NK cells, CD56+ T cells and cytotoxic T cells (CTLs), were shown in Fig 2A. Pre-treatment NKG2D expression of total lymphoid cells and of NK cells were significantly higher in cases that developed early HCC after IFN-free DAA treatment. Additionally, the pre-treatment frequency of CD56++CD16dim NK cells was significantly higher, and that of CD16++CD56dim NK cells was significantly lower in cases that developed early HCC after IFN-free DAAs (Fig 2B). Higher pre-treatment NKG2D expression on NK cells and CTLs correlated to previous HCC history, but did not correlated to SVR or FIB-4 scores (Fig 3A). As the study subjects were restricted to advanced fibrosis (FIB-4 score over 3.25) or cirrhosis (APRI over 2.0), the tendencies of higher pre-treatment NKG2D expression remained (Fig 3B), and the expression did not differ significantly when stratified by previous HCC history (Fig 3C). The analysis of the correlations of various biochemical studies and the NKG2D expression of individual immune cell types showed that higher pre-treatment NKG2D expression of NK cells correlated significantly to decreased liver protein synthesis, including a lower serum albumin levels or a more prolonged PT-INR (Table 4). Notably, NKG2D expression of NK cells, CD56+ T cells and CTLs all correlated slightly to serum AFP levels (Table 4).
Fig 2. Pre-treatment NKG2D expression and frequencies of natural killer cell compartments stratified by early emergence of HCC or not (IFN-/DAA group).
Filled circles represent cases with early emerging HCC and open circles represent cases without. Statistics were shown as mean with SD. *, P< 0.05; **, P< 0.01.
Fig 3. Background pre-treatment characteristics and NKG2D expression.
Pre-treatment NKG2D expression was, (a) compared by sex, SVR status, FIB-4 score, and HCC past history with case numbers shown below each group; (b) compared by early emergence of HCC or not in cases restricted to advanced fibrosis (FIB-4 score over 3.25; left panel) and cirrhosis (APRI over 2.0; right panel); (c) compared between cases with or without HCC past history as cases restricted to advanced fibrosis (FIB-4 score over 3.25). Filled circles represent cases with early-emerging HCC and open circles represent cases without. Statistics were shown as mean with SD. *, P< 0.05; NS, not significant.
Table 4. Various pre-treatment factors and their correlation to pre-treatment NKG2D expression.
| Pre-treatment Variables | Total NKG2D | NKG2D on NK | NKG2D on CD56+ T | NKG2D on CTLs | |
|---|---|---|---|---|---|
| Age | R | 0.04 | 0.06 | 0.1 | 0.18 |
| P | 0.69 | 0.56 | 0.35 | 0.07 | |
| AST | R | 0.14 | 0.09 | 0.17 | -0.15 |
| P | 0.17 | 0.36 | 0.08 | 0.12 | |
| T-Bil | R | 0.09 | 0.11 | 0.06 | 0.03 |
| P | 0.37 | 0.25 | 0.49 | 0.77 | |
| Albumin | R | -0.03 | -0.25 | -0.26 | -0.08 |
| P | 0.34 | 0.01* | <0.01** | 0.49 | |
| PI-INR | R | 0.17 | 0.23 | 0.17 | 0.07 |
| P | 0.10 | 0.03* | 0.09 | 0.48 | |
| Total cholesterol | R | 0.03 | 0.06 | 0.09 | 0.09 |
| P | 0.73 | 0.52 | 0.37 | 0.36 | |
| Platelet | R | -0.20 | -0.08 | -0.03 | 0.00 |
| P | 0.04* | 0.42 | 0.33 | 0.98 | |
| 4COL7s | R | 0.11 | 0.12 | 0.20 | -0.02 |
| P | 0.29 | 0.22 | 0.05 | 0.81 | |
| HCV-RNA | R | -0.08 | -0.10 | -0.03 | -0.04 |
| P | 0.43 | 0.31 | 0.71 | 0.68 | |
| AFP | R | 0.30 | 0.21 | 0.23 | -0.24 |
| P | <0.01** | 0.04* | 0.02* | 0.01* |
*, P< 0.05
**, P< 0.01.
Abbreviations: 4COL7s: type 4 collagen 7s domain; AFP: alpha-fetoprotein; G-GTP, gamma- glutamyl transpeptidase; HCV: hepatitis C virus; R, correlation coefficient; T-Bil, total bilirubin.
These results accord with previous studies which showed that background liver fibrosis status might greatly influence HCC development, in both untreated HCV patients[26] and SVR patients treated by PEG-IFN/RBV[27]. With standard surveillance according to the guidelines, nine out of twelve (75%) of the early-emerging HCCs in this current study were of early-stage diseases (BCLC 0-A), and most of the lesions could be ideally controlled, as shown in S1 Table and S1 Fig.
NKG2D expression decreased significantly at EOT for patients on IFN-free DAAs
In most cases of IFN−/DAA group, peripheral blood HCV-RNA decreased below 1.2 Log IU/ml soon after treatment with IFN-free DAAs (S3A Fig). The pre-treatment and EOT biochemical studies showed that IFN-free DAA administration significantly normalized AST and ALT, regardless of whether patients developed early-emerging HCC or not (S3B Fig).
