Abstract
Background
A calcineurin inhibitor (CNI)-based immunosuppression combined with mammalian target of rapamycin inhibitors (mTORs) seems to be attractive in patients after heart transplantation (HTX) in special clinical situations, for example, in patients with adverse drug effects of prior immunosuppression. Previous studies in patients after HTX detected advantageous effects regarding renal function of a tacrolimus (TAC)-based vs cyclosporine-A (CSA)-based immunosuppression (in combination with mycophenolate mofetil). However, data regarding renal function after HTX in mTOR/CNI patients remain limited.
Aim
Primary end point of the present study was to analyze renal function in HTX patients 1 year after switch to an mTOR/CNI-based immunosuppression.
Methods
Data of 80 HTX patients after change to mTOR/CNI-based immunosuppression were retrospectively analyzed. Renal function was assessed by measured serum creatinine and by estimated glomerular filtration rate (eGFR) calculated from Modification of Diet in Renal Disease equation.
Results
Twenty-nine patients received mTOR/CSA-based treatment and 51 patients received mTOR/TAC-based therapy. At time of switch and at 1-year follow-up, serum creatinine and eGFR did not differ significantly between both study groups (all P=not statistically significant). Analysis of variances with repeated measurements detected a similar change of renal function in both study groups.
Conclusion
The present study detected no significant differences between both mTOR/CNI study groups, indicating a steady state of renal function in HTX patients after switch of immunosuppressive regimen.
Keywords: heart transplantation, cyclosporine A, tacrolimus, risk factors
Introduction
Calcineurin inhibitor (CNI)-based immunosuppression in combination with mycophenolate mofetil (MMF) is the most frequently used immunosuppression in patients after heart transplantation (HTX).1–4 As a result of remodeling of renal arterioles and tubuli, interstitial fibrosis, and glomerular sclerosis, CNI-based immunosuppression is associated with irreversible renal damage.5–8 For this reason, a further deterioration of renal function parameters by maintenance CNI therapy is often observed.8 Although both CNIs suppress the immune system via a similar mechanism, differences in their side-effect profile can be observed.3,9 One important reason for the better renal function parameters in patients on tacrolimus (TAC)-based immunosuppression can possibly be explained by the 100 times lower serum concentration of TAC.10
After introduction to clinical practice in 2004, mammalian target of rapamycin inhibitors (mTORs) are presently used in about 10% of HTX patients.1,11 Due to its antiproliferative effects, mTOR-based immunosuppression appears to be favorable regarding development and progression of cardiac allograft vasculopathy (CAV).11–14 Moreover, posttransplant malignancy and CNI minimization,11,14,15 for example, to avoid further deterioration of renal function, are important reasons for mTOR-based immunosuppression.
However, application of a completely CNI-free immunosuppressive regimen may not be suitable in all clinical situations, for example, in patients with recurrent rejection episodes.16 Thus, the choice of concomitant immunosuppression is of enormous clinical interest. In patients on concomitant MMF therapy, especially gastrointestinal disorders, like diarrhea, and changes in blood count, for example, leukopenia, are often observed.17,18 Side effects of mTOR-based immunosuppression are dyslipidemia, leukopenia, and thrombopenia.11,19
The present study focused on renal function in patients on mTOR therapy in combination with a CNI, which may be indicated in special clinical situations, for example, intolerance of MMF. As previous studies demonstrated differences in renal function parameters between different CNIs,3,8,20–22 primary endpoint of this retrospective, observational study was renal function assessed by serum creatinine and estimated glomerular filtration rate (eGFR) calculated from Modification of Diet in Renal Disease (MDRD) equation 1 year after change of immunosuppressive regimen.
Patients and methods
Patients
In total, data of 80 adult HTX patients with mTOR-based immunosuppressive therapy in combination with a CNI were retrospectively analyzed. In all patients, HTX was performed at Heidelberg Heart Transplantation Center (Heidelberg, Germany). According to center’s routine protocol, primary immunosuppressive regimen after HTX consisted of a CNI, which was changed from cyclosporine A (CSA) to TAC in February 2006, as part of a dual immunosuppressive regimen.4 Steroids are routinely given for 6 months after HTX.4 To prevent adverse clinical outcomes in the early posttransplant period, like pericardial effusion and wound-healing problems,11 mTOR inhibitors were not started de novo after HTX.
Main inclusion criterion was an mTOR-based immunosuppressive regimen combined with a CNI, that is, CSA or TAC. All patients had to be on adequate and stable immunosuppression and had to be at least 2 months after HTX. Furthermore, patients had to be on mTOR/CNI therapy for at least 4 months after change of immunosuppressive regimen. Patients with a previous change of immunosuppressive therapy were consequently excluded from analysis.
