Abstract
BACKGROUND AND OBJECTIVES:
Pulmonary exacerbations lead to significant morbidity and mortality in patients with cystic fibrosis (CF). National consensus guidelines exist, but few studies report current practice in the treatment and monitoring of pulmonary exacerbations. The goal of this study was to characterize consistency and variability in the inpatient management of CF-related pulmonary exacerbations. We focused on the use of guideline-recommended maintenance therapies, antibiotic selection and treatment regimens, use of systemic corticosteroids, and frequency of lung function testing. We hypothesized that significant variability in these treatment practices exists nationally.
METHODS:
This trial was a retrospective cross-sectional study. It included patients with CF aged ≤18 years hospitalized for pulmonary exacerbations between July 1, 2010, and June 30, 2015, at hospitals within the US Pediatric Health Information System database that are also Cystic Fibrosis Foundation–accredited care centers. One exacerbation per patient was randomly selected over the 5-year study period.
RESULTS:
From 38 hospitals, 4827 individual pulmonary exacerbations were examined. Median length of stay was 10.0 days (interquartile range, 6–14.0 days). Significant variation was seen among centers in the use of hypertonic saline (11%–100%), azithromycin (5%–83%), and systemic corticosteroids (3%–61%) and in the frequency of lung function testing. Four different admission antibiotic regimens were used >10% of the time, and the most commonly used admission antibiotic regimen comprised 2 intravenous antibiotics with no additional oral or inhaled antibiotics (29%).
CONCLUSIONS:
Significant variation exists in the treatment and monitoring of pulmonary exacerbations across Pediatric Health Information System–participating, Cystic Fibrosis Foundation–accredited care centers. Results from this study can inform future research working toward standardized inpatient pulmonary exacerbation management to improve CF care for children and adolescents.
What’s Known on This Subject:
Pulmonary exacerbations have adverse effects on quality of life, lung function, and survival in cystic fibrosis. Although national guidelines for treatment of pulmonary exacerbations exist, there are limited data on current provider treatment practices.
What This Study Adds:
Using the Pediatric Health Information System database, we characterized inpatient management of pulmonary exacerbations. We found significant variability in antibiotic selection, chronic therapy use, and lung function monitoring. These findings can inform future studies targeting standardization of pulmonary exacerbation management.
Cystic fibrosis (CF) is an autosomal recessive disorder characterized clinically by respiratory, gastrointestinal, and reproductive tract involvement. The significant morbidity and mortality in patients with CF result from pulmonary exacerbations.1–3 Although lacking an established consensus definition in clinical practice, the clinical features of exacerbations include worsening cough, increased sputum expectoration, chest pain, shortness of breath, fatigue, weight loss, and decline in lung function.2,4,5 Pulmonary exacerbations have been negatively linked with physical and psychosocial quality of life outcomes,6 failure to return to baseline lung function,7 and survival.8 Although Cystic Fibrosis Foundation (CFF) expert guidelines on the management of pulmonary exacerbations exist,9 few studies have described current national trends in pulmonary exacerbation treatment and monitoring. A study of pulmonary exacerbations found that oral (rather than intravenous [IV]) antibiotics were more often given to younger, healthier patients.10 Heltshe et al5 used prospective data from 123 patients with CF to characterize the heterogeneity of pulmonary exacerbation care, correlating treatment practices with clinical response measures. A recent pilot study was conducted to examine willingness of CF providers to enroll in future interventional studies on exacerbation treatment standardization,11 and a prospective, interventional trial in adults is currently planned to evaluate optimal IV antibiotic duration in pulmonary exacerbations.
The Pediatric Health Information System (PHIS) database includes clinical and resource utilization data for inpatient, emergency department, ambulatory surgery, and observation unit patient encounters from 49 US children’s hospitals. PHIS data have been used extensively in projects on clinical effectiveness, resource utilization, and antimicrobial stewardship; however, to our knowledge, only 1 published CF-related study (focused on sinus surgery) has used this database.12 The goal of the present study was to use the PHIS database to characterize CF-related inpatient pulmonary exacerbations from national CFF-accredited children’s hospitals over a 5-year period to describe current exacerbation treatment practices. We hypothesized that significant variation exists across centers in inpatient pulmonary exacerbation treatment and monitoring.