Because HCV is thought to be noncytopathic[28], rapid AST/ALT normalization might be a result of rapid depression, whether quantitatively or functionally, of intrahepatic cytotoxic inflammatory cells.
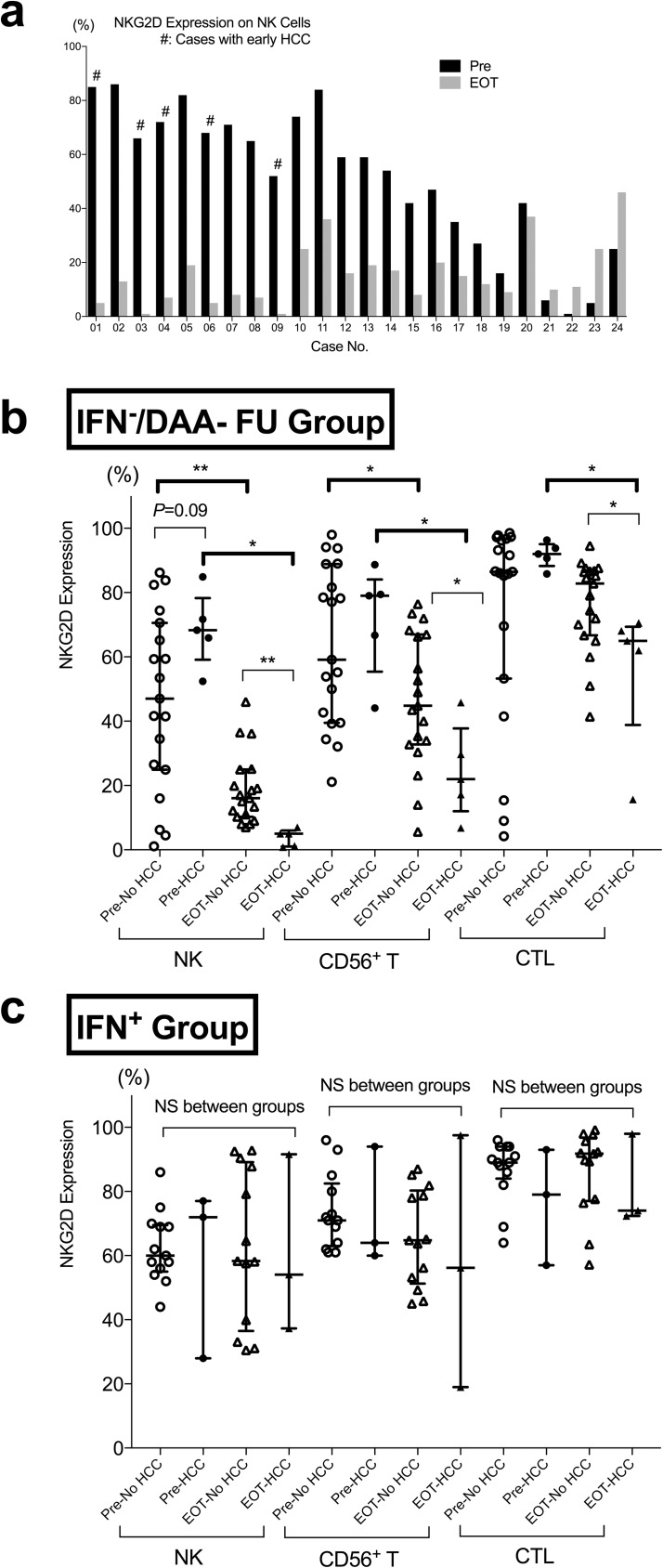
Patient background characteristics of the IFN−/DAA-FU group were shown in Table 2. The factors that were significantly correlating to early HCC emergence were similar to those in the IFN−/DAA group (Table 1). As shown in Fig 4A, in IFN−/DAA-FU group, NKG2D expression on NK cells was significantly decreased generally from pre-treatment to EOT. NKG2D expression of NK cells decreased more significantly (18.6% vs 4.5%, P = 0.003, Fig 4B), in cases that developed early HCC. NKG2D expression of CD56+ T cells and CTLs had the same trend of changes (Fig 4B). On the other hand, the frequency of CD56++CD16dim NK cells tended to (P = 0.11) decrease in cases without early HCC, but stayed relatively the same in cases with early HCC. There were no significant changes in frequencies of NK cells and the CD16++CD56dim compartment (S4 Fig). Contrarily, in IFN+ group, NKG2D expression of NK cells remained relatively unchanged, without significant differences and stayed almost the same (Fig 4C), even for 12 to 24 weeks after EOT (S6A Fig).
Fig 4. Pre- and End of Treatment NKG2D Expression of NK cells in IFN−/DAA-FU or IFN+ group.
(a) Pre and EOT NKG2D expression of NK cells in each cases of IFN−/DAA-FU group. # represents cases with early-emerging HCC. (b, c) NKG2D Expression of NK cells in IFN−/DAA-FU group and in IFN+ group were compared between cases with or without early emergence of clinically evident HCC. Filled circles (pre) or triangles (EOT), cases with HCC; open circles (pre) or triangles (EOT), cases without HCC. Statistics were shown as median with interquartile ranges. *, P< 0.05; **, P< 0.01; NS, not significant.
