Abstract
We found that ethyl gallate purified from a dried pod of tara (Caesalpinia spinosa) intensified β-lactam susceptibility in methicillin-resistant and methicillin-sensitive strains of Staphylococcus aureus (MRSA and MSSA strains, respectively). This compound and several known alkyl gallates were tested with MRSA and MSSA strains to gain new insights into their structural functions in relation to antimicrobial and β-lactam susceptibility-intensifying activities. The maximum activity of alkyl gallates against MRSA and MSSA strains occurred at 1-nonyl and 1-decyl gallate, with an MIC at which 90% of the isolates tested were inhibited of 15.6 μg/ml. At concentrations lower than the MIC, alkyl gallates synergistically elevated the susceptibility of MRSA and MSSA strains to β-lactam antibiotics. Such a synergistic activity of the alkyl gallates appears to be specific for β-lactam antibiotics, because no significant changes were observed in the MICs of other classes of antibiotics examined in this study. The length of the alkyl chain was also associated with the modifying activity of the alkyl gallates, and the optimum length was C5 to C6. The present work clearly demonstrates that the length of the alkyl chain has a key role in the elevation of susceptibility to β-lactam antibiotics.
The occurrence and proliferation of methicillin-resistant Staphylococcus aureus (MRSA) strains are a cause for major concern not only in the clinical environment but also in community life, since few agents can treat infection by this organism (3, 7, 24). In fact, MRSA is resistant to virtually all kinds of β-lactams (27). Although vancomycin and teicoplanin are the two glycopeptides presently used in clinics for the treatment of multiresistant infections by gram-positive organisms, vancomycin-resistant S. aureus has arisen in the United States (4, 5, 6, 8). In addition, resistance to recently developed antistaphylococcal agents, including the oxazolidinone- and streptogramin-type antibiotics, has emerged (14, 15, 37, 39). The development of new classes of anti-MRSA compounds is therefore of significant importance if we are to avoid situations similar to that which occurred with the glycopeptides, for which no backup lead compounds were available for development into agents active against multidrug-resistant bacterial strains (13).
Many studies have found that the efficacy of β-lactams can be improved by combining them with chemical substances, such as baicalin (23), diterpenes (25), epigallocatechin gallate (16, 41), epicatechin gallate (33), polidocanol (2), polyoxotungstates (12, 40), tellimagrandin I (34), corilagin (32), triazine dye (35), tripeptide (10), and Triton X-100 (18, 19, 20, 36). We also found that flavone and its derivatives have unique synergistic actions with the β-lactams; despite their very weak antimicrobial effects on MRSA and methicillin-sensitive S. aureus (MSSA), flavone and its derivatives dramatically intensified the susceptibility to β-lactams in MRSA and MSSA. We named these compounds ILSMRs (for “intensifiers of β-lactam-susceptibility in MRSA”) and their effects “ILSMR effects” (29, 30, 31).
Interest in plants as sources of antimicrobial agents is growing. This is because plant-derived medicines have been part of traditional health care in most parts of the world and because the antimicrobial properties of plant-derived compounds are well documented (9). In our continuing project to identify plant-derived compounds that modulate β-lactam resistance in MRSA, we screened an extract from dried pods of Caesalpinia spinosa (Caesalpinioideae); this extract, in combination with β-lactams, reduced the MICs of these antibiotics for MRSA. C. spinosa, commonly known as tara, is native to the Cordillera region of Bolivia, Peru, and northern Chile and occurs as well in Ecuador, Colombia, Venezuela, and Cuba. It is also cultivated in most of these countries. The plant is a shrub or tree, with spreading, grey-barked, leafy branches. The pods of tara are flat and about 10 cm long and 2.5 cm wide; they contain four to seven large round seeds, which are black when mature (11).
The present work deals with the isolation of ILSMRs from the tara pods and the effects of their interaction, in combination with antibiotics, with multiple strains of drug-sensitive and drug-resistant S. aureus.
MATERIALS AND METHODS
Chemicals.
The gallic acid used was from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Among the alkyl esters, methyl, ethyl, n-propyl, and lauryl esters were also from Wako, while n-butyl, cetyl, isoamyl, isobutyl, n-octyl, and stearyl esters were from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan) and hexyl ester was from Apin Chemicals, Ltd. (Abingdon, Oxon, United Kingdom). p-Hydroxybenzoic acid, m-hydroxybenzoic acid, and 2′,3′,4′-trihydroxyacetophenone were obtained from Wako Pure Chemical Industries, Ltd. 4-Hydroxybenzoic acid isoamyl ester and n-amyl ester were from Tokyo Kasei Kogyo Co., Ltd. n-Decyl alcohol, 2,5-dihydroxybenzoic acid, dimethyl sulfoxide, 5-hydroxyisophthalic acid, n-nonyl alcohol, and 2′,4′,5′-trihydroxybutyrophenone were from Kanto Kagaku Co., Ltd. (Tokyo, Japan). 1-Undecanol was from Kishida Chemical Co., Ltd. (Osaka, Japan). Cephapirin, oxacillin, norfloxacin, and tetracycline were obtained from Sigma Chemical Co. (St. Louis, Mo.). Other reagents were of the highest grade commercially available.
Bacterial strains.
