Abstract
Purpose
Two individual phase 3 conjunctival allergen challenge (CAC) studies of similar design have assessed the efficacy and safety of olopatadine hydrochloride (HCl) 0.77% for the treatment of allergic conjunctivitis. The purpose of this study is to evaluate the integrated efficacy and safety of olopatadine HCl 0.77% from a larger dataset by pooling data from the two individual CAC studies.
Methods
Data were pooled from two phase 3, randomized, multicenter, double-masked, active- and vehicle-controlled CAC studies. The primary comparison was on ocular itching scores between olopatadine HCl 0.77% versus vehicle (at onset and 24 hours) and olopatadine HCl 0.77% versus olopatadine 0.2% (at 24 hours). Additional end points included conjunctival redness, total redness, and proportion of itching responders at onset and 24-hour duration of CAC. For both primary and secondary analysis, mixed model repeated measures analysis was used, except for proportion of ocular itching responders. Sensitivity analyses were carried out using a two-sample t-test.
Results
This analysis included 448 patients. Olopatadine HCl 0.77% was superior to vehicle (P<0.0001) at onset and 24-hour duration of action (difference in means: −1.14 to −1.52) and to olopatadine 0.2% (P=0.0009) at 24-hour duration of action in relieving ocular itch. Additionally, olopatadine HCl 0.77% substantially reduced conjunctival redness and total redness over vehicle and olopatadine 0.2% at onset and 24-hour duration of action. At 24 hours CAC, there were a higher proportion of itching responders with olopatadine HCl 0.77% compared to vehicle or olopatadine 0.2% (difference in proportion of responders: 43.17%, P<0.0001, and 17.25%, P=0.0012, respectively). No safety concerns were identified.
Conclusion
This analysis confirms the findings from the individual studies. The rapid onset and prolonged duration of action (for 24 hours) of olopatadine HCl 0.77% supports once-daily dosing in the treatment of allergic conjunctivitis.
Keywords: olopatadine, allergic conjunctivitis, conjunctival allergen challenge, ocular itching, conjunctival redness
Introduction
Allergic conjunctivitis, caused by immunoglobulin E-mediated inflammatory reaction to an allergen, is the most common form of ocular allergy.1,2 Several epidemiological reports estimate its prevalence ranging from 15% to 20% worldwide, depending on the geographic location and patients’ age.3 According to the National Health and Nutrition Examination Survey III data, annually, ~40% of the population in the US has at least one occurrence of ocular symptoms indicative of allergic conjunctivitis,4 with the most common being ocular itching, redness, eyelid swelling, chemosis, and tearing.1,2 Several treatment options with different mechanisms of action are available for the treatment of allergic conjunctivitis. Olopatadine, an antiallergic agent, exhibits its effects through selective antagonism of histamine H1 receptors, mast cell stabilization, and prevention of histamine-induced inflammatory cytokine production.5–9
Olopatadine HCl ophthalmic solution at concentrations of 0.1% and 0.2% (Patanol® and Pataday®, respectively; Alcon Research Ltd, Fort Worth, TX, USA) has been approved for the management of allergic conjunctivitis in over 100 countries, including the US and Canada, as twice-daily and once-daily treatments, respectively. Once-daily doses of olopatadine 0.2% and alcaftadine 0.25% reduce signs and symptoms of allergic conjunctivitis for no more than 16 hours.1,10,11
Despite the efficacy of available formulations of olopatadine and other topical ocular antiallergy treatments, there is a need for products that address the incomplete symptom relief and/or provide longer duration of symptom relief such that it lasts for at least 24 hours between two consecutive doses with once-daily administration.
To ensure symptom relief over a longer duration, we assessed olopatadine administered once daily at a higher strength. This new formulation contains olopatadine HCl at a concentration of 0.77% (7.76 mg/mL), which is equivalent to 0.7% olopatadine as a free base.12 Olopatadine HCl 0.77% was developed with the rationale of expanding the benefits offered by olopatadine 0.2%, particularly superior and long-lasting relief over a period of 24 hours with once-daily dosing, while maintaining its safety and patient comfort.
Two phase 3 studies were conducted using the CAC model to assess the safety and efficacy of olopatadine HCl 0.77% in patients with allergic conjunctivitis.12–15 The outcomes of these two studies supported the recent approval (January 30, 2015) of olopatadine HCl ophthalmic solution 0.77% (olopatadine HCl 0.77%) by the FDA for the treatment of ocular itching associated with allergic conjunctivitis. The results from these two CAC studies demonstrated superiority of olopatadine HCl 0.77% over vehicle at onset and 24 hours post-dosing and over olopatadine 0.2% at 24 hours post-dosing for the treatment of ocular itching in patients with allergic conjunctivitis.16–18 In the current article, we report the pooled analysis of these two randomized phase 3 studies to evaluate the integrated efficacy and safety findings from a larger dataset.