In order to clarify the contribution of high levels of pre-NKG2D and the decreased levels of EOT-NKG2D, we did a linear regression analysis between “ΔNKG2D,” “pre-treatment NKG2D,” and “EOT NKG2D” on NK cells. Pre-treatment NKG2D highly correlated to ΔNKG2D (S5A Fig), however, EOT-NKG2D also significantly correlated to ΔNKG2D (S5A Fig). Therefore, significant “down-regulation,” as shown in Fig 4B, rather than merely “normalization,” of the NKG2D expression levels, contributed to ΔNKG2D. Furthermore, pre-NKG2D showed no significant correlations to EOT-NKG2D (S5B Fig).
An increased compartment of CD25high helper T cells correlated to NKG2D decrease on NK cells After IFN-free DAA
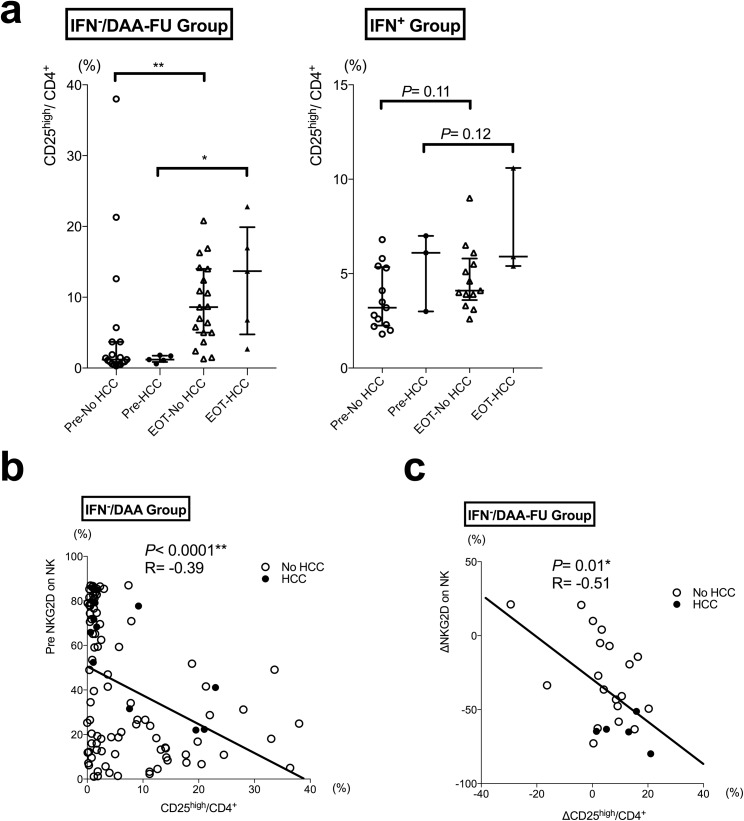
Since increased intrahepatic TGF-β has been reported in HCV-related liver fibrosis[29], we further focused our analysis on the influence of IFN-free DAA treatment on CD25-expressing helper T cells (defined as CD25highCD127−CD4+ T cells, suggested to be a major source of TGF-β[30]). In the IFN−/DAA group, the compartment of CD25high helper T cells significantly elevated in both the non-HCC group and the HCC group (Fig 5A). Furthermore, when we looked on correlations between NKG2D expression of NK cells and the compartment of CD25high helper T cells, pre-treatment NKG2D expression on NK cells significantly inversely correlated to the compartment of CD25high helper T cells (Fig 5B). We also noticed that the pre-treatment and EOT differences in NKG2D expression (ΔNKG2D) on NK cells significantly correlated to the differences of CD25high helper T cells (ΔCD25high/CD4+), in IFN−/DAA group (Fig 5C), but not in IFN+ group (S6B Fig).
Fig 5. CD25high helper T cells and the correlation to NKG2D expression of NK cells.
(a) CD25high helper T cells in IFN−/DAA-FU group and IFN+ group were compared between cases with or without early emerging HCC. (b) Correlations between pre-treatment NKG2D expression of NK cells and the compartment of CD25high helper T cells in IFN−/DAA group. (c) Correlations between pre- and end of treatment changes in NKG2D expressions (ΔNKG2D) of NK cells and the changes in the compartment of CD25high helper T cells (ΔCD25high/CD4+), in IFN−/DAA-FU group. Filled circles (pre) or triangles (EOT), cases with HCC; open circles (pre) or triangles (EOT), cases without HCC. Statistics were shown as median with interquartile ranges. *, P< 0.05; **, P< 0.01.
On-treatment decreased of NKG2D correlated to early-emerging HCC
Of the 12 patients with early-emerging HCC in this study, ten (83%) showed reduced or stably low levels of serum AFP (S1 Table). Serum AFP is widely used to help predict post-SVR HCC emergence after IFN-combined therapies[31]. However, as serum AFP is also related to the altered hepatocyte architectures in CHC[32], rapid normalization of serum AFP after IFN-free DAAs could be misleading; cautious re-evaluation of this useful clinical tumor marker is needed.
