SUMMARY
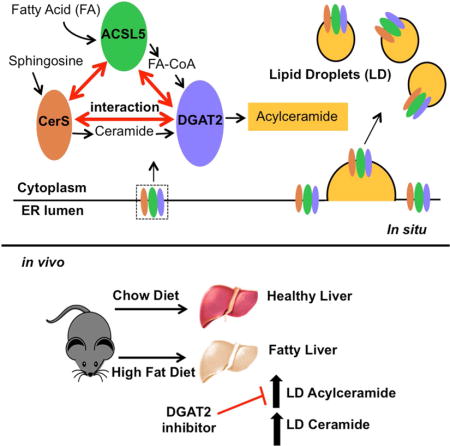
In an approach aimed at defining interacting partners of ceramide synthases (CerS), we found that fatty acyl CoA synthase ACSL5 interacts with all CerS. We demonstrate that ACSL5 generated FA-CoA was utilized with de novo ceramide for the generation of acylceramides, poorly studied ceramide metabolites. Functionally, inhibition of ceramide channeling to acylceramide enhanced accumulation of de novo ceramide and resulted in augmentation of ceramide-mediated apoptosis. Mechanistically, we show that acylceramide generation is catalyzed by diacylglycerol acyltransferase 2 (DGAT2) on lipid droplets. In summary, this study identifies a metabolic pathway of acylceramide generation and its sequestration in LD in cells and in livers of mice on a high fat diet. The study also implicates this novel pathway in ceramide-mediated apoptosis, and has implications in co-regulation of triglyceride and sphingolipid metabolisms.
eTOC Blurb
XXX et al identify a novel pathway whereby ceramide is converted to acylceramides by a CerS-ACSL-DGAT complex in lipid droplets for storage. These results raise interesting questions as to the metabolic interplay of TG/DAG and ceramide/acylceramide and the roles of ACSL5 and CerS in regulating these balances.
INTRODUCTION
Ceramide, the central molecule in the sphingolipid pathway, regulates various cellular responses such as growth arrest, senescence, apoptosis, and autophagy (Hannun and Obeid, 2008; Mullen et al., 2012; Russo et al., 2012; Sentelle et al., 2012). De novo ceramide generation is carried out by Ceramide Synthases (CerS). Six CerS isoforms (CerS1-6) preferentially utilize fatty acyl-CoAs with different chain lengths to generate distinct ceramides (Hannun and Obeid, 2011; Park et al., 2014). CerS were shown to mediate cell death in response to chemotherapeutic agents (Beverly et al., 2013; Min et al., 2007; Senkal et al., 2007), TRAIL (White-Gilbertson et al., 2009) and UV (Mullen et al., 2011). The activity of CerS proteins can be regulated by homo- and hetero-dimerization (Jensen et al., 2014; Laviad et al., 2012). However, there is little mechanistic insight into the regulation CerS. We therefore undertook an approach aimed at defining interacting partners of CerS’.
The human genome contains 26 acyl-CoA synthases, five of which are distinct isoforms of long-chain acyl-CoA synthases (Acsl) that convert long-chain FA to acyl-CoA (Li et al., 2010; Soupene and Kuypers, 2008; Watkins et al., 2007). ACSL5, which has a wide range of preference for the generation of FA-CoAs, has been implicated in fatty acid uptake and partitioning to triacylglycerol (TG) synthesis (Grevengoed et al., 2014; Mashek et al., 2006).
We have identified ACSL5 as one of the interacting partners of CerS. We initially hypothesized that ACSL5 can channel fatty acids towards ceramide synthesis. However, interestingly, our results implicate ACSL5 in the generation of acylceramides from ceramides. Moreover, inhibition of acylceramide synthesis enhanced accumulation of de novo ceramide and resulted in augmentation of ceramide-mediated apoptosis. Mechanistically, we show that acylceramide generation is catalyzed by DGAT2 and involves the formation of an ACSL5-CerS-DGAT2 complex on lipid droplets in cultured cells and liver tissue.
RESULTS
ACSL5 Associates with CerS
To study CerS regulation we set out to identify CerS associated proteins. Inducible HCT116 cell lines stably expressing FLAG tagged CerS isoforms 1, 2, 4, 5 and 6 (Figure S1A) were generated and cellular CerS activities were confirmed (Figure S1B). Next, after immunoprecipitation (IP) with anti-FLAG antibody, proteins that associate with expressed CerS isoforms were identified using LC-MS/MS. MS/MS data identified 41 probable protein hits. ACSL5 was one of the CerS associated proteins (Figure S2A). ACSL5 synthesizes C12–C20 chain lengths FA-CoAs. Functionally, knock-down of ACSL5 decreased FA incorporation into glycerolipids and cholesterol esters (Bu and Mashek, 2010; Mashek et al., 2006). Since ACSL5 has no known role in ceramide metabolism, the significance of this putative interaction between ACSL5 and CerS proteins was pursued. First we confirmed the interaction of ACSL5 and CerS using anti-FLAG IP and western blotting in digitonin and triton X-100 solubilized cell lysates since detergent strength can affect the degree of interaction (Vallee and Riezman, 2005). While the association of ACSL5 with CerS1 and CerS4 appeared stronger compared to other CerS isoforms, all CerS isoforms tested associated with ACSL5 significantly. In addition, inducible expression of CerS proteins did not change ACSL5 levels, and ACSL5 associated with CerS proteins regardless of detergent strength (Figures 1A and 1B). Next, to determine the possible association of other ACSL members with CerS’, the levels of ACSL1 in the anti-FLAG immunoprecipitated samples were determined. ACSL1 also IPed with CerS’ (Figure 1A), suggesting that ACSL family proteins can associate with CerS proteins. In addition, data from reverse IP using HA-tagged ACSL5 showed that HA-ACSL5 interacted with CerS proteins (Figure 1C). Furthermore, C-terminal V5 tagged CerS1 also associated with ACSL5 (Figure S2B). Consistent with the association, HA-ACSL5 appeared to co-localize with FLAG-CerS6 and the ER marker calnexin (Figure 1D), as well as other CerS isoforms (Figure S2C). Proximity ligation assay (PLA) resulted in positive spots (Figure 1E, red puncta), suggesting that ACSL5 and CerS proteins are within 40 nm proximity (Soderberg et al., 2006).
Figure 1. Determination of CerS-ACSL5 Association.
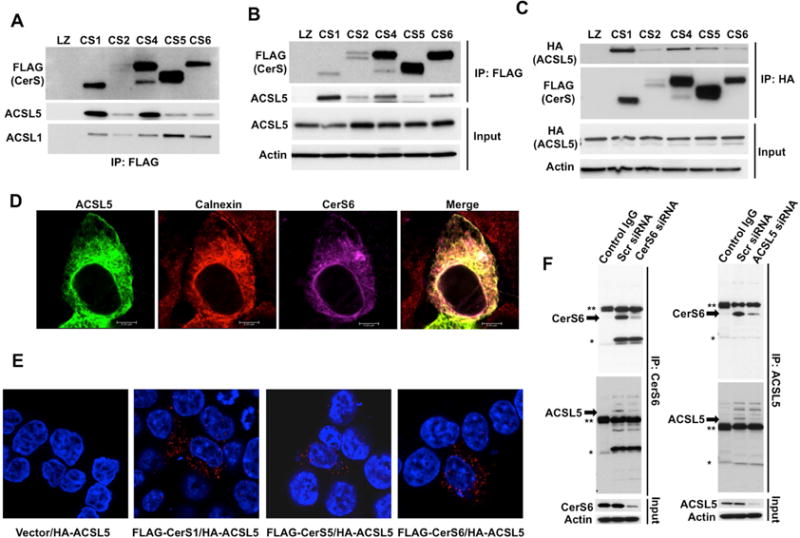
(A) FLAG-tagged CerS1, 2, 4, 5 and 6 were immunoprecipitated after lysis in digitonin containing buffer, and interactions of ACSL1 and ACSL5 with CerS was determined by Western Blotting.
