Abstract
This phase 1 study evaluated the safety and tolerability of antiangiogenic therapy using vandetanib and metronomic cyclophosphamide and methotrexate in metastatic breast cancer. Eligible patients had metastatic breast cancer with 0–4 prior chemotherapy regimens. All received cyclophosphamide 50 mg daily, methotrexate 2.5 mg days 1–2 weekly, and vandetanib daily in 3 dose-escalation cohorts: 100 mg (C1), 200 mg (C2), and 300 mg (C3). The primary endpoint was safety and tolerability; secondary endpoints included response rate and evaluation of platelet-associated proteins. Twenty three patients were treated and evaluable for toxicity. Common mild toxicities included nausea, vomiting, LFTs abnormalities, fatigue, and rash. Three episodes of dose-limiting toxicity occurred in C3. In all cohorts, 1/3 of patients required vandetanib dose reduction, and 22 % ended therapy for toxicity. Of the 20 response-evaluable patients, 10 % demonstrated partial response and 15 % stable disease ≥24 weeks. Proteomic analyses demonstrated changes in platelet content of angiogenesis regulators, including vascular endothelial growth factor and platelet factor 4, with exposure to therapy. This regimen was tolerable at a maximum vandetanib dose of 200 mg; modest clinical activity was observed in this heavily pretreated population. Changes in the platelet proteome may serve as pharmacodynamic markers of angiogenesis inhibition. Metronomic chemotherapy is an attractive partner with biologics and deserves further study in metastatic breast cancer.
Keywords: Angiogenesis inhibition, Breast cancer, Metronomic chemotherapy, Proteomics, Vandetanib
Introduction
Angiogenesis mediated through the vascular endothelial growth factor (VEGF) pathway is thought to play a relevant role in breast cancer [1, 2]. VEGF interaction with its receptor (VEGFR 1–3) leads to proliferation signaling in endothelial and possibly tumor cells as well [3]. Combining the anti-VEGF agent bevacizumab with chemotherapy has demonstrated activity in the treatment of multiple solid tumor types, including HER2-negative metastatic breast cancer (MBC) [4–6]. Small molecule tyrosine kinase inhibitors directed against targets in the angiogenic pathway, including VEGFR, platelet-derived growth factor receptor (PDGFR), and c-kit, have been under investigation in MBC and other solid tumors.
Vandetanib is an orally bioavailable 4-anilinoquinazoline which selectively inhibits the tyrosine kinase activity of VEGFR2 as well as epidermal growth factor receptor (EGFR) and rearranged during transfection (RET) receptor. Phase I monotherapy trials demonstrated satisfactory tolerability with a maximum tolerated dose (MTD) of 300 mg; common adverse effects (AEs) included rash, diarrhea, and QTc prolongation [7]. In non-small cell lung cancer (NSCLC), the addition of vandetanib to chemotherapy improves survival endpoints [8, 9]. Activity has also been observed in medullary thyroid cancer, likely through RET inhibition [10, 11], leading to FDA approval in April 2011. A phase II study of vandetanib monotherapy in pretreated MBC demonstrated tolerability, but limited single agent clinical activity (no responses, one prolonged stable disease) [12].
Metronomic chemotherapy, defined by a frequent, continuous dosing schedule, is thought to work through inhibition of tumor angiogenesis [13]. Actively dividing endothelial cells are more sensitive to chemotherapy than tumor cells; exposure to a frequent low-dose schedule can compromise the endothelial cell repair system with less systemic toxicity, leading to antiangiogenic effects and ultimate tumor death [14]. Preclinical data suggest synergistic activity from the combination of metronomically dosed chemotherapy with a biologic agent targeting VEGFR2 in both tumor xenografts and transgenic mouse models [15, 16]. In MBC, metronomic chemotherapy alone has demonstrated antitumor activity with minimal toxicity [17–19]. Combination antiangiogenic therapy by means of metronomic chemotherapy and the anti-VEGF antibody bevacizumab has been explored in both MBC and ovarian cancer with or without additional chemotherapy; in each setting, the combination provides efficacy with minimal toxicity [20–24].
Contemporary evaluation of angiogenesis inhibition requires study of potential biomarkers which identify successful drug targeting and predict treatment benefit. To date, the assessment of circulating angiogenesis regulators has not demonstrated consistent correlation with response. Another avenue for investigation is the evaluation of platelet proteomics. Platelets actively and selectively sequester circulating angiogenesis regulatory proteins [25], including VEGF, basic fibroblast growth factor (bFGF), PDGF, platelet factor 4 (PF4), connective tissue activating peptide (CTAPIII), and endostatin [26, 27]. In physiologic and tumor angiogenesis, this organization facilitates the controlled release of angiogenic regulators from tumor- or wound-aggregated platelets [25, 28, 29]. Platelet content of angiogenesis regulators is constant under normal, physiological, conditions [30], but changes significantly under pathological conditions, such as in the presence of a tumor [26, 27]. The platelet angiogenic profile is more inclusive than any single biomarker, can be examined in a quantifiable manner [30], and therefore is an attractive candidate biomarker for angiogenesis inhibitor therapy.
