Abstract
Bloodstream infection is a hallmark of sepsis, a medically emergent condition requiring rapid treatment. However, upregulation of host defense proteins through toll-like receptors and NFκB requires hours after endotoxin detection. Using confocal pulmonary intravital microscopy, we identified that the lung provides a TLR4-Myd88-and abl tyrosine kinase-dependent niche for immediate CD11b-dependent neutrophil responses to endotoxin and Gram-negative bloodstream pathogens. In an in vivo model of bacteremia, neutrophils crawled to and rapidly phagocytosed Escherichia coli sequestered to the lung endothelium. Therefore, the lung capillaries provide a vascular defensive niche whereby endothelium and neutrophils cooperate for immediate detection and capture of disseminating pathogens.
Introduction
Sepsis is a deleterious host response to an infection that continues to have an unacceptably high mortality(1). Of particular concern is that sepsis with bacteremia, or bloodstream infection, has even worse outcomes in humans (2). Bloodstream infections are common both in the community (3–5) and in the hospital (6–9). Escherichia coli, is a major overall causative organism found in community acquired and hospital acquired bloodstream infections (3, 10). Alarmingly, gram-negative bacteria are rapidly developing resistance to broad-spectrum antibiotics and in some circumstances have become pan-antibiotic resistant (11, 12). It is now recognized that sepsis is a medical emergency, similar to heart attacks and strokes, whereby every hour of untreated infection dramatically decreases the likelihood of patient survival (13). Likewise, the innate immune system must provide immediate protection when pathogens enter the bloodstream until the administration of effective antibiotics.
Lipopolysaccharide (LPS) is the major pathogen associated molecular pattern of gram-negative bacteria that binds and stimulates TLR4 (14). The signalling pathway is extremely well established and includes the activation of Myd88, IRAK-4 leading to NFκB translocation to the nucleus, and subsequent transcription and translation of various pro-inflammatory molecules (15–17). Indeed, macrophage begin to synthesize TNF-alpha and numerous chemokines while endothelium begins to synthesize E-selectin, ICAM-1 and VCAM-1 and additional chemokines in an attempt to recruit the main effector cell of innate immunity, namely the neutrophil (18). Neutrophils do not require protein synthesis to perform phagocytosis, oxidant production, degranulation or NET release when reacting to pathogens (19). Each of these effector functions occur in seconds following bacteria stimulation and has been attributed to opsonisation and rapid production of pro-inflammatory complement fragments (20). LPS, by contrast, classically requires hours for effector function to be fully elicited through transcription and translation. Contrary to this classical view, but for the most part ignored, is the observation that LPS can induce increases in neutrophil cell surface adhesion molecules and binding in minutes (21, 22). The biological relevance of this rapid non-transcriptional response to host defence in vivo is limited.
Conventionally, the capture of free-flowing particles and circulating pathogens is attributed to stationary resident mononuclear cells within the liver and spleen (23–25). This resident cellular defense system does not require active pursuit or tracking of the pathogen but absolutely requires that the bug remain in the free flow circulation. The liver is home to resident intravascular macrophages called Kupffer cells, which catch free flowing bacteria via the complement receptor Ig (CRIg) as they filter through the sinusoids (26–28). Despite the clear role of resident macrophages in clearing blood borne pathogens, the major risk factor for acquiring a bloodstream infection is not macrophage dysfunction but neutropenia (29). As well, the best prognostic factor in bacteremic cancer patients is the recovery of neutrophil counts (30). Both humans and mice do not have pulmonary intravascular macrophages 21, 22, however pathogens can sequester within the massive surface area of the capillary network potentially evading removal (26); therefore, it is unclear how the host detects and removes bloodstream pathogens within this macrophage free vascular labyrinth.
The prototypical neutrophil recruitment cascade is a multistep process requiring the endothelium to express molecules (selectins) to slow circulating leukocytes (tethering), followed by vascular rolling and firm adhesion (selectins and integrins)(31). Finally, leukocytes transmigrate the endothelium in a process termed emigration(32). However, this canonical recruitment paradigm may notbe true for the lung. The capillary vessels, distinct from larger venules, do not require known molecules involved in rolling, such as the selectins, during inflammation (33–35). Additionally, although integrins can mediate firm adhesion in lung capillaries, the prevailing understanding is that neutrophils lose cellular deformability and become physically stuck within the small vessels and subsequently emigrate into the alveolus (36–38). It remains unclear if physically stuck neutrophils provide any biological function other than emigrating into the lung tissue. Emigration in the lung is a distinct process mediated by both CD11/CD18 dependent and independent processes contingent on the initial stimulant (39–41). A previous report demonstrated that at baseline a substantial number of extravascular neutrophils inhabited the lung and that following bacterial stimulation vascular neutrophils were rapidly arrested in the microvasculature and the resident neutrophils crawled throughout the extravascular space (interstitial and alveolus), yet intravascular crawling was not reported (42). The existence of resident extravascular tissue neutrophils in the lung remains controversial and not consistently supported (43, 44). Despite extensive research to delineate molecules required during the neutrophil recruitment cascade leading to tissue emigration, intravascular neutrophil behaviors and functions, remain unexplored.
Using pulmonary intravital microscopy, we have discovered that vascular neutrophils immediately become alerted to the presence of endotoxin or bloodstream infection through TLR4 and Myd88 signaling. After intravenous endotoxin challenge, neutrophils sequestered within the capillaries but not larger venules, polarized, and crawled throughout the endothelium in a CD11b-dependent process. Importantly, lung-sequestered bacteria were removed from circulation by the capillary neutrophils. Therefore, we have discovered a host defense system involving the pulmonary capillary circulation and neutrophils that eliminates bloodstream pathogens that are no longer in free-flowing circulation and thus not amendable to capture by resident macrophages.
Results
Neutrophils rapidly upregulate the expression of CD11b after LPS exposure
Non-transcriptional alterations inneutrophil behavior after endotoxin exposure are reported, yet the mechanisms and biological consequences remain less defined (22, 38, 45). Under untreated conditions, neutrophils, defined by flow cytometry as Ly6G+ (fig. S1), expressed both CD11b and L-selectin. Mouse neutrophils treated with either LPS (5μg/ml) or a strong chemical pro-inflammatory stimulus, N-formyl-met-leu-phe (fMLP1μM-postive control), revealed substantial upregulation of cell surface CD11b (Fig. 1A) and decrease in L-selectin within minutes, thus replicating previous reports (Fig. 1B) (21). As a positive control, the CD11b expression increased with fMLP and L-selectin shed within 4minutes of stimulation. LPS treated neutrophils increased expression of CD11b and shed L-selectin at the 15-minute time point suggesting some delay compared to the fMLP pathway. This effect continued into the 30-minute time point. CD11b was assessed in permeabilized cells and the total CD11b was unaffected suggesting shuttling of protein from internal granules to the surface (fig. S2). As shown later, these events led to rapid phenotypic changes in neutrophil behavior in vivo and could not be affected by transcription and translational inhibitors.
Fig. 1. LPS induces rapid cell surface increases in neutrophil CD11b in mouse neutrophils and in neutrophils from humans treated with intravenous LPS.
Peripheral blood leukocytes from wildtype mice were treated with either saline, fMLP (1μM) or LPS (5μg/ml) for 4, 15, and 30 min and flow cytometry was performed to assess levels of cell surface expression of (A) CD11b and (B) L-selectin on Ly6G+ cells (n = 3). Five healthy human volunteers received intravenous LPS and peripheral blood was obtained over time. (C) Flow cytometry was performed to assess the level of cell surface CD11b pre-and post LPS and the gate demonstrates CD11b high expressing neutrophils. (D) The total number of peripheral neutrophils from five individuals treated with LPS (left axis, mean ± SEM, ** P = 0.022, n = 5, one way ANOVA with Dunnett’s correction) is graphed along with the percentage of CD11b high cells from these individuals (right axis, mean ± SEM, * p = 0.02, n = 5, one way ANOVA with Dunnett’s correction). (E) Human peripheral blood leukocytes were treated with LPS (100 ng/ml, 15 minutes) and adhesion was assessed using a flow chamber lined with primary human pulmonary endothelium (n = 3, mean ± SD, ** p = 0.002, unpaired two tailed T test).