This study was approved by the Ethics Committee of the University of Heidelberg. It was based upon the ethical principles of the Declaration of Helsinki (2013). Analyzed data were taken from clinical routine. Patient data confidentiality was warranted. As only clinical routine data were used for this study, no additional written informed consent was required from the patients.
Renal function
Renal function was analyzed by means of measured serum creatinine levels and by eGFR calculated from MDRD equation.23 Differences in renal function were analyzed by comparing values at time of switch to mTORs and at month 4, 8, and 1 year after introduction of mTORs. All follow-up parameters were obtained during routine follow-up.
Laboratory testing and immunosuppressive drug level monitoring
Laboratory parameters were collected during routine follow-up visits, including blood count, lipid profile, liver function parameters, and clinical routine data, for example, resting heart rate and blood pressure. Immunosuppressive medication was adapted according to center’s routine protocol.4 Trough levels of mycophenolic acid, CNIs, and mTOR are routinely monitored. Targeted immunosuppressive drug trough levels are described in Table 1. Immunosuppressive drug trough levels at the time of switch were collected at first laboratory control after medication switch.
Table 1.
Targeted immunosuppressive drug trough levels according to Heidelberg Heart Transplantation Center’s routine protocol
| Immunosuppressive Drug | Months 1–2 after HTX | Months 3–6 after HTX | Months 7–12 after HTX | Months 13–24 after HTX | >24 months after HTX |
|---|---|---|---|---|---|
| CSA (µg/L) | 125–175 | 100–125 | 90–110 | 70–90 | 50–70 |
| TAC (µg/L) | 9–11 | 7–9 | 6–8 | 4–6 | 3–5 |
| mTOR (µg/L) | 4–6, 5 | 3–5 | |||
Note: Data from Helmschrott et al.4
Abbreviations: HTX, heart transplantation; CSA, cyclosporine A; TAC, tacrolimus; mTOR, mammalian target of rapamycin inhibitor.
Acute rejection episodes
All endomyocardial biopsies were performed according to the center’s routine protocol, time after HTX, and occurrence of previous acute rejection episodes (AREs). Results of endomyocardial biopsies were graded according to the revised International Society for Heart and Lung Transplantation classification.24 An endomyocardial biopsy ≥2 R was consequently treated as ARE, making an additional steroid treatment necessary.
Statistical analysis
Data were statistically analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Numerical data were expressed as mean value ± SD or were described as absolute numbers (n) or percentage (%). Differences between both study groups were analyzed by means of chi-square test and Student’s t-test. For further analysis of renal function, analysis of variances with repeated measurements was used. A P-value of <0.05 was estimated to be statistically significant. Statistical graphics were used to illustrate findings, whenever suitable.
Results
Patient demographic and baseline characteristics
Eighty patients were included in this present retrospective, observational study. Twenty-nine patients (36.3% of total) received an mTOR immunosuppression in combination with CSA and 51 patients (63.8% of total) in combination with TAC. Both groups were balanced regarding gender distribution (P=0.78 not statistically significant[ns]) and regarding reason for HTX (P=0.49ns). However, inclusion of patients with CSA as a concomitant immunosuppressive drug was significantly later after HTX (P=0.0093) and patients were significantly younger in mTOR/TAC group (P=0.0414). HTX was performed between 06/1991 and 10/2013 in mTOR/CSA patients and between 12/1999 and 07/2013 in mTOR/TAC patients. Patients’ demographic data are described in Table 2.
Table 2.
Patients’ baseline demographic data
| Characteristics | mTOR/CSA | mTOR/TAC | P-value |
|---|---|---|---|
| Included patients (n/% of total) | 29/36.3 | 51/63.8 | na |
| Age at baseline (mean ± SD, years) | 55.4±9.7 | 50.3±11.9 | 0.0414 |
| Time after HTX (mean ± SD, days) | 1,507.3±1,904.3 | 479.4±855.0 | 0.0093 |
| Male patient (n/% of group) | 19/65.5 | 35/68.3 | 0.78ns |
| Reason for HTX (n) | 0.49ns | ||
| NCM | 15 | 27 | |
| CAD | 11 | 13 | |
| Valvular heart disease | 1 | 2 | |
| Cardiac amyloidosis | 2 | 5 | |
| Other | 0 | 4 | |
| Total death (n/%) | 2/6.9 | 1/2.0 | 0.26ns |
| Change of immunosuppressive regimen (n/%) | 7/24.1 | 16/31.4 | 0.49ns |
Abbreviations: CSA, cyclosporine A; TAC, tacrolimus; HTX, heart transplantation; NCM, non-ischemic cardiomyopathy; CAD, coronary artery disease; n, total number; na, not applicable; ns, not statistically significant; mTOR, mammalian target of rapamycin inhibitor; SD, standard deviation.