S. aureus strains 1 to 22 (clinical isolates of MRSA) and 1003 to 1032 (clinical isolates of MSSA), strain COL, and strain RN4220 were kindly supplied by the late Toru Usui (Kyoto Microbiological Institute, Kyoto, Japan), John J. Iandolo (University of Oklahoma Health Sciences Center, Tulsa, Okla.), and Hitoshi Komatsuzawa (Hiroshima University Graduate School of Biomedical Sciences, Hiroshima, Japan), respectively. Some properties of the clinical strains used in the present study were determined previously (31).
Extraction and isolation.
Extraction of target substances from fruit pods of C. spinosa was monitored by thin-layer chromatography on precoated silica gel plates (Merck, Darmstadt, Germany) with chloroform-methanol (9:1 [vol/vol]) as the mobile phase. Antimicrobial activity against MRSA strains was examined by a disk-diffusion method.
The pods of C. spinosa were collected in Brazil in March 2002 and were identified by K. Murakami. One voucher specimen of the plant has been retained in our laboratory for future reference. After collection, the fruit pods were washed, dried under shade, and pulverized by a mechanical grinder. The pulverized materials (29.4 g) were extracted with 50% ethanol. The extracts (22.7 g) were partitioned between ethyl acetate and water. Only the fraction (15.3 g) extracted in ethyl acetate remarkably intensified oxacillin's anti-MRSA activity. This fraction was chromatographed on a SiO2 column eluted stepwise with n-hexane and n-hexane-ethyl acetate (1:1) and, finally, ethyl acetate. Fractions that showed the ILSMR effect were further separated on the same column eluted with chloroform-methanol and yielded an active fraction (76.8 mg).
Synthesis.
Commercially unavailable alkyl gallates were synthesized as described by Kubo et al. (22) with slight modifications. To a solution of 2 mM gallic acid and 2 mM alcohol in tetrahydrofuran, cooled at 0°C, an equal volume of a solution of 4.2 mM N,N′-dicyclohexyl carbodiimide in tetrahydrofuran was added. After the solution was stirred for 20 h, the solvent was removed under reduced pressure. The residue was extracted with ethyl acetate several times and filtered. The filtrate was washed successively with dilute aqueous citric acid solution, saturated aqueous NaHCO3 solution, and water, dried over MgSO4, and evaporated. The crude products were purified by SiO2 column chromatography (elution with 98:2 [vol/vol] CHCl3-MeOH). The structures of the synthesized esters were established by spectroscopic methods.
n-Heptyl gallate (10) data are as follows (numbers identifying chemical structures of gallic acid and its related compounds correspond to numbering in Fig. 1). Colorless plate, mp 95 to 96°C. 1H-NMR (CD3OD): δ 7.07 (2H, s, H-2, 6), 4.24 (2H, t, J = 6.5 Hz, H-8), 1.75 (2H, m, H-9), 1.35 to 1.46 (8H, m, H-10 to 13), 0.93 (3H, t, J = 6.9 Hz, H-14). EI-MS m/z (%): 268 (14) [M]+, 170 (100).
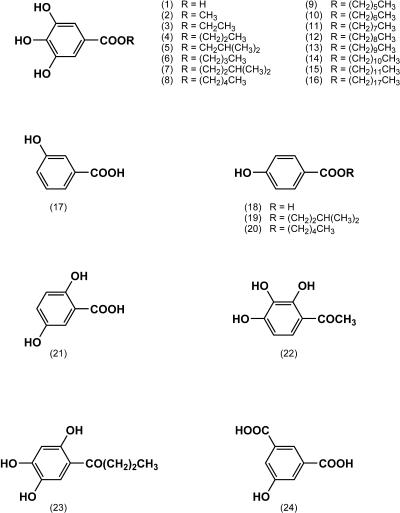
FIG. 1.
Chemical structures of gallic acid and its related compounds. The compounds examined are as follows (numbers in parentheses correspond to numbers in parentheses in the figure): gallic acid (1), methyl gallate (2), ethyl gallate (3), n-propyl gallate (4), isobutyl gallate (5), n-butyl gallate (6), isoamyl gallate (7), n-pentyl gallate (8), n-hexyl gallate (9), n-heptyl gallate (10), n-octyl gallate (11), n-nonyl gallate (12), n-decyl gallate (13), n-undecyl gallate (14), n-dodecyl gallate (15), n-stearyl gallate (16), m-hydroxybenzoic acid (17), p-hydroxybenzoic acid (18), 4-hydroxybenzoic acid isoamyl ester (19), 4-hydroxybenzoic acid n-amyl ester (20), 2,5-dihydroxybenzoic acid (21), 2′,3′,4′-trihydroxyacetophenone (22), 2′,4′,5′-trihydroxybutyrophenone (23), and 6-hydroxyisophthalic acid (24).
n-Nonyl gallate (12) data are as follows. Colorless plate, mp 92 to 93°C. 1H-NMR (CD3OD): δ 7.07 (2H, s, H-2, 6), 4.24 (2H, t, J = 6.5 Hz, H-8), 1.75 (2H, m, H-9), 1.32 to 1.47 (12H, m, H-10 to 15), 0.91 (3H, t, J = 7.0 Hz, H-16). EI-MS m/z (%): 296 (11) [M]+, 170 (100).
n-Decyl gallate (13) data are as follows. Colorless plate, mp 96 to 97°C. 1H-NMR (CD3OD): d 7.07 (2H, s, H-2, 6), 4.24 (2H, t, J = 6.5 Hz, H-8), 1.75 (2H, m, H-9), 1.31 to 1.47 (14H, m, H-10 to 16), 0.91 (3H, t, J = 7.0 Hz, H-17). EI-MS m/z (%): 310 (16) [M]+, 170 (100).
n-Undecyl gallate (14) data are as follows. Colorless plate, mp 94 to 95°C. 1H-NMR (CD3OD): 7.07 (2H, s, H-2, 6), 4.24 (2H, t, J = 6.5 Hz, H-8), 1.75 (2H, m, H-9), 1.31 to 1.47 (16H, m, H-10 to 17), 0.91 (3H, t, J = 7.0 Hz, H-18). EI-MS m/z (%): 324 (14) [M]+, 170 (100).