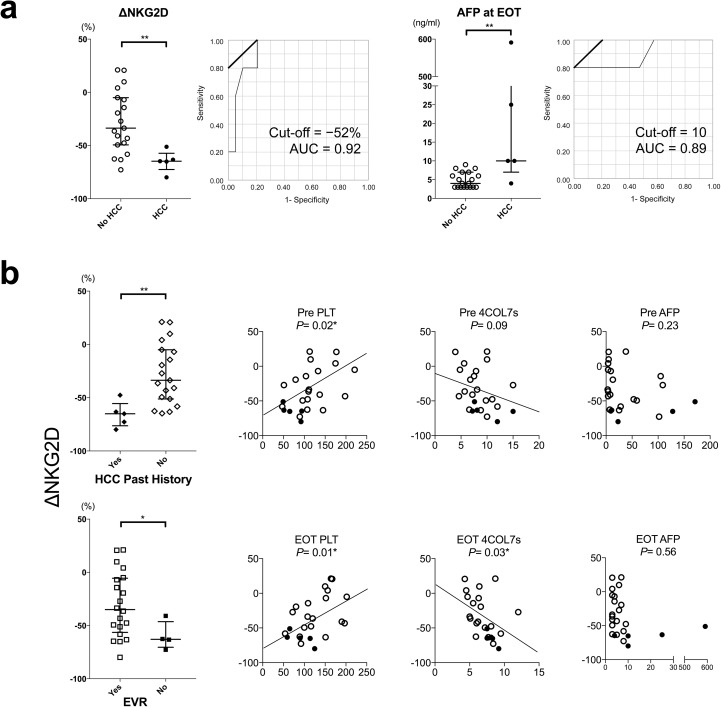
Because we noticed a significantly greater decrease in NKG2D expression of NK cells from pre-treatment to EOT (ΔNKG2D) in patients with early-emerging HCC (Fig 4B), we conducted ROC analysis of IFN−/DAA-FU group if ΔNKG2D predicted early-emerging HCC. The analysis showed that a cut-off of 52% decrease or more apparently predicted early-emerging HCC with an AUC = 0.92 in this model. Additionally, serum AFP at EOT had an AUC = 0.89 in the ROC analysis of IFN−/DAA-FU group at a cut-off value of 10ng/ml (Fig 6A). A more profound decrease of on-treatment NKG2D correlated to past history of HCC, EVR, and platelet count before treatment and at EOT, but didn’t correlate to AFP levels before treatment or at EOT (Fig 6B). On-treatment dynamics NKG2D might be a useful predictor of early-emerging HCC after IFN-free DAAs.
Fig 6. On-treatment decreased of NKG2D correlated to early-emerging HCC.
(a) Changes of NKG2D expression from pre to EOT (ΔNKG2D) were compared in the IFN−/DAA-FU group. The ROC analysis of ΔNKG2D and AFP at EOT as a predictive factor to early HCC emergence were shown. (B) Correlations of ΔNKG2D to other background characteristics and biochemical factors in the IFN−/DAA-FU group were shown. Y-axis: ΔNKG2D expression on NK cells. Filled circles (pre) or triangles (EOT), cases with HCC; open circles (pre) or triangles (EOT), cases without HCC. Statistics were shown as median with interquartile ranges. *, P< 0.05; **, P< 0.01.
Discussions
To our knowledge, this is the first real-world practice-based study to focus on immunological changes that correlate with early HCC emergence, with regard to IFN-free vs. IFN-combined regimens. Pre-treatment higher NKG2D expression and more profound NKG2D decrease on-treatment were noticed in patients with early-emerging HCC after IFN-free DAAs.
Previous studies[8–11] discussed the possibility of clinically undetectable HCCs or mistaken initial staging before administering IFN-free DAAs. In cirrhotic livers, transforming cells with genetic damages might have existed before they become transformed and large enough to be clinically detectable[33]. Additionally, subclinical HCCs could be cleared by host immunosurveillance in an early stage[34] before they become evident. Golden-Mason et al. demonstrated increased hepatic interleukin-15 (IL-15) promotes intrahepatic NK homeostasis, in chronic HCV infection[35]. IL-15 has also been showed to promote MICA/B- NKG2D interaction in CHC[36]. Therefore, higher pre-treatment levels of NKG2D (as shown in Table 1 and Fig 2) could result from such imbalance of inflammatory cytokines from both immune cells or other hepatic non-parenchymal cells such as hepatic stellate cells, especially in highly fibrotic livers, like the cases in IFN−/DAA group of this current study, even if no HCC was clinically evident at the time point of start of IFN-free DAA administration. Recently, Prenner et al. showed in a retrospective study that the existence of active HCC might reduce the rate to achieve SVR by IFN-free DAAs[37]. As we also showed in Table 1 that non-SVR correlated to early emergence of clinically evident HCC, pre-treatment existence of clinically undetectable HCC may be suggested as a causative factor for treatment failure.