Main reasons for introduction of mTOR-based immunosuppressive therapy were nephroprotection (n=29), adverse drug effects of prior immunosuppressive regimen (n=22), rejection profile (n=14), development of CAV (n=9), malignancies (n=4), infection (n=1), and left ventricular hypertrophy (n=1). Sixteen of 22 patients with adverse drug effects of prior immunosuppression were switched because of side effects of MMF.
Renal function
At time of switch and after observation period, no statistically significant differences regarding renal function between both mTOR/CNI study groups were detectable. At time of switch, serum creatinine in mTOR/CSA patients was 1.9±1.6 mg/dL vs 1.6±1.0 mg/dL in mTOR/TAC patients (P=0.44ns). At the end of study, serum creatinine was 2.4±2.0 mg/dL in mTOR/CSA group and 1.8±0.8 mg/dL in mTOR/TAC group (P=0.25ns). Analysis of renal function by eGFR showed an eGFR of 50.1±20.5 mL/min/1.73 m2 (mTOR/CSA) vs 57.9±27.3 mL/min/1.73 m2 (mTOR/TAC) at the time of switch (P=0.16ns). After the study period eGFR was 44.1±21.1 mL/min/1.73 m2 (mTOR/CSA) vs 50.9±26.6 mL/min/1.73 m2 (mTOR/TAC) (P=0.32ns). Analysis of variances with repeated measurements detected significant difference regarding renal function parameters over time (P=0.0067) in both study groups, but no interaction of treatment and time. In summary, analysis of variances with repeated measurement detected a comparable deterioration of renal function parameters in both study groups. Detailed information is shown in Table 3 and Figures 1 and 2.
Table 3.
Renal function parameters during study period
| Characteristics, all parameters, mean ± SD | Month | mTOR/CSA | mTOR/TAC | P-value |
|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) | 0a | 50.1±20.5 | 57.9±27.3 | 0.16ns |
| 4 | 37.6±15.7 | 54.4±24.2 | 0.0017 | |
| 8 | 45.1±23.9 | 54.3±26.3 | 0.18ns | |
| 12 | 44.1±21.1 | 50.9±26.6 | 0.32ns | |
| Serum creatinine (mg/dL) | 0a | 1.9±1.6 | 1.6±1.0 | 0.44ns |
| 4 | 2.6±2.8 | 1.7±1.1 | 0.14ns | |
| 8 | 2.4±2.1 | 1.7±0.9 | 0.17ns | |
| 12 | 2.4±2.0 | 1.8±0.8 | 0.25ns |
Notes:
Time of medication switch to mTOR/CNI.
Abbreviations: CNI, calcineurin inhibitor; CSA, cyclosporine A; eGFR, estimated glomerular filtration rate; mTOR, mammalian target of rapamycin inhibitor; ns, not statistically significant; SD, standard deviation; TAC, tacrolimus.
Figure 1.
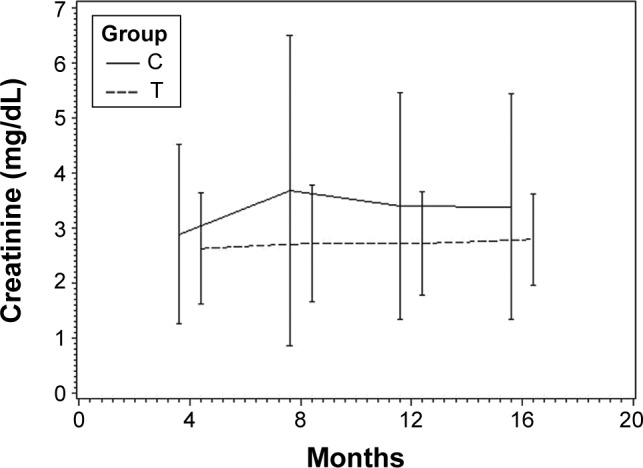
Renal function assessed by serum creatinine during study period.
Abbreviations: C, mTOR/cyclosporine A; T, mTOR/tacrolimus; mTOR, mammalian target of rapamycin inhibitor.
Figure 2.