Methods
Study design
Data were pooled from two similarly designed, phase 3, randomized, multicenter, double-masked, active- and vehicle-controlled, parallel-group studies. The studies used the CAC model (Ora-CAC®; Ora Inc., Andover, MA, USA), involving instillation of allergen directly into the eye under controlled conditions, for observing acute allergic responses.17,18 The trials were primarily designed to assess the efficacy of olopatadine HCl 0.77% compared with vehicle and olopatadine 0.2% at onset of action and 24-hour duration of action based on ocular itching scores (evaluated at 3, 5, and 7 minutes post-CAC). Details of the study design, inclusion and exclusion criteria, and assessments of the two individual studies have been published.17,18
In brief, the designs of both studies were identical, except that one study had an additional efficacy evaluation visit at 16 hours17 and the other included olopatadine 0.1% as an additional active comparator.18
In this pooled analysis, only the treatment arms and efficacy evaluation visits common to the studies were included, that is, assessments of olopatadine HCl 0.77%, olopatadine 0.2%, and vehicle arms at onset of action and 24-hour duration of action. The studies were conducted in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice, including the archiving of essential documents. The study protocol and consent form were written in accordance with the standards of the International Conference of Harmonization guidelines for Structure and Content of Clinical Study Reports. The studies are registered with ClinialTrials.gov as NCT01479374 and NCT01743027.
Ethics approval and consent to participate
Before enrolling any patient, an Independent Ethics Committee or Institutional Review Board (Alpha IRB [San Clemente, CA]) reviewed and approved the protocol and informed consent form and provided a copy to the site and Alcon. All patients were informed about the study and provided the opportunity to ask questions. Patients, or their legal representatives, read, signed, and dated the consent form before taking part in any study activity.
Patients
The study eligibility criteria were similar in both studies.17,18 Key inclusion criteria were that patients must be aged ≥18 years with a history of seasonal or perennial allergic conjunctivitis for at least 1 year prior to Visit 1 and have had a diagnostic skin test indicative of allergy for seasonal or perennial allergens within 24 months of Visit 1. Patients had to be willing to discontinue wearing contact lenses for ≥72 hours prior to the first study visit and throughout the study.
Enrolled patients had a positive bilateral CAC response to allergen at Visits 1 and 2. At Visit 1, a positive CAC response was defined as a score, in each eye, of ≥2 for itching and ≥2 for redness in two of the three vessel beds within 10 minutes of the last titration challenge. At Visit 2, a positive bilateral CAC response was defined as a score, in each eye, of ≥2 for itching and ≥2 for redness in two of the three vessel beds in at least two of the three post-CAC time points. Patients who had participated in any previous clinical trials with olopatadine HCl solution 0.77% were excluded from this study.
Study treatment
In one CAC study,17 patients were randomized (1:1:1) to receive one drop per eye of olopatadine HCl 0.77%, olopatadine HCl 0.2%, or vehicle at Visits 3A (Day 0), 4A (Day 14±2), and 5 (Day 21±3) prior to CAC and at Visits 3B (24 hours after Visit 3A), 4B (16 hours after Visit 4A), and 5 (27 minutes after treatment instillation).
In the other CAC study,18 patients were randomized (2:2:2:1) to receive one drop per eye of olopatadine HCl 0.77%, olopatadine HCl 0.2%, olopatadine HCl 0.1%, or vehicle at Visits 3A (Day 0) and 4 (Day 14±2) prior to CAC at 27 minutes (±1 minute) posttreatment.
Study objectives
The primary hypothesis for this pooled analysis was that olopatadine HCl 0.77% was superior to vehicle at onset of action and 24-hour duration of CAC and to olopatadine 0.2% at 24-hour duration of CAC in relieving ocular itching associated with allergic conjunctivitis. Additional end points included conjunctival redness, total redness, and ocular itching responders at onset of action and 24-hour duration of CAC for olopatadine HCl 0.77% compared with vehicle and olopatadine 0.2%.
Secondary analyses included the comparison of ciliary redness, episcleral redness, chemosis, eyelid swelling, and tearing assessed at onset of action and 24-hour post-dosing for olopatadine HCl 0.77% compared with vehicle and olopatadine 0.2%.
Efficacy assessments
Efficacy assessments included patient-evaluated symptoms and investigator-evaluated signs of allergic conjunctivitis. The symptoms included ocular itching and tearing assessed on a 0–4 scale with 0.5-unit increments (0= none, 4= very severe), and eyelid swelling assessed on a 0–3 scale with 1-unit increments (0= none, 3= severe). Investigator-evaluated signs included conjunctival redness, ciliary redness, episcleral redness, and chemosis all assessed on a 0–4 scale with 0.5-unit increments (0= none, 4= very severe).
Total redness score, ranging from 0 to 12, was the sum of the conjunctival, ciliary, and episcleral redness scores. Ocular itching and itching responders were assessed at 3, 5, and 7 minutes post-CAC. Conjunctival redness and total redness were assessed at 7, 15, and 20 minutes post-CAC. An ocular itching responder was defined as a patient with zero itch (a score of 0 on ocular itching for both the eyes) or with a ≥2-unit reduction in ocular itching relative to a baseline CAC score.
Safety assessments
The safety of olopatadine HCl 0.77% was assessed by monitoring BCVA, slit-lamp examination, IOP, dilated fundus examination, and all TEAEs. All TEAEs were coded to system organ class and preferred terms using the Medical Dictionary for Regulatory Activities version 15.0.