(B) Interactions of ACSL5 and CerS were determined as in A using Triton X-100 containing lysis buffer.
(C) HA-tagged ACSL5 was expressed in cells stably transfected with FLAG-tagged CerS. ACSL5-CerS interaction was determined by Western Blotting with anti-FLAG antibody after immunoprecipitation (IP) with anti-HA antibody.
(D) Co-localization of HA-ACSL5 with FLAG-CerS6 and calnexin was imaged by confocal microscopy.
(E) FLAG-tagged CerS and HA-ACSL5 interaction was identified using Proximity Ligation Assay (PLA).
(F) Interaction of endogenous CerS6 with ACSL5 was detected by IP with anti-CerS6 or anti-ACSL5 antibodies followed by Western Blotting after Control (Scr), CerS6, or ACSL5 siRNA transfections. Arrows indicate specific bands. Single and double asterisks indicate light and heavy chains of IP antibodies, respectively. Isotype matched IgG was used as negative control.
Data are representative of three independent experiments.
Furthermore, endogenous ACSL5 was found to be associated with endogenous CerS6 in basal conditions, and this association was decreased when either interacting partner was down-regulated with siRNA (Figure 1F). To evaluate specificity of CerS-ACSL interaction, serine palmitoyltransferase (SPT), which localizes on ER and is involved in sphingolipid metabolism or perilipin2 (PLIN2) which localizes to lipid droplets (see below) was tested in IP experiments. Neither SPT, nor PLIN2 associated with CerS6 in control or oleate treated cells (Figure S2D). In addition, two other ER resident proteins identified as hits in MS/MS, prolyl 4-hydroxylase (P4HB) and DDRGK domain-containing protein 1 (DDRGK1) did not associate with CerS (Figure S2E). These results suggest the existence of a previously unidentified specific association between CerS enzymes and ACSL5.
In order to investigate protein regions that are important for CerS-ACSL5 association, we generated multiple mutants of HA-ACSL5 (Figure 2A, right panel) and tested their interaction with CerS1. All mutants of ACSL5 (Red), except for the combined deletion of N-terminal (amino acids 1–66) with predicted transmembrane (TM, amino acids 67–88) (ΔN-ΔTM), co-localized with calreticulin (Green) (Figure 2A, left panel) consistent with ER localization. While ΔN-ΔTM mutant of ACSL5 had greatly reduced interaction with CerS1, other mutants associated with CerS1 similar to wild type ACSL5 (Figure 2B). This result suggests that the first 88 amino acids of ACSL5 may be required for the localization of ACSL5 to ER and association to CerS’. To test this, the amino acids 1–88 of ACSL5 were cloned to N-terminus of enhanced green fluorescent protein (eGFP) (Figure 2C) and this chimera (N+TM-GFP) associated with CerS1 (Figure 2D). This interaction between CerS1 and N+TM-GFP is not due to ER localization of binding partners, since ER-GFP did not associate with CerS1 (Figure 2E). Taken together, these data demonstrate that the N-terminus and TM domain of ACSL5 are involved in the association with CerS’.
Figure 2. Identification of Domains in ACSL5 Required for CerS-ACSL5 Association.
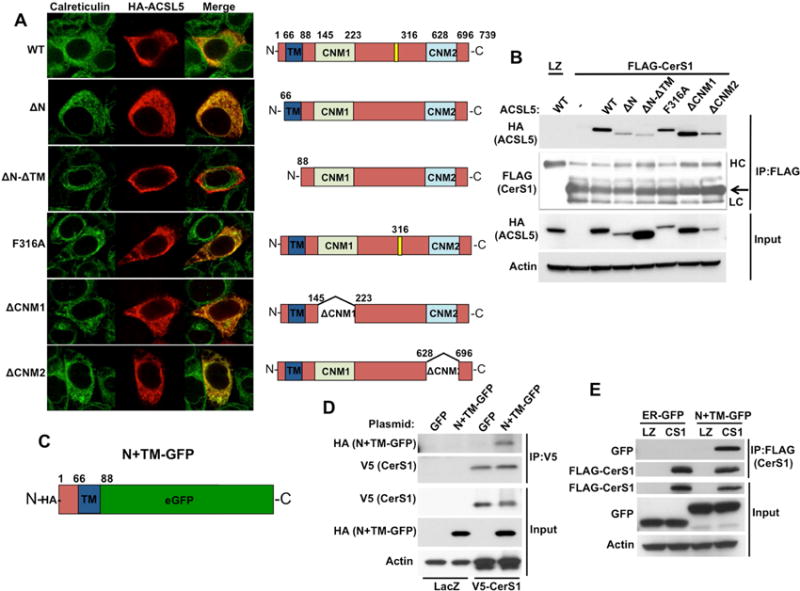
(A) Left; Cellular localization of wild type (WT) and mutants of ACSL5 was imaged. Right; Diagrams of ACSL5 protein with domains that were modified. N terminal: amino acids 1–66, TM: Putative transmembrane domain, CNM1: Common No Motif 1, CNM2: Common No Motif 2, Domain I: AMP binding domain.
(B) Levels of WT and ACSL5 mutants determined by Western blotting using anti-HA antibody (left panel). Association of WT and mutants of HA-tagged ACSL5 with FLAG-tagged CerS1 was determined by anti-FLAG IP and Western blotting using anti-HA antibody (right panel). HC and LC: Heavy and light chains of IP antibody. Arrow: FLAG-CerS1.
(C) Diagram of HA-tagged chimeric protein (N+TM-GFP) containing the first 88 amino acids of ACSL5 (N+TM) upstream of eGFP.
(D) Interaction of HA-tagged N+TM-GFP with V5-tagged CerS1 was identified by anti-V5 IP and Western blotting with anti-HA antibody.
(E) Interaction of FLAG-CerS1 with N+TM-GFP and ER-GFP were determined by anti-FLAG IP and Western blotting with anti-GFP antibody.
Data are representative of three independent experiments.
ACSL5 and CerS Generate Substrates for Acylceramide Production
Next, the biochemical significance of CerS-ACSL5 interaction was investigated. Since ACSL5 generates FA-CoAs, and CerS utilize FA-CoAs to generate ceramide, our initial hypothesis was that ACSL5-generated FA-CoAs are consumed directly by CerS. To test this, we used ACSL5 siRNA and measured ceramide concentrations. Unexpectedly, down-regulation of ACSL5 caused a significant increase in ceramide and dihydroceramide levels (Figures 3A and 3B). In addition siRNA silencing of ACSL1, that associated with CerS (Figure 1A), also induced ceramide accumulation without significant changes in S1P or Dihydro-S1P (Figures 3C and S3A). Next, to find the source of ceramide accumulation, we tested whether the CerS activity is induced after ACSL1 or ACSL5 knock down. Down-regulation of ACSL1 or ACSL5 did not have any significant effect on in situ CerS activity (Figure S3B), indicating that ceramide accumulation is not due to increased CerS’ activities. Moreover, ACSL1 or ACSL5 down regulation did not induce any significant change in sphingomyelin (Figures 3D and S3C), but induced accumulation of hexosyl-ceramide (Figure S3D) levels possibly due to metabolic flux of ceramide. However, inhibition of de novo ceramide generation by myriocin or FB1 prevented the effects of knock down of ACSL1 or ACSL5 on ceramide generation (Figure 3E). These data suggest that down-regulation of ACSL1/5 cause ceramide accumulation stemming from the de novo pathway via CerS proteins.
Figure 3. Silencing of ACSL’s Induce Ceramide Accumulation.