There is considerable interest in the exploration of multi-agent antiangiogenic blockade—a strategy which could provide increased activity over single agents alone and could potentially overcome resistance to single agent therapy [31]. Metronomic chemotherapy offers a defined antiangiogenic mechanism of action and a tolerable side effect profile, making it an attractive partner for combination antiangiogenic therapy, especially with another oral antiangiogenic agent. This phase I dose-escalation study was designed to explore the safety and tolerability of the all-oral combination regimen of metronomic cyclophosphamide and methotrexate with the VEGFR inhibitor vandetinib in MBC, and to monitor the effect of exposure to antiangiogenesis therapy on the platelet angiogenic profile.
Patients and methods
Patients
Eligible patients had MBC of any histologic subtype (estrogen and/or progesterone receptor positive, HER2 positive, and/or triple-negative) with up to 4 prior chemotherapy regimens, and no prior exposure to VEGFR or EGFR kinase inhibitors. Prior exposure to bevacizumab was permitted. At least 2 weeks washout from prior therapy was required. Other eligibility criteria included ECOG performance status ≤2, adequate hepatic [AST/ALT < 2.5 × upper limit of normal (ULN), total bilirubin ≤ 1.5 × ULN], renal (serum creatinine ≤ 1.5 × ULN), and hematologic parameters (absolute neutrophil count ≥1,500/mcL; platelets ≥100,000/mcL; hemoglobin ≥9 g/dL; prothrombin time ≤ ULN). Patients were required to have adequate cardiac function (LVEF > 45 %), no evidence of QTc prolongation (≥480 ms), and no use of concomitant QTc-prolonging medication. Measurable disease was not required. Patients were excluded for uncontrolled blood pressure, therapeutic anticoagulation, recent clinically significant cardiac event, or untreated CNS metastases.
All treatment occurred within the facilities of the Dana-Farber/Harvard Cancer Center (DF/HCC); the study protocol was approved by the Institutional Review Committee of the DF/HCC and all patients gave informed consent.
Study design and treatment plan
This was an investigator-initiated phase 1 trial designed to treat 3 sequential cohorts of 8 patients each with escalating doses of daily oral vandetanib (C1, 100 mg; C2, 200 mg; C3, 300 mg) and continuous metronomic chemotherapy (cyclophosphamide 50 mg PO qd, and methotrexate 2.5 mg PO bid, day 1 and 2 of each week). Treatment was administered in a 28-day cycle, and accrual into the next cohort commenced once all the subjects in the previous cohort safely completed 1 cycle of therapy with ≤2 experiencing dose-limiting toxicity (DLT). Patients with progressive disease before the completion of cycle 1 were to be replaced. Protocol treatment was planned to continue until time of tumor progression or undue toxicity.
Safety and tolerability
Evaluation for adverse events utilized the National Cancer Institute common toxicity criteria for adverse events (CTCAE) v3.0. Subjects were monitored for toxicity every other week for the first 8 weeks (2 cycles) of therapy. Safe completion of protocol therapy was defined as ≤2 patients experiencing DLT. DLT was defined during cycle 1 of therapy and included refractory hypertension despite adequate drug therapy, excessive QTc prolongation, any grade 3/4 non-hematologic toxicity, any grade 4 hematologic toxicity with clinical sequelae, grade 4 hematologic toxicity without clinical sequelae which failed to resolve in 1 week when treatment was withheld, or toxicity not resolving to ≤grade 1 within 3 weeks when treatment was withheld. If ≥3 DLTs occurred at any dose level, accrual was to cease and the study would terminate. EKGs to monitor QTc interval were performed every 2 weeks for the first 2 cycles and then once per cycle; QTc prolongation was defined as a single QTc value of ≥550 ms or an increase of ≥100 ms from baseline. Vandetanib dose reductions were permitted for ≥grade 3 toxicity; however, metronomic chemotherapy doses remained fixed. Specific text and tables were included in the protocol to optimize early and aggressive management of hypertension.
Tumor response evaluation
Baseline imaging was performed within 3 weeks before the initiation of study treatment. Response was assessed every 2 cycles by response evaluation criteria in solid tumors (RECIST) [32].
Correlative studies
Platelet proteomics
SELDI-ToF mass spectroscopy of platelet extracts was performed to prospectively evaluate platelet angiogenic profiles. Blood samples were collected at baseline and after 4 and 8 weeks of treatment. Platelets were prepared as previously described [27]. Briefly, platelets were isolated from 1 mL of plasma by centrifugation and lysed in 100 μL 9 M urea (U9 buffer, BioRad, Hercules CA). The platelet lysates were then fractionated by strong anionic exchange by means of a stepwise pH gradient as per the Serum Fractionation Kit instructions (BioRad). Fraction 1, containing the flow through and pH9 wash, was placed onto chips coated with copper utilizing the IMAC ProteinChip Arrays (BioRad) and then analyzed by SELDI-ToF MS on a ProteinChip System 4000 Enterprise Auto-biomarker System (PCS4000, BioRad). Resultant spectra were analyzed for differential expression of proteins by ProteinChip Data Manager Software 3.5.0 (BioRad).
ELISA quantification of VEGF, PF-4, and human actin
In order to quantify protein levels, platelet pellets were lysed in 100 μL 0.05 % Triton-X 100 and diluted with 900 μL of PBS, pH 7.2, to make a 10× dilution stock. VEGF and PF-4 DuoSet® sandwich ELISA kits (R&D systems, Minneapolis, MN) were used according to the suggested protocol using R&D reagents and plates. Samples were diluted and loaded in duplicate with each protein assay repeated. Results were assayed using a Victor 3 multi label reader (PerkinElmer, Waltham, MA) at 450 nm with a 650 nm correction. Data were exported into Excel for analysis. Total actin levels in 1 mL of PRP were quantified using PathScan® actin ELISA kits (Cell Signaling, Danvers, MA) and used to normalize VEGF and PF-4 data.