Statistical analysis
MMRM analysis of variance was used as the primary analysis method for the pooled efficacy analysis. MMRM analysis included the score from each eye as the dependent variable and fixed-effects terms of study, treatment, eye type, time, and treatment-by-treatment interaction. Estimate of the treatment difference at each post-CAC time point, the average treatment difference (over 3, 5, and 7 minutes post-CAC time points) between olopatadine HCl 0.77% versus vehicle and olopatadine 0.2%, and the associated 95% confidence intervals and P-values were obtained from the MMRM model. For each primary and secondary comparison, the criterion for statistical success was significant at 5% level in majority of the post-CAC time points; that is, for each comparison, significance was required in at least two out of three time points. The same MMRM model was used for all other secondary end points, except proportion of ocular itching responders, where the analysis was based on proportions and used the chi-squared test. Sensitivity analyses were conducted using a two-sample t-test for comparing each post-CAC time point.
The efficacy analysis set was the pooled primary efficacy analysis set from each individual study. Both studies used the ITT set as the primary efficacy analysis set. The ITT set and safety analysis set included all randomized patients who received study treatment.
Results
Patient demographics and baseline characteristics
A total of 448 patients were included in this pooled analysis. Of these, 164 received olopatadine HCl 0.77%, 167 received olopatadine 0.2%, and 117 received vehicle (Figure 1). The mean (±standard deviation) age of patients was 40.9 (±13.2) years, 60% were females, and 77.5% were Caucasians. Overall, the patient baseline and demographic characteristics were similar across all treatment groups (Table 1).
Figure 1.
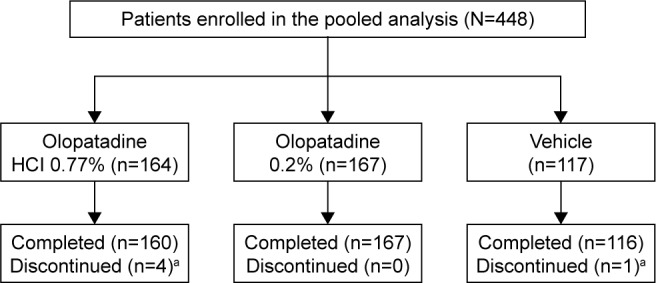
Patient disposition.
Notes: aDue to adverse events not related to the drug, n (%): olopatadine HCl 0.77%, 4 (2.4); vehicle, 1 (0.9).
Table 1.
Demographic and baseline characteristics (ITT population)
| Parameters | Olopatadine HCl 0.77% (n=164) | Olopatadine 0.2% (n=167) | Vehicle (n=117) |
|---|---|---|---|
| Age (years) | |||
| Mean (±SD) | 39.7 (12.9) | 41.3 (13.9) | 41.7 (12.8) |
| 18–64, n (%) | 161 (98.2) | 159 (95.2) | 114 (97.4) |
| ≥65, n (%) | 3 (1.8) | 8 (4.8) | 3 (2.6) |
| Gender, n (%) | |||
| Male | 60 (36.6) | 70 (41.9) | 46 (39.3) |
| Female | 104 (63.4) | 97 (58.1) | 71 (60.7) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 26 (15.9) | 20 (12.0) | 12 (10.3) |
| Not Hispanic or Latino | 137 (83.5) | 147 (88.0) | 105 (89.7) |
| Race, n (%) | |||
| Caucasian | 131 (79.9) | 124 (74.3) | 92 (78.6) |
| African American | 27 (16.5) | 37 (22.2) | 17 (14.5) |
| Asian | 2 (1.2) | 2 (1.2) | 3 (2.6) |
| American Indian or Alaska Native | 2 (1.2) | 1 (0.6) | 3 (2.6) |
| Multiracial | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Other | 1 (0.6) | 3 (1.8) | 2 (1.7) |
| Allergen, n (%) | |||
| Cat dander | 17 (10.4) | 11 (6.6) | 13 (11.1) |
| Grass | 66 (40.2) | 74 (44.3) | 45 (38.5) |
| Ragweed | 32 (19.5) | 16 (9.6) | 27 (23.1) |
| Trees | 21 (12.8) | 24 (14.4) | 10 (8.5) |
| Dust mites | 24 (14.6) | 36 (21.6) | 19 (16.2) |
| Cockroach | 2 (1.2) | 2 (1.2) | 2 (1.7) |
| Dog dander | 2 (1.2) | 4 (2.4) | 1 (0.9) |
Note: Olopatadine HCl 0.77% refers to olopatadine HCl 0.77% (equivalent to 0.7% olopatadine free base) treatment group.
Abbreviations: ITT, intent-to-treat; SD, standard deviation.