Methods
This retrospective observational study included PHIS inpatient data on CF-related pulmonary exacerbations over a 5-year period from July 1, 2010, through June 30, 2015.13 This time period was selected to postdate published 2009 CFF pulmonary exacerbation guidelines.9 PHIS data were defined and extracted by using the PHIS Cohort Builder Tool and include International Classification of Diseases, Ninth Revision (ICD-9), and Clinical Transaction Classification diagnostic codes to denote individual diseases, medications, and procedures. Laboratory data including respiratory microbiology results are not included in the PHIS database, nor are results of lung function testing. Similarly, discharge medications, including home IV antibiotics, are not recorded.
Children and adolescents aged ≤18 years at time of discharge were included if they fit 1 of 2 possible CF pulmonary exacerbation case definitions. The first definition comprised an ICD-9 code for CF with pulmonary manifestations and IV antibiotic use within the first 24 hours of admission. The second definition comprised 1 of the CF-related ICD-9 codes (CF without mention of meconium ileus, CF with gastrointestinal manifestations, or CF with other manifestations, in each case with IV antibiotic use within the first 24 hours of admission) and 1 of the disease-specific ICD-9 codes (acute upper respiratory tract infections of multiple or unspecified sites, acute bronchitis and bronchiolitis, pneumonia and influenza, bronchitis and chronic bronchitis, or bronchiectasis).
Exclusion criteria included transfer to or from another facility during an exacerbation, history of organ transplant or malignancy, unknown sex, or 1 of the following ICD-9 diagnostic codes: complications of transplant or other postoperative complication, acute pancreatitis, or mechanical complication of gastrostomy, colostomy, enterostomy tube, insulin pump, or vascular device. Hospitals were excluded if not CFF-accredited by July 1, 2010 (accreditation verified by the CFF), had incomplete oral medication information, or had systematic coding discrepancies making data unreliable. Only CFF-accredited centers were included because these sites are more likely to be aware of published CFF pulmonary exacerbation guidelines. CFF accreditation of CF centers is established and maintained by periodic CFF center committee site visits that rigorously evaluate staffing, facilities, clinical and teaching practices, and research programs. Detailed annual progress reports are submitted to maintain accreditation between site visits.
Once all pulmonary exacerbations were identified, a single exacerbation was randomly selected from each individual. Data on demographic variables, medications, lung function testing frequency, and respiratory support were collected from ICD-9 and Clinical Transaction Classification diagnostic codes (Supplemental Table 3). Oral azithromycin maintenance therapy was defined as administration between 25% and 75% of hospital days; these frequencies were used to evaluate for thrice weekly administration per CFF guidelines and to minimize misclassification of patients as chronic azithromycin users who were being treated acutely with daily azithromycin for a pulmonary exacerbation. Characteristics for the cohort were summarized by using descriptive statistics.
Data handling was performed in R versions 3.2.3 to 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria; 2016). This study was approved by the institutional review board at Seattle Children’s Hospital, Seattle, Washington.
Results
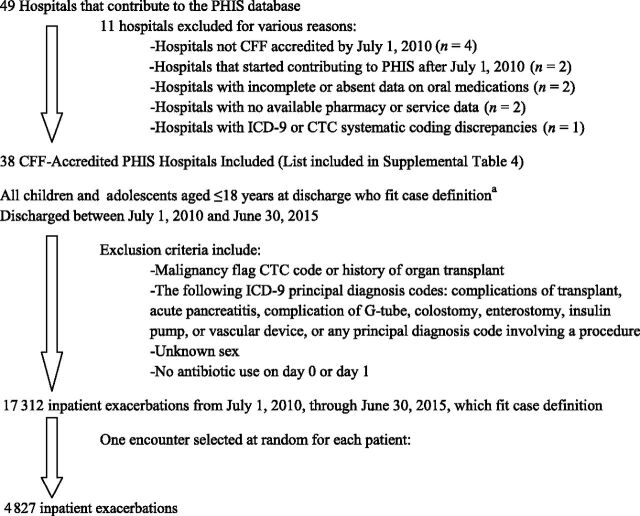
Figure 1 illustrates hospital and study participant selection. Of the 49 hospitals providing data to PHIS, 38 met inclusion criteria and contributed patient data to the study (Supplemental Table 4). A total of 17 312 pulmonary exacerbations were identified; the mean ± SD exacerbation number per patient was 3.8 ± 4.3, with 37% of patients experiencing only a single exacerbation, and 46% experiencing between 2 and 6 exacerbations over the 5 years. After randomly selecting a single exacerbation from each eligible subject, 4827 individual pulmonary exacerbations were included for analysis.