To confirm that modulation of CD11b occurs in vivo in humans, healthy volunteers received intravenous highly-purified LPS (2 ng/kg) and blood was drawn over time to assess neutrophil numbers and how they coincided with CD11b+ expression on neutrophils (Fig. 1C and D). After 30 minutes of LPS, the number of circulating neutrophils dropped by 50% and simultaneously there was evidence of an increase in CD11b expression. This drop in neutrophil counts and increase of CD11b persisted for at least 90 minutes. Therefore, human peripheral neutrophil counts dropped as CD11b increased, analogous to our findings later in mice, where neutrophils increased CD11b following LPS and accumulated in the lung. It was impossible to examine the lung vasculature in humans; however, primary human pulmonary endothelium was cultured to confluence and LPS stimulated (15 min) human neutrophils, or control untreated cells, were assayed within a flow chamber to mimic a surrogate lung microvasculature. Neutrophil adhesion on lung endothelium occurred at baseline without stimulation, a phenomenon we have not observed previously using dermal human microvascular cells (46). Conventionally, investigations have pre-treated HUVEC for a minimum of 4 hours prior to leukocyte adhesion assays to allow time for the synthesis of adhesion molecules such as E-selectin and ICAM-1(47, 48). However, adhesion was significantly increased following 15 min of LPS stimulation of human neutrophils independent of endothelial pre-treatment (Fig. 1E). Importantly, the parallel plate flow chamber provides a physical space between the endothelium and chamber that is much larger than a capillary dimension; thus, the LPS treated neutrophils cannot become physically stuck or lodged in this assay, suggesting a different mechanism of rapid adhesion compared to cytoskeleton stiffening (38). Collectively, these data support the concept that LPS induces a rapid neutrophil response in humans’ in vitro and in vivo leading to modulation of cell surface adhesion molecules. Although rapid changes in integrin and selectins on the surface of neutrophil have been demonstrated, the biological significance and underlying mechanism in vivo that contribute to host defense remain uncertain (21). To understand the biology of these observations we moved to mouse models and imaged the lung vasculature in vivo.
Behaviours of lung neutrophils
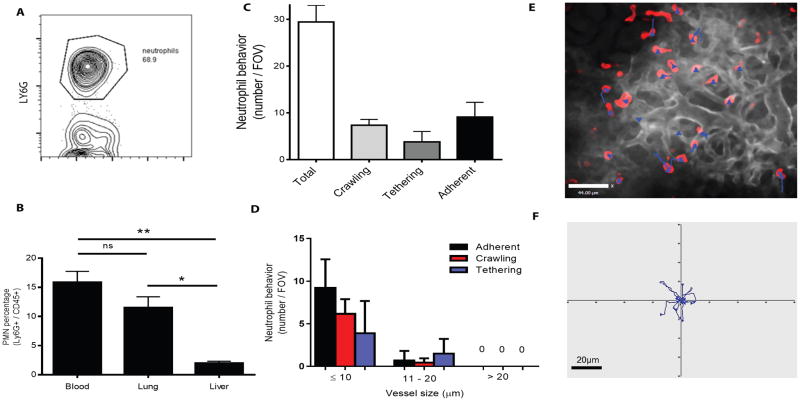
We quantified Ly6G+ neutrophils using flow cytometry in whole flushed mouse lungs exsanguinated of peripheral blood and found the percentage of neutrophils to total leukocytes was significantly higher than in flushed liver, another highly vascular organ (Fig. 2A and B). Using an established flow cytometry method to characterize neutrophil anatomic localization, we observed that most lung neutrophils were intravascular, with a minimum number of extravascular neutrophils under baseline conditions (Fig. S3). This agrees with other reports showing the vast majority of neutrophils in the lung are intravascular (43, 44). Using pulmonary intravital microscopy, we directly visualized neutrophils within the lung vasculature and found a distribution of three behavioral phenotypes: tethering, crawling and firm adhesion. Under basal conditions, most interactions occurred within the capillaries (vessel ≤ 10μm diameter) and no interactions were observed in vessels larger than 20μm (Fig. 2C and D). Strikingly, some neutrophils demonstrated tethering, but none of these capture events translated into rolling. We did not observe rolling cells in these capillaries. One third of neutrophils crawled short distances along the vessel walls, an uncommon behavior in other untreated vascular beds (49–51). Lung capillary neutrophils were manually tracked over a 10-minute duration; however, all imaging experiments were performed for at least 1 hour per mouse to allow evaluation of various fields of view (movie S1). Examples of tracks are displayed for individual neutrophils as blue lines superimposed on an intravital image or from one field of view plotted with a common origin point (Fig. 2E and F). At baseline, capillary neutrophils crawled distances less than 20μm (3–4 cell lengths) and 35% of neutrophils did not crawl but remained stationary (adherent).
Fig. 2. Defining neutrophil behavior within the pulmonary circulation in vivo using intravital microscopy.
(A) Ly6G+ cells were quantified using flow cytometry of exsanguinated untreated C57BL/6J whole lungs. (B) Ly6G+ cells were compared in blood, lung and liver of untreated C57BL/6J mice (mean ± SD, ** p = 0.0017, * p = 0.011, n=3, one way ANOVA). (C) Three neutrophil phenotypes, crawling, tethering and adhesion, were identified in vivo using pulmonary intravital microscopy (n=3). (D) Quantification of lung neutrophil behaviors during untreated conditions in vivo in relation to vessel diameter (n = 3). (E) Manually tracked pulmonary neutrophils in an unstimulated mouse. Neutrophils (red, intravenous fluorescently conjugated anti-Ly6G, clone 1A8), vasculature (greyscale, intravenous fluorescently conjugated anti-CD31, clone 390) and blue tracks displaying 10 minutes of tracking time. (F) Individual tracks displayed from a representative mouse over 10 minutes, compared from a central origin point.
LPS rapidly induced neutrophil crawling within the pulmonary circulation
Crawling, which occurred at baseline in a third of neutrophils, rapidly and significantly increased in terms of the number of cells crawling and the distance they crawled following intravenous stimulation with LPS. Baseline crawling distance averaged 20 μm (10 min of tracking), but within 10 minutes of a low dose of LPS (10 μg i.v.), neutrophil crawling distance significantly increased to an average of 38 μm (10 min of tracking) and further to 50 μm (10 min of tracking) 20 minutes after LPS stimulation (Fig. 3A). Tracking data was obtained in 10-minute interval periods; however, each individual imaging experiment lasted at least 1 hour per mouse. Neutrophils accumulated within the field of view and were significantly increased 30 minutes after LPS (Fig. 3B). Vascular neutrophils rapidly polarized with a lead pseudopod and a uropod tail, a phenotype that is essential prior to cellular mobility. Manual tracking between the 20–30 min. post LPS interval revealed very long crawling tracks and vascular neutrophils were not observed to emigrate out of the microvessels (Fig. 3C and D and movie S2).
Fig. 3. Lung neutrophils rapidly begin crawling throughout the vasculature following LPS exposure.
Lung neutrophils were tracked for 10 minutes prior to stimulation to quantify baseline neutrophil characteristics. (A) LPS (10μg i.v.) was administered and neutrophils were tracked over time. Each symbol represents an individual neutrophil combined from three separate experiments. Statistical testing was performed by averaging crawling distance of all neutrophils tracked from each mouse and using this as a single n value (mean ± SD, * p= 0.0169, ** p = 0.0014, one way ANOVA, n = 3). (B) Total neutrophil accumulation was quantified per field of view prior to and 30 minutes following LPS intravenous administration (mean ± SD, *** p = 0.0001, n = 5, unpaired two tailed T test). (C) A representative pulmonary intravital image with neutrophils tracked between 20–30min post-LPS. Neutrophils (red, i.v. fluorescently conjugated anti-Ly6G, clone 1A8, 3.5μg/mouse), vasculature (greyscale, i.v. fluorescently conjugated anti-CD31, clone 390, 5μg/mouse) and blue tracks displaying 10 minutes of tracking time. (D) Individual tracks displayed from the experiment depicted in panel C. Tracks are plotted from a central common origin point.