Natural killer cell can be classified as immunoregulatory (cytokine-productive) CD56++CD16dim and cytotoxic CD16++CD56dim compartments[38]. Previous reports have showed an increased immunoregulatory CD56++CD16dim compartment[39] and a decreased cytotoxic CD16++CD56dim compartment[40] in chronic hepatitis C. To our knowledge, this current study is the first to demonstrate the significant differences of the two compartments between cases that developed HCC after treatment or not (Fig 2B).
In this study, various possible mechanisms might explain why on-treatment immune cells behave differently than in those undergoing IFN-free DAAs and IFN-combined therapy. First, the external administration of IFN-α might play a role. Reportedly, ISGs can be greatly decreased in patients treated with IFN-free DAAs[12], thus external IFN might explain why NKG2D expression on NK cells is maintained in IFN-combined therapy. Stegmann et al. showed that ex vivo stimulation of NK cells by IFN-α helped increase recognition of HCV-infected hepatoma cells[41]. Second, NKG2D ligands have been implicated in immune escape of tumor cells[42] and viral-induced HCC[16]. Mechanisms such as increased soluble NKG2D ligand shedding from HCC[43] could be possible, and should be further studied. Third, an increased compartment of CD25high helper T cells, as shown in the current study, may down-modulate NKG2D-expressing NK cells through TGF-β[44–46]. Recently, Langhans et al. reported that peripheral FOXP3+CD25+CD4+ regulatory T cells may increase persistently after successful DAA treatment[47]. However, whether and how TGF-β changes during the rapid eradication of HCV by IFN-free DAAs still need to be investigated.
In earlier clinical trial-based PBMC studies, Spaan et al. demonstrated, in 12 patients who received DCV/ASV, a significant decrease of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on NK cells from day 0 to 12W—i.e., half of the treatment course[14]. Serti et al. showed a rapid decrease in viremia and levels of inflammatory cytokines were associated with decreased activation of intrahepatic and blood NK cells in 13 patients who received DCV/ASV[13]. However, these studies showed relatively no significant change in NKG2D expression as we show here. The discrepancies in NKG2D expression between studies in chronic HCV infection are thought to reflect background host factors, such as degree of background fibrosis, or possible clinically undetectable pre-cancerous lesions in this real-world practice setting. In this current study, pre-treatment fibrotic status of the liver, implicated as higher pre-treatment FIB-4 scores (Tables 1 and 3), decreased hepatic protein synthesis (Table 4), and lower platelet counts (Fig 6B), were suggested to correlate to the differences in NKG2D expression.
Decreased NKG2D (Fig 4) and increased compartment of CD25high helper T cells (Fig 5) are generally thought to be unfavorable to immunosurveillance of cancer cells[45]; these events might demonstrate how the influence on host immune response when a persistent infectious factor (in this case, HCV) is rigorously eradicated. Our study here suggested that rapid viral suppression by IFN-free DAAs may depress cytotoxic inflammatory cells, thus leading to a state of temporal relative immunosuppression due to immune reconstitution, not only to transformed cells as shown in this study, but also to cells co-infected with viruses such as hepatitis B virus (HBV) or herpes viruses, as increased reactivations of these viruses have been reported in patients who undergo IFN-free DAA treatment[48, 49]. However, in this study, successful HCV eradication (leading to SVR or not) was still an optimistic predictor of HCC development (Table 3). As with SVR after IFN-combined therapies that were effective for a second recurrence of HCC, but not for the first[50], we must still manage HCCs that may have existed before administration of IFN-free DAAs, even if they are not clinically evident. As IFN-free DAAs effectively lead to viral eradication, we might use IFN-free DAAs, if we are careful, in patients who are suspected to be at higher HCC risk, such as those with advanced fibrosis, or past history of HCC. Additionally, because immune cells might react quite differently to treatments with or without external IFN-α, what we have learned from previous IFN-combined therapies, including post-treatment follow-up parameters, intervals or durations, might also need to be re-evaluated.
A major limitation of this study is its observational design that is exploratory in nature. Subsequent studies with larger subject numbers will be needed to solidify the associations described in this study. We cannot conclude if there is a causal relationship between DAA administration, the on-treatment NKG2D decease, and HCC emergence. Again, we have to emphasize that this current study alone is not enough for indicating whether IFN-free DAAs increase the emergence of HCC or not. Additionally, due to IFN tolerability, background characteristics vary between study cohort (IFN-/DAA group) and the control (IFN+ group) in liver fibrotic status (S3 Table). Besides external IFN, the less fibrotic liver may also contribute to the maintenance of NKG2D expression in IFN+ group. Because only four cases with de novo early HCC were included in this study, our current data along may not be sufficient to conclude if there is any difference compared with early recurrence.
In conclusion, In this study based on real-world clinical practice, we found that early emergence of clinically evident HCC after IFN-free DAA treatment correlated to significant and profound decrease of NKG2D expression on NK cells, compared with that of patients who were treated with IFN-combined therapy. Stricter monitoring and use of novel non-invasive markers to predict fibrosis or HCC development are warranted. On-treatment dynamics of peripheral blood NKG2D expression could be a useful predictor of HCC in patients receiving IFN-free DAAs.