FIGURE 1.
Flow diagram for patients included in the study. a(1) Discharge ICD-9 code for CF with pulmonary manifestations and IV antibiotic use within the first 24 hours of admission (98% of cases); or (2) discharge CF-related ICD-9 code and IV antibiotic use within the first 24 hours of admission plus acute respiratory illness (2% of cases). CTC, Clinical Transaction Classification.
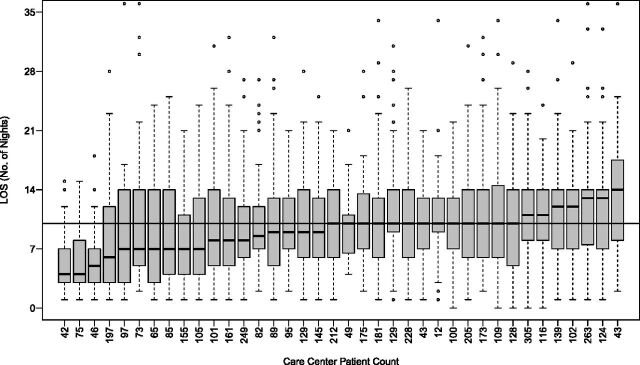
Characteristics of the study cohort are described in Table 1. The majority of patients were female (51.1%), non-Hispanic white (76.3%), and pancreatic insufficient (90.9%). Figure 2 displays length of stay (LOS) according to center, ranging from a mean of 5.2 ± 3.7 days to 13.2 ± 7.0 days. Among all patients, the median LOS was 10.0 days (interquartile range, 6–14.0 days); 13% of the cohort had an LOS ≤3 days, 71.8% had an LOS 4 to 14 days and 14.2% of individuals were hospitalized for >14 days.
TABLE 1.
Demographic Variables for Study Cohort
| Male | Female | |||
|---|---|---|---|---|
| n = 2362 (48.9%) | n = 2465 (51.1%) | |||
| Race/ethnicity | White | Latino | Black | Other/unknown |
| n = 3682 (76.3%) | n = 632 (13.1%) | n = 225 (4.7%) | n = 288 (5.9%) | |
| Payer | Employer | Government | Other/unknown | Self |
| n = 2330 (48.3%) | n = 2259 (46.8%) | n = 188 (3.9%) | n = 50 (1.0%) | |
| Age | Pancreatic Insufficient (yes) | Ursodiol Use (yes) | Insulin Use (yes) | Gastrostomy Present (yes) |
| <2 y | 430 (88.1%) | 26 (5.3%) | 2 (0.4%) | 28 (5.7%) |
| n = 488 (10.1%) | ||||
| 2–5 y | 636 (91.6%) | 71 (10.2%) | 5 (0.7%) | 92 (13.3%) |
| n = 694 (14.4%) | ||||
| 6–11 y | 1193 (91.5%) | 199 (15.3%) | 104 (8.0%) | 253 (19.4%) |
| n = 1304 (27.0%) | ||||
| 12–18 y | 2131 (91.0%) | 526 (22.5%) | 538 (23.0%) | 389 (16.6%) |
| n = 2341 (48.5%) | ||||
| Entire cohort | 4390 (90.9%) | 822 (17.0%) | 649 (13.4%) | 762 (15.8%) |
| N = 4827 (100%) |
FIGURE 2.
LOS distribution according to CFF-accredited care center in CF-related pulmonary exacerbations. The numbers on the x-axis indicate number of patients included from each center. The black horizontal line represents the median LOS for each center, and the boxes above and below indicate the upper and lower interquartile ranges.