Basal and enhanced rapid vascular crawling behavior requires CD11b
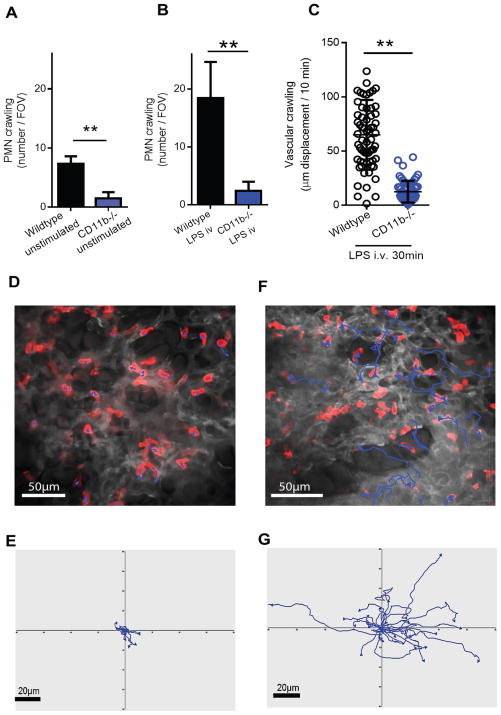
The adhesion molecules mediating either baseline crawling or rapid LPS stimulated crawling in the lung are unknown; however, β2-integrins mediate crawling in inflamed muscle vasculature (50). The number of neutrophils demonstrating crawling under unstimulated conditions was significantly lower in CD11b-deficient animals (Fig. 4A) and profoundly attenuated at 30 minutes of LPS stimulation (Fig. 4B and movie S3), while the phenotype for CD11a-deficiency was less obvious (fig. S4). Crawling distance of LPS treated CD11b-deficient mice remained lower (Fig. 4C, D, E and movie S3) then LPS treated wildtype mice (Fig. 4F and G). Therefore, in relation to rapid upregulation of CD11b in mouse and human neutrophils (Fig. 1), we now have a specific biological reason for this, namely crawling within the lung microcirculation following pathogen related stimulation.
Fig. 4. CD11b mediates baseline and LPS induced rapid pulmonary crawling in vivo.
Neutrophil crawling was compared between wildtype C57BL/6 and Cd11b-deficient mice under (A) control (saline i.v. 30 min, mean ± SD, ** p = 0.0013, n = 4 C57BL/6 and n = 3 for Cd11b-deficient, unpaired two tailed T test) and (B) LPS (10μg i.v. 30 min, mean ± SD, ** p = 0.0025, n = 4, unpaired two tailed T test) treated conditions. (C) Neutrophil crawling distance was compared between wildtype and Cd11b-deficient mice following stimulation (LPS 10μg i.v. 30min, mean ± SD, * p = 0.01, n = 3, unpaired two tailed T test). Neutrophil tracks from LPS (10μg i.v. 30 min) treated Cd11b-deficient mice are depicted in (D) and (E) from a representative intravital experiment. Neutrophil tracks from LPS (10μg i.v. 30 min) treated wildtype mice are depicted in (F) and (G) from a representative intravital experiment.
LPS-induced rapid crawling requires TLR4 and Myd88 in vivo
Reports indicate that LPS may have non-TLR4 intracellular receptors (52, 53); and perhaps these mediate the non-transcriptional phenotypic changes in cellular behaviour in vivo. Therefore, we initially tested if the rapid LPS mediated crawling is downstream of TLR4. Using pulmonary intravital microscopy, we directly visualized neutrophils in the capillaries of Tlr4-deficient mice during LPS intravenous challenge. Indeed, there was no increase in neutrophil crawling behaviour after LPS (Fig. 5A and B), confirming that TLR4 is the major physiological pattern recognition receptor involved in this process. Myd88 is the major downstream signalling molecule for all toll-receptors except TLR3, and is essential for linking membrane signalling to activation of nuclear receptors and modulation of nuclear transcription. To test whether LPS induced rapid crawling (which occurs temporally faster than gene transcription, translation and expression) occurs via this pathway we examined neutrophils in lungs of Myd88-deficient mice using pulmonary intravital microscopy and observed very little increase in basal crawling level in these animals (Fig. 5C and D). Myd88-deficient mice had higher basal crawling than either wildtype or Tlr4-deficient mice, however this was not due to differences in baseline levels of lung neutrophil CD11b expression (fig. S5).
Fig. 5. Rapid neutrophil crawling is TLR4 and Myd88 dependent.
Pulmonary intravital microscopy was used to directly assess the molecular requirement of rapid neutrophil crawling following LPS stimulation. Tlr4-deficient mice were imaged and (A) neutrophil crawling was quantified pre-and post LPS (10μg i.v. mean ± SD, n = 3, one way ANOVA, not significant). (B) Neutrophil tracking is displayed from a representative experiment between 20–30 min post LPS. Myd88-deficient mice were imaged and (C) neutrophil crawling was quantified pre-and post LPS (10μg i.v. mean ± SD, n = 3, one way ANOVA, not significant). (D) Neutrophil tracking is displayed from a representative experiment between 20–30 min post LPS.
Rapid LPS induced neutrophil phenotype changes require abl-kinase
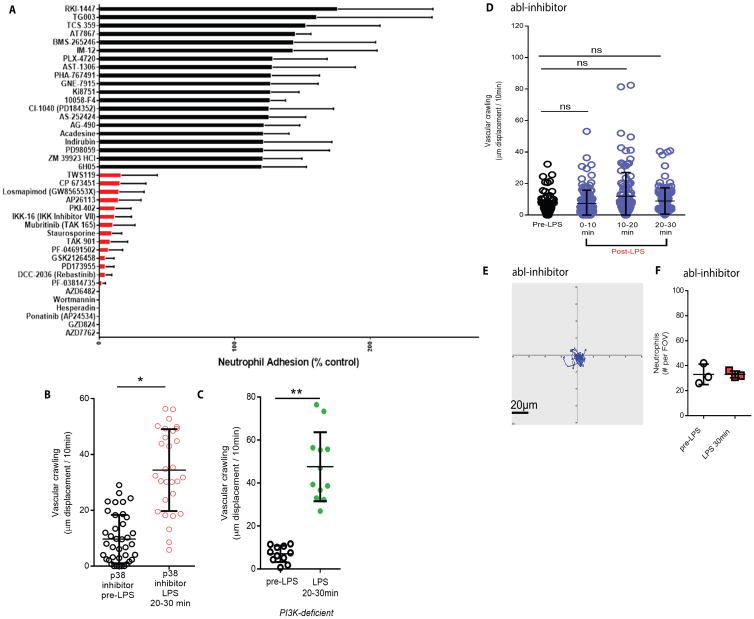
To better understand the pathway linking Myd88 and rapid changes in CD11b and crawling, we performed a large screen of defined pharmacological inhibitors to determine candidate targets that could inhibit LPS mediated rapid adhesion in isolated human neutrophils (Fig. 6A). We first confirmed that rapid LPS mediated adhesion did not require new transcription or translation (fig. S6). We next screened a pharmacologicalinhibitor library. The adhesion data are ordered from the lowest to highest effect on adhesion and the least effective (black), and most effective (red) 20 compounds are demonstrated in triplicate. Five compounds eliminated rapid LPS-activated neutrophil binding including two different PI3K inhibitors. Additionally, several inhibitors of p38 MAPK had moderate inhibitory effects. These pathways mediate neutrophil chemotaxis making these likely targets (54–56). Two compounds, GZD824 and ponatinib, are direct and potent inhibitors of the non-receptor tyrosine kinase abl. Interestingly, abl is one of the few tyrosine kinases able to directly bind and modulate both cytoskeletal elements and integrins in an inside-out activation model (57, 58).
Fig. 6. The abl-tyrosine kinase mediates rapid vascular crawling in vivo following LPS stimulation.
(A) A pharmacological inhibitor screen (1 μM) was performed to assess molecules involved in rapid neutrophil adhesion following 30 minutes of LPS stimulation in vitro. Adhesion levels were rank ordered and only the 20 least effective (black) and 20 most effective inhibitors (red or absent bars) are displayed. (B) A specific and potent p38-MAP kinase inhibitor (SB 239063, 10 μg/gram) was administered intravenously prior to LPS and vascular crawling was quantified (mean ± SD, * p = 0.018, n = 3, unpaired two tailed T test). (C) Mice deficient in PI3K underwent pulmonary intravital prior to and following intravenous LPS. Vascular crawling displacement was quantified (mean ± SD, ** p = 0.066, n = 3, unpaired two tailed T test). (D) Wildtype mice received GZD824, a specific abl-kinase inhibitor (5 μg/gram i.v.) 30 minutes prior to baseline pulmonary intravital imaging. Treated mice were imaged and neutrophil crawling was quantified (mean ± SD, n = 3, one way ANOVA, not significant) and (E) tracking determined. (F) Neutrophil accumulation was quantified in mice pretreated with abl-inhibitor before and after LPS administration (mean ± SD, n = 3, unpaired two tailed T test, not significant).