Supporting information
The start of each case shows the patient’s last established active HCC before receiving IFN-free DAAs. Gd-EOB-DTPA, gadolinium ethoxybenzyl diethylene triamine pentaacetic acid; RFA, radiofrequency ablation; TACE, trans-arterial chemoembolization. (For other abbreviations, please see the main text.).
(TIFF)
(TIFF)
Viral dynamics (a) and biochemial changes (b) stratified by early HCC emergence in IFN−/DAA group.
Statistics were shown as mean with SD. *, P< 0.05; **, P< 0.01. Units: HCV-RNA: LogIU/ml; AST: IU/L; ALT: IU/L; AFP: ng/ml. VBT, viral breakthrough.
(TIFF)
Statistics were shown as mean with SD. *, P< 0.05; **, P< 0.01. NS, not significant.
(TIFF)
(a) Linear regression analyses of ΔNKG2D and pre- or EOT NKG2D expressions. (b) Linear regression analyses of pre- and EOT NKG2D expressions. Filled circles represent cases with early emerging HCC and open circles represent cases without. R, correlation coefficient; *, P< 0.05; **, P< 0.01.
(TIFF)
(TIFF)
CPT, Child-Pugh-Turcotte scores; PS, performance status; R/O, recurrence or occurrence; RFA, radiofrequency ablation. TACE, trans-arterial chemoembolization. (For other abbreviations, please see the main text.).
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank Miss Kyoko Watanabe, for her technical and secretarial assistances.
Abbreviations
- 4COL7s
7s domain of type 4 collagen
- AFP
alpha-fetoprotein
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ASV
asunaprevir
- BCLC
Barcelona Clinic Liver Cancer
- CHC
chronic hepatitis C
- CTL
cytotoxic T cells
- DAA
direct antiviral agent
- DCV
daclatasvir
- EOT
end-of-treatment
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IFN
interferon
- ISG
interferon-stimulated gene
- LDV
ledipasvir
- MICA
MHC class I chain-related protein A
- NK
natural killer
- NKG2D
natural killer group 2, member D
- PBMC
peripheral blood mononuclear cell
- PEG
pegylated
- RBV
ribavirin
- SMV
simeprevir
- SOF
sofosbuvir
- SVR
sustained viral response
- TGF
transforming growth factor
- Treg
regulatory T cell
- VBT
viral breakthrough
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by grants-in-aid from the Keio Gijuku Academic Development Funds and Japan Agency for Medical Research and Development (grant number: 15fk0210037h0101). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025 ; PubMed Central PMCID: PMC2866629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association for Study of L. EASL Recommendations on Treatment of Hepatitis C 2015. Journal of hepatology. 2015;63(1):199–236. doi: 10.1016/j.jhep.2015.03.025 . [DOI] [PubMed] [Google Scholar]
- 3.Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatology international. 2016. doi: 10.1007/s12072-016-9717-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2010;8(3):280–8, 8 e1. doi: 10.1016/j.cgh.2009.11.018 . [DOI] [PubMed] [Google Scholar]
- 5.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Annals of internal medicine. 2013;158(5 Pt 1):329–37. doi: 10.7326/0003-4819-158-5-201303050-00005 . [DOI] [PubMed] [Google Scholar]
- 6.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr., et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149(3):649–59. doi: 10.1053/j.gastro.2015.05.010 . [DOI] [PubMed] [Google Scholar]
- 7.van der Meer AJ, Berenguer M. Reversion of disease manifestations after HCV eradication. Journal of hepatology. 2016;65(1 Suppl):S95–S108. doi: 10.1016/j.jhep.2016.07.039 . [DOI] [PubMed] [Google Scholar]
- 8.Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected early tumor recurrence in patients with hepatitis C virus -related hepatocellular carcinoma undergoing interferon-free therapy: a note of caution. Journal of hepatology. 2016. doi: 10.1016/j.jhep.2016.04.008 . [DOI] [PubMed] [Google Scholar]
- 9.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early Occurrence and Recurrence of Hepatocellular Carcinoma in HCV-related Cirrhosis Treated with Direct Acting Antivirals. Journal of hepatology. 2016. doi: 10.1016/j.jhep.2016.06.015 . [DOI] [PubMed] [Google Scholar]
- 10.Yang JD, Aqel BA, Pungpapong S, Gores GJ, Roberts LR, Leise MD. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. Journal of hepatology. 2016;65(4):859–60. doi: 10.1016/j.jhep.2016.06.023 . [DOI] [PubMed] [Google Scholar]
- 11.Pol S. Lack of evidence of an effect of Direct Acting Antivirals on the recurrence of hepatocellular carcinoma: The ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CIRVIR and CO23 CUPILT cohorts). Journal of hepatology. 2016. doi: 10.1016/j.jhep.2016.05.045 . [DOI] [PubMed] [Google Scholar]
- 12.Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. The Journal of clinical investigation. 2014;124(8):3352–63. doi: 10.1172/JCI75938 ; PubMed Central PMCID: PMC4109554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015;149(1):190–200 e2. doi: 10.1053/j.gastro.2015.03.004 ; PubMed Central PMCID: PMC4523392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HL, et al. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. The Journal of infectious diseases. 2016;213(2):216–23. doi: 10.1093/infdis/jiv391 . [DOI] [PubMed] [Google Scholar]