Efficacy outcomes
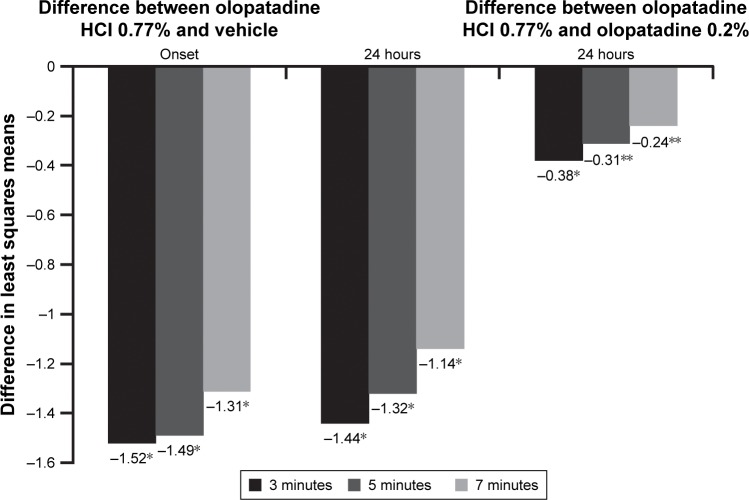
Analysis of the pooled data demonstrated superiority of olopatadine HCl 0.77% over vehicle (P<0.0001) in relieving ocular itch at all three post-CAC time points (3, 5, and 7 minutes) at onset of action and 24-hour duration of action (P<0.0001 for all; Figure 2). The differences in means were clinically significant (P<0.0001), that is, >1 unit at all post-CAC time points at onset of action and 24-hour duration of action. Additionally, at 24 hours, olopatadine HCl 0.77% was superior to olopatadine 0.2% (P=0.0009; Figure 2) in relieving ocular itch at all three post-CAC time points. Overall, olopatadine HCl 0.77% demonstrated a lasting ability to relieve ocular itching for a minimum of 24 hours.
Figure 2.
Ocular itching: treatment differences in least squares means at onset and 24 hours post-CAC.
Notes: *P<0.0001 overall and at all time points versus vehicle; **P<0.05 versus olopatadine 0.2%. The differences in least squares means for ocular itching between olopatadine 0.77% and vehicle were significant (P<0.0001) at all three post-CAC time points at onset and 24 hours. The differences in least squares means for ocular itching between olopatadine 0.77% and olopatadine 0.2% were significant (P<0.05) at all three post-CAC time points at 24 hours.
Abbreviation: CAC, conjunctival allergen challenge.
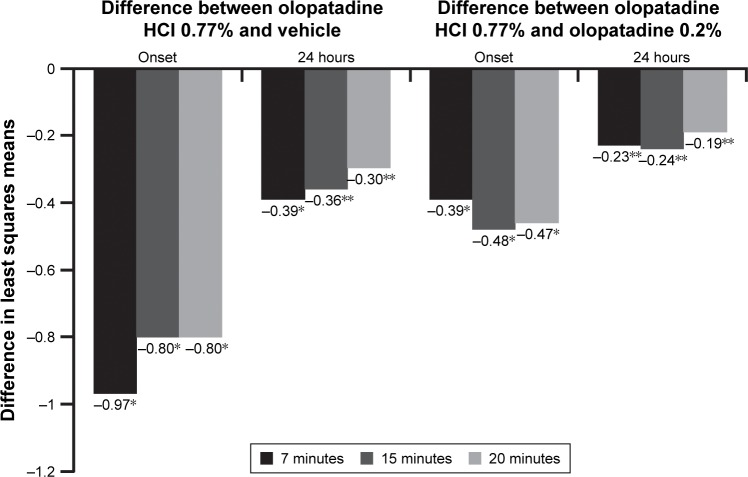
For conjunctival redness, olopatadine HCl 0.77% was superior to vehicle and olopatadine 0.2% at onset of action and 24 hours post-dosing. The means for conjunctival redness were significantly lower for olopatadine HCl 0.77% versus vehicle at all three post-CAC time points (7, 15, and 20 minutes) at onset (P<0.0001) and 24 hours (P=0.0001; Figure 3). Similarly, olopatadine HCl 0.77% was superior to olopatadine 0.2% at onset of action (P<0.0001) and 24 hours post-CAC (P<0.05) in relieving conjunctival redness at all three post-CAC time points (Figure 3).
Figure 3.
Conjunctival redness: treatment differences in least squares means at onset and 24 hours post-CAC.
Notes: *P<0.0001 overall and at all other time points versus vehicle; **P<0.05 overall and at all time points versus olopatadine 0.2%. The differences in least squares means for conjunctival redness between olopatadine 0.77% and both vehicle and olopatadine 0.2% were significant (P<0.05) at all three post-CAC time points at 7, 15, and 20 minutes.
Abbreviation: CAC, conjunctival allergen challenge.
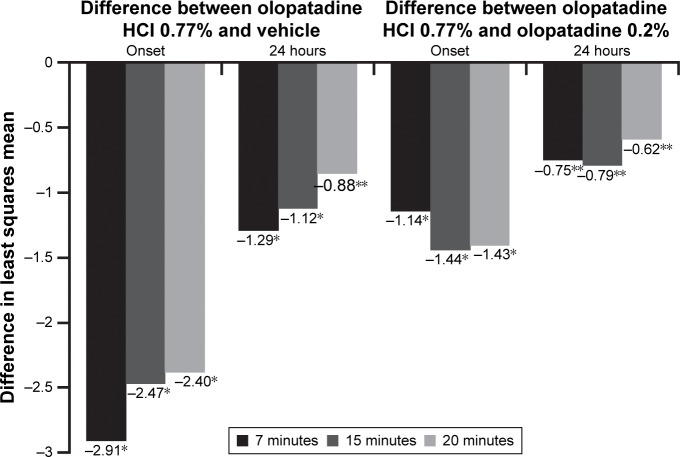
Olopatadine HCl 0.77% was also superior to vehicle and olopatadine 0.2% in relieving total redness at onset of action and 24 hours post-dosing. The means for total redness were significantly lower for olopatadine HCl 0.77% versus vehicle at all three post-CAC time points at onset and 24 hours (both P<0.0001; Figure 4). Similarly, olopatadine HCl 0.77% was superior to olopatadine 0.2% in relieving total redness at onset of action (P<0.0001) and 24 hours post-dosing (P=0.0036; Figure 4).