Cardiac analysis
Correlative analysis of the effect of vandetanib and metronomic chemotherapy on blood pressure, markers of vascular nitric oxide production, and in vivo vascular function was performed on a subset of patients, and the results have been previously described [33].
Statistical considerations
The primary objective of the study was to determine the safety and toxicity of combination therapy with vandetanib and metronomic chemotherapy in MBC. Planned accrual was to stop at any time if ≥3 patients in a cohort experienced DLT, since the probability of observing ≥3 patients with a DLT would be 0.11, 0.20, 0.78, or 0.86 for a true DLT rate of 15, 20, 45, or 50 %, respectively. At that time, the lower dose level would be declared the MTD.
Secondary endpoints included overall clinical response rate (ORR) and the determination of treatment effects on the platelet angiogenesis profile. Confidence intervals for percents are based on the exact Binomial method. Toxicities were described by frequency and grade. Statistical techniques for the platelet proteomic analysis were performed as previously described [34]. Comparisons of proteins peaks were performed by rank sum t tests. Student t test and ANOVA were used to assign significance to the ELIZA analysis. Both SAS (version 6.12, SAS Institute, Cary, NC) and SPSS (version 16.0, SPSS Inc., Chicago, IL) software packages were utilized.
Results
A total of 25 patients were enrolled between July 2007 and April 2008. One patient in C2 received <7 days of therapy and then went to hospice; she did not contribute toxicity information and was replaced. A patient in C3 was enrolled, but did not initiate therapy and was not included in the analysis. Characteristics of the remaining 23 patients are described in Table 1. Most patients had visceral metastases (83 %) and were heavily pretreated: 91 % had prior chemotherapy for MBC with a median of 2 prior chemotherapy regimens and/or 2 prior hormonal therapy regimens. Nine patients (39 %) had prior bevacizumab exposure; of those, 7 patients had bevacizumab included in their most recent regimen, and 5 had at least a 4 week bevacizumab washout since last dose (mean 5.1 weeks). One patient had prior exposure to the VEGFR inhibitor cediranib; although this was a protocol violation, she received treatment and is included in the analysis.
Table 1.
Baseline patient and tumor characteristics
| Characteristic | No. of patients (%) N = 23 |
|---|---|
| Median age in years (range) | 49 (29–71) |
| Median ECOG performance status (range) | 0 (0–2) |
| 0 | 15 (65 %) |
| 1–2 | 8 (35 %) |
| HR positive | 16 (70 %) |
| TNBC | 7 (30 %) |
| HER2 positive (IHC 3+ or FISH amplified) | 1 (4 %) |
| Sites of metastases | |
| Chest wall/soft tissue/lymph nodes | 4 (17 %) |
| Visceral (lung or liver) | 19 (83 %) |
| CNS | 1 (4 %) |
| Prior adjuvant chemotherapy | 18 (78 %) |
| Prior therapies for metastatic disease | |
| Prior chemotherapy for metastatic disease | 21 (91 %) |
| Median number chemotherapy regimens (range) | 2 (0–4) |
| Prior biologic therapy for metastatic disease | |
| Bevacizumab | 9 (39 %) |
| Cetuximab | 2 (9 %) |
| Cediranib | 1 (4 %) |
| Prior trastuzumab or lapatinib exposure* | 2 (9 %) |
| Prior hormonal therapy for metastatic disease | 14 (61 %) |
| Median number hormonal therapy regimens (range) | 2 (0–4) |
ECOG Eastern Cooperative Oncology Group, TNBC triple negative breast cancer (ER–/PR–/HER2–)
One patient had previously received trastuzumab for HER2-negative breast cancer
Enrollment
Of the remaining 23 patients, there were 8 patients in C1, 9 in C2, and 6 in C3. Three patients came off study before first restaging for reasons other than progressive disease, and did not contribute response information. The median cycles of therapy completed by all patients was 2 (mean 3.6, range of 0–11). The median number of weeks on study was 8 (mean 13.8, range of 2–44).
A single episode of Cycle 1 DLT was observed in C1 [diarrhea, vomiting, and elevated liver function tests (LFTs)]. No DLT was observed in C2. Three episodes of DLT were observed in C3 (two with elevated asymptomatic LFTs and one with mucositis and rash). Given the observed frequency of DLT in C3, enrollment to the trial was stopped and the study was closed. Vandetanib 200 mg qd was identified as the MTD in combination with metronomic chemotherapy.
Toxicity
Full toxicity information by cohort is presented in Table 2. The most frequently reported toxicities (any grade) for all patients included elevated LFTs, 16 (70 %); diarrhea, 15 (65 %); nausea/vomiting, 15 (65 %), and hyperglycemia, 15 (65 %). The most frequent reported grade 3 or 4 toxicities included elevated LFTs, 4 (17 %); rash, 3 (13 %); diarrhea, 2 (9 %), and fatigue, 2 (9 %). The single patient in C1 who experienced Cycle 1 DLT contributed almost all grade 3/4 toxicity in that cohort. The majority of grade 3/4 events occurred in C3, the highest dose level.
Table 2.