Our initial focus was on p38 MAPK and PI3K. Inhibition of these molecules, either by administration of a pharmacological inhibitor for p38 MAPK (Fig. 6B) or by using the PI3K-deficient mouse did not inhibit LPS-dependent rapid neutrophil crawling in vivo (Fig. 6 B and C). This is very interesting as we have previously reported an important role for p38 MAPK and PI3K in fMLP and chemokine dependent crawling respectively in vitro and in vivo, suggesting that the LPS pathway is distinct from these chemotactic pathways(54, 55, 59, 60). We next tested if inhibiting abl disrupted rapid LPS induced crawling in vivo. Mice received intravenous GZD824 (5 μg/gram, i.v.), currently the most potent and specific pharmacological inhibitor of abl (61, 62), thirty minutes prior to LPS administration. Remarkably, neutrophils were incapable of enhanced crawling even after 30 min. of LPS exposure (Fig. 6 D and E). Additionally, neutrophil accumulation was attenuated at 30 min. post LPS, unlike in LPS treated mice that did not receive the abl inhibitor (Fig. 6F). In vitro, mouse blood neutrophils did not increase cell surface CD11b after LPS exposure when abl was inhibited (fig. S7). Clearly, abl mediates the rapid LPS dependent upregulation of CD11b.
Abl-kinase links LPS cellular activation to CD11b expression on neutrophils in vivo
To determine if abl was directly influencing CD11b rapid expression following LPS in vivo, we used pulmonary intravital microscopy to visualize CD11b expression in Ly6G+ neutrophils in mice pretreated with the abl-inhibitor. Following twenty minutes of intravenous LPS, Ly6G+ cells became CD11b-bright and overlapping Ly6G+/CD11b+ staining wasvisualized as yellow (Fig. 7A and movie S4). CD11b-staining was predominantly observed as it accumulated in the tail and pseudopod of crawling cells as previously reported in vitro(63). Animals pretreated with intravenous abl-inhibitor had lower visual evidence of Ly6G+/CD11b+ overlap (Fig. 7B and movie S4). Moreover, all images between 20–40 min. post LPS, were quantified for CD11b positivity using two different strategies. First, we measured the total area of overlapping Ly6G+ and CD11b+ per field of view of each image and averaged this over 20 minutes of imaging (Fig. 7C). Then we quantified the number of individual Ly6G+ cells that were also CD11b+ by imaging (Fig. 7D). Abl-inhibition resulted in significant impairment of CD11b increases in lung neutrophils; thereby directly linking abl-function to rapid neutrophil behavior in vivo through CD11b.
Fig. 7. Abl-kinase mediates rapid CD11b dependent hunting during endotoxemia in vivo.
Pulmonary intravital microscopy compared LPS induced CD11b upregulation in abl-inhibitor treated versus saline treated mice. (A) LPS enhanced CD11b expression (intravenous fluorescently conjugated monoclonal antibody clone M1/70, 2.5 μg, green) on lung neutrophils (intravenous fluorescently conjugated monoclonal antibody clone 1A8, 3.5 μg, red), which appear yellow when overlapped (n = 3). Image displayed is following 20 minutes of LPS. (B) Abl-inhibitor pre-treatment (GZD824, 5 μg/gram i.v.) attenuated CD11b-bright neutrophils after 20 minutes of LPS (n = 3). (C) Abl-inhibitor pre-treated or control mice were quantified over twenty minutes of imaging between 20–40 min. post LPS. The area, averaged over 20 minutes of video, of CD11b bright/Ly6G+ overlap is displayed (mean ± SD, n = 3, one way ANOVA, * p = 0.039 and # p = 0.022). (D) Additionally, the number of CD11b bright neutrophils was averaged over twenty minutes of video between 20–40 min. post LPS (mean ± SD, n = 3, one way ANOVA, ** p = 0.008 and ## p = 0.0043).
Rapid lung neutrophil crawling facilitates capture of bloodstream bacteria in vivo
Most importantly, we hypothesized that the increased vascular crawling phenotype may be a surveillance system providing immediate detection and capture of lung sequestered bacteria, no longer in free circulation. Therefore, we tested if rapid pulmonary neutrophil crawling facilitated the capture of blood borne pathogens. E. coli remains the most common cause of bacteremia in humans and it is the prototypical pathogen associated with LPS-TLR4 mediated host defense. A simple model of bacteremia, employing a live fluorescently conjugated human clinical E. coli isolate (64) injected intravenously, was directly imaged in the lung circulation. Bacteria rapidly sequestered within the lung vasculature by adhering to the vessel wall (Fig. 8A and movie S5). More than 50% of the total visualized pathogens adhered to the vessel wall during the first minute (Fig. 8B). Macrophages are classically involved in sequestering circulating particles and pathogens (28). However, mice and humans do not have resident intravascular pulmonary macrophage (65, 66). Indeed, we could not detect any intravascular macrophage using pulmonary intravital and either fluorescently labelled antibodies against macrophage or in the lysm-EGFP transgenic mice; nevertheless, we depleted all macrophages using systemic clodronate liposomes and E. coli continued to adhere to the vessel walls (Fig. 8C). Deletion of CRIg, a receptor reported on intravascular macrophage and the molecule critical for bacterial capture by macrophage in liver (26, 28), again failed to prevent bacterial binding to pulmonary endothelium (Fig. 8D). Additionally, following neutrophil depletion, bacteria remained unperturbed by any other immune cells within the vasculature for as long as we could image (Fig. 8E). As such, the data suggest that the bacteria were in fact adhering to the endothelium and not macrophage or neutrophils and that neutrophils were chiefly responsible for immediate capture.
Fig. 8. The lung provides a niche for rapid neutrophil surveillance and capture of bloodstream bacteria in vivo.
(A) Crawling neutrophil behavior was directly examined during a model of E. coli gram-negative bacteremia. Fluorescently labelled transgenic E. coli was administered (1 × 10 7 CFU i.v.) at the time of imaging using the lymEGFP mouse. The sequence of images demonstrates endothelial capture of E. coli and subsequently two separate neutrophil capture events. Arrows highlight bacteria, while the corresponding neutrophil is marked with color-coded asterisks. (B) Bacteria trapped along the vasculature of lysmEGFP during the first-pass are compared to the total number of bacteria visualized during the first-pass (mean ± SD, n = 3, unpaired two tailed T test, not significant). (C) E. coli vascular sequestration was observed in macrophage depleted C57/BL6 or control mice (mean ± SD, n = 5, unpaired two tailed T test, not significant). (D) Bacteria trapped along the vasculature of CRIg-deficient mice during the first-pass are compared to the total number of bacteria visualized during the first-pass (mean ± SD, n = 5, unpaired two tailed T test, not significant). (E) E. coli sequestration in neutrophil depleted versus control C57BL/6 (mean ± SD, n = 5, unpaired two tailed T test, *** p = 0.001). E. coli captured by lung neutrophils compared to the total bacteria per FOV was quantified after 60 minutes of bacteria administration in either (F) lysmEGFP (mean ± SD, n = 3, unpaired two tailed T test, not significant) or (G) CRIg-deficient mice (mean ± SD, n = 3, unpaired two tailed T test, not significant). Following 60 minutes of bacteria administration, the number of E. coli remaining adhered to the vessel wall versus phagocytosed by neutrophils is demonstrated in (H) lysmEGFP (mean ± SD, n = 3, unpaired two tailed T test, ** p = 0.0049) and (I) CD11b-deficient mice (mean ± SD, n = 3, unpaired two tailed T test, not significant). (J) Neutrophil crawling was quantified as vascular distance between 20–30min following intravenous E. coli in control C57BL/6 mice versus mice pretreated with the abl-inhibitor (GZD824, 5 μg/gram i.v. mean ± SD, n = 3, unpaired two tailed T test, * p = 0.032). (K) The number of freely circulating uncaptured bacteria was quantified in control and abl inhibitor treated mice after 30 min of bacteremia (mean ± SD, n = 3, unpaired two tailed T test, * p = 0.018). (L) Bacteria capture by neutrophils was determined following 60 minutes of E. coli administration in control mice versus macrophage depleted mice (mean ± SD, n = 3, unpaired two tailed T test, ** p = 0.009).