Figure 4.
Total redness: treatment differences in least squares means at onset and 24 hours post-CAC.
Notes: *P<0.0001 overall and at all other time points versus vehicle; **P<0.05 overall and at all time points versus olopatadine 0.2%. The differences in least squares means for total redness between olopatadine 0.77% and both vehicle and olopatadine 0.2% were significant (P<0.05) at all three post-CAC time points at 7, 15, and 20 minutes.
Abbreviation: CAC, conjunctival allergen challenge.
The observed proportions of ocular itching responders for olopatadine HCl 0.77%, olopatadine 0.2%, and vehicle were 73.2%, 69.1%, and 11.1%, respectively, at onset of action and 45.7%, 28.5%, and 2.6%, respectively, at 24 hours post-dosing. At onset of action, the proportion of ocular itching responders with olopatadine HCl 0.77% was 62.06% higher than vehicle (P<0.0001) and 4.08% higher than olopatadine 0.2% (P=0.4142; Figure 5). At 24 hours, the proportion of ocular itching responders with olopatadine HCl 0.77% was 43.17% higher than vehicle (P<0.0001) and 17.25% higher compared with olopatadine 0.2% (P=0.0012; Figure 5).
Figure 5.
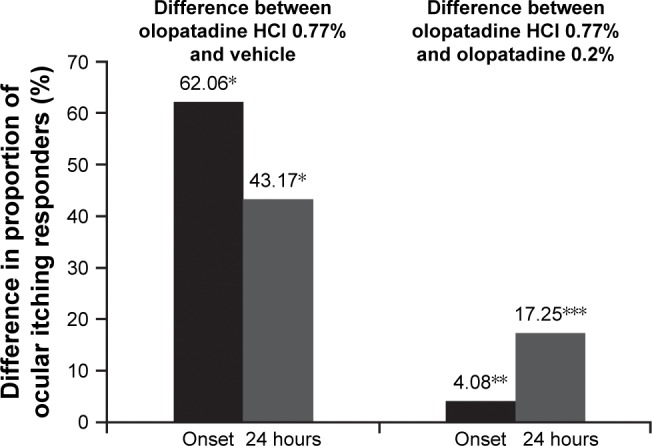
Itching responders: treatment differences in proportion of responders at 24 hour duration of action CAC or CAC 24 hours post treatment.
Notes: *P<0.0001 overall and at all other time points versus vehicle; **P=0.4142 at onset of action; ***P=0.0012 at 24 hours of CAC versus olopatadine 0.2%. The differences in least squares means for proportion of ocular itching responders between olopatadine 0.77% and vehicle at onset and 24 hours (P<0.0001) and between olopatadine 0.77% and olopatadine 0.2% at 24 hours (P=0.0012) were significant. A responder was defined as a patient with zero itch (a score of 0 on ocular itching for both the eyes) or with at least a 2-unit reduction in ocular itching relative to a baseline score. Two subjects (one in olopatadine 0.77% group, one in vehicle group) had missing data and were considered as nonresponders in this analysis. Ocular itching score was averaged across both the eyes and over the three post-CAC assessments (3, 5, and 7 minutes) for the calculation of unit reduction.
Abbreviation: CAC, conjunctival allergen challenge.
In this pooled analysis, the differences in means for the additional signs and symptoms associated with allergic conjunctivitis favored olopatadine HCl 0.77% over vehicle and olopatadine 0.2% at onset of action and 24-hour duration of action (Table 2). The differences in means for ciliary redness, episcleral redness, chemosis, eye lid swelling, and tearing between olopatadine 0.77% and vehicle were significant (P<0.05) at all three post-CAC time points (7, 15, and 20 minutes; Table 2).
Table 2.