Adverse events and laboratory toxicities in all cohorts
| Adverse event | Vandetanib dose (mg)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 100 mg (n = 8) | 200 mg (n = 9) | 300 mg (n = 6) | All (n = 23) | |||||
| CTCAE event | All | Grade 3/4 | All | Grade 3/4 | All | Grade 3/4 | All | Grade 3/4 |
| AST/ALT elevation | 4 | 1 | 7 | 0 | 5 | 3 | 16 | 4 |
| Diarrhea | 5 | 1 | 5 | 0 | 5 | 1 | 15 | 2 |
| Nausea/vomiting | 5 | 1 | 6 | 0 | 4 | 0 | 15 | 1 |
| Hyperglycemia | 7 | 0 | 6 | 0 | 4 | 0 | 15 | 0 |
| Fatigue | 5 | 1 | 6 | 0 | 2 | 1 | 13 | 2 |
| QTc prolongation | 4 | 0 | 5 | 0 | 4 | 1 | 13 | 1 |
| Anemia | 4 | 0 | 5 | 0 | 2 | 0 | 11 | 0 |
| Hypertension | 3 | 0 | 4 | 0 | 3 | 1 | 10 | 1 |
| Rash | 2 | 1 | 3 | 1 | 4 | 1 | 9 | 3 |
| Proteinuria | 1 | 0 | 4 | 0 | 4 | 0 | 9 | 0 |
| Mucositis | 3 | 0 | 2 | 0 | 1 | 1 | 6 | 1 |
| Neutropenia | 3 | 1 | 1 | 0 | 2 | 0 | 6 | 1 |
A total of 5 (22 %) patients withdrew from the study due to severe or persistent toxicity. Reasons for withdrawal included reversible cerebrovascular event (1), pulmonary embolus (1), body rash and mucositis (1), persistent transaminase elevation (1), and asymptomatic myocarditis (1).
There were 9 vandetanib dose reductions involving 8 of the 23 patients (35 %). Reasons for dose reductions included rash (2), mucositis (2), hypertension (1), elevated LFTs (1), and vomiting (1). The percentage of patients requiring dose reduction was higher with increasing dose (25 % C1, 30 % C2, and 50 % C3).
Response
A total of 20 patients were considered evaluable for response; results are summarized in Table 3. Two patients (10, 95 %CI 1 to 32 %) were confirmed to have a partial response. The first, in C1, had triple negative disease and completed 13 weeks on study before removal for a minor cerebrovascular event. The other, in C2, had ER +/PR +/HER2− breast cancer and completed 47 weeks on therapy before documented progressive disease. A total of 13 patients exhibited stable disease as their best response: 5 patients with stable disease ≥4 months and 3 with prolonged stable disease ≥6 months (two ER+/PR+/HER2−, one triple negative). A total of 4 patients (20, 95 % CI 6 to 44 %) had progressive disease. Figure 1 presents maximum decrease in tumor dimension as a waterfall plot.
Table 3.
Best Response by RECIST (n = 20 evaluable)
| No. of patients (%) | |
|---|---|
| Complete response | (0) |
| Partial response | 2 (10 %) |
| Stable disease | 13 (65 %) |
| Stable disease ≥4 months | 5 (25 %) |
| Stable disease ≥6 months | 3 (15 %) |
| Progressive disease | 5 (25 %) |
Fig. 1.
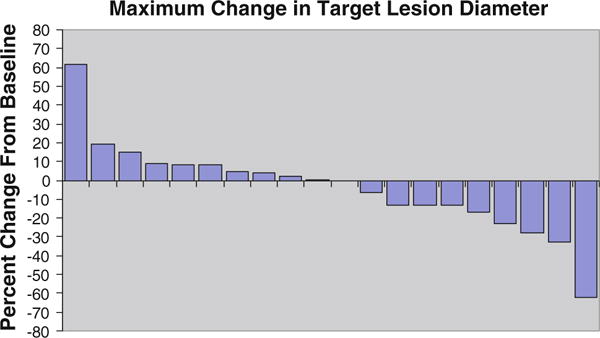
Waterfall plot describing maximum change in target lesion diameter. A total of 20 patients contributed more than one imaging evaluation. The best overall response is demonstrated
Correlative proteomic analysis
Exploratory platelet proteomic analysis was performed in a subset of patients. There was no thrombocytopenia observed during the study, and peripheral platelet counts were stable with treatment exposure. Platelet lysates from 6 of 23 patients were of suitable quality for SELDI-ToF analysis (due to variability in collection techniques) [35]. Testing was performed at baseline and after 4 and 8 weeks of drug exposure (for 5 and 3 of the patient samples, respectively), and the results were compared to reference values for age-and gender-matched healthy controls. Twenty-six protein peaks exhibited significantly differential levels; of those, 10 were associated with drug exposure, suggesting a drug-responsive pattern. The other 16 peaks varied across the cycles of therapy, were interpreted as potentially due to inter-individual variability, and were therefore excluded from the analysis. Figure 2 demonstrates two examples of proteins with differential levels associated with drug exposure. A peak, representing a polypeptide of approximately 12.5 kD, was found to be lower in patients at baseline when compared to controls (Fig. 2a). At 4 weeks, the 12.5 kD polypeptide, while still lower than controls, increased 2.5-fold over the baseline. At 8 weeks, the 12.5 kD polypeptide was further elevated (fourfold over baseline, p < 0.05) with levels comparable to controls. In contrast, a peak representing a protein of approximately 53.9 kD decreased upon treatment (Fig. 2b). At baseline, the 53.9 kD protein was approximately 16-fold greater in patients over controls (p < 0.05). At 4 weeks, the 53.9-kD protein decreased by 66 % to a level approximately 5.5-fold greater than controls. At 8 weeks, the levels in all but one outlier patient did not differ significantly between patients and controls. The sample quantity was insufficient for the identification of the proteins by sequencing. Owing to the small sample size under evaluation, correlation between changes in platelet proteomics and either response or toxicity could not be performed.