- 15.Chu PS, Ebinuma H, Nakamoto N, Sugiyama K, Usui S, Wakayama Y, et al. Genotype-Associated Differential NKG2D Expression on CD56+CD3+ Lymphocytes Predicts Response to Pegylated-Interferon/Ribavirin Therapy in Chronic Hepatitis C. PloS one. 2015;10(5):e0125664 doi: 10.1371/journal.pone.0125664 ; PubMed Central PMCID: PMC4428701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto K, Kato N. MICA SNPs and the NKG2D system in virus-induced HCC. Journal of gastroenterology. 2015;50(3):261–72. doi: 10.1007/s00535-014-1000-9 . [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Lu S, Wang X, Page ST, Higano CS, Plymate SR, et al. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. The Journal of clinical investigation. 2013;123(10):4410–22. doi: 10.1172/JCI69369 ; PubMed Central PMCID: PMC3784540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. The Journal of clinical investigation. 2011;121(9):3609–22. doi: 10.1172/JCI45816 ; PubMed Central PMCID: PMC3171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konjevic G, Mirjacic Martinovic K, Jurisic V, Babovic N, Spuzic I. Biomarkers of suppressed natural killer (NK) cell function in metastatic melanoma: decreased NKG2D and increased CD158a receptors on CD3-CD16+ NK cells. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2009;14(4):258–70. doi: 10.1080/13547500902814658 . [DOI] [PubMed] [Google Scholar]
- 20.Giuliani E, Vassena L, Desimio MG, Buonomini AR, Malagnino V, Andreoni M, et al. Expression and Function of NKG2D Is Impaired in CD8+ T Cells of Chronically HIV-1-Infected Patients Without ART. Journal of acquired immune deficiency syndromes. 2015;70(4):347–56. doi: 10.1097/QAI.0000000000000792 . [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Kubo S, Takayasu K, Sakamoto M, Tanaka M, Ikai I, et al. Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version). Hepatology research: the official journal of the Japan Society of Hepatology. 2010;40(7):686–92. doi: 10.1111/j.1872-034X.2010.00674.x . [DOI] [PubMed] [Google Scholar]
- 22.Kudo M, Ueshima K, Kubo S, Sakamoto M, Tanaka M, Ikai I, et al. Response Evaluation Criteria in Cancer of the Liver (RECICL) (2015 Revised version). Hepatology research: the official journal of the Japan Society of Hepatology. 2016;46(1):3–9. doi: 10.1111/hepr.12542 . [DOI] [PubMed] [Google Scholar]
- 23.Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41(6):1376–82. doi: 10.1002/hep.20717 . [DOI] [PubMed] [Google Scholar]
- 24.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6. doi: 10.1002/hep.21669 . [DOI] [PubMed] [Google Scholar]
- 25.Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36(4 Pt 1):986–92. doi: 10.1053/jhep.2002.36128 . [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Annals of internal medicine. 1999;131(3):174–81. . [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of Hepatocellular Carcinoma after Sustained Virologic Response in Veterans with HCV-infection. Hepatology. 2016. doi: 10.1002/hep.28535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annual review of pathology. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230 . [DOI] [PubMed] [Google Scholar]
- 29.Clemente M, Nunez O, Lorente R, Rincon D, Matilla A, Salcedo M, et al. Increased intrahepatic and circulating levels of endoglin, a TGF-beta1 co-receptor, in patients with chronic hepatitis C virus infection: relationship to histological and serum markers of hepatic fibrosis. Journal of viral hepatitis. 2006;13(9):625–32. doi: 10.1111/j.1365-2893.2006.00733.x . [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. The Journal of experimental medicine. 2006;203(7):1701–11. doi: 10.1084/jem.20060772 ; PubMed Central PMCID: PMC2118339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, et al. alpha-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58(4):1253–62. doi: 10.1002/hep.26442 . [DOI] [PubMed] [Google Scholar]
- 32.Goldstein NS, Blue DE, Hankin R, Hunter S, Bayati N, Silverman AL, et al. Serum alpha-fetoprotein levels in patients with chronic hepatitis C. Relationships with serum alanine aminotransferase values, histologic activity index, and hepatocyte MIB-1 scores. American journal of clinical pathology. 1999;111(6):811–6. . [DOI] [PubMed] [Google Scholar]
- 33.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–55. doi: 10.1136/gutjnl-2013-306627 ; PubMed Central PMCID: PMC4337888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subleski JJ, Scarzello AJ, Alvord WG, Jiang Q, Stauffer JK, Kronfli A, et al. Serum-based tracking of de novo initiated liver cancer progression reveals early immunoregulation and response to therapy. Journal of hepatology. 2015;63(5):1181–9. doi: 10.1016/j.jhep.2015.06.021 ; PubMed Central PMCID: PMC4615530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golden-Mason L, Kelly AM, Doherty DG, Traynor O, McEntee G, Kelly J, et al. Hepatic interleuklin 15 (IL-15) expression: implications for local NK/NKT cell homeostasis and development. Clinical and experimental immunology. 2004;138(1):94–101. doi: 10.1111/j.1365-2249.2004.02586.x ; PubMed Central PMCID: PMC1809196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. Journal of immunology. 2003;171(10):5423–9. . [DOI] [PubMed] [Google Scholar]