Ocular signs and symptoms: treatment difference in means at onset and 24 hours post-CAC
| Secondary endpoints | Difference in means between olopatadine HCl 0.77% versus vehicle and olopatadine 0.2% at onset of action
|
Difference in means between olopatadine HCl 0.77% versus vehicle and olopatadine 0.2% at 24-hour duration of action
|
||||
|---|---|---|---|---|---|---|
| 7 minutes | 15 minutes | 20 minutes | 7 minutes | 15 minutes | 20 minutes | |
| Ciliary rednessa | ||||||
| Vehicle | −1.01* | −0.91* | −0.87* | −0.51* | −0.41* | −0.30** |
| Olopatadine 0.2% | −0.36* | −0.50* | −0.49* | −0.24** | −0.28** | −0.21** |
| Episcleral rednessa | ||||||
| Vehicle | −0.94* | −0.78* | −0.75* | −0.39* | −0.35** | −0.27** |
| Olopatadine 0.2% | −0.38* | −0.46* | −0.47* | −0.26** | −0.25** | −0.20** |
| Chemosisb | ||||||
| Vehicle | −0.42* | −0.49* | −0.49* | −0.36* | −0.44* | −0.45* |
| Olopatadine 0.2% | −0.06 | −0.18** | −0.18** | −0.07 | −0.14 | −0.12 |
| Eyelid swellingc | ||||||
| Vehicle | −0.50* | −0.40* | −0.30* | −0.50* | −0.40** | −0.30** |
| Olopatadine 0.2% | −0.10 | −0.10 | −0.10 | −0.20** | −0.10 | −0.10 |
| Tearingd | ||||||
| Vehicle | −0.50* | −0.40* | −0.30† | −0.50* | −0.30** | −0.20 |
| Olopatadine 0.2% | 0 | −0.10 | −0.10 | −0.10 | −0.10 | −0.10 |
Notes:
Ciliary and episcleral redness each were assessed on a 0–4 scale by 0.5-unit increments: 0= none and 4= extremely severe;
chemosis was assessed on a 0–4 scale by 0.5-unit increments: 0= none and 4= severe;
eyelid swelling was assessed on a 0–3 scale with 1-unit increments: 0= none and 3= severe; and
tearing was assessed on a 0–4 scale by 1-unit increments: 0= none and 4= very severe.
P<0.0001 overall and at all other time points versus vehicle;
P<0.05 overall and at all time points versus olopatadine 0.2%.
Abbreviation: CAC, conjunctival allergen challenge.
Safety
No clinically relevant differences in safety were noted across the treatment groups in this pooled analysis (Table 3).
Table 3.
AEs (≥1% in any treatment group; safety set)
| AE category (MedDRA PT presented by SOC), n (%) | Olopatadine HCl 0.77% (n=164) | Olopatadine 0.2% (n=167) | Vehicle (n=117) |
|---|---|---|---|
| Eye disorders | |||
| Eye irritation | 2 (1.2) | 0 | 1 (0.9) |
| Vision blurred | 0 | 1 (0.6) | 2 (1.7) |
| VA reduced | 2 (1.2) | 1 (0.6) | 0 |
| Infections and infestations | |||
| Gastroenteritis viral | 3 (1.8) | 0 | 0 |
| Nervous system disorders | |||
| Dysgeusia | 2 (1.2) | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | |||
| Cough | 0 | 0 | 2 (1.7) |
Note: Olopatadine HCl 0.77% refers to olopatadine HCl 0.77% (equivalent to 0.7% olopatadine free base) treatment group.
Abbreviations: AEs, adverse events; MedDRA, Medical Dictionary for Regulatory Activities; PT, preferred term; SOC, system organ class; VA, visual acuity.
A review of AEs did not show any safety concerns with olopatadine HCl 0.77% compared with vehicle and olopatadine 0.2%. Five patients discontinued the study due to TEAEs. Four of these patients had received olopatadine HCl 0.77% (three discontinuations due to viral gastroenteritis and one due to influenza), and one patient received vehicle (discontinuation due to ear infection). None of these TEAEs were serious or related to the study treatment. No deaths were reported during the study period. Additionally, no clinically meaningful differences were reported for safety parameters evaluated using BCVA, slit-lamp examination, IOP, or dilated fundus examination.
Discussion
In this pooled analysis, olopatadine HCl 0.77% demonstrated superiority over vehicle (P<0.0001) both at onset of action and after 24 hours and over olopatadine 0.2% at 24 hours in relieving ocular itch. The differences in means for ocular itching between olopatadine HCl 0.77% and vehicle were >1 unit at all post-CAC time points. A 1-unit difference compared with vehicle is considered clinically relevant by the FDA.
Prior to the US approval of olopatadine HCl 0.77%, the available treatment options for the management of allergic conjunctivitis included dual-acting antihistamines, mast cell stabilizers, and nonpharmacological agents, such as cold compresses or eye lubricants.2,11 Olopatadine at a lower concentration of 0.1% (Patanol®), given twice daily, and 0.2% (Pataday®), given once daily, is effective in relieving symptoms associated with allergic conjunctivitis with a good safety profile.12–15 The once-daily olopatadine 0.2%, the active comparator in this study, was found to be better than many other available antiallergic treatments in terms of its efficacy and tolerability.1,5,19,20 However, none of these medications provided relief over 24 hours from ocular itching; the coverage lasted only for 16 hours post-dosing.17,18 Therefore, there is a medical need for a treatment that is effective for at least 24 hours without necessitating a second dose, particularly in patients experiencing moderate-to-severe symptoms or those with incomplete relief from symptoms. Availability of a once-daily dosing regimen would help maintain efficacy through a full 24-hour period, improve treatment compliance, and reduce exposure to preservatives, which may lower the risk of developing dry eye symptoms in patients with allergic conjunctivitis.19,20
The aqueous solubility of olopatadine at neutral pH is a limiting factor in formulating olopatadine-containing ophthalmic solutions. This new formulation allows olopatadine to remain dissolved in a stable solution at a free base concentration of 0.7%. This improves the concentration of drug in the ocular tissue upon instillation (Cmax and area under the curve of olopatadine in the conjunctiva), thereby increasing the magnitude of effect and duration of action in the treatment of allergic conjunctivitis.11 Olopatadine HCl 0.77% has shown long-lasting efficacy for a minimum of 24 hours post-dose in relieving ocular itch associated with allergic conjunctivitis. When dosed in the morning, this 24-hour duration of efficacy offers a lasting, clinically relevant benefit to patients throughout the day and night. This ensures complete coverage between two consecutive doses of medication administered 24 hours apart.