Antimicrobial activity.
The agar disk diffusion method was used to screen the antimicrobial activities of the plant extracts, a series of alkyl gallates, and related compounds. Samples were dissolved in dimethyl sulfoxide at a concentration of 10 mg/ml.
To screen for antimicrobial activity, MRSA strains 5 (a clinical isolate) and COL (a reference strain) were routinely used. An overnight liquid culture of the strain maintained at 37°C in Mueller-Hinton broth (MHB) was diluted with saline and finally adjusted with MHB supplemented with 25 μg of Ca2+/ml, 50 μg of Mg2+/ml, 2% NaCl, and 0.8% agar (semisolid cation adjusted-Mueller-Hinton agar [CA-MHA]) to obtain a final concentration of 105 CFU/ml.
The assay plates (No. 2 plate; Eiken Kizai, Tokyo, Japan) were composed of two layers: a 20-ml base layer and an 8-ml seed layer. The base layer was made from MHA supplemented with 25 μg of Ca2+/ml, 50 μg of Mg2/ml+, and 2% NaCl (CA-MHA), and the seed layer was prepared by use of semisolid CA-MHA with the test organism. For each strain, two plates were routinely prepared: one was CA-MHA, and the other was CA-MHA containing 10 μg of oxacillin/ml as the base layer. Sterile blank disks (Whatman AA disk; Whatman International Ltd., Maidstone, United Kingdom) (6 mm in diameter) were placed on the solidified agar surface. Then, 10 μl of each sample solution was transfused into the disks. The reference drugs used the antibiotics cephapirin, norfloxacin, and tetracycline. After 20 h of incubation at 37°C, the plates were screened for growth inhibition zones.
The MICs of the chemicals and antibiotics were determined by the twofold plate-dilution method with CA-MHA. Overnight cultures of test strains at 37°C in MHB were diluted with 0.85% NaCl, and the bacteria (about 106 CFU/ml) were applied with an inoculator onto the surfaces of 10-ml agar layers containing the test materials in the presence or absence of alkyl gallates. The plates were read after 20 h incubation at 37°C.
FIC testing.
Fractional inhibitory concentrations (FICs) were determined by the following formula: (MIC of oxacillin in combination/MIC of oxacillin alone) + (MIC of drug x in combination/MIC of drug x alone), where x is any of the drugs used in combination with oxacillin. The average FIC index was calculated from individual FICs. Synergism was defined as an FIC index ≤ 0.5, antagonism was defined as an FIC index > 4.0, and indifference was defined as an FIC index from >0.5 to 4.0 (17).
RESULTS
Screening test.
Antimicrobial screening tests of crude extracts (ethyl acetate, butanol, and water extracts) obtained from a 50% ethanol extract of dried tara pods were carried out by using two MRSA strains, S. aureus COL (a reference strain) and clinical isolate 5. Among the extracts, only the ethyl acetate fraction exhibited zones of inhibition with both strains. The zones on the medium containing 10 μg of oxacillin/ml were significantly larger than those on the antibiotic-free medium, demonstrating that the fraction displayed an ILSMR effect as well as antimicrobial activity. Activity-guided chromatography of the extract led to the isolation of active compounds, one of which was ethyl gallate. Other active compounds isolated from the extract, including 3,4,5-tri-O-galloylquinic acid, will be described elsewhere (21). The antimicrobial activity of ethyl gallate was reported previously (28), and recently Kubo et al. (22) also reported the antimicrobial activity of alkyl esters of gallic acid.
Antimicrobial activity.
In the present work, we first examined the antimicrobial effects of gallic acid and its related compounds (Fig. 1), because ethyl gallate is a congener of alkyl gallates. The MICs of the alkyl gallates for MRSAs and MSSAs were determined to compare the relative activity levels of these compounds. Table 1 shows the MIC ranges, the MICs at which 50% of isolates were inhibited (MIC50s), and those at which 90% of isolates were inhibited (MIC90s). Alkyl esterification of gallic acid resulted in compounds with a variety of antimicrobial activity levels. The MIC90s ranged between 15.6 and >250 μg/ml. The maximum activity against MRSA occurred in the nonyl gallate with an MIC90 of 15.6 μg/ml. Against MSSA, the optimum activity was found in the nonyl and decyl gallates, with the MIC90s of both being 15.6 μg/ml. m- and p-Hydroxybenzoic acid, 2,5-dihydroxybenzoic acid, and 5-hydroxyisophthalic acid at 250 μg/ml showed no antimicrobial activity against any of the strains of S. aureus used in the present work. On the other hand, the alkyl esters of 4-hydroxybenzoic acid (n-amyl and isoamyl esters) were effective. As reported previously (28), 2′,4′,5′-trihydroxybutyrophenone and 2′,3′,4′-trihydroxyacetophenone were also effective.