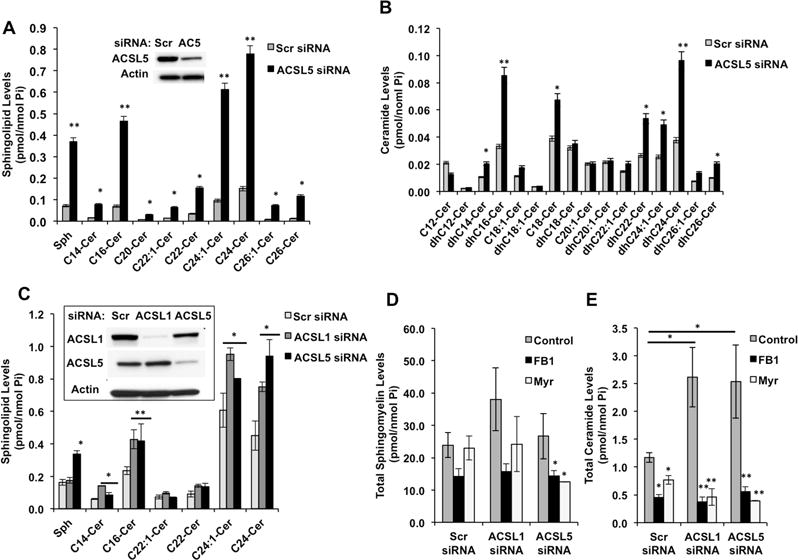
(A) Cellular sphingosine and ceramide levels were measured by LC/MS after ACSL5 silencing. ACSL5 abundance after siRNA transfections (inset).
(B) Dihydroceramide levels were measured in cells transfected with Scr (control) or ACSL5 siRNAs.
(C) Sphingosine and ceramide levels were measured in cells transfected with All Star (control), ACSL1, or ACSL5 siRNAs individually. Down regulation of ACSL1 and ACSL5 was confirmed by Western Blotting (inset).
(D and E) Total sphingomyelin and ceramide levels of cells transfected with indicated siRNAs and treated with PBS (Control), Fumonisin B1 (FB1), or Myriocin (Myr) were measured by LC/MS.
Results are expressed as mean of three independent experiments with error bars representing ± SEM. *p <0.05 and ** p< 0.01.
The unexpected increase in ceramide levels upon ACSL silencing did not fit the initial hypothesis of ACSL5 generated FA-CoA being utilized by CerS to generate ceramide. We next tested gain of function effects of ACSL5. Cells stably expressing ACSL5 exhibited lower levels of ceramide (Figures 4A and 4B).
Figure 4. Effects of ACSL5 and DGAT2 Modulation on Ceramide and Acylceramide Levels.
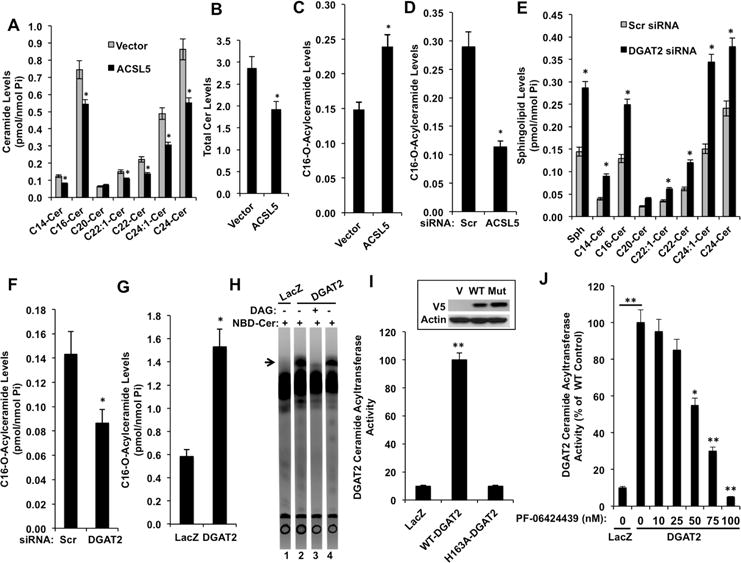
Species specific (A) and total (B) ceramide content of cells stably expressing vector control or HA-ACSL5 was measured by LC/MS.
(C and D) C16-acylceramide levels (Mild base sensitive) of cells stably expressing vector control or ACSL5 (C) and cells transfected with Scr (control) or ACSL5 siRNAs
(D) was measured by LC/MS.
(E) Sphingosine and ceramide levels of cells transfected with Scr (Control) or DGAT2 siRNAs was measured using LC/MS.
(F and G) C16-acylceramide levels (Mild base sensitive) of cells transfected with Scr (control) or DGAT2 siRNAs (F) and cells stably expressing LacZ (control) or DGAT2
(G) was measured by LC/MS.
(H) In vitro activity of DGAT2 for generation of acylceramide was determined as described in Materials and Methods. O-acyl-NBD-C12-ceramide band is indicated with an arrow.
(I) In vitro activity of wild type (WT) and H163A mutant (Mut) DGAT2 for generation of acylceramide was determined.
(J) Microsomes from LacZ or DGAT2 expressing cells were incubated with PF-06424439 and their in vitro ceramide acyltransferase activities were determined.
Results are expressed as mean of three independent experiments with error bars representing ± SEM. *p <0.05 and ** p< 0.01.
The increase and decrease of ceramide levels upon silencing and over expression of ACSL5, respectively, without major effects on established pathways of ceramide clearance suggested that ACSL5 might divert ceramide to another metabolite. The best candidate would be 1-O-acyl-ceramide since its formation requires the generation of ceramide followed by acylation on the 1-hydroxy position by an acyltransferase (Figure S3E), a reaction identified more than three decades ago (Okabe and Kishimoto, 1977), but not mechanistically characterized. To test this, selective hydrolysis of ester-linked but not amide-linked fatty acids by mild base treatment of lipid extracts was utilized. This results in hydrolysis of O-acylceramides and generates ceramide by cleavage of the ester bond (arrowhead, Figure S3E). Cells stably expressing ACSL5 had significantly increased (about 40 %) base sensitive O-acylceramide content (Figure 4C). On the other hand, silencing of ACSL5 caused a significant reduction (about 80%) in the O-acylceramide levels (Figure 4D), suggesting that ACSL5 generated FA-CoA is likely consumed in acylation of ceramide to generate O-acylceramides.
Biochemically, O-acylation of ceramide can be accomplished by an enzyme that has a similar reaction as diacylglycerol (DAG) acyl transferase activity due to the analogous chemical structures between DAG and ceramide. Interestingly, reduced total acylceramide generation in DGAT2 knockout mice has been reported (Stone et al., 2004). Additionally, DGA1 (yeast orthologue of DGAT2) is shown to be involved in generation of 1-O-acylceramides in Saccharomyces cerevisiae (Voynova et al., 2012). Therefore, we next tested whether modulation of DGAT2 activity could affect ceramide levels by controlling its conversion to acylceramides (Figure S3E). DGAT2 siRNA transfection decreased DGAT2 abundance by about 95% (Figure S3F). More importantly, while DGAT2 silencing induced significant ceramide accumulation, acylceramide levels were decreased (Figures 4E and 4F). Moreover, over expression of DGAT2 induced accumulation of acylceramides (Figure 4G). To establish that DGAT2 can utilize FA-CoA and ceramide to generate acylceramide, and that the increase in the acylceramide levels after DGAT2 expression is directly due to elevated acylceramide generation by DGAT2, in vitro acyltransferase activity of DGAT2 towards ceramide was tested. As shown in Figure 4H, incubation of microsomes from cells stably expressing DGAT2 with palmitoyl-CoA and NBD-C12-Ceramide resulted in the formation of NBD-Acylceramide (Figure 4H, lane 2, arrow). Additionally, NBD-Acylceramide generation was diminished in the presence of DAG (Figure 4H, lane 3), suggesting that DGAT2 has ceramide acyltransferase activity which can be competed off by DAG. Moreover, microsomes from cells stably expressing (Figure 4I, inset) catalytically inactive DGAT2 (Stone et al., 2006) did not have activity towards acylceramide generation (Figure 4I). Furthermore, acylceramide generation was prevented by the DGAT2 specific inhibitor PF-06424439 (Futatsugi et al., 2015) in a dose dependent manner in vitro (Figure 4J). Belonging to a MBOAT family, distinct from DGAT2, DGAT1 was shown to have DGAT, MGAT, and wax ester synthase activities (Yen et al., 2005a; Yen et al., 2005b), therefore we tested the ability of DGAT1 to generate acylceramide in vitro. Murine DGAT1 was able to utilize ceramide and FA-CoA to generate acylceramide similar to DGAT2 (Figure S4A) consistent with similar activities of DGAT1 and DGAT2 for generation of TG (Yen et al., 2005b). These data suggest that ACSL5 and CerS cooperate to produce, FA-CoA and ceramide, respectively, which are utilized by DGAT for the generation of acylceramides.