Like LPS treatment, neutrophils in the capillary vessels rapidly polarized and began to crawl throughout the vasculature ultimately targeting the sequestered E. coli. Fig. 8A demonstrates two examples of neutrophil rapidly initiating crawling leading to bacterial capture. Crawling that resulted in capture is demonstrated on the left side of the screen and marked by a blue asterisk and arrow, while the right side demonstrates the second neutrophil crawling and capturing event highlighted by a white asterisk and arrow (Fig. 8A and movie S5). The number of E. coli adhered to vessels decreased over time because the clear majority of visualized bacteria were phagocytosed by vascular neutrophils (Fig. 8F). Neutrophils in CRIg-deficient mice also efficiently phagocytosed sequestered bacteria (Fig. 8G). Furthermore, few bacteria remained free of neutrophil capture along the vessel wall at 1 hour in lysmEGFP mice as the majority were now phagocytosed (Fig. 8h). However, in CD11b-deficient mice, where neutrophils could not crawl to efficiently reach the E.coli, low numbers of bacteria were phagocytosed (Fig. 8I). Of note, we studied the process of neutrophil mediated rapid crawling and E. coli capture in both transgenically labeled myeloid cells (lysmEGFP, Fig. 8A, 8B, 8F, 8H and movie S5) and using fluorescently labelled specific antibodies toward neutrophils (clone 1A8) and no differences were observed. The abl-inhibitor impaired E. coli induced rapid crawling in vivo (Fig. 8J), similarly to our previous LPS experiments. Importantly, the abl-inhibitor also impaired the ability of the capillary neutrophils to detain vascular bacteria leading to an increase in uncaptured but vascular-adhered E. coli (Fig. 8K). In a final experiment, depletion of macrophage did not impair the neutrophil phagocytosis in the lung (Fig. 8L). Taken together, we have demonstrated a host defence system whereby neutrophils are rapidly activated upon pathogen detection to survey the pulmonary vasculature for bloodstream infections sequestered in the pulmonary microvasculature. Hence, the lung capillaries provide a host defence niche for immediate neutrophil patrolling and capture of bloodstream bacteria.
Discussion
Systemic infections, whereby the pathogen enters the bloodstream, have worse outcomes than focal and localized infections. Even for sepsis, which has an incredibly high mortality, the outcomes are worse if bacteremia occurs (2). Conventional paradigms consider most bloodstream invaders are cleared via specialized stationary mononuclear phagocytes in the liver and spleen. Yet, the major risk factor for developing a bloodstream infection is the lack of, or dysfunction of, neutrophils (29, 30). Here, we report two discoveries concerning neutrophil mediated host defense and establish the lung as an important organ involved in vascular defense with unique contributions that differ from conventional macrophage dominant defense in the liver and spleen.
Endotoxin triggers immediate changes in host physiology; however, functionally relevant non-transcriptional cellular behaviors following LPS-TLR4 activation either in vivo or in vitro are underreported. In humans and mice treated with LPS, changes in vital signs, such as fever or tachycardia, occurs after 2 hours (67–70). Additionally, the appearance of proinflammatory cytokines IL-6 or TNF-α or changes in leukocyte counts also requirehours. In contrast to the reported downstream mechanisms of LPS-TLR4 that require hours, we discovered a physiologically relevant in vivo host defense mechanism that occurs within minutes of LPS and requires TLR4. In vivo, neutrophils were immediately triggered to search the vast capillary network in a CD11b dependent manner. Following TLR4 and Myd88 signal transduction, CD11b mediated crawling required abl-kinase. The role of this kinase was revealed using a pharmacological inhibition library to assess unbiased pathways in rapid LPS mediated adhesion. Although other kinases are also involved in neutrophil adhesion and migration, including Src and Syk (71–74), the functional relevance of kinase activation directly linked to rapid behavioural activation in vivo is less appreciated. We observed that abl mediates an inside-out neutrophil activation pathway, leading to CD11b-dependent crawling, that is important in immediate vascular host defense. Further confirmation of this rapid TLR4-dependent neutrophil activation was the shedding of L-selectin cells rapidly. Neutrophils adhere rapidly to artificial substrata when given LPS (22, 45). Recently, the macrophage secretosome was mapped downstream of LPS stimulation. Using proteomics, it was discovered that numerous proteins were non-transcriptionally secreted following LPS however this only peaked after 8 hours(75). Nevertheless, these studies support our view that TLR4 can mediate cellular responses, both transcriptionally and non-transcriptionally. Interestingly, we previously discovered that bacteria could trigger NETosis via TLR4 on platelets(64, 76), yet using pulmonary intravital microscopy we could not observe any NET structures in the capillaries after bacterial stimulation.
Neutrophils can marginate and recruit to the lung under basal conditions (77, 78), yet the functional relevance of this large cohort of innate cells has remained obscure. Our data demonstrates the large populations of transient and marginated neutrophils are poised to immediately eliminate sequestered pathogens. Therefore, we have discovered an in vivo host defense system that deals with bloodstream infections independent of the mononuclear phagocyte system and supports the clinical finding that neutropenia predisposes to disseminated bloodstream infection.
CD11b is critical to neutrophil mediated host defense. A major role of this integrin is to mediate neutrophil adhesion and migration. Selective β2-integrin mediated recruitment is regulated by multiple mechanisms, including increased cell surface expression and changes in the extracellular activation state(79). Examples exist demonstrating the importance of deficiencies in cell surface CD11b as well as impairments in activation of CD11b. Patients with severely depressed β2-integrin cellsurface levels develop severe invasive bloodstream infections and sepsis (80). In humans or mice with Wiskott-Aldrich syndrome (WAS), a primary immunodeficiency characterized by recurrent bacterial infections, neutrophils have both impairment in integrin induced activation and an impaired ability of CD11b to properly polarize to the uropod thereby impairing adhesion and cell migration (81). Similarly, in leukocyte adhesion deficiency type III, a human condition in which a molecule kindlin-3 that binds the common integrin beta chain, mediates CD11b activation and adhesion in vitro is missing, also is characterized by bacterial infections(82–84). Our in vivo imaging identified that increases in cell surface CD11b are required for rapid crawling and patrolling behaviors in the lung a common place for infections. Although both our human and mouse in vivo and our in vitro model demonstrate overall increases in CD11b, we cannot rule out the possibility that the activation state may also be rapidly changed following LPS.
Abl-kinase has not specifically been shown to mediate rapid CD11b mediated neutrophil host defense, yet there is precedence linking this kinase to integrin function and neutrophil adhesion. The abl-non-receptor tyrosine kinase is notable for its role in chronic myelogenous leukemia (CML) as BCR-abl. Under non-malignant conditions, abl is only one of two kinases that are known to directly bind and interact with the cytoskeleton and mediate cell migration and polarity(57). Growth factors stimulate abl through Src-kinase activation and β2-integrin adhesion can stimulate abl via Src and Syk. Interestingly, the Wiskott-Aldrich syndrome (WAS) proteins, implicated in CD11b impairment, are activated via abl kinase(57). Moreover, abl-kinases can activate Vav1, a molecule known to mediate neutrophil migration(49), at the pseudopod leading edge during β2-integrin crawling in neutrophils and inhibiting abl impairs recruitment in a peritonitis model (72, 73). Dasatinib, a clinical drug used to treat CML, inhibits several tyrosine kinases, including abl and Src. This drug inhibits neutrophil chemotaxis and adhesion mediated functions, although the relationship to integrin function is unclear (71). In our model, abl-kinase was necessary to increase CD11b expression presumably through granule mobilization and secretion where CD11b is stored.
Several limitations of this study require further investigation. The mechanistic differences observed in the pulmonary vasculature compared to other vascular beds, in terms of sequestering bacteria and supporting rapid neutrophil crawling, remain obscure. Specifically, we could not identify how bacteria adhere to the endothelium and if this is related to adhesion molecules or vascular architecture. Additionally, the endothelial molecules responsible for mediating rapid neutrophil recruitment in the lung, but not in other microvascular beds such as dermal endothelium has not been resolved. Clearly, endothelium is heterogenous and diverse throughout the different organs of the body. Another limitation is that it remains unclear what the exact relationship is between Myd88, abl kinase and the process of CD11b upregulation. Direct measurements of abl-kinase activity in primary, lung neutrophils from mice is challenging and so far the only example of measuring abl-kinase activity in granulocytes has required HL-60 cell lines in vitro (72). Finally, deficiency of CD11b, a subunit of the β2-integrin adhesion molecule family, could lead to compensatory changes in the other chains, such as CD11a, CD11c and CD11d. However, we have not methodically tested if compensatory expression of other β2-integrin chains could be impacted in the CD11b-deficient mice.
Overall, this study opens new areas of host defense and inflammation research and highlights a neutrophil surveillance and capture behavior in vivo. Additionally, it establishes that the lung microvascular system is not solely involved in gas exchange but is an important host defence niche for neutrophils distinct from the conventional monophagocytic systems of the liver and spleen.