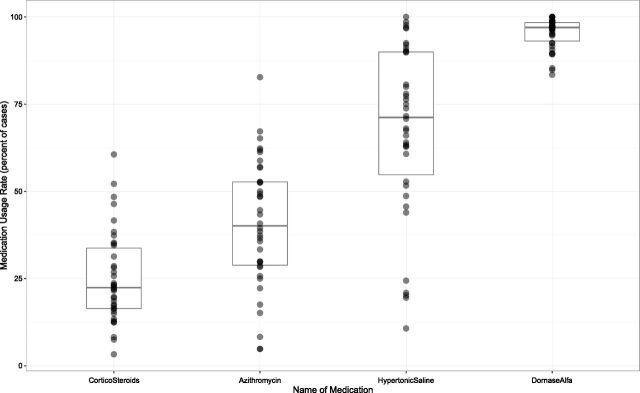
Figure 3 illustrates the frequency, according to center, of dornase alfa, hypertonic saline, maintenance azithromycin, and systemic corticosteroid use, limited to children aged ≥6 years (no guidelines exist for use of these medications in children aged <6 years).9 Dornase alfa was used most frequently (range, 83%–100%), and there was wide variation among centers in maintenance azithromycin (range, 5%–83%) and hypertonic saline (range, 11%–100%) use. Among all patients, 14.5% received systemic corticosteroids on admission, and the median rate of systemic corticosteroid use at any time during hospitalization was 23% (interquartile range, 18%–32%) but ranged from 3% to 61% between centers.
FIGURE 3.
Systemic corticosteroid, maintenance azithromycin, hypertonic saline, and dornase alfa use according to care center in children aged ≥6 years. The middle line represents the median percent medication use, and the boxes above and below indicate the upper and lower interquartile ranges. Each dot represents a single care center. Gray dots represent a single care center; darker dots reflect multiple superimposed data points.
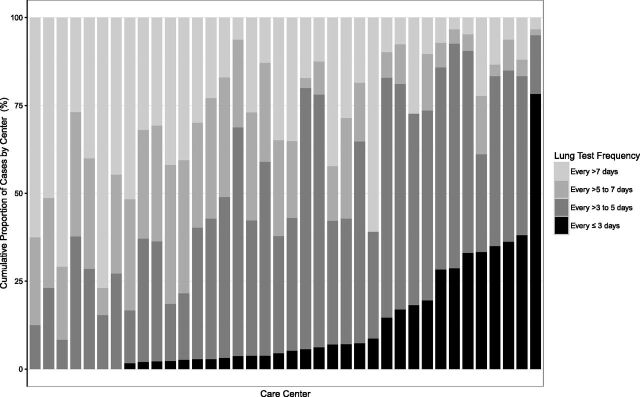
Figure 4 illustrates variability in spirometry frequency according to center among children aged ≥6 years with an LOS ≥7 days. Among centers, 7 had >30% of patients perform spirometry every 3 days or less, whereas 5 centers had >50% of patients complete spirometry <1 time per week. Among all patients, the most common spirometry frequency was every 4 to 5 days (42%), followed by more than every 7 days (25%) and every 6 to 7 days (21%).
FIGURE 4.
Frequency of lung function testing according to individual care center in children aged ≥6 years with an LOS ≥7 days. The numbers on the x-axis indicate number of patients included from each center. Center order differs from Fig 2.
Table 2 describes the different admission antibiotic regimens. IV antibiotics alone were used at admission in 49.9% of exacerbations, whereas IV, oral, and inhaled antibiotics were all used simultaneously on admission in 8.5% of exacerbations. In terms of IV antibiotics, 2 IV agents were used at admission most commonly (56.6%); 3 IV antibiotics were used in 22.8% of exacerbations. Supplemental Tables 5 through 10 list the specific antibiotics and most common antibiotic combinations used. Tobramycin (58.5%) and ceftazidime (27.3%) were the most frequently used admission IV antibiotics; trimethoprim-sulfamethoxazole was the most commonly used oral antibiotic (46.2% of oral antibiotics prescribed); and tobramycin was the most commonly used inhaled antibiotic (87.4% of inhaled antibiotics prescribed).
TABLE 2.