Results from the supportive pooled efficacy analyses have demonstrated superiority of olopatadine HCl 0.77% over vehicle and olopatadine 0.2% for the treatment of ocular redness (both conjunctival and total redness) at onset and after 24 hours. These findings are consistent with the outcomes observed in the individual CAC studies.17,18 The benefit of a higher concentration of olopatadine was demonstrated by an increase in the proportion of itching responders at 24 hours (~17% greater) compared with olopatadine 0.2%, which was evident because of the 24-hour-lasting efficacy of olopatadine HCl 0.77%, unlike olopatadine 0.2% (16 hours), and the concentration differences. This suggests the beneficial effect of olopatadine HCl 0.77% in providing longer duration of action than olopatadine 0.2%, without losing its potent onset of action.
In addition, olopatadine HCl 0.77% had lower mean scores compared with vehicle and olopatadine 0.2% for all other measured signs and symptoms post-CAC at onset of action and 24-hour duration of action. This data support the overall benefit of olopatadine HCl 0.77% over olopatadine 0.2% in relieving signs and symptoms associated with allergic conjunctivitis, lasting for 24 hours. The similarities in study population and study design justify this pooled analysis, and the consistency of the results across multiple measures supports the overall benefit of olopatadine HCl 0.77% over olopatadine 0.2% in relieving signs and symptoms associated with allergic conjunctivitis lasting for 24 hours.
No serious AEs were reported with olopatadine HCl 0.77% regardless of the treatment duration (onset of action and 24 hours). Overall, the safety profile of olopatadine HCl 0.77% was comparable to the vehicle and the well-established safety profile of olopatadine 0.2%.
Conclusion
This pooled analysis reinforces the findings from the two individual CAC studies demonstrating superiority of olopatadine HCl 0.77% over vehicle and olopatadine 0.2% for the treatment of allergic conjunctivitis. Olopatadine HCl 0.77% provided superior and longer duration of relief from itching that persisted over a period of 24 hours. No safety concerns were identified with once-daily olopatadine HCl 0.77%. The safety profile of olopatadine HCl 0.77% was comparable to that of olopatadine 0.2%. The rapid onset and prolonged duration of action (at least 24 hours) of olopatadine HCl 0.77% further support its once-daily dosing in the treatment of allergic conjunctivitis.
Acknowledgments
This study was sponsored by Novartis Pharmaceutical Corporation (Fort Worth, TX, USA). Novartis Pharmaceutical Corporation participated in the design of the studies, analysis of the data, and approval of the manuscript. In addition, Ora Inc. provided support with the design and conduct of the studies. Alcon Research sponsored this study. Medical writing and editorial assistance was given by Rhutika Desai and Usha Gutti (Scientific Services Practice-Product Lifecycle Service, Novartis Healthcare Pvt. Ltd., Hyderabad, India) toward the development of this manuscript.
Abbreviations
- AEs
adverse events
- BCVA
best-corrected visual acuity
- CAC
conjunctival allergen challenge
- FDA
US Food and Drug Administration
- HCl
hydrochloride
- IOP
intraocular pressure
- ITT
intent-to-treat
- MMRM
mixed model repeated measures
- TEAEs
treatment-emergent adverse events
Footnotes
Disclosure
Eugene McLaurin has received research grants from the following companies: Aciex, Acucela, Alcon Research Ltd., Allergan, AstraZeneca, Bausch & Lomb, Inotek Pharma, InSite Vision, Lexicon Pharma, Mimetogen, and Ocular Therapeutix. Mark Bergmann is a study investigator; he has received consultancy fees from Ora, Inc. Abhijit Narvekar was a former employee, and Adeniyi Adewale is an employee of Alcon Research Ltd. Paul Gomes is the vice president of Allergy and an employee at Ora, Inc., an organization that consults and conducts research and clinical trials in the field of ocular allergy and other areas of ophthalmology and allergic diseases. Gail Torkildsen is a study investigator; she has received consultancy fees from Ora, Inc., reimbursement of meeting traveling expenses from Alcon Research Ltd., and research grants from Allergan. The authors report no other conflicts of interest in this work.