TABLE 1.
MICs of gallic acid and its related compounds for MRSA and MSSA strains
| Compounda | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| MRSA (n = 18)
|
MSSA (n = 8)
|
|||||
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | |
| Gallic acid (1) | 31.3-62.5 | 62.5 | 62.5 | 31.3-62.5 | 62.5 | 62.5 |
| Methyl gallate (2) | 62.5-250 | 250 | 250 | 62.5-250 | 250 | 250 |
| Ethyl gallate (3) | 62.5-125 | 125 | 125 | 62.5-125 | 125 | 125 |
| Propyl gallate (4) | 62.5-125 | 125 | 125 | 62.5-125 | 125 | 125 |
| isoButyl gallate (5) | 62.5-125 | 125 | 125 | 62.5-125 | 125 | 125 |
| Butyl gallate (6) | 62.5-125 | 125 | 125 | 62.5-125 | 125 | 125 |
| isoAmyl gallate (7) | 31.3-125 | 62.5 | 125 | 62.5-125 | 62.5 | 125 |
| Pentyl gallate (8) | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 |
| Hexyl gallate (9) | 31.3-62.5 | 31.3 | 62.5 | 31.3-62.5 | 62.5 | 62.5 |
| Heptyl gallate (10) | 31.3 | 31.3 | 31.3 | 31.3 | 31.3 | 31.3 |
| Octyl gallate (11) | 15.6-31.3 | 31.3 | 31.3 | 31.3 | 31.3 | 31.3 |
| Nonyl gallate (12) | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 |
| Decyl gallate (13) | 15.6-31.3 | 15.6 | 31.3 | 15.6 | 15.6 | 15.6 |
| Undecyl gallate (14) | 31.3 | 31.3 | 31.3 | 31.3 | 31.3 | 31.3 |
| Dodecyl gallate (15) | 15.6-62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 |
| Stearyl gallate (16) | >250 | >250 | >250 | >250 | >250 | >250 |
| m-Hydroxybenzoic acid (17) | >250 | >250 | >250 | >250 | >250 | >250 |
| p-Hydrobenzoic acid (18) | >250 | >250 | >250 | >250 | >250 | >250 |
| 4-Hydroxybenzoic acid isoamyl ester (19) | 31.3-62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 |
| 4-Hydroxybenzoic acid n-amyl ester (20) | 31.3-62.5 | 62.5 | 62.5 | 31.3-62.5 | 62.5 | 62.5 |
| 2, 5-Dihydroxybenzoic acid (21) | >250 | >250 | >250 | >250 | >250 | >250 |
| 2′,3′,4′-Trihydroxyacetophenone (22) | 31.3-62.5 | 62.5 | 62.5 | 31.3-62.5 | 62.5 | 62.5 |
| 2′,4′,5′-Trihydroxybutyrophenone (23) | 62.5-125 | 125 | 125 | 62.5-125 | 125 | 125 |
| 5-Hydroxyisophthalic acid (24) | >250 | >250 | >250 | >250 | >250 | >250 |
Compounds listed correspond to the chemical structures in Fig. 1 which are marked with the same numbers as those shown in parentheses.
ILSMR effect.
A series of alkyl gallates was screened for ILSMR activity by use of four β-lactams and nine other classes of antibiotics. To demonstrate the typical reactivity of alkyl gallates, we selected isoamyl gallate, since the compound was one of the most effective ILSMRs found in the present study. Table 2 shows the MIC ranges, MIC50s, and MIC90s of β-lactams and other classes of antibiotics used alone and in combination with a 25 μg/ml concentration of isoamyl gallate for MRSAs and MSSAs. At that concentration, isoamyl gallate showed no antimicrobial activity against any of the S. aureus strains examined (Table 1). When isoamyl gallate was incorporated into the medium, the MICs of the β-lactams tested decreased markedly. In contrast, the compound had no such effect on the MICs of other classes of antibiotics. The same result held good for other effective gallates (data not shown).
TABLE 2.