ACSL5/DGAT2 Sequester Biologically Active Ceramide into Acylceramide
Having established the biochemical significance of ACSL5-CerS interaction, we next focused on biological significance of acylceramide generation. Accumulation of ceramide in response to cellular stress is generally associated with growth inhibition and activation of cell death pathways. Since ceramide accumulation upon down-regulation of ACSLs was observed (Figure 3), cellular growth after siRNA mediated silencing of ACSLs was determined. Silencing ACSL1 or ACSL5 caused growth inhibition (~60 %) (Figure 5A). Since 5FU was shown to induce ceramide accumulation (Realini et al., 2013), the effects of ACSL5 silencing on 5FU-induced apoptosis were determined. ACSL5 knock down (Figure 5B, inset) significantly increased 5FU-induced caspase 3/7 activation (Figure 5B). Likewise, ACSL5 silencing increased growth inhibition by other chemotherapeutics such as gemcitabine and methotrexate (Figure S5A). To investigate the effects of ACSL5 modulation on de novo ceramide generated by CerS directly, ACSL5 was silenced in CerS1 over-expressing cells. Silencing of ACSL5 enhanced CerS1-induced caspase activation about 2.5 fold (Figure 5C). These data suggest that ACSL5 maybe involved in regulating a biologically active pool of de novo ceramide.
Figure 5. Modulation of Bioactive Ceramide Generation via ACSL5/DGAT2 Silencing and Overexpression.
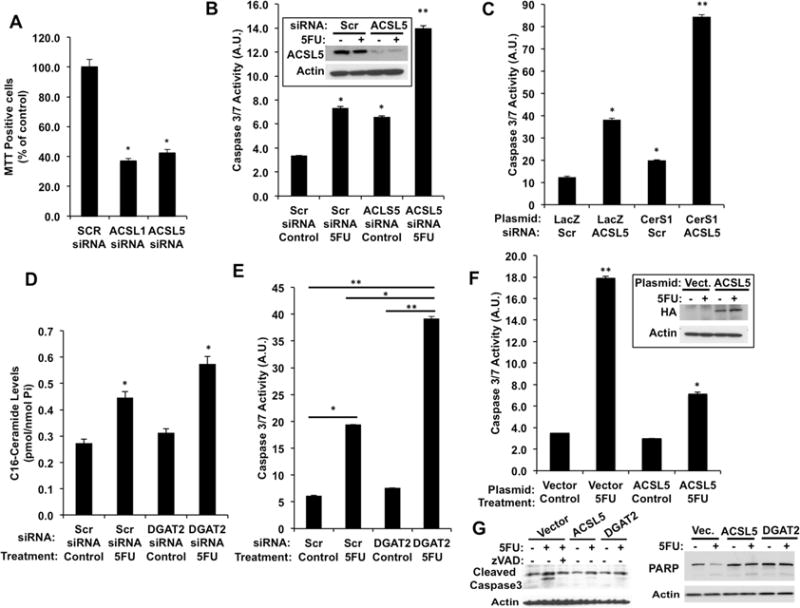
(A) Cellular growth was measured in cells transfected with Scr (control), ACSL1, or ACSL5 siRNAs.
(B) Cells were transfected with Scr (control) or ACSL5 siRNAs and treated with DMSO (control) or 5-FU (0.3 mM) and Caspase 3/7 activity was measured. ACSL5 silencing was confirmed by Western blotting (inset).
(C) Caspase 3/7 activity was measured in cells stably expressing LacZ or CerS1 after ACSL5 silencing.
(D) C16-Ceramide levels were measured in cells transfected with Scr or DGAT2 siRNAs and treated with DMSO (control) or 5FU.
(E) Cells were transfected and treated as in D, and caspase 3/7 activity was measured.
(F) Cells stably expressing control (Vector) or HA-ACSL5 were treated with 5-FU and Caspase 3/7 activity was measured. HA-ACSL5 expression was confirmed by Western blotting (inset). Results are expressed as mean of three independent experiments with error bars representing ± SEM. *p <0.05 and ** p< 0.01.
(G) Caspase 3 activation and cleavage of PARP in response to 5-FU treatment was detected by Western blotting in cells expressing control vector, ACSL5, or DGAT2.
We next investigated the biological significance of DGAT2 regulation of ceramide metabolism. Similar to silencing of ACSL5, DGAT2 silencing enhanced 5FU induced ceramide accumulation (Figure 5D), caspase activation, and PARP cleavage (Figures 5E and S5B). Hence, these data may lend support to the notion that DGAT2, like ACSL5, regulates a biologically active pool of ceramide.
Reciprocally, ACSL5 or DGAT2 over expressing cells, with decreased ceramide levels (Figures 4A and 4B) did not show changes in CerS2 or CerS6 protein levels (Figure S5C) and were resistant to 5FU-induced apoptosis as determined by caspase activation (Figures 5F and 5G, left panel) and PARP cleavage (Figure 5G, right panel). Taken together, these results also lend support to the view that DGAT2 and ACSL5 regulate a biologically active pool of ceramide.
DGAT2 Produces Acylceramide at Lipid Droplets
Recently, Caenorhabditis elegans orthologues of DGAT2 and FATP1 FA-CoA synthetase were shown to interact and be important for lipid droplet (LD) expansion on the ER-LD interface (Xu et al., 2012). Therefore, the possibility of physical interaction of DGAT2 and ACSL5 was tested. The results showed that DGAT2 associated with ACSL5 as determined by co-IP (Figure 6A). Since data suggested that ACSL5 and DGAT2 channel de novo ceramide to acylceramide, we tested the presence of an ACSL5-DGAT2-CerS complex. The results showed that both ACSL5 and DGAT2 co-IP’ed with FLAG-CerS1 (Figure 6B). These data suggest that ACSL5, DGAT2 and CerS associate, possibly to form a complex that is involved in acylceramide generation.
Figure 6. ACSL5, DGAT2, and CerS Interact on Lipid Droplet/ER interface.
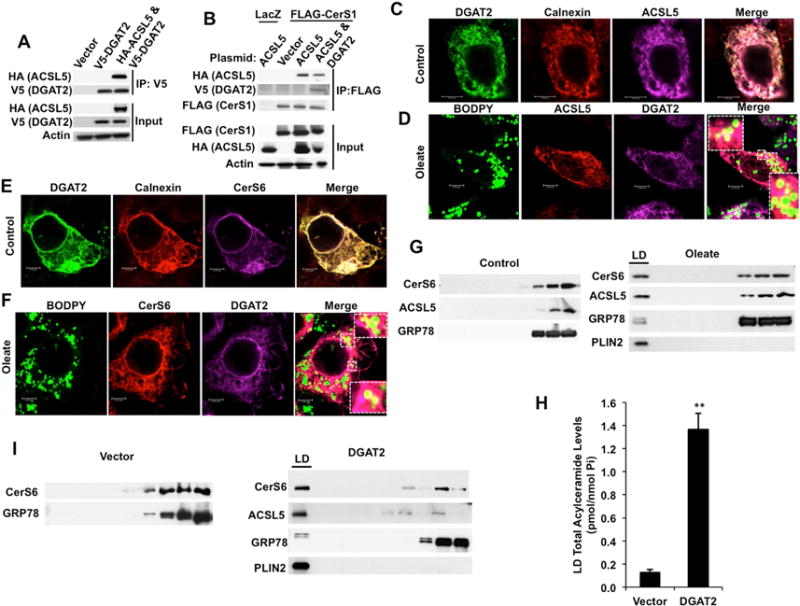
(A) Interaction of HA-tagged ACSL5 with V5-tagged DGAT2 was identified by anti-V5 IP and Western blotting with anti-HA and anti-V5 antibodies.