- 37.Prenner S, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, Kulik L. Hepatocellular Carcinoma Decreases the Chance of Successful Hepatitis C Virus Therapy with Direct-Acting Antivirals. Journal of hepatology. 2017. doi: 10.1016/j.jhep.2017.01.020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagiwa S, Kamimura H, Ichida T. Natural killer cell receptors and their ligands in liver diseases. Medical molecular morphology. 2009;42(1):1–8. doi: 10.1007/s00795-008-0434-7 . [DOI] [PubMed] [Google Scholar]
- 39.Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N, et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. Journal of hepatology. 2009;51(3):458–67. doi: 10.1016/j.jhep.2009.05.030 . [DOI] [PubMed] [Google Scholar]
- 40.Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43(3):573–80. doi: 10.1002/hep.21073 . [DOI] [PubMed] [Google Scholar]
- 41.Stegmann KA, Bjorkstrom NK, Ciesek S, Lunemann S, Jaroszewicz J, Wiegand J, et al. Interferon alpha-stimulated natural killer cells from patients with acute hepatitis C virus (HCV) infection recognize HCV-infected and uninfected hepatoma cells via DNAX accessory molecule-1. The Journal of infectious diseases. 2012;205(9):1351–62. doi: 10.1093/infdis/jis210 ; PubMed Central PMCID: PMC3690562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–8. doi: 10.1038/nature01112 . [DOI] [PubMed] [Google Scholar]
- 43.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annual review of immunology. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951 ; PubMed Central PMCID: PMC4244079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sene D, Levasseur F, Abel M, Lambert M, Camous X, Hernandez C, et al. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS pathogens. 2010;6(11):e1001184 doi: 10.1371/journal.ppat.1001184 ; PubMed Central PMCID: PMC2978723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunological reviews. 2006;214:229–38. doi: 10.1111/j.1600-065X.2006.00445.x . [DOI] [PubMed] [Google Scholar]
- 46.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. The Journal of experimental medicine. 2005;202(8):1075–85. doi: 10.1084/jem.20051511 ; PubMed Central PMCID: PMC2213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langhans B, Dieter Nischalke H, Kramer B, Hausen A, Dold L, van Heteren P, et al. Increased peripheral cd4+ regulatory t cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. Journal of hepatology. 2016. doi: 10.1016/j.jhep.2016.12.019 . [DOI] [PubMed] [Google Scholar]
- 48.Collins JM, Raphael KL, Terry C, Cartwright EJ, Pillai A, Anania FA, et al. Hepatitis B Virus Reactivation During Successful Treatment of Hepatitis C Virus With Sofosbuvir and Simeprevir. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;61(8):1304–6. doi: 10.1093/cid/civ474 . [DOI] [PubMed] [Google Scholar]
- 49.Perello MC, Fernandez-Carrillo C, Londono MC, Arias-Loste T, Hernandez-Conde M, Llerena S, et al. Reactivation of Herpesvirus in Patients With Hepatitis C Treated With Direct-Acting Antiviral Agents. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2016. doi: 10.1016/j.cgh.2016.05.016 . [DOI] [PubMed] [Google Scholar]
- 50.Hagihara H, Nouso K, Kobayashi Y, Iwasaki Y, Nakamura S, Kuwaki K, et al. Effect of pegylated interferon therapy on intrahepatic recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. International journal of clinical oncology. 2011;16(3):210–20. doi: 10.1007/s10147-010-0150-x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The start of each case shows the patient’s last established active HCC before receiving IFN-free DAAs. Gd-EOB-DTPA, gadolinium ethoxybenzyl diethylene triamine pentaacetic acid; RFA, radiofrequency ablation; TACE, trans-arterial chemoembolization. (For other abbreviations, please see the main text.).
(TIFF)
(TIFF)
Viral dynamics (a) and biochemial changes (b) stratified by early HCC emergence in IFN−/DAA group.
Statistics were shown as mean with SD. *, P< 0.05; **, P< 0.01. Units: HCV-RNA: LogIU/ml; AST: IU/L; ALT: IU/L; AFP: ng/ml. VBT, viral breakthrough.
(TIFF)
Statistics were shown as mean with SD. *, P< 0.05; **, P< 0.01. NS, not significant.
(TIFF)
(a) Linear regression analyses of ΔNKG2D and pre- or EOT NKG2D expressions. (b) Linear regression analyses of pre- and EOT NKG2D expressions. Filled circles represent cases with early emerging HCC and open circles represent cases without. R, correlation coefficient; *, P< 0.05; **, P< 0.01.
(TIFF)
(TIFF)
CPT, Child-Pugh-Turcotte scores; PS, performance status; R/O, recurrence or occurrence; RFA, radiofrequency ablation. TACE, trans-arterial chemoembolization. (For other abbreviations, please see the main text.).
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.