Effect of isoamyl gallate on antibacterial effects of β-lactams and other antibiotics on MRSA and MSSA strains
| Strain group and antibiotic | MIC (μg/ml) of antibiotic:
|
|||||
|---|---|---|---|---|---|---|
| Alone
|
With isoamyl gallate (25 μg/ml)
|
|||||
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | |
| MRSA | ||||||
| β-Lactam | ||||||
| Penicillin G | 16-64 | 32 | 64 | 0.06-8 | 1 | 8 |
| Ampicillin | 8-64 | 32 | 32 | 0.25-8 | 2 | 4 |
| Cephapirin | 16-128 | 64 | 128 | <0.06-16 | 0.5 | 4 |
| Oxacillin | 32->256 | 256 | >256 | <0.06-64 | 1 | 16 |
| Other antibiotic | ||||||
| Norfloxacin | 1-256 | 64 | 256 | 0.5->256 | 64 | 256 |
| Erythromycin | 0.25->256 | >256 | >256 | 0.25->256 | >256 | >256 |
| Kanamycin | 16->256 | >256 | >256 | 16->256 | >256 | >256 |
| Streptomycin | 64->256 | 64 | 128 | 16->256 | 32 | 64 |
| Arbekacin | 8-32 | 8 | 32 | 4-16 | 8 | 16 |
| Vancomycin | 1-4 | 1 | 2 | 1-2 | 2 | 2 |
| Chloramphenicol | 4-32 | 8 | 16 | 2-32 | 4 | 8 |
| Fosfomycin | 32->256 | >256 | >256 | 16->256 | >256 | >256 |
| Tetracyclin | 0.5-256 | 1 | 256 | 0.25-256 | 0.5 | 256 |
| MSSA | ||||||
| β-Lactam | ||||||
| Penicillin G | <0.06-8 | 0.13 | 8 | <0.06-0.25 | 0.06 | 0.25 |
| Ampicillin | 0.06-16 | 0.5 | 16 | 0.03-0.5 | 0.13 | 0.5 |
| Cephapirin | 0.06-0.5 | 0.25 | 0.5 | <0.06-0.06 | 0.06 | 0.06 |
| Oxacillin | 0.06-4 | 0.25 | 4 | 0.06-0.5 | 0.25 | 0.5 |
| Other antibiotic | ||||||
| Norfloxacin | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 |
| Erythromycin | 0.13-0.25 | 0.25 | 0.25 | 0.06-0.25 | 0.13 | 0.25 |
| Kanamycina | 8->256 | 32 | 32 | 0.06->256 | 16 | 32 |
| Streptomycin | 16-64 | 64 | 64 | 32-64 | 32 | 64 |
| Arbekacin | 4-32 | 8 | 32 | 2-16 | 4 | 16 |
| Vancomycin | 1-2 | 1 | 2 | 1-2 | 1 | 2 |
| Chloramphenicol | 2-8 | 4 | 8 | 2-4 | 2 | 4 |
| Fosfomycinb | 4->256 | 16 | 256 | 1-256 | 16 | 64 |
| Tetracyclinc | 0.5-16 | 1 | 1 | 0.25-16 | 0.25 | 0.5 |
Resistant strain, 1010 (MIC, >256 μg/ml).
Resistant strain, 1003 (256 μg/ml).
Resistant strains, 1020 (>256 μg/ml) and 1023 (16 μg/ml).
Table 3 summarizes the activities of a series of alkyl gallates in combination with oxacillin from their in vitro interactions against MRSAs and MSSAs. FIC results are shown only for those strains for which FICs could be calculated from on-scale results. The minimum and maximum FICs and the interpretations of the activities of the methyl to decyl gallates against MRSAs predominantly showed synergism. In particular, the ethyl to hexyl gallates, when combined with oxacillin, were synergistic against all or almost all strains of MRSA. In this study, pentyl gallate was found to be the most effective ILSMR (FIC index range for synergism, 0.07 to 0.40). The FIC index interpretations for the activities of the undecyl and dodecyl gallates against MRSAs predominantly showed indifference. In this case, the interpretations produced four determinations of synergism and two determinations of antagonism. That is, the undecyl and dodecyl gallates in combination with oxacillin were each synergistic against two MRSAs (FIC index ranges for synergism, 0.16 to 0.26); the undecyl gallate combined with oxacillin was antagonistic against two MRSAs (FIC indices for antagonism, 4.05). It is interesting that the FIC results for alkyl hydroxybenzoates and 2′,4′,5′-trihydroxybutyrophenone combined with oxacillin with respect to activity against MRSAs predominantly showed indifference. The interpretations produced two determinations of synergism and a determination of antagonism; 2′,4′,5′-trihydroxybutyrophenone combined with oxacillin was synergistic against two MRSAs (FIC indices for synergism, 0.40 and 0.43), and 4-hydroxybenzoic acid isoamyl ester in combination with oxacillin was antagonistic against one MRSA (FIC index for antagonism, 4.2).
TABLE 3.
FIC indices of combinations of gallic acid-related compounds and oxacillin for MRSA and MSSA strainsa
| Compoundb | Σ FIC index
|
|||
|---|---|---|---|---|
| MRSA
|
MSSA
|
|||
| Rangec | Average | Rangec | Average | |
| Methyl gallate (2) | 0.16-1.20 (11/18) | 0.51 ± 0.29 | 0.30-2.05 (1/8) | 1.24 ± 0.69 |
| Ethyl gallate (3) | 0.13-0.60 (17/18) | 0.32 ± 0.13 | 0.30-2.05 (1/8) | 1.23 ± 0.70 |
| Propyl gallate (4) | 0.13-0.45 (18/18) | 0.27 ± 0.13 | 0.30-2.10 (1/8) | 1.48 ± 0.78 |
| Isobutyl gallate (5) | 0.13-0.90 (17/18) | 0.30 ± 0.19 | 0.30-2.02 (1/8) | 1.12 ± 0.62 |
| Butyl gallate (6) | 0.12-0.70 (17/18) | 0.27 ± 0.15 | 0.30-2.05 (2/8) | 1.19 ± 0.75 |
| isoAmyl gallate (7) | 0.10-0.45 (18/18) | 0.19 ± 0.10 | 0.33-2.10 (2/8) | 1.17 ± 0.76 |
| Pentyl gallate (8) | 0.07-0.40 (18/18) | 0.16 ± 0.08 | 0.35-2.02 (2/8) | 1.08 ± 0.63 |
| Hexyl gallate (9) | 0.08-0.41 (17/17) | 0.19 ± 0.09 | 0.35-4.02 (1/8)d | 1.48 ± 1.20 |
| Heptyl gallate (10) | 0.12-1.20 (14/18) | 0.39 ± 0.32 | 0.33-2.02 (2/8) | 1.05 ± 0.67 |
| Octyl gallate (11) | 0.10-2.05 (13/18) | 0.42 ± 0.47 | 0.23-2.02 (3/8) | 0.99 ± 0.72 |
| Nonyl gallate (12) | 0.05-2.05 (11/17) | 0.42 ± 0.53 | 0.23-2.02 (3/8) | 0.99 ± 0.72 |
| Decyl gallate (13) | 0.05-2.05 (11/17) | 0.54 ± 0.65 | 0.23-2.02 (3/8) | 0.99 ± 0.72 |
| Undecyl gallate (14) | 0.16-4.05 (2/17)e | 1.38 ± 1.13 | 0.30-2.05 (3/8) | 1.14 ± 0.78 |
| Dodecyl gallate (15) | 0.18-1.20 (2/18) | 0.82 ± 0.33 | 0.45-2.02 (1/8) | 1.22 ± 0.53 |
| 4-Hydroxybenzoic acid isoamyl ester (19) | 1.20-4.20 (0/17)d | 1.39 ± 0.73 | —f | |
| 4-Hydroxybenzoic acid n-amyl ester (20) | 0.53-1.80 (0/18) | 0.97 ± 0.36 | — | |
| 2′,4′,5′-Trihydroxybutyrophenone (23) | 0.40-1.40 (2/18) | 1.03 ± 0.29 | — | |
Results for FIC indices ≥0.5 are synergistic, those for FIC indices > 0.5 to 4.0 are indifferent, and those for FIC indices >4.0 are antagonistic.