(B) Cells expressing FLAG-CerS1 were transfected with control vector, HA-ACSL5, or V5-DGAT2 in combination with HA-ACSL5 and formation of ACSL5-DGAT2-CerS1 complex was determined by anti-FLAG IP and Western blotting with anti-HA, -V5 and -FLAG antibodies. Results are representative of three independent experiments.
(C and D) Co-localization of HA-ACSL5 with V5-DGAT2 on ER (Calnexin) and lipid droplets (BODIPY) was imaged using confocal microscopy in the absence (C) and presence of 0.5 mM oleate (D). Inset: Magnified images of indicated areas.
(E and F) Co-localization of FLAG-CerS6 with V5-DGAT2 on ER (Calnexin) and lipid droplets (BODIPY) was imaged in the absence (E) and presence of 0.5 mM oleate (F). Inset: Magnified images of indicated areas.
(G) Localization of endogenous CerS6 and ACSL5 in lipid droplets upon oleate treatment was detected using cellular fractionation followed by Western blotting. LD: lipid droplet fractions.
(H) Total acylceramide content of lipid droplets isolated from cells stably expressing vector control or DGAT2 was measured by LC/MS.
(I) Localization of CerS6 in lipid droplet fractions was determined by Western blotting in cells stably transfected with control vector (left panel) or DGAT2 (right panel). LD: Lipid droplet fractions. Results are representative of at least three independent experiments.
Several enzymes involved in lipid and triacylglycerol synthesis are shown to localize to LD fractions of oleate-loaded cells (Brasaemle et al., 2004; Fujimoto et al., 2007; Poppelreuther et al., 2012; Wilfling et al., 2013). Therefore, we hypothesized that acylceramide generation can take place at the ER/LD interface and tested the presence of CerS in the LD. Consistent with previous reports (Harris et al., 2011; McFie et al., 2014), confocal microscopy revealed that transiently expressed ACSL5 and DGAT2 co-localized with calnexin at the ER (Figure 6C) and on the ER/LD interface upon oleate loading (Figure 6D). Similar to ACSL5, CerS6 and DGAT2 co-localized on ER membranes in control cells (Figure 6E) and on the LD membranes upon oleate loading as detected by microscopy (Figure 6F) and cellular fractionation (Figures 6G and S6A). Next, the presence of CerS6 in the LDs of DGAT2 stably over expressing cells which have an increased number of LDs (Figure S6B) and elevated LD associated acylceramide content (Figure 6H) was tested. CerS6 was localized to LD fractions in the DGAT2 stable over-expressing cells (Figures 6I and S6C), Moreover, we observed significant ceramide acyltransferase activity in isolated LD from DGAT2 expressing cells (Figure S6D). These data suggest that acylceramide generation can take place on the ER/LD interface.
Lipid Droplet Acylceramide Generation in Liver is Induced with High Fat Diet
To investigate the physiological relevance of acylceramide generation, we used a diet induced liver steatosis mouse model. After four weeks of feeding mice an oleate enriched high fat diet (HFD), livers of HFD group mice showed formation of steatosis and significant LD accumulation (Figure 7A). We next investigated the presence of an ACSL5-DGAT2-CerS complex in the liver tissues of control and HFD fed mice. ACSL5 and DGAT2 co-IP’ed with CerS6 in lysates from liver tissue (Figure 7B). SPT or alkaline ceramidase 1 (ACER1) did not associate with CerS6 (Figure 7B), showing the specificity of ACSL5-DGAT2-CerS association. Of note, there was no significant change in the amounts of ACSL5, DGAT2, or CerS6 in the possible complex in the HFD group compared to controls. HFD fed mice livers had a slight but significant accumulation of ceramides (Figure 7C), but not of acylceramide after 4 weeks of HFD (Figure 7D). Interestingly, isolated LDs from these livers showed 4- and 7-fold increases in ceramide and acylceramide levels, respectively (Figures 7E and 7F). We next investigated the contribution of DGAT2 to the accumulation of acylceramides in an 8-week HFD experimental model using the DGAT2 inhibitor PF-06424439 (PF). Acylceramides significantly accumulated in the livers and lipid droplets isolated from livers of the HFD group. Importantly, these increased were significantly blunted when the mice were treated with PF (Figures 7G and 7H, respectively). These data hint that DGAT2 may be responsible for elevated acylceramide synthesis and storage in liver upon HFD.
Figure 7. Acylceramides Accumulate in Liver Lipid Droplets of HFD Fed Mice.
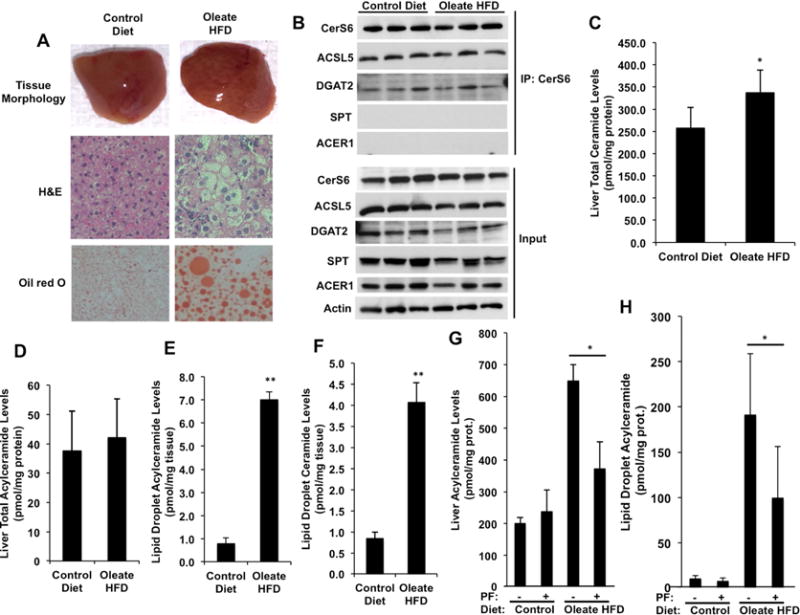
(A) Liver sections of control diet or oleate HFD (4 weeks) fed mice were stained with H&E or Oil red O and visualized.
(B) Interaction of CerS6, ACSL5, and DGAT2 in liver lysates of control diet or oleate HFD (4 weeks) fed mice (n=3 for each group) was detected by IP with anti-CerS6 antibody followed by Western Blotting. SPT and ACER1 blotting was used as controls representing other ER resident proteins.
(C and D) Total ceramides and acylceramides were measured in livers of mice that were fed a control diet or oleate enriched HFD for four weeks using LC/MS. (E and F) Total ceramide and acylceramide levels were measured in lipid droplets from the livers of control diet or oleate enriched HFD (4 weeks) fed mice.
(G and H) Total acylceramide levels were measured in livers (G) and lipid droplets from the livers (H) of mice that were fed a control diet or oleate enriched HFD for 8 weeks with or without PF-06424439 treatment.
Results are expressed as means (n=4 for each group in 4 week study) (n=3 for each group in 8 week study) with error bars representing ± SEM. *p <0.05 and ** p< 0.01.