Methods and Materials
Study Design
This study used an in vivo mouse model of intravital imaging to assess neutrophil host defense. A sample size calculation was not performed a priori since the effect sizes of our observations and interventions could not be determined prior to experimentation. This study was not randomized and was not blinded.
Reagents
Highly purified LPS from Escherichia coli O111:B4 was purchased from List Biological Industries Inc. Fluorescently conjugated anti-Ly6G-antibodies (clone 1A8, Alexa594 and Alexa647), anti-CD31-antibodies (clone 390, Alexa594 and Alexa 647) were purchased from Biolegend, eBioscience and BD biosciences. Escherichia coli, isolated from a patient with meningitis, were previously transfected with an mTomato-fluorescent reporter (64). The Abl-specific inhibitor GZD824 Dimesylate was purchased from Selleck Chemicals. Previous in vivo studies found abl-inhibition with 5 to 10 μg/g/day for 6 days, or 25μg/g/day for two weeks (61, 62).
Inhibitor screen
Human neutrophils (5 × 106/mL) were labeled with calcein-AM (1 μM final concentration; Molecular Probes) for 30 minutes at 37°C, washed twice, and resuspended in Hepes buffer at a concentration of 2 × 106/mL. Adhesion was determined in an uncoated 96-well MaxiSorp plate (Nunc), which has a high affinity for proteins. After pre-incubation with DMSO or the different compounds at the indicated concentrations for 15 min at 37°C, calcein-labeled cells (2 × 105/well, final volume 100 μL) were stimulated with 20 ng/mL bacterial TLR4 ligand lipopolysaccharide (isolated from Escherichia coli strain 055:B5, Sigma Aldrich) in the presence of 50 ng/mL lipopolysaccharide-binding protein (R&D Systems). Plates were incubated for 30 minutes at 37°C and washed 3 times with PBS. Adherent cells were lysed in 0.5% (w/v) Triton X-100 in H2O for 5 minutes at room temperature. Fluorescence was measured with a Genios plate reader at an excitation wavelength of 485 nm and an emission wavelength of 535 nm(82).
LPS administration to human volunteers
Subjects were enrolled after screening and prehydrated as previously reported (85). US reference E. coli endotoxin (lot Ec-5; Center for Biologic Evaluation and Research, Food and Drug Administration, Bethesda, Maryland, USA) was used in this study. Endotoxin was reconstituted in 5 ml saline and injected as single i.v. bolus during 1 minute at t = 0. Blood samples anticoagulated with sodium heparin were taken from the arterial catheter. The study protocol concerning the human endotoxemia challenges was approved by the Ethics Committee of the Radboud University Nijmegen Medical Centre and complies with the Declaration of Helsinki and the Good Clinical Practice guidelines. Volunteers gave written informed consent. The Ethics Committee of the University Medical Center Utrecht approved the study protocol. Patients gave informed consent after sampling of the blood according to the research protocol. For human flow cytometry, WBC differential counts were performed on a Cell-Dyn Emerald haematocytometer. After blood withdrawal, RBC were lysed immediately (150 mM NH4Cl, 10 mM KHCO3 and 0.1 mM NA2EDTA), followed by antibody staining in PBS2+ (0.32% trisodium citrate and 10% human pasteurized plasma solution) followed by fixation (1% formaldehyde). FACS was performed on a BD Fortessa with the following fluorescently conjugated antibodies against CD35, CD16 (Becton Dickinson) and CD11b (Dako).
Animals
Mice were housed at the University of Calgary in a specific-pathogen free facility and used under a specific IRB approved ethics protocol. C57BL/6J were purchased from The Jackson Laboratories (Maine, USA). CD11b, Tlr4, and Myd88 deficient and lysm-EGFP colonies were maintained at the University of Calgary. Mice (20–35 g, 6–10 weeks old) were housed in a pathogen-free environment and had access to food and water ad libitum. All procedures performed were approved by the University of Calgary Animal Care Committee and were in accordance with the Canadian Guidelines for Animal Research.
Pulmonary Intravital microscopy
Stabilized pulmonary intravital microscopy has been previously reported (86). This technique was learned at UCSF in the Krummel lab and performed with minor alterations related to fluorescently conjugated antibodies and the microscopes used at the University of Calgary. Briefly, anaesthetized mice (ketamine and xylazine) receive a right internal jugular intravenous catheter to administer fluorescent antibodies, anaesthetics and inhibitors. Some experiments utilized lysm-EGFP mice to visualize neutrophils and monocytes. To visualize the endothelium 5μg of fluorescently conjugate anti-CD31 antibody (clone 390, Biolegend, either Alex594 or Alexa647) was administered intravenously 10 minutes prior to imaging. For experiments using non-fluorescent mice (C57BL/6J CD11b, Tlr4, and Myd88 deficient), 3.5μg of fluorescently conjugated anti-Ly6G antibody (clone 1A8, Biolegend either Alexa594 or Alexa647) was injected intravenously, to specifically visualize neutrophils at the same time as fluorescently conjugated anti-CD31 antibody. A tracheostomy is performed and the mouse is ventilated at 10μl/gram tidal volume and a rate between 120–130 with entrained oxygen and a PEEP of 4 cmH2O. To avoid volutrauma and barotrauma mice in our experiments are subjected to tidal volumes between 180 and 240μl, compared to previous reports that used 500 μl per tidal volume(42). The left lung is exposed following a thoracotomy and rib resection and the vacuum chamber is applied to facilitate imaging. Intravital microscopy was performed with either a spinning disk (Quorum) or a resonant scanning confocal (Leica) microscope. Spinning-disk confocal intravital microscopy was performed using an Olympus BX51 (Olympus) upright microscope equipped with a 20×/0.95 XLUM Plan Fl water immersion objective. The microscope was equipped with a confocal light path (WaveFx, Quorum) based on a modified Yokogawa CSU-10 head (Yokogawa Electric Corporation). Laser excitation at 488, 561 and 649 was used in rapid succession and fluorescence in green, red and blue channels was visualized with the appropriate long pass filters (Semrock). A 512×512 pixels back-thinned EMCCD camera (C9100–13, Hamamatsu) was used for fluorescence detection. Resonant scanning (8Hz) confocal was performed with an upright Leica SP8 equipped with a white light laser and three HyD spectral detectors and a 25× water objective with a 0.9NA. All imaging experiments were carried out for a minimum of one continuous hour. Images were acquired every 10 seconds and a new field of view was chosen after 20 minutes of observation, therefore at least three fields of view were observed for every 1 hour imaging experiment. Images were processed and analyzed in Volocity 4.20.
Neutrophil behavior analysis
Neutrophil behavior was analysed off line using Volocity 4.20. Cellular enumeration and tracking was performed manually. For phenotypic quantification, tethering was defined as a discreet neutrophil interaction with the vascular wall, which arrests its circulatory movement for less than 30 seconds. Crawling was defined as continuous interaction of a neutrophil with the vascular wall for more than 30 seconds, which involves a polarized cell that does not remain stationary. Adhesion was defined as a stationary neutrophil that was not mobile and remained static for at least 30 seconds (46, 49, 50, 87, 88). Velocity, distance and track crawling for individual neutrophils was determined manually using Volocity software. Individual neutrophils were tracked for 10-minute intervals.
Statistics
Data was analyzed using GraphPad Prism software for Windows version 6.02. Data is presented as either mean ± SEM or mean ± SD. For figures comparing two groups of data a two-tailed unpaired t-test was utilized. For figures comparing more than two groups, one way ANOVA with Tukey’s multiple comparisons tests were utilized. For the human intravenous LPS experiments, data was analyzed using repeated measures ANOVA with Dunnett’s correction for multiplicity. Flow cytometry data was analysed using FlowJo V10. For all experiments, n represents the number of individual experiments performed.
Supplementary Material
Acknowledgments
We thank the Mouse Phenomics Resources Laboratory, funded by the Snyder Institute, and the flow cytometry core facility at the University of Calgary.
Funding: Supported by operating grants from the Canadian Institutes of Health Research (CIHR) (RS-342013), the Dept. of Critical Care Medicine and bridge funding from the University of Calgary Medical Group (UCMG) to B.G.Y. Infrastructure funding was provided by the Canadian Foundation for Innovation-John R. Evans Leaders fund with matching support from the Alberta Enterprise and Advanced Education Research Capacity Program. B.G.Y is a tier II Canada Research Chair in Pulmonary Immunology, Inflammation and Host Defence. P.K is supported by a CIHR Team Grant: Health Challenges in Chronic Inflammation Initiative. P.K is a tier I Canada Research Chair Leukocyte Recruitment in inflammatory disease. L.K is supported by a grant from the Duth Lung Foundation 3.2.10.052.