References
- 1.Abelson MB, Gomes PJ. Olopatadine 0.2% ophthalmic solution: the first ophthalmic antiallergy agent with once-daily dosing. Expert Opin Drug Metab Toxicol. 2008;4(4):453–461. doi: 10.1517/17425255.4.4.453. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez MC, Parra Fernández B, Matheu V, et al. Allergic conjunctivitis. J Investig Allergol Clin Immunol. 2011;21(Suppl 2):1–19. [Google Scholar]
- 3.Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2011;11:471–476. doi: 10.1097/ACI.0b013e32834a9676. [DOI] [PubMed] [Google Scholar]
- 4.Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988–1994. J Allergy Clin Immunol. 2010;126(4):778.e6–783.e6. doi: 10.1016/j.jaci.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Uchio E. Treatment of allergic conjunctivitis with olopatadine hydrochloride eye drops. Clin Ophthalmol. 2008;2(3):525–531. doi: 10.2147/opth.s3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGill JI. A review of the use of olopatadine in allergic conjunctivitis. Int Ophthalmol. 2004;25(3):171–179. doi: 10.1007/s10792-004-1818-x. [DOI] [PubMed] [Google Scholar]
- 7.Sharif NA, Xu SX, Miller ST, Gamache DA, Yanni JM. Characterization of the ocular anti-allergic and antihistaminic activity of olopatadine (AL-4943A), a novel drug for treating ocular allergic diseases. J Pharmacol Exp Ther. 1996;278(3):1252–1261. [PubMed] [Google Scholar]
- 8.Yanni JM, Stephens DJ, Miller ST, et al. The in vitro and in vivo ocular pharmacology of olopatadine (AL-4943A), an effective anti-allergic/antihistaminic agent. J Ocul Pharmacol Ther. 1996;12(4):389–400. doi: 10.1089/jop.1996.12.389. [DOI] [PubMed] [Google Scholar]
- 9.Yanni JM, Weimer LK, Sharif NA, Xu SX, Gamache DA, Spellman JM. Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch Ophthalmol. 1999;117(5):643–647. doi: 10.1001/archopht.117.5.643. [DOI] [PubMed] [Google Scholar]
- 10.Greiner JV, Edwards-Swanson K, Ingerman A. Evaluation of alcaftadine 0.25% ophthalmic solution in acute allergic conjunctivitis at 15 minutes and 16 hours after instillation versus placebo and olopatadine 0.1% Clin Ophthalmol. 2011;5:87–93. doi: 10.2147/OPTH.S15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackerman S, D’Ambrosio F, Jr, Greiner JV, Villanueva L, Ciolino JB, Hollander DA. A multicenter evaluation of the efficacy and duration of action of alcaftadine 0.25% and olopatadine 0.2% in the conjunctival allergen challenge model. J Asthma Allergy. 2013;6:43–52. doi: 10.2147/JAA.S38671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer GR, Cason MM, Womble SW, Li G, Chastain JE. Ocular pharmacokinetics comparison between 0.2% olopatadine and 0.77% olopatadine hydrochloride ophthalmic solutions administered to male New Zealand white rabbits. J Ocul Pharmacol Ther. 2015;31(4):204–210. doi: 10.1089/jop.2014.0140. [DOI] [PubMed] [Google Scholar]
- 13.Abelson MB, Gomes PJ, Vogelson CT, et al. Clinical efficacy of olopatadine hydrochloride ophthalmic solution 0.2% compared with placebo in patients with allergic conjunctivitis or rhinoconjunctivitis: a randomized, double-masked environmental study. Clin Ther. 2004;26(8):1237–1248. doi: 10.1016/s0149-2918(04)80065-1. [DOI] [PubMed] [Google Scholar]
- 14.Berdy GJ, Spangler DL, Bensch G, Berdy SS, Brusatti RC. A comparison of the relative efficacy and clinical performance of olopatadine hydrochloride 0.1% ophthalmic solution and ketotifen fumarate 0.025% ophthalmic solution in the conjunctival antigen challenge model. Clin Ther. 2000;22(7):826–833. doi: 10.1016/S0149-2918(00)80055-7. [DOI] [PubMed] [Google Scholar]
- 15.Spangler DL, Bensch G, Berdy GJ. Evaluation of the efficacy of olopatadine hydrochloride 0.1% ophthalmic solution and azelastine hydrochloride 0.05% ophthalmic solution in the conjunctival allergen challenge model. Clin Ther. 2001;23(8):1272–1280. doi: 10.1016/s0149-2918(01)80106-5. [DOI] [PubMed] [Google Scholar]
- 16.Vogelson CT, Abelson MB, Pasquine T, et al. Preclinical and clinical antiallergic effect of olopatadine 0.2% solution 24 hours after topical ocular administration. Allergy Asthma Proc. 2004;25(1):69–75. [PubMed] [Google Scholar]
- 17.Torkildsen G, Narvekar A, Bergmann M. Efficacy and safety of olopatadine hydrochloride 0.77% in patients with allergic conjunctivitis using a conjunctival allergen-challenge model. Clin Ophthalmol. 2015;9:1703–1713. doi: 10.2147/OPTH.S83263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaurin E, Narvekar A, Gomes P, Adewale A, Torkildsen G. Phase 3 randomized double-masked study of efficacy and safety of once-daily 0.77% olopatadine hydrochloride ophthalmic solution in subjects with allergic conjunctivitis using the conjunctival allergen challenge model. Cornea. 2015;34(10):1245–1251. doi: 10.1097/ICO.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 19.Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge: a clinical approach to studying allergic conjunctivitis. Arch Ophthalmol. 1990;108(1):84–88. doi: 10.1001/archopht.1990.01070030090035. [DOI] [PubMed] [Google Scholar]
- 20.Mah FS, Rosenwasser LJ, Townsend WD, Greiner JV, Bensch G. Efficacy and comfort of olopatadine 0.2% versus epinastine 0.05% ophthalmic solution for treating itching and redness induced by conjunctival allergen challenge. Curr Med Res Opin. 2007;23(6):1445–1452. doi: 10.1185/030079907X188206. [DOI] [PubMed] [Google Scholar]