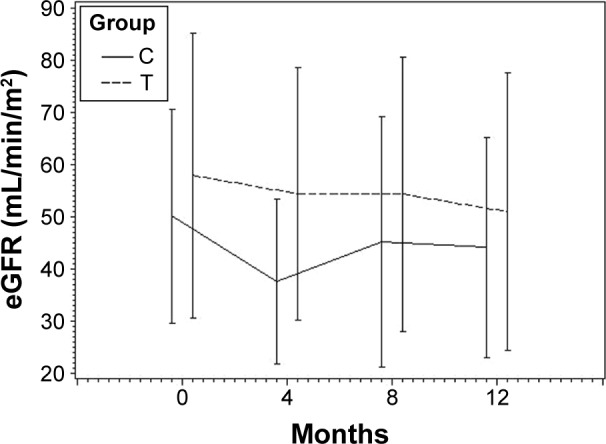
Renal function assessed by estimated glomerular filtration rate during study period.
Abbreviations: C, mTOR/cyclosporine A; T, mTOR/tacrolimus; mTOR, mammalian target of rapamycin inhibitor.
Survival
In total, three patients of included study patients (3.8% of total) died during observation period. In mTOR/CSA group two patients and in mTOR/TAC patients one patient died (P=0.26ns). Reasons for deaths were cardiopulmonary arrest (n=1, mTOR/CSA group) and infection (n=1, in each group).
ARE
An ARE was found in 10 patients in the mTOR/CSA group (34.5% of group) and in 12 patients in the mTOR/TAC group (24.0% of group) during study period (P=0.32ns). One patient in the mTOR/TAC group received no endomyocardial biopsy during observation period.
Laboratory findings and physical data
Analysis of laboratory values during study period detected no significant differences regarding serum triglycerides, blood cholesterol, and high-density lipoprotein. Significant higher low-density lipoprotein values at beginning (P=0.0179) and after study period (P=0.0386) were seen in patients with mTOR/CSA immunosuppression. Detailed laboratory findings regarding lipid profile and liver function parameters are shown in Table 4.
Table 4.
Lipid profile and liver function parameters
| Characteristics, all parameters, mean ± SD | Month | mTOR/CSA | mTOR/TAC | P-value |
|---|---|---|---|---|
| Triglycerides (mg/dL) | 0a | 142.6±69.1 | 169.8±102.0 | 0.20ns |
| 4 | 198.6±133.7 | 246.8±317.5 | 0.44ns | |
| 8 | 217.9±129.5 | 199.4±95.7 | 0.59ns | |
| 12 | 223.8±229.3 | 187.6±87.0 | 0.59ns | |
| Blood cholesterol (mg/dL) | 0a | 196.8±40.4 | 175.2±46.6 | 0.0482 |
| 4 | 209.3±55.9 | 192.5±38.8 | 0.26ns | |
| 8 | 219.4±58.5 | 193.5±38.3 | 0.09ns | |
| 12 | 216.8±40.3 | 194.6±34.9 | 0.10ns | |
| HDL (mg/dL) | 0a | 53.3±17.3 | 51.7±19.1 | 0.72ns |
| 4 | 49.1±12.1 | 49.1±15.2 | 0.99ns | |
| 8 | 53.9±17.8 | 65.7±79.1 | 0.43ns | |
| 12 | 53.0±13.3 | 53.1±24.6 | 0.99ns | |
| Low-density lipoprotein (mg/dL) | 0a | 111.5±34.7 | 90.2±31.5 | 0.0179 |
| 4 | 117.9±46.3 | 103.2±32.8 | 0.28ns | |
| 8 | 119.9±49.0 | 97.3±28.1 | 0.13ns | |
| 12 | 132.2±41.3 | 100.8±29.2 | 0.0386 | |
| ASAT (U/L) | 0a | 20.4±7.3 | 25.3±9.5 | 0.0236 |
| 4 | 27.7±10.5 | 36.9±22.4 | 0.0464 | |
| 8 | 28.1±15.0 | 33.6±19.6 | 0.2556ns | |
| 12 | 32.8±20.8 | 34.1±18.0 | 0.8366ns | |
| ALAT (U/L) | 0a | 19.8±8.7 | 29.8±23.9 | 0.0158 |
| 4 | 24.4±9.5 | 48.8±57.0 | 0.0139 | |
| 8 | 24.7±8.3 | 36.8±25.8 | 0.0140 | |
| 12 | 35.3±27.4 | 35.2±15.4 | 0.99ns |
Note:
Time of medication switch to mTOR/CNI.
Abbreviations: ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; CSA, cyclosporine A; HDL, high-density lipoprotein; mTOR, mammalian target of rapamycin inhibitor; ns, not statistically significant; TAC, tacrolimus.