DISCUSSION
In this study, we identified ACSL5 as a binding partner of CerS. Mechanistically, we demonstrate that, acylceramide generation is carried out by a CerS-ACSL5-DGAT2 enzyme complex on the ER-LD interface. Functionally, generation of acylceramide by utilization of ceramide instigated resistance to chemotherapy-induced apoptosis in cancer cells. On the other hand, inhibition of acylceramide generation caused elevated ceramide levels and sensitized cells to chemotherapy. To our knowledge, this is the first report identifying the pathway of acylceramide generation and shows the biological importance of this novel pathway in ceramide sequestration in LD and in apoptotic cell death.
Metabolically, the results show that acylceramide generation from ceramide and FA-CoA produced by CerS and ACSL, respectively, required the enzymatic activity of DGAT2. Mechanistically, the results suggest that CerS, ACSL, and DGAT2 may form a multi-enzyme complex on the ER-LD interface. Canonically, DGAT1 and DGAT2 catalyze the formation of TG from 1,2-diacylglycerol and FA-CoA (Cases et al., 2001); however, ceramide acyltransferase activity of DGAT2 has not been described previously. The current results show that DGAT1 and DGAT2 display in vitro acyltransferase activity towards ceramide (Figure S4A), and down-regulation of DGAT2 results in accumulation of ceramide (Figure 4E). This is supported by in vivo studies showing that mice lacking DGAT2 demonstrated about 60 % reduction in total acylceramide content in the skin and die shortly after birth due to defects in sources for energy metabolism (Stone et al., 2004). Taken with our results, this suggests that DGAT2 is likely involved in the synthesis of acylceramides in vivo. While dgat1 −/− mice are viable (Buhman et al., 2002), cardiomyocytes of thsese mice had ceramide accumulation that contributed to heart failure (Liu et al., 2014), possibly due to inefficient routing of ceramides to acylceramides. Therefore, tissue and cell type specific generation of acylceramides might be closely linked to the tissue distribution of DGAT enzymes.
Moreover, these results connect formation of acylceramides to LD. The components of the multi-enzyme complex, CerS, ACSL, and DGAT2, are found at the ER/LD interface (Figure 6). LD are organelles where metabolic energy is stored in the form of TG and sterol esters. The core of LD is surrounded by a monolayer of phospholipids (Farese and Walther, 2009), and the LD surface contains several structural proteins, such as perilipins (Hsieh et al., 2012) and metabolic enzymes that are involved in the formation and mobilization of TG. Amongst the enzymes localized on LD and involved in the TG production are glycerol-3-phosphate O-acyltransferase 4 (GPAT4) (Wilfling et al., 2013) and monoacylglycerol acyltransferase 2 (MGAT2). On the other hand, adipose triglyceride lipase (ATGL) is shown to be involved in lipid mobilization from LD (Olzmann et al., 2013). DGAT2 is involved in bulk TG synthesis, and its overexpression in mouse liver or hepatoma cells induces TG formation in LD (Monetti et al., 2007). Fatty acid treatment also induces DGAT2 to associate with LD and take part in LD expansion (Harris et al., 2011; Jin et al., 2014; McFie et al., 2014; Wilfling et al., 2013; Xu et al., 2012). In addition to TG storage and control of energy metabolism, LD are involved in cancer cachexia and cancer cell growth (Qi et al., 2013; Yue et al., 2014). We now can add acylceramide to this group of storage lipids, and suggest that hydrolysis of acylceramide at its ester link by lipases may constitute a source of ceramide generation.
Our results raise interesting questions as to the metabolic interplay of TG/DAG and ceramide/acylceramide and possible roles of the interaction of ACSL5 and CerS in regulating these ‘balances’. Whereas ceramide is utilized by DGAT2 to generate acylceramide, DAG successfully competed for ceramide as a substrate for DGAT2 in vitro (Figure 4H). Therefore, cellular DAG levels might dictate the amount of acylceramide depending on the affinity of the enzyme towards the substrates and their spatial availability. On the other hand, formation of TG may be controlled indirectly by the availability of ceramide as a substrate for DGAT2. Moreover, since FA-CoA is required for formation of both acylceramides and TG, the diversion of FA-CoA used for acylceramide versus TG generation may be controlled by the degree of interaction between ACSL and CerS such that more interaction would favor acylceramide over TAG and lower interaction would lead to the opposite.
Functionally, this study identifies acylceramide as a sink for diverting ceramide from a bioactive pool into a storage pool. Thus, induction of this pathway results in attenuation of ceramide and ceramide-mediated apoptosis whereas blockade of it results in augmented accumulation of ceramide and exaggeration of ceramide-mediated 5FU-induced apoptosis. In an unbiased siRNA screen, down regulation of both ACSL5 and DGAT2 was found to induce ceramide accumulation, defects in cell division and growth (Atilla-Gokcumen et al., 2014), suggesting that pathway of acylceramide generation is the likely target. On the other hand, increased generation of acylceramides may be a mechanism for oncogenic transformation. In fact, ACSL4 and ACSL5 have both been implicated in regulation of cell growth, whereby; expression of ACSL4 in breast cancer cells increased cell growth and conferred resistance to lapatinib (Wu et al., 2013). Conversely inhibition of ACSL5 in glioma cells enhanced the apoptotic effects of etoposide (Mashima et al., 2009). The data presented here identify the novel mechanism by which ACSL5-CerS interaction controls cellular ceramide/acylceramide levels and implicates this interaction in cell death pathways. These results suggest that pro-apoptotic pool of de novo ceramide can be modulated by regulating the generation of acylceramide.
Manipulating this novel pathway may also have therapeutic implications. As such, inhibition of DGAT2 or ACSL5 may augment ceramide-mediated biology. However, these targets may affect both DAG and ceramide. Moreover, identifying the determinants of ACSL-CerS binding may lead to the development of inhibitors of this interaction and thus prevent acylceramide generation and be a novel strategy for cancer therapy that selectively targets ceramide clearance.
Oleate treatment or DGAT2 expression (both enhance LD formation) induced redistribution of CerS6 to LD. Importantly, increased LD formation has been identified as a feature of high fat diet (HFD)-induced obesity. Moreover, recently a role for de novo C16-ceramide/CerS6 has been proposed in the pathogenesis of HFD-induced obesity. It was shown that, while wild type mice on HFD had elevated C16-ceramide accumulation in liver and adipose tissues and suffer from insulin resistance, CerS6 knockouts display decreased blood insulin, and increased glucose sensitivity (Turpin et al., 2014). Additionally, heterozygous CerS2 knockout mice, in which C16-ceramide is elevated, exhibited increased liver weight, TG content, and insulin resistance in stark contrast to the CerS6 knockouts (Raichur et al., 2014). Our data showed enhanced accumulation of ceramide and acylceramide preferentially in LD from steatotic livers of oleate HFD fed mice likely formed by CerS-ACSL-DGAT complex (Figure 7). It is possible that CerS localization to LD and generation of acylceramides is a protective mechanism, which removes ceramide from cellular membranes and increases the oxidative capacity of mitochondria. Hence, acylceramide generation might be a therapeutic link for treatment of metabolic syndrome.
In summary, we describe a molecular pathway generating acylceramide and shed light on its sequestration in LD, and its biological significance in apoptosis by controlling bioactive ceramide levels. Further studies will define the functions of ceramide-acylceramide shunt in obesity, atherosclerosis, aging and cancer.
EXPERIMENTAL PROCEDURES
Cell Lines, Growth Conditions, and Plasmids
HCT116 colorectal carcinoma cells were provided by Dr. Richard J. Youle (NINDS, NIH, USA). Cells were grown in McCoys’ 5A Media supplemented with 10% FBS (Life Technologies Corporation, Grand Island, NY) without antibiotics at 37°C humidified incubator. Possible mycoplasma contaminations were monitored regularly by MycoAlert mycoplasma detection kit (Lonza). See Supplemental Experimental Procedures (SEP).
Immunoprecipitation and Determination of Proteins by LC/MS-MS
Anti-FLAG immunoprecipitations were carried out using Anti-FLAG M2 Affinity gel (Sigma, St. Louis, MO) as described by the manufacturer. Where indicated, 1% (w/v) digitonin was used instead of Triton X-100 in lysis buffer. LC/MS-MS was performed as described in SEP.