Different Antibiotic Regimens
| IV Antibiotic Regimen | IV Antibiotic Alone | Plus Oral Antibiotics | Plus Inhaled Antibiotics | Plus Oral and Inhaled Antibiotics | Total |
|---|---|---|---|---|---|
| 1 IV antibiotic | 507 (10.5%) | 309 (6.4%) | 100 (2.15%) | 81 (1.7%) | 997 (20.7%) |
| 2 IV antibiotics | 1400 (29.0%) | 790 (16.4%) | 335 (6.9%) | 207 (4.3%) | 2732 (56.6%) |
| ≥3 IV antibiotics | 504 (10.4%) | 274 (5.7%) | 197 (4.1%) | 123 (2.5%) | 1098 (22.7%) |
| Total | 2411 (49.9%) | 1373 (28.4%) | 632 (13.1%) | 411 (8.5%) | 4827 (100%) |
Discussion
In this study of ∼5000 patients with CF cared for at 38 US CFF-accredited care centers in the PHIS database, we found substantial variability in current inpatient CF pulmonary exacerbation treatment and monitoring practices. In particular, there was marked variability in LOS, spirometry frequency, maintenance therapies, systemic corticosteroid use, and choice of inpatient antibiotic regimens across centers. The CF centers included in this study represent 31.4% of all CFF-accredited US pediatric centers and are well distributed geographically (West, 26.3%; Midwest, 23.7%; Northeast, 18.4%; and South, 31.6%).14 We thus believe these results are generalizable to CFF-accredited care centers across the country.
The demographic characteristics of the study cohort are similar to those of patients in the 2014 CFF National Patient Registry, and a comparison can provide a framework to understanding our results. The rate of pancreatic insufficiency (90.9% vs 87.4%) was similar between data sets.15 Higher proportions of gastrostomies for enteral feeding (15.8% vs 11.4%), use of ursodiol (as a marker of CF-related liver disease, 17.0% vs 12.6%), and use of insulin (as a marker of CF-related diabetes, 13.4% vs 6.7%) in our cohort compared with the 2014 CFF registry likely reflect the fact that sicker patients are more likely admitted for IV antibiotic treatment of a pulmonary exacerbation.
Median LOS was almost identical in our data set (10.0 days; interquartile range, 6.0–14.0 days) compared with the 2014 CFF registry data for children aged <18 years (median hospital stay duration, 9.7 days), which encouragingly supports our pulmonary exacerbation case definition. Lack of information in the PHIS database regarding discharge home IV antibiotics made it impossible to determine overall duration of IV antimicrobial therapy. In the present cohort, 13% of patients had an LOS ≤3 days; these patients were more likely to have completed IV antibiotic treatment at home. This proportion is similar to 2014 CFF registry data for individuals aged <18 years, among whom 82.4% of total treatment duration for pulmonary exacerbations took place within a hospital.15 Thus, our LOS data likely reflect total IV antibiotic duration for the majority of exacerbations. Consensus on best location or duration of antibiotic treatment of pulmonary exacerbations has not been established, and future studies are needed to determine whether a similar variation in treatment patterns exists for patients treated with home IV antibiotics for exacerbations.
With respect to dornase alfa, maintenance azithromycin, and hypertonic saline, CFF guidelines recommend these medications as chronic therapies for the maintenance of lung health in patients with CF aged ≥6 years16 and do not recommend medication discontinuation during a pulmonary exacerbation.9 Our data illustrate that in children aged ≥6 years, all centers reported >75% use of dornase alfa, but hypertonic saline and maintenance azithromycin use varied between centers. Median hypertonic saline use (71%) was similar to CFF registry data from clinic encounters for chronic home therapies among children aged ≥6 years (65.7% in 2014), suggesting that in-hospital hypertonic saline administration could reflect continuation of maintenance therapy. The variability in hypertonic saline administration in our study could be due to a variety of factors, including withholding this therapy for bronchospasm or hemoptysis in the acute setting, lack of perceived benefit, medication intolerance, or provider prescription variability. Median maintenance azithromycin use in the inpatient setting was 40%, with significant between-center variation. Similar to data regarding use of hypertonic saline, these findings could be due to prescriber prescription variation but also to the presence or absence of Pseudomonas aeruginosa respiratory culture positivity, which cannot be ascertained from the PHIS data. Another possible source of variability in inpatient chronic therapy use is variability in the use of these medications in the outpatient setting. 2014 CFF registry data illustrate heterogeneity in maintenance azithromycin (range, 17.6%–100%) and hypertonic saline (range, 6.7%–97.1%) use according to center15; the variability in chronic therapy use in our study could reflect outpatient management, because chronic therapies (if not prescribed as an outpatient) are rarely started in the hospital during a pulmonary exacerbation.