Analysis of hematological parameters detected no statistically significant differences at the time of study inclusion and after study period. In particular, hemoglobin, leukocytes, and thrombocytes did not differ significantly at the time of switch and during study period (all P=ns). Moreover, analysis of clinical parameters detected no significant differences in both study groups. Resting heart frequency was 85.6±12.6/min (mTOR/CSA) vs 84.3±12.9/min (mTOR/TAC) at the time of switch (P=0.69ns), and at the end of study 87.1±12.2/min (mTOR/CSA) vs 80.6±13.6/min (mTOR/TAC) (P=0.13ns). Regarding systolic blood pressure no statistically significant differences were seen during the study period (all P=ns).
Immunosuppressive regimen and measured drug levels
Immunosuppressive therapy before switch to mTOR/CNI was TAC/MMF in 48 patients, CSA/MMF in 25 patients, CSA/azathioprine in three patients, and TAC/azathioprine in two patients. One patient was switched from a CNI-free immunosuppression and one patient was switched from a single CSA-based regimen.
Immunosuppressive therapy was monitored by immunosuppressive drug trough levels (therapeutic drug monitoring). At the time of switch, mean dose of mTOR was 5.7±2.6 mg in mTOR/CSA patients vs 4.3±2.2 in mTOR/TAC patients (P=0.28ns). During the study period no statistically significant differences in mTOR drug trough levels between both study groups were seen (all P=ns); however, significantly higher mTOR doses in patients on mTOR/TAC treatment were seen. Moreover, a reduction in CNI exposure was detectable, as described in Table 5. Discontinuation rate in both study groups did not differ significantly (P=0.49ns). For detailed information see Tables 2, 5, and 6.
Table 5.
Immunosuppressive drug trough levels
| Month | Characteristics, all parameters, mean ± SD | mTOR/CSA | mTOR/TAC | P-value |
|---|---|---|---|---|
| 0a | mTOR (µg/L) + CNI (µg/L) | 5.7±2.6 | 4.3±2.2 | 0.2762ns |
| 139.1±58.3 | 9.7±4.8 | na | ||
| 4 | mTOR (µg/L) + CNI (µg/L) | 6.2±2.0 | 6.8±3.4 | 0.3382ns |
| 104.6±62.5 | 7.5±2.4 | na | ||
| 8 | mTOR (µg/L) + CNI (µg/L) | 6.4±2.8 | 6.1±3.3 | 0.6763ns |
| 88.2±37.4 | 7.6±3.1 | na | ||
| 12 | mTOR (µg/L) + CNI (µg/L) | 7.4±3.8 | 6.6±2.9 | 0.4069ns |
| 90.23 | 6.6±5.7 | na |
Note:
Time of medication switch to mTOR/CNI.
Abbreviations: CSA, cyclosporine A; CNI, calcineurin inhibitor; mTOR, mammalian target of rapamycin inhibitor; na, not applicable; ns, not statistically significant; TAC, tacrolimus.
Table 6.
Immunosuppressive drug doses
| Month | Characteristics all doses (mg) | mTOR/CSA (mean ± SD) | mTOR/TAC (mean ± SD) | P-value |
|---|---|---|---|---|
| 0a | mTOR + CNI | 1.6±0.6 | 2.3±0.9 | 0.0006 |
| 205.7±97.3 | 7.2±5.0 | na | ||
| 4 | mTOR + CNI | 1.7±0.7 | 3.1±1.5 | <0.0001 |
| 147.3±59.7 | 6.1±4.6 | na | ||
| 8 | mTOR + CNI | 1.6±1.1 | 2.9±1.1 | <0.0001 |
| 143.8±54.5 | 5.1±3.5 | na | ||
| 12 | mTOR + CNI | 1.8±1.4 | 2.9±1.2 | 0.0019 |
| 132.8±52.3 | 5.1±3.6 | na |
Note:
Time of medication switch to mTOR/CNI.
Abbreviations: CSA, cyclosporine A; CNI, calcineurin inhibitor; mTOR, mammalian target of rapamycin inhibitor; na, not applicable; TAC, tacrolimus.
Discussion
Previously, advantageous renal function parameters in TAC patients compared to CSA patients were reported;3,8,20–22 therefore, primary endpoint of the present study was to evaluate renal function in patients with mTORs in combination with either CNI.8,20–22,25 Our data detected no significant differences regarding renal function at the time of switch and after study period in both mTOR/CNI groups assessed by measured serum creatinine levels and by eGFR calculated from MDRD equation. Further analysis of variances with repeated measurements detected a similar deterioration of renal function in both study groups during study period, which was independent of time after HTX. Recently published data suggest that expected effect on renal function is dependent on renal function at the time of mTOR introduction.26 Arora et al, detected posttransplant effects of mTOR introduction on renal function in patients with pretransplant renal failure.26 In general, study patients in our current study can be classified to the stage of moderate renal insufficiency. In line with present publications we see similar changes of renal function parameters in both study groups.26,27 However, inclusion of patients with mTOR/CSA was significantly later after HTX.