Immunofluorescence Confocal Microscopy and Proximity Ligation Assay
Confocal microscopy was performed in cells that were plated on 35 mm dishes with glass bottoms (MatTek Corp., Ashland, MA) and transfected with indicated plasmids as described in Supplemental Experimental Procedures. Proximity ligation assays (PLA) were performed using Duolink II Fluorescence kit (Olink Bioscience, Uppsala, Sweden) with anti-HA and anti-FLAG antibodies as described by the manufacturer. The PLA spots were detected with 554 nm excitation and 579 nm emission filters in a Leica TSC SP8 laser scanning confocal microscope.
In Situ and In Vitro CerS Activity, Determination of Endogenous Ceramide and Acylceramide Levels
In situ ceramide synthase activity was measured by 17C-sphingosine labeling. After the indicated treatments, cells were treated with 1 μM of 17C-sphingosine (Avanti Polar Lipids, Alabaster, AL) and incorporation of 17C-sphingosine was determined as described in (Mullen et al., 2011). In vitro CerS activity was determined in microsomes as described previously (Siskind et al., 2010). Ceramide levels were measured by HPLC coupled to mass spectrometry (LC/MS) as described (Bielawski et al., 2006). Acylceramide content of the samples was determined by mild base hydrolysis after Bligh-Dyer lipid extraction. Briefly, lipids were incubated with 2 mL of 0.2 N KOH in methanol at 37°C for 1h. After neutralization with 1 mL of 0.4 N HCL, lipids were extracted using another round of Bligh-Dyer extraction and dried down under N2 stream. The ceramide content of the resulting lipids were determined using LC/MS. The acylceramide amounts were calculated by subtracting ceramide levels of control samples from base-hydrolyzed samples.
In Vitro DGAT2 Acyltransferase Activity Assay
Activity of DGAT2 to generate acylceramide was determined by modifying a previously described method (McFie and Stone, 2011; Yen et al., 2005a). Briefly, microsomes (100 μg) from cells stably expressing LacZ or DGAT2 were incubated with palmitoyl-CoA (25 μM) and NBD-C12-ceramide (100 μM) in the absence or presence of DAG (1,2-dioleoyl-sn-glycerol, 100 μM) in a buffer containing 200 mM sucrose, 100 mM Tris-HCl (pH 7.2), 20 mM MgCl2, and 1.25 mg/mL fatty acid free BSA for 15 min at 37°C. Lipids were extracted using Bligh-Dyer method and resolved on TLC plates as described (Takagi et al., 2004). O-acyl-NBD-C12-ceramide was visualized and quantitated using Typhoon FLA 7000 imaging system (GE Healthcare Life Sciences, Piscataway, NJ). 1-O-Acylceramide was used as standard.
Visualization and Isolation of Lipid Droplets
Lipid droplet formation was enhanced by incubating cells with 0.5 mM oleic acid (Sigma, St. Louis, MO) for 16h. Cells were fixed in 3.5 % (w/v) paraformaldehyde in 1X PBS for 10 min. After washes with 1X PBS and blocking in 0.3% (w/v) fatty acid free BSA containing 1X PBS for 1h, cells were incubated BODIPY-493/503 (Life Technologies Corporation, Grand Island, NY) in BSA solution for 30 min. Dishes were washed, mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and visualized using Leica TSC SP8 laser scanning confocal microscope. Lipid droplet fractions were isolated as described (Krahmer et al., 2013). See SEP.
High Fat Diet Induced Liver Steatosis
All animal procedures were approved by the Stony Brook University Institutional Animal Care and Use Committee (IACUC) and followed the guidelines of the American Veterinary Medical Association. C57BL/6 mice were fed either a control (12.6% calories from fat; TD.05230) or a diet enriched with oleate (41.7% calories from fat; TD.140589) for four or eight weeks and euthanized prior to tissue collection. PF-064244399 (PF) treatment of mice was carried out as described (Futatsugi et al., 2015). Briefly, at the end of the 8 weeks of oleate HFD, mice were treated with vehicle (0.5 % methylcellulose) or PF at a dose of 30 mg/kg BID for 4 days (7 doses). See SEP.
Statistical Analysis
Statistical significance was determined by Student’s two-tailed t-test for single comparisons and by two-way ANOVA with Bonferroni post test for multiple comparisons. p ≤0.05 was considered statistically significant.
Supplementary Material
Research Highlights.
Ceramide synthases (CerS) associate with fatty acyl-CoA synthase ACSL5.
CerS-ACSL-DGAT catalyze acylceramide generation from ceramide in lipid droplets.
High fat diet induces accumulation of acylceramides in liver lipid droplets.
Acylceramide formation in lipid droplets acts as a storage for ceramide.
Acknowledgments
We thank Dr. Chiara Luberto for overall advice. We also thank Stony Brook Lipidomics Core for analysis of sphingolipids and Medical University of South Carolina Lipidomics Core for acylceramide standards. Study was supported by: NIH grants F32GM108384 (NAR), GM062887, P01CA097132, and Veterans Affairs Merit Award (LMO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
C.E.S., Y.A.H., and L.M.O. designed research. C.E.S., M.F.S., A.J.S., J.J.A., N.A.R., A.K., Y.A.H., and L.M.O. performed experiments and/or analyzed data. C.E.S., Y.A.H., and L.M.O. wrote the manuscript.
SUPPLEMENTAL INFORMATION
Supplemental information includes six supplemental figures and SEP.