CFF guidelines note that insufficient evidence exists to recommend for or against systemic corticosteroid use in pulmonary exacerbations.9 Our data revealed significant variability in systemic corticosteroid use for pulmonary exacerbations. A randomized controlled trial in children evaluated IV hydrocortisone use as pulmonary exacerbation adjunct therapy and noted sustained improvement in lung function after hospitalization.17 A more recent randomized study, however, of oral prednisone versus placebo in pulmonary exacerbations found no difference in lung function or markers of sputum inflammation between the groups.18 The variability in prescribing patterns in the present study likely reflects the dearth of published data on this subject. Further studies are needed to determine the utility of adjunctive systemic corticosteroid therapy in pulmonary exacerbations.
Frequency of lung function testing also varied between centers. There is no consensus on frequency of spirometry testing during inpatient stays, and CFF guidelines make no recommendations. Spirometry is useful for monitoring treatment improvement in exacerbations, particularly because studies report that up to 25% of patients fail to return to lung function baseline after a pulmonary exacerbation.7,19 However, the manner in which spirometric measures are incorporated into medical decision-making is not standardized. Johnson et al20 examined dissimilarities in lung health between US CF care sites and noted that sites reporting the highest forced expiratory volume in 1 second values also obtained measurements of lung function in the outpatient setting most frequently. Although spirometry testing can help guide day-to-day exacerbation management (ie, when to change antibiotic regimen, start systemic corticosteroids), very frequent testing could lead to patient and/or caregiver anxiety if no improvements are seen, and spirometric results must be interpreted in addition to other markers of overall improvement (including weight gain and respiratory and systemic symptoms).
In contrast to LOS, maintenance therapies, and spirometry frequency, admission antibiotic regimens were described without center stratification to maximize an understanding of pulmonary exacerbation admission antibiotic choices and to minimize the overwhelming number of tables and figures that would be required to describe between-center variation for each antibiotic regimen. Provider selection of admission antibiotic regimens was variable, as 4 different antibiotic regimens were started on admission >10% of the time. Antibiotics are almost universally used in pulmonary exacerbations, although, surprisingly, evidence that antibiotics are beneficial in pulmonary exacerbations is sparse and derived largely from 2 small randomized trials.21,22 Respiratory culture findings typically guide antibiotic choices on admission. Although a limitation of this data set is the absence of microbiologic data, the antibiotic regimens reported in the PHIS data provide a clue regarding respiratory microbiology. For example, the top 3 pairs of admission IV antibiotics (ceftazidime and tobramycin, piperacillin and tazobactam/tobramycin, and ticarcillin and clavulanate/tobramycin) comprise 50.3% of all IV admission antibiotic regimens when 2 IV antibiotics were used. These 3 combinations are frequently used to “double cover” P aeruginosa, an organism known to cause significant morbidity and mortality in patients with CF.23 The use of 2 antipseudomonal drugs for exacerbations is the standard approach to reduce selection of resistant organisms,24 although antibiotics in CF are generally believed to select for resistance,25 and CFF guidelines note that there is insufficient evidence to recommend single versus double coverage of P aeruginosa for exacerbation treatment.9 Although we cannot verify culture status in our cohort, among the top 10 antibiotics used when a single IV antibiotic was used on admission, only 1 specific antipseudomonal (IV ceftazidime) was prescribed (and in only 5.7% of cases), suggesting most patients with P aeruginosa in our data set were likely treated with at least 2 antipseudomonal agents.