As chronic renal failure is associated with mortality and morbidity in patients after HTX, CNI reduction in terms of nephroprotection is of particular interest.1 As the use of mTORs offers the possibility of CNI reduction, positive effects on renal function may be assumed in the current study, as immunosuppressive drug trough levels were reduced during study period.11
Due to its antiproliferative effects,12,28–30 mTOR-based immunosuppression is an attractive option in patients with CAV,16 posttransplant malignancies,11,14,15,19 or in patients with impaired renal function.11,19 In accordance with the present publications, main reasons for mTOR introduction in our study population were avoidance of further deterioration of renal function, rejection profile, development of posttransplant CAV, and malignancies.11 However, combination of mTORs with CNIs may also be indicated in patients intolerant to MMF, allowing CNI reduction and minimization of consecutive possible dose-dependent adverse drug effects of CNIs, like nephrotoxicity and tremor.11,15,31 In the present study, a large percentage of patients were switched to CNI/mTOR because of adverse drug effects of previous immunosuppressive regimens. Especially application of MMF is often not possible because of gastrointestinal disorders and changes in blood count, especially leukopenia.17,18
Present studies reported advantageous effects of CNI in preventing AREs.32 Thus, in patients with recurrent rejection episodes, complete CNI avoidance is not possible and concomitant immunosuppressive medication has to be chosen carefully. Regarding rejection profile, noninferiority of CNI/mTOR regimen vs MMF/mTOR was described before.33,34 As favorable effects of immunosuppression based on TAC versus CSA were seen, combination of TAC and mTORs is of special interest.3,4,8,19–22,25 Analysis of occurrence of AREs demonstrated comparable rejection profiles in both mTOR study groups. Recently published consensus report showed that mTOR trough levels >3 ng/mL provide protection against AREs.35 In our study cohort, trough levels >3 ng/mL were seen at all study visits. Consequently, we can underline data reporting effectiveness of mTORs in combination with CNIs in preventing AREs.36
Analysis of concomitant laboratory values detected no statistically significant differences regarding blood count and physical data between both study groups. Significantly higher cholestasis parameters, that is, gamma glutamyl transferase and serum bilirubin, were seen in patients with mTOR and TAC at the time of switch. After study period no statistically significant differences regarding cholestasis parameters were detectable. As higher cholestasis parameters in patients with TAC-based immunosuppression were reported before, we interpret this as effects of CNI reduction.37,38 In line with the present publications, a trend to higher liver function parameters in patients receiving TAC was seen; however, statistically significance was not reached.39 However, higher lipid levels in patients with CSA-based immunosuppression were reported before.4,40–42 As introduction of mTORs can possibly cause further deterioration of lipid status, close monitoring of mTOR/CNI patients appears warranted.43
In both study arms, rate of drug discontinuation was high, but similar in comparison to other studies.44,45 In a prospective randomized trial by Eisen et al, main reasons for discontinuation of mTOR therapy were hematological, gastrointestinal, and neurological disorders because of antiproliferative effects of mTORs.45 In line with the present publications, we see significant higher mTOR doses in patients on TAC/mTOR treatment.46,47
Limitations
Findings have to be interpreted carefully because of the retrospective and observational character of current data. Moreover, data were collected in a comparatively small study cohort. Time after HTX was significantly different in both study groups, consequently an era effect cannot be completely denied. However, a possible better renal function of patients with mTOR/TAC could be assumed, as immunosuppressive drug trough levels tend to be higher in early period after HTX. For this reason, analysis of variances with repeated measurements was performed and detected similar deterioration of renal function in both groups. Additionally, renal function at study inclusion did not differ significantly and consequent immunosuppressive drug monitoring was performed.
In summary, our data should be considered as hypothesis-generating. To evaluate the impact of immunosuppressive effects on renal function of mTOR-based therapy, randomized, controlled clinical trials should be planned.
Conclusion
The present study demonstrated no statistically significant differences in renal function parameters in both mTOR/CNI study groups. Moreover, both mTOR/CNI groups were safe regarding occurrence of AREs, changes in concomitant laboratory values, and physical data.