Footnotes
This manuscript has been accepted for publication in Science Immunology. This version has not undergone final editing. Please refer to the complete version of record at http://immunology.sciencemag.org. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Competing interests: The authors declare no competing interests
Author Contributions: Experiments were conducted and planned by B.G.Y, J.H.K, R.L, L.D.Z, B.P, N.S, M.H, V.G.S, T, K, L.K, P.P, A.T.J.T, T.W.K, T.K.V, M.R.L, M.F.K and P.K. The manuscript was written and revised by B.G.Y and P.K. Statistical analysis was performed by B.G.Y, J.H.K and A.T.J.T. Funding was provided by B.G.Y and P.K.
References and Notes
- 1.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 2.Mansur A, Klee Y, Popov AF, Erlenwein J, Ghadimi M, Beissbarth T, Bauer M, Hinz J. Primary bacteraemia is associated with a higher mortality risk compared with pulmonary and intra-abdominal infections in patients with sepsis: a prospective observational cohort study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laupland KB. Incidence of bloodstream infection: a review of population-based studies. Clin Microbiol Infect. 2013;19:492–500. doi: 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- 4.Biondi E, Evans R, Mischler M, Bendel-Stenzel M, Horstmann S, Lee V, Aldag J, Gigliotti F. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132:990–996. doi: 10.1542/peds.2013-1759. [DOI] [PubMed] [Google Scholar]
- 5.Deen J, von Seidlein L, Andersen F, Elle N, White NJ, Lubell Y. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis. 2012;12:480–487. doi: 10.1016/S1473-3099(12)70028-2. [DOI] [PubMed] [Google Scholar]
- 6.Metersky ML, Waterer G, Nsa W, Bratzler DW. Predictors of in-hospital vs postdischarge mortality in pneumonia. Chest. 2012;142:476–481. doi: 10.1378/chest.11-2393. [DOI] [PubMed] [Google Scholar]
- 7.Magret M, Lisboa T, Martin-Loeches I, Manez R, Nauwynck M, Wrigge H, Cardellino S, Diaz E, Koulenti D, Rello J. Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: a prospective and observational multicenter study. Crit Care. 2011;15:R62. doi: 10.1186/cc10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sligl W, Taylor G, Brindley PG. Five years of nosocomial Gram-negative bacteremia in a general intensive care unit: epidemiology, antimicrobial susceptibility patterns, and outcomes. Int J Infect Dis. 2006;10:320–325. doi: 10.1016/j.ijid.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003;41:3655–3660. doi: 10.1128/JCM.41.8.3655-3660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Retamar P, Lopez-Prieto MD, Natera C, de Cueto M, Nuno E, Herrero M, Fernandez-Sanchez F, Munoz A, Tellez F, Becerril B, Garcia-Tapia A, Carazo I, Moya R, Corzo JE, Leon L, Munoz L, Rodriguez-Bano J, Rodriguez-Lopez F, Garcia MV, Fernandez-Galan V, del Arco A, Perez-Santos MJ, Sanchez Porto A, Torres-Tortosa M, Martin-Aspas A, Arroyo A, Garcia-Figueras C, Acosta F, Florez C, Navas P, Escobar-Lara T. Reappraisal of the outcome of healthcare-associated and community-acquired bacteramia: a prospective cohort study. BMC Infect Dis. 2013;13:344. doi: 10.1186/1471-2334-13-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 12.Savard P, Perl TM. A call for action: managing the emergence of multidrug-resistant Enterobacteriaceae in the acute care settings. Curr Opin Infect Dis. 2012;25:371–377. doi: 10.1097/QCO.0b013e3283558c17. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 14.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol. 2009;9:364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 19.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 20.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 22.Worthen GS, Avdi N, Vukajlovich S, Tobias PS. Neutrophil adherence induced by lipopolysaccharide in vitro. Role of plasma component interaction with lipopolysaccharide. J Clin Invest. 1992;90:2526–2535. doi: 10.1172/JCI116146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halpern BN. The Role and Function of the Reticulo-Endothelial System in Immunological Processes*. Journal of Pharmacy and Pharmacology. 1959;11:321–338. doi: 10.1111/j.2042-7158.1959.tb12560.x. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins SJ, Hume DA. Homeostasis in the mononuclear phagocyte system. Trends Immunol. 2014;35:358–367. doi: 10.1016/j.it.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Helmy KY, Katschke KJ, Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Z, Surewaard BG, Wong CH, Geoghegan JA, Jenne CN, Kubes P. CRIg Functions as a Macrophage Pattern Recognition Receptor to Directly Bind and Capture Blood-Borne Gram-Positive Bacteria. Cell Host Microbe. 2016;20:99–106. doi: 10.1016/j.chom.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 30.Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 31.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 32.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 33.Kubo H, Doyle NA, Graham L, Bhagwan SD, Quinlan WM, Doerschuk CM. L-and P - selectin and CD11/CD18 in intracapillary neutrophil sequestration in rabbit lungs. Am J Respir Crit Care Med. 1999;159:267–274. doi: 10.1164/ajrccm.159.1.9709011. [DOI] [PubMed] [Google Scholar]
- 34.Mizgerd JP, Meek BB, Kutkoski GJ, Bullard DC, Beaudet AL, Doerschuk CM. Selectins and neutrophil traffic: margination and Streptococcus pneumoniae-induced emigration in murine lungs. J Exp Med. 1996;184:639–645. doi: 10.1084/jem.184.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickey MJ, Westhorpe CL. Imaging inflammatory leukocyte recruitment in kidney, lung and liver--challenges to the multi-step paradigm. Immunol Cell Biol. 2013;91:281–289. doi: 10.1038/icb.2012.83. [DOI] [PubMed] [Google Scholar]
- 36.Doerschuk CM. The role of CD18-mediated adhesion in neutrophil sequestration induced by infusion of activated plasma in rabbits. Am J Respir Cell Mol Biol. 1992;7:140–148. doi: 10.1165/ajrcmb/7.2.140. [DOI] [PubMed] [Google Scholar]
- 37.Downey GP, Worthen GS, Henson PM, Hyde DM. Neutrophil sequestration and migration in localized pulmonary inflammation. Capillary localization and migration across the interalveolar septum. Am Rev Respir Dis. 1993;147:168–176. doi: 10.1164/ajrccm/147.1.168. [DOI] [PubMed] [Google Scholar]
- 38.Worthen GS, Schwab B, 3rd, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 39.Doerschuk CM, Winn RK, Coxson HO, Harlan JM. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- 40.Burns AR, Doerschuk CM. Quantitation of L-selectin and CD18 expression on rabbit neutrophils during CD18-independent and CD18-dependent emigration in the lung. J Immunol. 1994;153:3177–3188. [PubMed] [Google Scholar]
- 41.Doerschuk CM, Tasaka S, Wang Q. CD11/CD18-dependent and -independent neutrophil emigration in the lungs: how do neutrophils know which route to take? Am J Respir Cell Mol Biol. 2000;23:133–136. doi: 10.1165/ajrcmb.23.2.f193. [DOI] [PubMed] [Google Scholar]
- 42.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proceedings of the National Academy of Sciences. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barletta KE, Cagnina RE, Wallace KL, Ramos SI, Mehrad B, Linden J. Leukocyte compartments in the mouse lung: Distinguishing between marginated, interstitial, and alveolar cells in response to injury. Journal of Immunological Methods. 2012;375:100–110. doi: 10.1016/j.jim.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel BV, Tatham KC, Wilson MR, O’Dea KP, Takata M. In vivo compartmental analysis of leukocytes in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2015;309:L639–652. doi: 10.1152/ajplung.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erzurum SC, Downey GP, Doherty DE, Schwab B, 3rd, Elson EL, Worthen GS. Mechanisms of lipopolysaccharide-induced neutrophil retention. Relative contributions of adhesive and cellular mechanical properties. J Immunol. 1992;149:154–162. [PubMed] [Google Scholar]
- 46.Yipp BG, Andonegui G, Howlett CJ, Robbins SM, Hartung T, Ho M, Kubes P. Profound differences in leukocyte-endothelial cell responses to lipopolysaccharide versus lipoteichoic acid. J Immunol. 2002;168:4650–4658. doi: 10.4049/jimmunol.168.9.4650. [DOI] [PubMed] [Google Scholar]
- 47.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 48.Lawrence MB, Smith CW, Eskin SG, McIntire LV. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990;75:227–237. [PubMed] [Google Scholar]