Fig. 2.
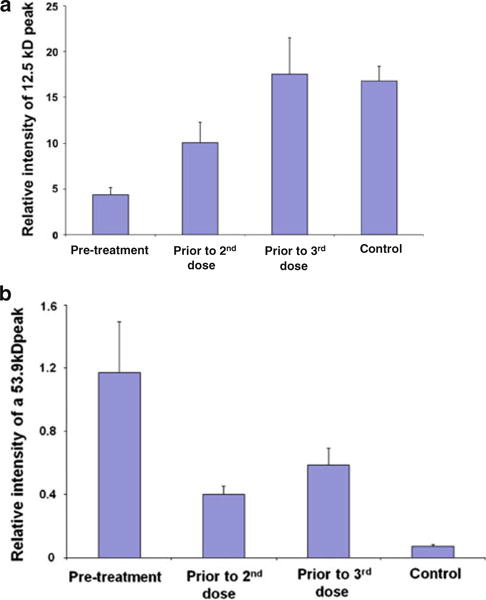
Longitudinal changes in platelet proteome with therapy. Figures illustrate examples of detectable changes in the levels of platelet-associated proteins by SELDI-ToF analysis. Two examples are presented for differentially expressed proteins in platelet lysates from 6 breast cancer patients treated with vandetanib and metronomic chemotherapy compared with matched normal controls. Time points are pre-treatment (baseline), 4-weeks drug exposure, and 8-weeks drug exposure. Both graphs represent mean ± SEM. a At baseline, levels of a 12.5-kD protein are low, but with subsequent exposure to protocol therapy, protein levels rise, reaching levels comparable to healthy controls at 8 weeks of therapy. b In contrast, the levels of a 53.9-kD polypeptide are high at baseline; by 8 weeks of therapy, levels of this protein decrease to about 50 % normal
Of the 10 proteins with differential expression between the baseline and subsequent cycles, two were matched in mass and isoelectric point to candidate biomarkers VEGF and PF4. VEGF- and PF4-specific antibodies were used to immunologically confirm their identity. Because SELDI ToF permits mass spectrometry on very small amounts of biologic sample, each patient’s platelets were analyzed separately (without pooling of samples) allowing for statistical analysis at each time point. As shown in Fig. 3, at baseline, the levels of intra-platelet VEGF were notably higher in platelets from patients than in those of controls. With each subsequent cycle of therapy, intra-platelet VEGF “normalized,” and by the third cycle approached normal levels (Fig. 3 a). Although plasma VEGF levels have been previously described to rise with exposure to antiangiogenic therapy [36, 37], here, platelet VEGF was observed to decrease. PF4, an endogenous inhibitor of angiogenesis, was also noted to be elevated at the baseline and declined with each subsequent cycle (Fig. 3b), reaching ~ 50 % of the normal by the 3rd cycle. Considering the consistent treatment-dependent decline in the protein levels with each subsequent cycle, it is unlikely that these changes reflect residual effects from previous therapies.
Fig. 3.
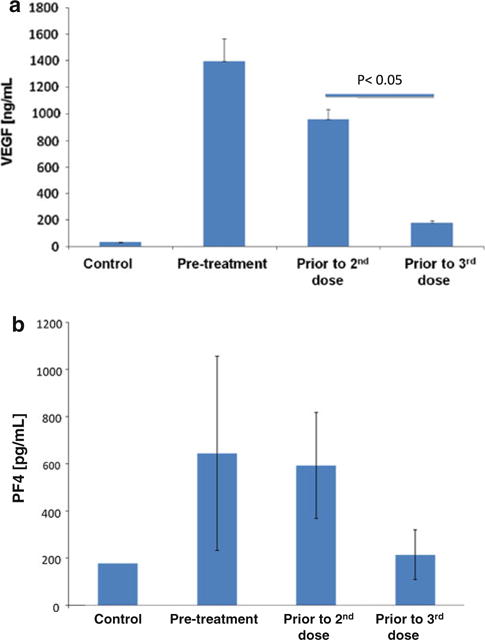
Levels of intra-platelet VEGF and PF4 “normalize” with exposure to therapy. VEGF and PF4 were identified as differentially expressed between platelets of normal subjects and patients with breast cancer, and demonstrated dynamic change with exposure to therapy. All measurements were repeated on 2 separate occasions, and the graph represents the mean of the two measurements ± SEM. At baseline, the levels of VEGF (Fig. 3a) and PF4 (Fig. 3b) were notably higher in platelets of patients with breast cancer than in those of normal controls. With each subsequent cycle, the levels of both of these proteins “normalized,” and by the third cycle VEGF closely approached levels in platelets of normal healthy subjects
Discussion
In this phase I study of vandetanib and metronomic chemotherapy in pretreated MBC, the all-oral combination was generally tolerable with commonly observed toxicities including nausea, vomiting, LFTs abnormalities, fatigue, and rash. These toxicities were generally mild and expected with a VEGFR antagonist and metronomic therapy. Important rare toxicities included a cerebrovascular event, pulmonary embolus, and myocarditis. As vascular toxicity has been observed with VEGFR inhibitors, these toxicities are possibly related to vandetanib exposure. The vandetanib MTD was 200 mg daily. Within this heavily pretreated cohort, including many with prior exposure to bevacizumab, modest activity from the combination was observed with 10 % demonstrating a response to therapy, 15 % with prolonged stable disease, and a clinical benefit rate of 25 %. Indirect comparisons suggest improved activity compared with single agent therapy [12].