In summary, we could show that the introduction of an mTOR/CNI immunosuppressive regimen was associated with a stabilization of renal function parameters in HTX patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report–2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):996–1008. doi: 10.1016/j.healun.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirtieth official adult heart transplant report–2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Helmschrott M, Rivinius R, Ruhparwar A, et al. Advantageous effects of immunosuppression with tacrolimus in comparison with cyclosporine A regarding renal function in patients after heart transplantation. Drug Design Devel Ther. 2015;9:1217–1224. doi: 10.2147/DDDT.S79343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmschrott M, Beckendorf J, Akyol C, et al. Superior rejection profile during the first 24 months after heart transplantation under tacrolimus as baseline immunosuppressive regimen. Drug Design Devel Ther. 2014;8:1307–1314. doi: 10.2147/DDDT.S68542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35(2):333–346. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 6.Kahan BD. Cyclosporine. N Engl J Med. 1989;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- 7.Kopp JB, Klotman PE. Cellular and molecular mechanisms of cyclosporin nephrotoxicity. J Am Soc Nephrol. 1990;1(2):162–179. doi: 10.1681/ASN.V12162. [DOI] [PubMed] [Google Scholar]
- 8.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 9.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 2013;191(12):5785–5791. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 10.Kino T, Hatanaka H, Miyata S, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) 1987;40(9):1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 11.Hirt SW, Bara C, Barten MJ, et al. Everolimus in heart transplantation: an update. J Transplant. 2013;2013:683964. doi: 10.1155/2013/683964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuler W, Sedrani R, Cottens S, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64(1):36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Boilson BA, Raichlin E, Park SJ, Kushwaha SS. Device therapy and cardiac transplantation for end-stage heart failure. Curr Probl Cardiol. 2010;35(1):8–64. doi: 10.1016/j.cpcardiol.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Rivinius R, Helmschrott M, Ruhparwar A, et al. Analysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapy. Drug Des Devel Ther. 2015;9:93–102. doi: 10.2147/DDDT.S75464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuckermann A, Wang SS, Epailly E, et al. Everolimus immunosuppression in de novo heart transplant recipients: what does the evidence tell us now? Transplant Rev (Orlando) 2013;27(3):76–84. doi: 10.1016/j.trre.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Stypmann J, Engelen MA, Eckernkemper S, et al. Calcineurin inhibitor-free immunosuppression using everolimus (Certican) after heart transplantation: 2 years’ follow-up from the University Hospital Munster. Transplant Proc. 2011;43(5):1847–1852. doi: 10.1016/j.transproceed.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 17.Kobashigawa J, Miller L, Renlund D, et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation. 1998;66(4):507–515. doi: 10.1097/00007890-199808270-00016. [DOI] [PubMed] [Google Scholar]
- 18.Vanhove T, Kuypers D, Claes KJ, et al. Reasons for dose reduction of mycophenolate mofetil during the first year after renal transplantation and its impact on graft outcome. Transpl Int. 2013;26(8):813–821. doi: 10.1111/tri.12133. [DOI] [PubMed] [Google Scholar]
- 19.Manito N, Delgado JF, Crespo-Leiro MG, et al. Clinical recommendations for the use of everolimus in heart transplantation. Transplant Rev (Orlando) 2010;24(3):129–142. doi: 10.1016/j.trre.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 21.Israni A, Brozena S, Pankewycz O, Grossman R, Bloom R. Conversion to tacrolimus for the treatment of cyclosporine-associated nephrotoxicity in heart transplant recipients. Am J Kidney Dis. 2002;39(3):E16. doi: 10.1053/ajkd.2002.31427. [DOI] [PubMed] [Google Scholar]
- 22.Kobashigawa JA, Miller LW, Russell SD, et al. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006;6(6):1377–1386. doi: 10.1111/j.1600-6143.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Canales M, Youssef P, Spong R, et al. Predictors of chronic kidney disease in long-term survivors of lung and heart-lung transplantation. Am J Transplant. 2006;6(9):2157–2163. doi: 10.1111/j.1600-6143.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 26.Arora S, Gude E, Sigurdardottir V, et al. Improvement in renal function after everolimus introduction and calcineurin inhibitor reduction in maintenance thoracic transplant recipients: the significance of baseline glomerular filtration rate. J Heart Lung Transplant. 2012;31(3):259–265. doi: 10.1016/j.healun.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Gullestad L, Iversen M, Mortensen SA, et al. Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation. 2010;89(7):864–872. doi: 10.1097/TP.0b013e3181cbac2d. [DOI] [PubMed] [Google Scholar]