Inhaled antibiotics were used on admission in 13.1% of exacerbations, and inhaled tobramycin was the most commonly used (87.4%). A 2008 study illustrated that as the usage of inhaled antibiotics increased over a 10-year period, the acute use in pulmonary exacerbation treatment decreased.26 This study (in which 66% of participants were aged <18 years) noted inhaled antibiotic use in 24.3% of exacerbations from 2003 to 2005, and inhaled tobramycin was the most commonly used agent. Compared with that study, the lower inhaled antibiotic use in our data set might reflect increasing chronic inhaled antibiotic use; in fact, 2014 CFF registry data noted chronic median inhaled tobramycin use of 71.4% in children and adults aged ≥6 years.15 Interestingly, IV and inhaled tobramycin were used together in 10.8% of all admissions in our data set. The simultaneous use of inhaled and IV antibiotics for pulmonary exacerbations is controversial, and CFF guidelines conclude that there is insufficient evidence to recommend for or against this practice.9 Using 2 routes of administration could theoretically lead to improved drug exposure, although no clinical improvement was seen in 1 study using antibiotic aerosols as adjunctive therapy to IV antibiotics.27 A 2014 study illustrated lung perfusion defects detectable according to MRI in children with pulmonary exacerbations,28 possibly discounting the assumption that IV antibiotics rather than inhaled antibiotics are superior for the treatment of pulmonary exacerbations. Ultimately, further studies are needed to address the utility of concomitant inhaled and IV antibiotic administration for pulmonary exacerbations.
Strengths of our study include the analysis of a large, national data set not previously used to describe pulmonary exacerbations. The PHIS data set includes many characteristics of inpatient pulmonary exacerbations not captured in the CFF registry, including antibiotics, other medications, and spirometry frequency. Almost 5000 individual patient encounters were included in the present analysis, representing a wide US geographic distribution, and we therefore believe that these results are generalizable to CF care centers across the country.
Limitations of our study, in addition to those described earlier, include absence of information on CF genotyping, respiratory microbiology, and lung function, precluding adjustment for disease severity. In addition, information on use of home IV or oral antibiotics was not available for analysis, thus making it impossible to determine overall duration of IV antimicrobial therapy, an important limitation because centers likely differ on the use of home IV antibiotics for pulmonary exacerbations. Unfortunately, there are no identifiers available with which to match patients in the PHIS database and the CFF registry. Despite attempts to exclude hospitals with low-quality data and review coding from all participating hospitals to create an exacerbation case definition, misclassification of CF or of pulmonary exacerbations could have occurred, and some of the observed between-hospital variability could be attributed to misclassification.
Conclusions
Our data reveal substantial variation in the treatment and monitoring of inpatient CF pulmonary exacerbations among 38 US CFF-accredited care centers. We hope results from this study can complement the already-robust CFF registry data in future retrospective studies targeting exacerbation management and augment the process of quality improvement at CF care sites across the country. The variability seen in the present study can provide opportunities to standardize care to improve CF-related outcomes. Standardization of care in medical practice has been associated with improved patient outcomes. A Cochrane Review comparing standardized clinical pathways versus usual care noted fewer in-hospital complications in the clinical pathway group,29 and another study illustrated a 4.9% improvement in forced expiratory volume in 1 second after the implementation of a standardized CF pulmonary exacerbation score.30 In an era of evidence-based medicine leading to standardization of care, we hope these results will spur future prospective studies focusing on standardization of pulmonary exacerbation treatment strategies to improve respiratory health and quality of life for children and adolescents with CF.
Supplementary Material
Acknowledgments
The authors thank Dr Tamara Simon and additional members of the Seattle Children’s PHIS working group for feedback regarding study methods and results.
Glossary
- CF
cystic fibrosis
- CFF
Cystic Fibrosis Foundation
- ICD-9
International Classification of Diseases, Ninth Revision
- IV
intravenous
- LOS
length of stay
- PHIS
Pediatric Health Information System
Footnotes
Dr Cogen conceptualized and designed the study, conducted some of the analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Oron conceptualized and designed the study, and conducted most of the analysis; Drs Gibson, Kronman, and Rosenfeld participated in study design, provided primary mentorship, and critically reviewed and revised the manuscript; and Drs Hoffman and Ong participated in study design and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
FUNDING: Dr Cogen received funding from the 2015 Safeway Outstanding Cystic Fibrosis Junior Clinical Research Award.