Multiple VEGFR inhibitors are either approved or in the development for the treatment of advanced solid tumors [38]. Trials of VEGFR monotherapy for MBC have suggested limited activity to date, [12, 37, 39, 40] although this lack of response may reflect refractory disease in pretreated patients, small sample size, or response not easily identified using traditional clinical tools. Disease stabilization rates in these studies have been higher, reflecting the dichotomy between radiologic response and disease stabilization often seen with biologic agents [41]. Combination therapy using VEGFR inhibitors and chemotherapy may hold more promise [42–44]; however, significant toxicity has been observed with these combinations, often requiring dose reduction [42]. A recent small randomized phase 2 trial combining vandetanib with docetaxel in pretreated MBC demonstrated tolerability of the combination although the addition of vandetanib at a dose of 100 mg did not improve clinical outcomes [45].
There has been interest in combining antiangiogenic therapy to target the VEGF pathway in a sequential fashion with a goal of increased activity and reduced resistance to therapy. However, studies combining VEGFR inhibitors and bevacizumab have been fraught with significant toxicity [46, 47]. Metronomic chemotherapy provides an attractive partner in combination with an anti-VEGF biologic given the favorable toxicity profile and activity against endothelial cells. In this study, although observed activity was modest, metronomic chemotherapy appears to have not contributed much additive toxicity, and thus remains a desirable partner for future study. It is possible that further study of vandetanib with either metronomic antiangiogenic chemotherapy or standard dose chemotherapy in less heavily pretreated populations may identify a more robust efficacy signal.
Multiple correlative studies have attempted to identify accurate serologic biomarkers which reflect target specificity and predict response to antiangiogenic therapy [48]. To date, measurement of serum VEGF has been a promising biomarker of exposure to anti-VEGF therapy, as elevated VEGF levels have been observed after treatment with VEGFR inhibitors [36, 37]. Furthermore, baseline VEGF level may be predictive of benefit from treatment with vandetanib in NSCLC [49]. However, the relationship between serum/plasma VEGF level and either response to therapy or exposure to drug has not been consistent. Circulating endothelial cells (CECs) have also been examined as a predictive tool for antiangiogenic therapy. However, results have been variable with studies showing both increases and decreases in CEC levels after drug exposure [23, 50, 51]. In addition, non-standardized methodology for specimen collection and analysis has complicated comparisons. Recent data have also suggested a role for pharmacogenomic or gene expression analysis as methods to identify the best candidates for antiangiogenic therapy [52, 53]. Overall, more robust markers predictive of response to angiogenesis inhibitors are needed.
In this study, an open-ended sequential proteomic analysis of platelets was conducted with no bias to angiogenesis-related proteins. Yet, in the total proteomic analysis, two of the proteins identified as differentially expressed were found to be angiogenesis regulators, VEGF and PF4. Both reflected longitudinal changes with therapy. This finding suggests that the analysis of platelet angiogenesis regulators may emerge as a marker of exposure to antiangiogenic therapies. Future larger studies are warranted to further explore the utility of platelet proteomics as a pharmacodynamic marker of exposure to treatment, and possibly as a marker of activity in patients lacking visible clinical response.
Antiangiogenesis therapy has had an established role in the care of patients with advanced solid tumors. VEGFR inhibitors have been the focus of multiple ongoing studies; however, their future role in the majority of malignancies is unclear at this time. Significant interest exists in combination therapy with more than one biologic, including angiogenesis inhibitors, although efforts to date have been limited by toxicity. In this phase 1 trial, the combination of vandetanib and metronomic chemotherapy was well tolerated at the MTD with modest activity in a pretreated patient population. Although response rates were limited, the lack of additional toxicity with the combination supports further exploration of the regimen. Combination therapy by means of metronomic dosing and biologics, or traditional chemotherapy, would be of interest in less heavily pretreated breast cancer populations.
Acknowledgments
This research was supported by a grant from the Investigator-Sponsored Study Program of AstraZeneca, Wilmington, DE
Footnotes
A portion of the study described herein was presented as a poster at the 31st San Antonio Breast Cancer Symposium, 2008.
www.clinicaltrials.gov identification number: NCT00496665.
Conflict of interest None.
Contributor Information
Erica L. Mayer, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215, USA Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Steven J. Isakoff, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Giannoula Klement, Tufts University School of Medicine, Boston, MA, USA.
Sean R. Downing, Foundation Medicine, Cambridge, MA, USA
Wendy Y. Chen, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215, USA Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Keri Hannagan, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215, USA.
Rebecca Gelman, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215, USA.