Compounds listed correspond to the chemical structures in Fig. 1 which are marked with the same numbers as those in the parentheses.
Figures in parentheses express the number of isolates for which drug combination resulted in synergism/number of isolates examined.
Drug combination resulted in antagonism for one strain.
Drug combination resulted in antagonism for two strains.
—, not determined.
FIC index interpretations of the activities of alkyl gallates against MSSAs predominantly showed indifference; the drug combinations were each synergistic for one to three strains of MSSA (FIC index range for synergism, 0.23 to 0.45). The interpretations of the activity of hexyl gallate combined with oxacillin produced a determination of antagonism (FIC index, 4.02).
DISCUSSION
MRSA is resistant to virtually all kinds of β-lactams, and it thereby threatens the most potent antibiotics we have (27). To recover the β-lactams' glory days, it will be desirable to identify novel compounds that dramatically intensify the susceptibility of MRSAs to β-lactams. We examined these requirements by screening extracts from a dried pod of C. spinosa (tara), yielding an active compound, ethyl gallate.
The results presented in Table 1 support and extend a previous finding in this laboratory, namely, that gallic acid and related compounds possess antimicrobial activity (28). With respect to the activities of the effective compounds, there are no differences between MRSAs and MSSA results. The length of the alkyl chain was found to be associated with antimicrobial activity, and the optimum chain length was C9 to C10. The data are in close agreement with those published by Kubo et al. (22).
In the present study, we found that some alkyl gallates at sub-MIC concentrations, in combination with β-lactams, modulated the activities of antibiotics by reducing the concentration of antibiotics needed to inhibit the growth of drug-resistant bacteria (Table 2 and Table 3). The synergistic activity of the alkyl gallates appears to be specific to β-lactams, because no significant changes were observed in the MICs of other classes of antibiotics examined in this study. Unlike the occurrence of antimicrobial activity described above, the presence of isoamyl gallate resulted in a modest increase in the susceptibility of MSSAs to β-lactams (e.g., the MIC50 change for ampicillin ranged from 0.5 to 0.13 μg/ml), whereas a much larger decrease in the MIC was found with MRSAs (e.g., the MIC50 change for ampicillin ranged from 32 to 2 μg/ml). These values reflect the magnitude of changes found for the β-lactams, i.e., the potency of the ILSMR. With some alkyl gallates (e.g., octyl, nonyl, and decyl gallates), their ILSMR potencies were equal to that of 6,7-dihydroxyflavone and they were more effective than other gallates (epicatechin gallate and epigallocatechin gallate) and than Triton X-100 (data not shown).
The molecule of an alkyl gallate consists of three sections: a lipophilic alkyl chain at one end is connected via an ester linkage to the galloyl group bearing the polar hydroxyl groups at the other end. This amphiphilic property makes the cell membrane of S. aureus one of the most likely target sites of the action of alkyl gallate.
Table 3 provides evidence that the length of the alkyl chain was also associated with the activity of ILSMR. In this case, however, the optimum length was C5 to C6, which differs from that for the antimicrobial activity described above. Thus, whether alkyl gallates work as antimicrobials or as ILSMRs appears to depend upon the chemical structure of the molecule's lipid moiety. Interestingly, however, no ILSMR-like activity was observed with n-amyl or isoamyl 4-hydroxybenzoate, despite a marked improvement in the antimicrobial activity of 4-hydroxybenzoic acid by the alkyl esterification (MICs were reduced from >250 to 31.3 μg/ml). This observation clearly demonstrates that the galloyl moiety of the molecules plays an important role in eliciting ILSMR activity. That the galloyl moiety plays a key role is supported by observations of other researchers that ILSMR-active substances, such as epicatechin gallate (33), epigallocatechin gallate (16, 41), tellimagrandin I (34), and corilagin (32), contain this moiety. Catechins without the galloyl moiety, such as epicatechin and epigallocatechin, had little ILSMR activity (data not shown).