References
- Atilla-Gokcumen GE, Muro E, Relat-Goberna J, Sasse S, Bedigian A, Coughlin ML, Garcia-Manyes S, Eggert US. Dividing cells regulate their lipid composition and localization. Cell. 2014;156:428–439. doi: 10.1016/j.cell.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly LJ, Howell LA, Hernandez-Corbacho M, Casson L, Chipuk JE, Siskind LJ. BAK activation is necessary and sufficient to drive ceramide synthase-dependent ceramide accumulation following inhibition of BCL2-like proteins. The Biochemical journal. 2013;452:111–119. doi: 10.1042/BJ20130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. The Journal of biological chemistry. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- Bu SY, Mashek DG. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. Journal of lipid research. 2010;51:3270–3280. doi: 10.1194/jlr.M009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV., Jr DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. The Journal of biological chemistry. 2002;277:25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV., Jr Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. The Journal of biological chemistry. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Itabe H, Kinoshita T, Homma KJ, Onoduka J, Mori M, Yamaguchi S, Makita M, Higashi Y, Yamashita A, et al. Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. Journal of lipid research. 2007;48:1280–1292. doi: 10.1194/jlr.M700050-JLR200. [DOI] [PubMed] [Google Scholar]
- Futatsugi K, Kung DW, Orr ST, Cabral S, Hepworth D, Aspnes G, Bader S, Bian J, Boehm M, Carpino PA, et al. Discovery and Optimization of Imidazopyridine-Based Inhibitors of Diacylglycerol Acyltransferase 2 (DGAT2) Journal of medicinal chemistry. 2015;58:7173–7185. doi: 10.1021/acs.jmedchem.5b01006. [DOI] [PubMed] [Google Scholar]
- Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA metabolism and partitioning. Annual review of nutrition. 2014;34:1–30. doi: 10.1146/annurev-nutr-071813-105541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews. Molecular cell biology. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Many ceramides. The Journal of biological chemistry. 2011;286:27855–27862. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, et al. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. Journal of lipid research. 2011;52:657–667. doi: 10.1194/jlr.M013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K, Lee YK, Londos C, Raaka BM, Dalen KT, Kimmel AR. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets. Journal of cell science. 2012;125:4067–4076. doi: 10.1242/jcs.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SA, Calvert AE, Volpert G, Kouri FM, Hurley LA, Luciano JP, Wu Y, Chalastanis A, Futerman AH, Stegh AH. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5682–5687. doi: 10.1073/pnas.1316700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, McFie PJ, Banman SL, Brandt C, Stone SJ. Diacylglycerol Acyltransferase-2 (DGAT2) and Monoacylglycerol Acyltransferase-2 (MGAT2) Interact to Promote Triacylglycerol Synthesis. The Journal of biological chemistry. 2014;289:28237–28248. doi: 10.1074/jbc.M114.571190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahmer N, Hilger M, Kory N, Wilfling F, Stoehr G, Mann M, Farese RV, Jr, Walther TC. Protein correlation profiles identify lipid droplet proteins with high confidence. Molecular & cellular proteomics: MCP. 2013;12:1115–1126. doi: 10.1074/mcp.M112.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviad EL, Kelly S, Merrill AH, Jr, Futerman AH. Modulation of ceramide synthase activity via dimerization. The Journal of biological chemistry. 2012;287:21025–21033. doi: 10.1074/jbc.M112.363580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochimica et biophysica acta. 2010;1801:246–251. doi: 10.1016/j.bbalip.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Trent CM, Fang X, Son NH, Jiang H, Blaner WS, Hu Y, Yin YX, Farese RV, Jr, Homma S, et al. Cardiomyocyte-specific loss of diacylglycerol acyltransferase 1 (DGAT1) reproduces the abnormalities in lipids found in severe heart failure. The Journal of biological chemistry. 2014;289:29881–29891. doi: 10.1074/jbc.M114.601864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashek DG, McKenzie MA, Van Horn CG, Coleman RA. Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle-RH7777 cells. The Journal of biological chemistry. 2006;281:945–950. doi: 10.1074/jbc.M507646200. [DOI] [PubMed] [Google Scholar]
- Mashima T, Sato S, Okabe S, Miyata S, Matsuura M, Sugimoto Y, Tsuruo T, Seimiya H. Acyl-CoA synthetase as a cancer survival factor: its inhibition enhances the efficacy of etoposide. Cancer science. 2009;100:1556–1562. doi: 10.1111/j.1349-7006.2009.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFie PJ, Jin Y, Banman SL, Beauchamp E, Berthiaume LG, Stone SJ. Characterization of the interaction of diacylglycerol acyltransferase-2 with the endoplasmic reticulum and lipid droplets. Biochimica et biophysica acta. 2014;1841:1318–1328. doi: 10.1016/j.bbalip.2014.06.004. [DOI] [PubMed] [Google Scholar]
- McFie PJ, Stone SJ. A fluorescent assay to quantitatively measure in vitro acyl CoA:diacylglycerol acyltransferase activity. Journal of lipid research. 2011;52:1760–1764. doi: 10.1194/jlr.D016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, Alexander S. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Molecular cancer research: MCR. 2007;5:801–812. doi: 10.1158/1541-7786.MCR-07-0100. [DOI] [PubMed] [Google Scholar]
- Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell metabolism. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. The Biochemical journal. 2012;441:789–802. doi: 10.1042/BJ20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen TD, Jenkins RW, Clarke CJ, Bielawski J, Hannun YA, Obeid LM. Ceramide synthase-dependent ceramide generation and programmed cell death: involvement of salvage pathway in regulating postmitochondrial events. The Journal of biological chemistry. 2011;286:15929–15942. doi: 10.1074/jbc.M111.230870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe H, Kishimoto Y. In vivo metabolism of ceramides in rat brain. Fatty acid replacement and esterification of ceramide. The Journal of biological chemistry. 1977;252:7068–7073. [PubMed] [Google Scholar]
- Olzmann JA, Richter CM, Kopito RR. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1345–1350. doi: 10.1073/pnas.1213738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochimica et biophysica acta. 2014;1841:671–681. doi: 10.1016/j.bbalip.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Poppelreuther M, Rudolph B, Du C, Grossmann R, Becker M, Thiele C, Ehehalt R, Fullekrug J. The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. Journal of lipid research. 2012;53:888–900. doi: 10.1194/jlr.M024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Fitchev PS, Cornwell ML, Greenberg J, Cabe M, Weber CR, Roy HK, Crawford SE, Savkovic SD. FOXO3 growth inhibition of colonic cells is dependent on intraepithelial lipid droplet density. The Journal of biological chemistry. 2013;288:16274–16281. doi: 10.1074/jbc.M113.470617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, et al. CerS2 Haploinsufficiency Inhibits beta-Oxidation and Confers Susceptibility to Diet-Induced Steatohepatitis and Insulin Resistance. Cell metabolism. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Realini N, Solorzano C, Pagliuca C, Pizzirani D, Armirotti A, Luciani R, Costi MP, Bandiera T, Piomelli D. Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Scientific reports. 2013;3:1035. doi: 10.1038/srep01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, Cowart LA. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. The Journal of clinical investigation. 2012;122:3919–3930. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, Jiang JC, Jazwinski SM, Hannun YA, Ogretmen B. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Molecular cancer therapeutics. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ, Szulc ZM, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nature chemical biology. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Mullen TD, Romero Rosales K, Clarke CJ, Hernandez-Corbacho MJ, Edinger AL, Obeid LM. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. The Journal of biological chemistry. 2010;285:11818–11826. doi: 10.1074/jbc.M109.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Experimental biology and medicine. 2008;233:507–521. doi: 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Levin MC, Farese RV., Jr Membrane topology and identification of key functional amino acid residues of murine acyl-CoA:diacylglycerol acyltransferase-2. The Journal of biological chemistry. 2006;281:40273–40282. doi: 10.1074/jbc.M607986200. [DOI] [PubMed] [Google Scholar]
- Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. The Journal of biological chemistry. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nakagawa H, Matsuo N, Nomura T, Takizawa M, Imokawa G. Biosynthesis of acylceramide in murine epidermis: characterization by inhibition of glucosylation and deglucosylation, and by substrate specificity. The Journal of investigative dermatology. 2004;122:722–729. doi: 10.1111/j.0022-202X.2004.22307.x. [DOI] [PubMed] [Google Scholar]
- Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, et al. Obesity-Induced CerS6-Dependent C16:0 Ceramide Production Promotes Weight Gain and Glucose Intolerance. Cell metabolism. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Vallee B, Riezman H. Lip1p: a novel subunit of acyl-CoA ceramide synthase. The EMBO journal. 2005;24:730–741. doi: 10.1038/sj.emboj.7600562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynova NS, Vionnet C, Ejsing CS, Conzelmann A. A novel pathway of ceramide metabolism in Saccharomyces cerevisiae. The Biochemical journal. 2012;447:103–114. doi: 10.1042/BJ20120712. [DOI] [PubMed] [Google Scholar]
- Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. Journal of lipid research. 2007;48:2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- White-Gilbertson S, Mullen T, Senkal C, Lu P, Ogretmen B, Obeid L, Voelkel-Johnson C. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene. 2009;28:1132–1141. doi: 10.1038/onc.2008.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Developmental cell. 2013;24:384–399. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Li Y, Wang J, Wen X, Marcus MT, Daniels G, Zhang DY, Ye F, Wang LH, Du X, et al. Long chain fatty Acyl-CoA synthetase 4 is a biomarker for and mediator of hormone resistance in human breast cancer. PloS one. 2013;8:e77060. doi: 10.1371/journal.pone.0077060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Zhang SO, Cole RA, McKinney SA, Guo F, Haas JT, Bobba S, Farese RV, Jr, Mak HY. The FATP1-DGAT2 complex facilitates lipid droplet expansion at the ER-lipid droplet interface. The Journal of cell biology. 2012;198:895–911. doi: 10.1083/jcb.201201139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CL, Brown CHT, Monetti M, Farese RV., Jr A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. Journal of lipid research. 2005a;46:2388–2397. doi: 10.1194/jlr.M500168-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CL, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. Journal of lipid research. 2005b;46:1502–1511. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell metabolism. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