Eric P. Winer, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215, USA Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Harold J. Burstein, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215, USA Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
References
- 1.Gasparini G. Angiogenesis in breast cancer: role in biology, tumor progression, and prognosis. In: Bowcock A, editor. Breast cancer: molecular genetics, pathogenesis and therapeutics. Humana Press; Totowa: 1999. pp. 347–371. [Google Scholar]
- 2.Sledge GW., Jr Vascular endothelial growth factor in breast cancer: biologic and therapeutic aspects. Semin Oncol. 2002;29:104–110. doi: 10.1053/sonc.2002.34062. [DOI] [PubMed] [Google Scholar]
- 3.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shaughnessy J, Miles D, Gray R, Dieras V, Perez E, Zon R, Cortes J, Zhou X, Phan S, Miller K. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC) J Clin Oncol. 2010;28:A1005. [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, Brahmer J, Schiller J, Dowlati A, Lilienbaum R, Johnson D. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, Rosenthal MA, Wheeler C, Barge A, Hurwitz HI. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol. 2005;16:1391–1397. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Sun Y, Eberhardt WE, Germonpre P, Saijo N, Zhou C, Wang J, Li L, Kabbinavar F, Ichinose Y, Qin S, Zhang L, Biesma B, Heymach JV, Langmuir P, Kennedy SJ, Tada H, Johnson BE. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-smallcell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heymach JV, Paz-Ares L, De Braud F, Sebastian M, Stewart DJ, Eberhardt WE, Ranade AA, Cohen G, Trigo JM, Sandler AB, Bonomi PD, Herbst RS, Krebs AD, Vasselli J, Johnson BE. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-smallcell lung cancer. J Clin Oncol. 2008;26:5407–5415. doi: 10.1200/JCO.2008.17.3138. [DOI] [PubMed] [Google Scholar]
- 10.Wells S, Gosnell J, Gagel R, Moley J, Pfister D, Sosa J, Skinner M, Krebs A, Hou J, Schlumberger M. Vandetanib in metastatic hereditary medullary thyroid cancer: follow-up results of an open-label phase II trial. J Clin Oncol. 2007;25:A6018. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells S, Robinson B, Gagel R, Dralle H, Fagin J, Santoro M, Baudin E, Vasselli J, Read J, Schlumberger M. Vandetanib (VAN) in locally advanced or metastatic medullary thyroid cancer (MTC): a randomized, double-blind phase III trial (ZETA) J Clin Oncol. 2010;28:A5503. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller KD, Trigo JM, Wheeler C, Barge A, Rowbottom J, Sledge G, Baselga J. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2005;11:3369–3376. doi: 10.1158/1078-0432.CCR-04-1923. [DOI] [PubMed] [Google Scholar]
- 13.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 14.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 15.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 17.Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, Peruzzotti G, Robertson C, Orlando L, Cinieri S, de BF, Viale G, Goldhirsch A. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 18.Orlando L, Cardillo A, Rocca A, Balduzzi A, Ghisini R, Peru-zzotti G, Goldhirsch A, D’Alessandro C, Cinieri S, Preda L, Colleoni M. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2006;17:961–967. doi: 10.1097/01.cad.0000224454.46824.fc. [DOI] [PubMed] [Google Scholar]
- 19.Wong NS, Buckman RA, Clemons M, Verma S, Dent S, Trudeau ME, Roche K, Ebos J, Kerbel R, Deboer GE, Sutherland DJ, Emmenegger U, Slingerland J, Gardner S, Pritchard KI. Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol. 2010;28:723–730. doi: 10.1200/JCO.2009.24.0143. [DOI] [PubMed] [Google Scholar]
- 20.Burstein HJ, Spigel D, Kindsvogel K, Parker LM, Bunnell CA, Partridge AH, Come SE, Ryan PD, Gelman R, Winer EP. Metronomic chemotherapy with and without bevacizumab for advanced breast cancer: a randomized phase II study. Breast Cancer Res Treat. 2005;94:A4. [Google Scholar]
- 21.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, Groshen S, Swenson S, Markland F, Gandara D, Scudder S, Morgan R, Chen H, Lenz HJ, Oza AM. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Saenz JA, Martin M, Calles A, Bueno C, Rodriguez L, Bobokova J, Custodio A, Casado A, Diaz-Rubio E. Bevacizumab in combination with metronomic chemotherapy in patients with anthracycline- and taxane-refractory breast cancer. J Chemother. 2008;20:632–639. doi: 10.1179/joc.2008.20.5.632. [DOI] [PubMed] [Google Scholar]
- 23.Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, Pietri E, Colleoni M. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 24.Jurado JM, Sanchez A, Pajares B, Perez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–586. doi: 10.1007/s12094-008-0254-7. [DOI] [PubMed] [Google Scholar]
- 25.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro-and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, Almog N, Kieran MW, Folkman J. Platelets actively sequester angiogenesis regulators. Blood. 2009;113:2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi A, Naumov GN, Bender E, Almog N, Italiano JE, Jr, Folkman J, Klement GL. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood. 2008;111:1201–1207. doi: 10.1182/blood-2007-04-084798. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Perini R, McKnight W, Dicay M, Klein A, Hollenberg MD, Wallace JL. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proc Natl Acad Sci USA. 2005;102:216–220. doi: 10.1073/pnas.0406682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30:95–108. doi: 10.1055/s-2004-822974. [DOI] [PubMed] [Google Scholar]