- 28.Bohler T, Waiser J, Budde K, et al. The in vivo effect of rapamycin derivative SDZ RAD on lymphocyte proliferation. Transplant Proc. 1998;30(5):2195–2197. doi: 10.1016/s0041-1345(98)00588-0. [DOI] [PubMed] [Google Scholar]
- 29.Schuurman HJ, Pally C, Weckbecker G, Schuler W, Bruns C. SDZ RAD inhibits cold ischemia-induced vascular remodeling. Transplant Proc. 1999;31(1–2):1024–1025. doi: 10.1016/s0041-1345(98)01885-5. [DOI] [PubMed] [Google Scholar]
- 30.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Medicine. 2002;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 31.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitorsparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22(1):1–15. doi: 10.1111/j.1399-0012.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Vilchez F, de Prada JA, Exposito V, et al. Avoidance of calcineurin inhibitors with use of proliferation signal inhibitors in de novo heart transplantation with renal failure. J Heart Lung Transplant. 2008;27(10):1135–1141. doi: 10.1016/j.healun.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Lehmkuhl HB, Arizon J, Vigano M, et al. 2411 Study Investigators Everolimus with reduced cyclosporine versus MMF with standard cyclosporine in de novo heart transplant recipients. Transplantation. 2009;88(1):115–122. doi: 10.1097/TP.0b013e3181aacd22. [DOI] [PubMed] [Google Scholar]
- 34.Rothenburger M, Zuckermann A, Bara C, et al. Recommendations for the use of everolimus (Certican) in heart transplantation: results from the second German-Austrian Certican Consensus Conference. J Heart Lung Transplant. 2007;26(4):305–311. doi: 10.1016/j.healun.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Shipkova M, Hesselink DA, Holt DW, et al. Therapeutic drug monitoring of everolimus: a consensus report. Ther Drug Monit. 2016;38(2):143–169. doi: 10.1097/FTD.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 36.Schaffer SA, Ross HJ. Everolimus: efficacy and safety in cardiac transplantation. Expert Opin Drug Saf. 2010;9(5):843–854. doi: 10.1517/14740338.2010.511611. [DOI] [PubMed] [Google Scholar]
- 37.Ganschow R, Albani J, Grabhorn E, Richter A, Burdelski M. Tacrolimus-induced cholestatic syndrome following pediatric liver transplantation and steroid-resistant graft rejection. Pediatric Transplant. 2006;10(2):220–224. doi: 10.1111/j.1399-3046.2005.00413.x. [DOI] [PubMed] [Google Scholar]
- 38.Oto T, Okazaki M, Takata K, et al. Calcineurin inhibitor-related cholestasis complicating lung transplantation. Ann Thorac Surg. 2010;89(5):1664–1665. doi: 10.1016/j.athoracsur.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 39.Sacher VY, Bejarano PA, Pham SM. Tacrolimus induced hepatotoxicity in a patient with bilateral lung transplant. Transpl Int. 2012;25(10):e111–e112. doi: 10.1111/j.1432-2277.2012.01546.x. [DOI] [PubMed] [Google Scholar]
- 40.Ye F, Ying-Bin X, Yu-Guo W, Hetzer R. Tacrolimus versus cyclosporine microemulsion for heart transplant recipients: a meta-analysis. J Heart Lung Transplant. 2009;28(1):58–66. doi: 10.1016/j.healun.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Grimm M, Rinaldi M, Yonan NA, et al. Superior prevention of acute rejection by tacrolimus vs. cyclosporine in heart transplant recipients–a large European trial. Am J Transplant. 2006;6(6):1387–1397. doi: 10.1111/j.1600-6143.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 42.Penninga L, Moller CH, Gustafsson F, Steinbruchel DA, Gluud C. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol. 2010;66(12):1177–1187. doi: 10.1007/s00228-010-0902-6. [DOI] [PubMed] [Google Scholar]
- 43.Moro J, Almenar L, Martinez-Dolz L, et al. mTOR inhibitors: do they help preserve renal function? Transplant Proc. 2007;39(7):2135–2137. doi: 10.1016/j.transproceed.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 44.Potena L, Prestinenzi P, Bianchi IG, et al. Cyclosporine lowering with everolimus versus mycophenolate mofetil in heart transplant recipients: long-term follow-up of the SHIRAKISS randomized, prospective study. J Heart Lung Transplant. 2012;31(6):565–570. doi: 10.1016/j.healun.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349(9):847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 46.Kovarik JM, Curtis JJ, Hricik DE, Pescovitz MD, Scantlebury V, Vasquez A. Differential pharmacokinetic interaction of tacrolimus and cyclosporine on everolimus. Transplant Proc. 2006;38(10):3456–3458. doi: 10.1016/j.transproceed.2006.10.092. [DOI] [PubMed] [Google Scholar]
- 47.Brandhorst G, Tenderich G, Zittermann A, et al. Everolimus exposure in cardiac transplant recipients is influenced by concomitant calcineurin inhibitor. Ther Drug Monit. 2008;30(1):113–116. doi: 10.1097/FTD.0b013e318161a335. [DOI] [PubMed] [Google Scholar]