- 49.Phillipson M, Heit B, Parsons SA, Petri B, Mullaly SC, Colarusso P, Gower RM, Neely G, Simon SI, Kubes P. Vav1 is essential for mechanotactic crawling and migration of neutrophils out of the inflamed microvasculature. J Immunol. 2009;182:6870–6878. doi: 10.4049/jimmunol.0803414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menezes GB, Lee WY, Zhou H, Waterhouse CC, Cara DC, Kubes P. Selective down-regulation of neutrophil Mac-1 in endotoxemic hepatic microcirculation via IL-10. J Immunol. 2009;183:7557–7568. doi: 10.4049/jimmunol.0901786. [DOI] [PubMed] [Google Scholar]
- 52.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 53.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L, Puri KD, Penninger JM, Kubes P. Leukocyte PI3Kgamma and PI3Kdelta have temporally distinct roles for leukocyte recruitment in vivo. Blood. 2007;110:1191–1198. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- 55.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heit B, Colarusso P, Kubes P. Fundamentally different rolesfor LFA -1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J Cell Sci. 2005;118:5205–5220. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 57.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci. 2009;122:3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Backert S, Feller SM, Wessler S. Emerging roles of Abl family tyrosine kinases in microbial pathogenesis. Trends Biochem Sci. 2008;33:80–90. doi: 10.1016/j.tibs.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Heit B, Liu L, Colarusso P, Puri KD, Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci. 2008;121:205–214. doi: 10.1242/jcs.020412. [DOI] [PubMed] [Google Scholar]
- 60.Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ, Jirik FR, Kubes P. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 61.Ye W, Jiang Z, Lu X, Ren X, Deng M, Lin S, Xiao Y, Lin S, Wang S, Li B, Zheng Y, Lai P, Weng J, Wu D, Ma Y, Chen X, Wen Z, Chen Y, Feng X, Li Y, Liu P, Du X, Pei D, Yao Y, Xu B, Ding K, Li P. GZD824 suppresses the growth of human B cell precursor acute lymphoblastic leukemia cells by inhibiting the SRC kinase and PI3K/AKT pathways. Oncotarget. 2016 doi: 10.18632/oncotarget.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren X, Pan X, Zhang Z, Wang D, Lu X, Li Y, Wen D, Long H, Luo J, Feng Y, Zhuang X, Zhang F, Liu J, Leng F, Lang X, Bai Y, She M, Tu Z, Pan J, Ding K. Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem. 2013;56:879–894. doi: 10.1021/jm301581y. [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Xu J, Perkins C, Guo F, Snapper S, Finkelman FD, Zheng Y, Filippi MD. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood. 2012;120:3563–3574. doi: 10.1182/blood-2012-04-426981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 65.Brain JD, Molina RM, DeCamp MM, Warner AE. Pulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 species. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1999;276:L146–L154. doi: 10.1152/ajplung.1999.276.1.L146. [DOI] [PubMed] [Google Scholar]
- 66.Schneberger D, Aharonson-Raz K, Singh B. Pulmonary intravascular macrophages and lung health: what are we missing? Am J Physiol Lung Cell Mol Physiol. 2012;302:L498–503. doi: 10.1152/ajplung.00322.2011. [DOI] [PubMed] [Google Scholar]
- 67.Suffredini AF, Noveck RJ. Human endotoxin administration as an experimental model in drug development. Clin Pharmacol Ther. 2014;96:418–422. doi: 10.1038/clpt.2014.146. [DOI] [PubMed] [Google Scholar]
- 68.Lynn M, Rossignol DP, Wheeler JL, Kao RJ, Perdomo CA, Noveck R, Vargas R, D’Angelo T, Gotzkowsky S, McMahon FG. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J Infect Dis. 2003;187:631–639. doi: 10.1086/367990. [DOI] [PubMed] [Google Scholar]
- 69.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D I. the Host Response to Injury. Acute Inflammatory Response to Endotoxin in Mice and Humans. Clinical and Diagnostic Laboratory Immunology. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson M, Blum R, Dandona P, Mousa S. Effects in humans of intravenously administered endotoxin on soluble cell-adhesion molecule and inflammatory markers: a model of human diseases. Clin Exp Pharmacol Physiol. 2001;28:376–380. doi: 10.1046/j.1440-1681.2001.03463.x. [DOI] [PubMed] [Google Scholar]
- 71.Futosi K, Nemeth T, Pick R, Vantus T, Walzog B, Mocsai A. Dasatinib inhibits proinflammatory functions of mature human neutrophils. Blood. 2012;119:4981–4991. doi: 10.1182/blood-2011-07-369041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tong H, Zhao B, Shi H, Ba X, Wang X, Jiang Y, Zeng X. c-Abl tyrosine kinase plays a critical role in beta2 integrin-dependent neutrophil migration by regulating Vav1 activity. J Leukoc Biol. 2013;93:611–622. doi: 10.1189/jlb.1012487. [DOI] [PubMed] [Google Scholar]
- 73.Cui L, Chen C, Xu T, Zhang J, Shang X, Luo J, Chen L, Ba X, Zeng X. c-Abl kinase is required for beta 2 integrin-mediated neutrophil adhesion. J Immunol. 2009;182:3233–3242. doi: 10.4049/jimmunol.0802621. [DOI] [PubMed] [Google Scholar]
- 74.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 75.Meissner F, Scheltema RA, Mollenkopf HJ, Mann M. Direct proteomic quantification of the secretome of activated immune cells. Science. 2013;340:475–478. doi: 10.1126/science.1232578. [DOI] [PubMed] [Google Scholar]
- 76.McDonald B, Kubes P. Neutrophils and intravascular immunity in the liver during infection and sterile inflammation. Toxicol Pathol. 2012;40:157–165. doi: 10.1177/0192623311427570. [DOI] [PubMed] [Google Scholar]
- 77.Doerschuk CM, Allard MF, Martin BA, MacKenzie A, Autor AP, Hogg JC. Marginated pool of neutrophils in rabbit lungs. J Appl Physiol. 1987;63:1806–1815. doi: 10.1152/jappl.1987.63.5.1806. [DOI] [PubMed] [Google Scholar]
- 78.Kuebler WM, Goetz AE. The marginated pool. Eur Surg Res. 2002;34:92–100. doi: 10.1159/000048894. [DOI] [PubMed] [Google Scholar]
- 79.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson DC, Schmalsteig FC, Finegold MJ, Hughes BJ, Rothlein R, Miller LJ, Kohl S, Tosi ME, Jacobs RL, Waldrop TC, Goldman AS, Shearer WT, Springer TA. The Severe and Moderate Phenotypes of Heritable Mac-1, LFA-1 Deficiency: Their Quantitative Definition and Relation to Leukocyte Dysfunction and Clinical Features. The Journal of Infectious Diseases. 1985;152:668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, Schaff UY, Green CE, Chen H, Sarantos MR, Hu Y, Wara D, Simon SI, Lowell CA. Impaired Integrin-Dependent Function in Wiskott-Aldrich Syndrome Protein-Deficient Murine and Human Neutrophils. Immunity. 2006;25:285–295. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van de Vijver E, Tool AT, Sanal O, Cetin M, Unal S, Aytac S, Seeger K, Pagliara D, Rutella S, van den Berg TK, Kuijpers TW. Kindlin-3-independent adhesion of neutrophils from patients with leukocyte adhesion deficiency type III. J Allergy Clin Immunol. 2014;133:1215–1218. doi: 10.1016/j.jaci.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 83.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, Wang HV, Sperandio M, Fassler R. Kindlin-3 is required for [beta]2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 84.Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, van den Berg TK, Moser M, Jakobs ME, Seeger K, Sanal O, Unal S, Cetin M, Roos D, Verhoeven AJ, Baas F. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. 2009;113:4740–4746. doi: 10.1182/blood-2008-10-182154. [DOI] [PubMed] [Google Scholar]
- 85.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. The Journal of Clinical Investigation. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. Stabilized imaging of immune surveillance in the mouse lung. Nat Methods. 2011;8:91–96. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnston B, Issekutz TB, Kubes P. The alpha 4-integrin supports leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo. J Exp Med. 1996;183:1995–2006. doi: 10.1084/jem.183.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanwar S, Bullard DC, Hickey MJ, Smith CW, Beaudet AL, Wolitzky BA, Kubes P. The association between alpha4-integrin, P-selectin, and E-selectin in an allergic model of inflammation. J Exp Med. 1997;185:1077–1087. doi: 10.1084/jem.185.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.