- 30.Peterson JE, Zurakowski D, Italiano JE, Jr, Michel LV, Fox L, Klement GL, Folkman J. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol. 2010;85:487–493. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
- 31.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 32.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 33.Mayer EL, Dallabrida SM, Rupnick MA, Redline WM, Hannagan K, Ismail NS, Burstein HJ, Beckman JA. Contrary effects of the receptor tyrosine kinase inhibitor vandetanib on constitutive and flow-stimulated nitric oxide elaboration in humans. Hypertension. 2011;58:85–92. doi: 10.1161/HYPERTENSIONAHA.110.168120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park Y, Downing SR, Kim D, Hahn WC, Li C, Kantoff PW, Wei LJ. Simultaneous and exact interval estimates for the contrast of two groups based on an extremely high dimensional variable: application to mass spec data. Bioinformatics. 2007;23:1451–1458. doi: 10.1093/bioinformatics/btm130. [DOI] [PubMed] [Google Scholar]
- 35.Downing S, Klement G. Isolation and proteomic analysis of platelets by SELDI-ToF MS. In: Clarke C, McCarthy D, editors. Methods in molecular biology: SELDI-ToF-MS: applications and protocols. Humana Press; Totowa: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM, Weinerman A, Emmenegger U, Ma L, Thorpe P, Davidoff A, Huber J, Hicklin DJ, Kerbel RS. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res. 2004;64:6616–6625. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 37.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 38.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6:569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 39.Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM, Jr, Wiesenfeld M, Flynn PJ, Fitch TR, Perez EA. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bianchi G, Loibl S, Zamagni C, Salvagni S, Raab G, Siena S, Laferriere N, Pena C, Lathia C, Bergamini L, Gianni L. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs. 2009;20:616–624. [PubMed] [Google Scholar]
- 41.Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004;22:4442–4445. doi: 10.1200/JCO.2004.07.960. [DOI] [PubMed] [Google Scholar]
- 42.Baselga J, Segalla JG, Roche H, Del Giglio A, Pinczowski H, Ciruelos EM, Filho SC, Gomez P, Van Eyll B, Bermejo B, Llombart A, Garicochea B, Duran MA, Hoff PM, Espie M, de Moraes AA, Ribeiro RA, Mathias C, Gil Gil M, Ojeda B, Morales J, Kwon Ro S, Li S, Costa F. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30:1484–1491. doi: 10.1200/JCO.2011.36.7771. [DOI] [PubMed] [Google Scholar]
- 43.Gradishar W, Kaklamani V, Prasad Sahoo T, Lokanatha D, Raina V, Bondarde S, Jain M, Schwartzberg L. A double-blind, randomized, placebo-controlled, phase 2b study evaluating the efficacy and safety of sorafenib (SOR) in combination with paclitaxel (PAC) as a first-line therapy in patients (pts) with locally recurrent or metastatic breast cancer (BC) Cancer Res. 2009;69:A44. doi: 10.1016/j.ejca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Rugo HS, Stopeck AT, Joy AA, Chan S, Verma S, Lluch A, Liau KF, Kim S, Bycott P, Rosbrook B, Bair AH, Soulieres D. Randomized, placebo-controlled, double-blind, phase II. Study of axitinib plus docetaxel versus docetaxel plus. Placebo in patients with metastatic breast cancer. J Clin Oncol. 2011;29:2459–2465. doi: 10.1200/JCO.2010.31.2975. [DOI] [PubMed] [Google Scholar]
- 45.Boer K, Lang I, Llombart-Cussac A, Andreasson I, Vivanco GL, Sanders N, Pover GM, Murray E. Vandetanib with docetaxel as second-line treatment for advanced breast cancer: a double-blind, placebo-controlled, randomized phase II study. Invest New Drugs. 2012;30:681–687. doi: 10.1007/s10637-010-9538-8. [DOI] [PubMed] [Google Scholar]
- 46.Mayer EL, Dhakil S, Patel T, Sundaram S, Fabian C, Kozloff M, Qamar R, Volterra F, Parmar H, Samant M, Burstein HJ. SABRE-B: an evaluation of paclitaxel and bevacizumab with or without sunitinib as first-line treatment of metastatic breast cancer. Ann Oncol. 2010;21:2370–2376. doi: 10.1093/annonc/mdq260. [DOI] [PubMed] [Google Scholar]
- 47.Rini BI, Garcia JA, Cooney MM, Elson P, Tyler A, Beatty K, Bokar J, Mekhail T, Bukowski RM, Budd GT, Triozzi P, Borden E, Ivy P, Chen HX, Dolwati A, Dreicer R. A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin Cancer Res. 2009;15:6277–6283. doi: 10.1158/1078-0432.CCR-09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown AP, Citrin DE, Camphausen KA. Clinical biomarkers of angiogenesis inhibition. Cancer Metastasis Rev. 2008;27:415–434. doi: 10.1007/s10555-008-9143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanrahan EO, Ryan AJ, Mann H, Kennedy SJ, Langmuir P, Natale RB, Herbst RS, Johnson BE, Heymach JV. Baseline vascular endothelial growth factor concentration as a potential predictive marker of benefit from vandetanib in non-small cell lung cancer. Clin Cancer Res. 2009;15:3600–3609. doi: 10.1158/1078-0432.CCR-08-2568. [DOI] [PubMed] [Google Scholar]
- 50.Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, Shalinsky DR, Demetri GD, Heymach JV. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 51.Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, Agliano A, Goldhirsch A, Shaked Y, Kerbel RS, Bertolini F. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willis S, Miller K, Young B, Perou C, Hu Z, Sparano J, Gray R, Sledge G, Davidson N, Leyland-Jones B. Association of a compact 13-gene VEGF signature with OS in E2100. J Clin Oncol. 2012;30:A1027. [Google Scholar]