References
- 1.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148(2):259–264 [DOI] [PubMed] [Google Scholar]
- 2.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waters V, Stanojevic S, Atenafu EG, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40(1):61–66 [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld M, Emerson J, Williams-Warren J, et al. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139(3):359–365 [DOI] [PubMed] [Google Scholar]
- 5.Heltshe SL, Goss CH, Thompson V, et al. Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. Thorax. 2016;71(3):223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(1):64–72 [DOI] [PubMed] [Google Scholar]
- 7.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182(5):627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flume PA, Mogayzel PJ Jr, Robinson KA, et al. ; Clinical Practice Guidelines for Pulmonary Therapies Committee . Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–808 [DOI] [PubMed] [Google Scholar]
- 10.Wagener JS, Rasouliyan L, VanDevanter DR, et al. ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis . Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48(7):666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West NE, Goss CH, VanDevanter DR, et al. Standardized Treatment of Pulmonary Exacerbations (STOP) Study: treatment goals for pulmonary exacerbations. J Cyst Fibros. 2015;14(S1):S13 [Google Scholar]
- 12.Virgin FW, Huang L, Roberson DW, Sawicki GS. Inter-hospital variation in the frequency of sinus surgery in children with cystic fibrosis. Pediatr Pulmonol. 2014;50(3):231–235 [DOI] [PubMed] [Google Scholar]
- 13.Data Source: Pediatric Health Information Systems (PHIS), Data Year: 2010-2015. Available at: https://www.childrenshospitals.org/programs-and-services/data-analytics-and-research/pediatric-analytic-solutions/pediatric-health-information-system
- 14.Agency for Healthcare Research and Quality. Healthcare cost and utilization project. Available at: http://www.ahrq.gov/research/data/hcup/index.html
- 15.Cystic Fibrosis Foundation . Cystic Fibrosis Foundation Patient Registry, 2014 Annual Data Report to the Center Directors. Bethesda, MD: Cystic Fibrosis Foundation; 2014 [Google Scholar]
- 16.Mogayzel PJ Jr, Naureckas ET, Robinson KA, et al. ; Pulmonary Clinical Practice Guidelines Committee . Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187(7):680–689 [DOI] [PubMed] [Google Scholar]
- 17.Tepper RS, Eigen H, Stevens J, et al. Lower respiratory illness in infants and young children with cystic fibrosis: evaluation of treatment with intravenous hydrocortisone. Pediatr Pulmonol. 1997;24(1):48–51 [DOI] [PubMed] [Google Scholar]
- 18.Dovey M, Aitken ML, Emerson J, McNamara S, Waltz DA, Gibson RL. Oral corticosteroid therapy in cystic fibrosis patients hospitalized for pulmonary exacerbation: a pilot study. Chest. 2007;132(4):1212–1218 [DOI] [PubMed] [Google Scholar]
- 19.Waters V, Atenafu EG, Salazar JG, et al. Chronic Stenotrophomonas maltophilia infection and exacerbation outcomes in cystic fibrosis. J Cyst Fibros. 2012;11(1):8–13 [DOI] [PubMed] [Google Scholar]
- 20.Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest. 2003;123(1):20–27 [DOI] [PubMed] [Google Scholar]
- 21.Hyatt AC, Chipps BE, Kumor KM, Mellits ED, Lietman PS, Rosenstein BJ. A double-blind controlled trial of anti-Pseudomonas chemotherapy of acute respiratory exacerbations in patients with cystic fibrosis. J Pediatr. 1981;99(2):307–314 [DOI] [PubMed] [Google Scholar]
- 22.Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis. 1990;141(4 pt 1):914–921 [DOI] [PubMed] [Google Scholar]
- 23.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100 [DOI] [PubMed] [Google Scholar]
- 24.Döring G, Conway SP, Heijerman HG, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J. 2000;16(4):749–767 [DOI] [PubMed] [Google Scholar]
- 25.Gilligan PH. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4(1):35–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz SM, Silva SJ, Mayer-Hamblett N, et al. ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis (ESCF) . Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol. 2008;43(9):874–881 [DOI] [PubMed] [Google Scholar]
- 27.Touw DJ, Brimicombe RW, Hodson ME, Heijerman HG, Bakker W. Inhalation of antibiotics in cystic fibrosis. Eur Respir J. 1995;8(9):1594–1604 [PubMed] [Google Scholar]
- 28.Wielpütz MO, Puderbach M, Kopp-Schneider A, et al. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med. 2014;189(8):956–965 [DOI] [PubMed] [Google Scholar]
- 29.Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;3(3):CD006632. [DOI] [PubMed] [Google Scholar]
- 30.Kraynack NC, McBride JT. Improving care at cystic fibrosis centers through quality improvement. Semin Respir Crit Care Med. 2009;30(5):547–558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.