In addition, we found that resveratrol 3-(6"-galloyl)-O-β-d-glucopyranoside possessed ILSMR activity, while resveratrol 3-(4"-acetyl)-O-β-d-glucopyranoside did not (26). These results are indicative of a feature common to the molecular structures of all the ILSMRs listed above: a lipophilic portion is connected via an ester linkage to the galloyl group bearing the polar hydroxyl groups. This amphiphilic property is presumably important for the establishment of a molecular interaction between the phospholipid acyl chains and some proteins that are membrane bound (1). The hydroxyl groups of the galloyl moiety would be placed near the lipid-water interface, where they could form hydrogen bonds with water and may also promote other types of interaction with the polar part of the phospholipid. These polar interactions will keep the alkyl gallate molecules in the upper part of the membrane. Such a location would perturb the membrane interiors, and the resulting restrictions on the fluidity of membrane components could sterically hinder substrate diffusion for PBPs, especially for PBP2a, and thereby decrease the kinetics of cell wall biosynthesis. Consequently, the alkyl gallates would cause a dramatic decrease in the MICs of β-lactams for MRSA strains. This is compatible with our hypothesis on the action mechanism of ILSMRs presented previously (29, 30).
Animal experiments performed with propyl, octyl, and dodecy gallates showed that no effects were observed at a dose level of 1,000 mg/kg of feed, a level that was adopted as the no-effect level by the FAO-WHO Joint Expert Committee on Food Additives in 1976 (38). In Japan, propyl and octyl gallates are recognized as quasi drugs by the Minister of Health, Labour and Welfare of Japan. These results indicate that gallates may be used safely not only as additives but also in clinical applications.
The results presented in this report highlight the potential of alkyl gallates to produce ILSMR leads with strong activity against a multidrug-resistant strain. Further work is presently under way to characterize the action mechanisms of these interesting compounds against multidrug-resistant S. aureus.
REFERENCES
- 1.Aranda, F. J., J. Villalain, and J. C. Gomez-Fernandez. 1995. Capsaicin affects the structure and phase organization of phospholipid membranes. Biochim. Biophys. Acta 1234:225-234. [DOI] [PubMed] [Google Scholar]
- 2.Bruns, W., H. Keppeler, and R. Baucks. 1985. Suppression of intrinsic resistance to penicillins in Staphylococcus aureus by polidocanol, a dodecyl polyethyleneoxid ether. Antimicrob. Agents Chemother. 27:632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2001. National Nosocomial Infections Surveillance (NNIS) report, data summary from January 1992-June 2001. Am. J. Infect. Control 29:404-421. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 7.Chang, F.-Y., B. B. MacDonald, J. E. Peacock, D. M. Musher, P. Triplett, J. M. Mylotte, A. O'Donnell, M. M. Wagener, and V. L. Yu. 2003. A prospective multicenter study of Staphylococcus aureus bacteremia. Incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine 82:322-332. [DOI] [PubMed] [Google Scholar]
- 8.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 9.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eid, C. N., N. G. Halligan, T. I. Nicas, D. L. Mullen, T. F. Butler, R. J. Loncharich. J. W. Paschal, C. J. Schofield, N. J. Westwood, and L. Cheng. 1997. Tripeptide LY301621 and its diastereomers as methicillin potentiators against methicillin resistant Staphylococcus aureus. J. Antibiot. 50:283-285. [PubMed] [Google Scholar]
- 11.Food and Agriculture Organization of the United Nations. 1995. Gums, resins and latexes of plant origin. Non-wood forest products 6. Food and Agriculture Organization of the United Nations. [Online.] http://www.fao.org/docrep/v9236e/V9236e00.htm. Accessed 17 May 2004.
- 12.Fukuda, N., T. Yamase, and Y. Tajima. 1999. Inhibitory effect of polyoxotungstates on the production of penicillin-binding proteins and β-lactamase against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 22:463-470. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons, S., B. Ohlendorf, and I. Johnsen. 2002. The genus Hypericum—a valuable resource of anti-staphylococcal leads. Fitoterapia 73:300-304. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 15.Hershberger, E., S. Donabedian, K. Konstantinou, and M. J. Zervos. 2004. Quinupristin-dalfopristin resistance in gram-positive bacteria: mechanism of resistance and epidemiology. Clin. Infect. Dis. 38:92-98. [DOI] [PubMed] [Google Scholar]
- 16.Hu, Z. Q., W. H. Zhao, N. Asano, Y. Yoda, Y. Hara, and T. Shimamura. 2002. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:558-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenberg, H. D. 1992. Clinical microbiology procedures handbook, vol. 1. American Society for Microbiology, Washington, D.C.
- 18.Komatsuzawa, H., M. Sugai, C. Shirai, J. Suzuki, K. Hiramatsu, and H. Suginaka. 1995. Triton X-100 alters the resistance level of methicillin-resistant Staphylococcus aureus to oxacillin. FEMS Microbiol. Lett. 134:209-212. [DOI] [PubMed] [Google Scholar]
- 19.Komatsuzawa, H., M. Sugai, K. Ohta, T. Fujiwara, S. Nakashima, J. Suzuki, C. Y. Lee, and H. Suginaka. 1997. Cloning and characterization of the fmt gene which affects the methicillin resistance level and autolysis in the presence of triton X-100 in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 41:2355-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsuzawa, H., J. Suzuki, M. Sugai, Y. Miyake, and H. Suginaka. 1994. The effect of Triton X-100 on the in-vitro susceptibility of methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 34:885-897. [DOI] [PubMed] [Google Scholar]
- 21.Kondo, K., Y. Takaishi, H. Shibata, and T. Higuti. 2004. ILSMRs (intensifier of β-lactam-susceptibility in methicillin-resistant Staphylococcus aureus) from tara [Caesalpinia spinosa (Molina) Kuntze]. Phytomed. (in press). [DOI] [PubMed]
- 22.Kubo, I., P. Xiao, and K. Fujita. 2002. Anti-MRSA activity of alkyl gallates. Bioorg. Med. Chem. Lett. 12:113-116. [DOI] [PubMed] [Google Scholar]
- 23.Liu, I. X., D. G. Durham, and R. M. Richards. 2000. Baicalin synergy with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other β-lactam-resistant strains of S. aureus. J. Pharm. Pharmacol. 52:361-366. [DOI] [PubMed] [Google Scholar]
- 24.Lowy, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 25.Nicolson, K., G. Evans, and P. W. O'Toole. 1999. Potentiation of methicillin activity against methicillin-resistant Staphylococcus aureus by diterpenes. FEMS Microbiol. Lett. 179:233-239. [DOI] [PubMed] [Google Scholar]
- 26.Okasaka, M., Y. Takaishi, K. Kogure, K. Fukuzawa, H. Shibata, and T. Higuti. 2004. New stilbene derivatives from Calligonum leucocladum. J. Nat. Products (in press). [DOI] [PubMed]
- 27.Projan, S. J. 2000. Antibiotic resistance in the staphylococci, p. 463-470. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 28.Sato, Y., H. Oketani, K. Singyouchi, T. Ohtsubo, M. Kihara, H. Shibata, and T. Higuti. 1997. Extraction and purification of effective antimicrobial constituents of Terminalia chebula RETS against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 20:401-404. [DOI] [PubMed] [Google Scholar]
- 29.Sato, Y., H. Shibata, N. Arakaki, and T. Higuti. 2004. 6,7-Dihydroxyflavone dramatically intensifies the susceptibility to β-lactam antibiotics in methicillin-resistant and -sensitive Staphylococcus aureus. Antimicrob. Agents Chemother. 48:1357-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato, Y., H. Shibata, T. Arai, A. Yamamoto, Y. Okimura, N. Arakaki, and T. Higuti. 2004. Variation in synergistic activity by flavone and its related compounds on the increased susceptibility of various strains of methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. Int. J. Antimicrob. Agents 24:28-35. [DOI] [PubMed] [Google Scholar]
- 31.Shibata, H., C. Shirakata, H. Kawasaki, Y. Sato, T. Kuwahara, Y. Ohnishi, N. Arakaki, and T. Higuti. 2003. Flavone markedly affects phenotypic expression of β-lactam resistance in methicillin-resistant Staphylococcus aureus strains isolated clinically. Biol. Pharm. Bull. 26:1478-1483. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu, M., S. Shiota, T. Mizushima, H. Ito, T. Hatano, T. Yoshida, and T. Tsuchiya. 2001. Marked potentiation of activity of β-lactams against methicillin-resistant Staphylococcus aureus by corilagin. Antimicrob. Agents Chemother. 45:3198-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiota, S., M. Shimizu, T. Mizushima, H. Ito, T. Hatano, T. Yoshida, and T. Tsuchiya. 1999. Marked reduction in the minimum inhibitory concentration (MIC) of β-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis). Biol. Pharm. Bull. 22:1388-1390. [DOI] [PubMed] [Google Scholar]
- 34.Shiota, S., M. Shimizu, T. Mizusima, H. Ito, T. Hatano, T. Yoshida, and T. Tsuchiya. 2000. Restoration of effectiveness of β-lactams on methicillin-resistant Staphylococcus aureus by tellimagrandin I from rose red. FEMS Microbiol. Lett. 185:135-138. [DOI] [PubMed] [Google Scholar]
- 35.Shirai, C., M. Sugai, H. Komatsuzawa, K. Ohta, M. Yamakido, and H. Suginaka. 1998. A triazine dye, cibacron blue F3GA, decreases oxacillin resistance levels in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 42:1278-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki, J., H. Komatsuzawa, M. Sugai, K. Ohta, K. Kozai, N. Nagasaka, and H. Suginaka. 1997. Effects of various types of Triton X on the susceptibilities of methicillin-resistant staphylococci to oxacillin. FEMS Microbiol. Lett. 153:327-331. [DOI] [PubMed] [Google Scholar]
- 37.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 38.Van der Heijden, C. A., P. J. Janssen, and J. J. Strik. 1986. Toxicology of gallates: a review and evaluation. Food Chem. Toxicol. 24:1067-1070. [DOI] [PubMed] [Google Scholar]
- 39.Wilson, P., J. A. Andrews, R. Charlesworth, R. Walesby, M. Singer, D. J. Farrell, and M. Robins. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186-188. [DOI] [PubMed] [Google Scholar]
- 40.Yamase, T., N. Fukuda, and Y. Tajima. 1996. Synergistic effect of polyoxotungstates in combination with β-lactam antibiotics on antibacterial activity against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 19:459-465. [DOI] [PubMed] [Google Scholar]
- 41.Zhao, W. H., Z. Q. Hu, S. Okubo, Y. Hara, and T. Shimamura. 2001. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]