Abstract
Introduction
Previous studies have suggested cost-savings using blue light cystoscopy (BLC) with hexaminolevulinate (HAL) compared to white light cystoscopy (WLC) during transurethral resection of bladder tumour (TURBT) for non-muscle-invasive bladder cancer (NMIBC), secondary to improvements in recurrence and progression rates; however, these studies have used ‘best case scenario’ recurrence rate probabilities, thus decreasing generalizability of the findings. The objective of this study was to perform a contemporary cost-effectiveness assessment of BLC compared to WLC at the time of TURBT.
Methods
A decision and cost-effectiveness model with a five-year time horizon following initial TURBT was used. The model was created from the healthcare payer perspective. Comprehensive literature review was performed to obtain contemporary recurrence and progression rates. These values were meta-analyzed for inclusion into the model. Cost variables included in the model were from three large Canadian bladder cancer centres. Model outputs were number of recurrences prevented, bed days saved, and overall costs. One-way sensitivity and scenario analyses were performed to assess model robustness.
Results
The five-year amortized cost of using BLC with HAL on all incident NMIBC compared to WLC assistance was $4 832,908 for Ontario (n=4696; $1372/patient); $1 168 968 for British Columbia (n=1204; $1295/patient); and $2 484, 872 (n=2680; $1236/patient) for Quebec. Use of BLC with HAL would result in 87 338 fewer recurrences annually. On sensitivity/scenario analyses for Ontario data, if BLC with HAL equipment were provided to the province at no cost, five-year costs would be $4 158 814 and $1181 cost per patient. If BLC with HAL were only used for cystoscopically appearing aggressive tumours, the five-year amortized cost would be $3 874 098, with a cost per patient of $1222. If there was a 20% or 50% improvement in progression rates with BLC plus HAL, the five-year amortized cost would be $2 660 529 and −$598 039 (cost-saving), respectively.
Conclusions
TURBT using BLC with HAL for patients with NMIBC is associated with a five-year cost of approximately $1–5 million for jurisdictions of 4–13 million people. Although this translates to a cost of $1200–1400 per patient for their initial TURBT, BLC with HAL improves patients care, reduces recurrences, and decreases the need for hospital beds after TURBT. If this diagnostic procedure eventually improves progression rates, there would be considerably improved cost-effectiveness.
Introduction
Bladder cancer (BCa) is the fourth and twelfth most common malignancy by incidence in Canadian men and women, respectively.1 In Canada, the lifetime probability of developing BCa is one in 27 men and one in 84 women.1 Most patients diagnosed with BCa have non-muscle-invasive BCa (NMIBC), which carries a high risk of recurrence (up to 78% within five years of initial resection) and a risk of progression of up to 45% at five years from diagnosis.2 Secondary to these risks, rigorous followup with periodic cystoscopy is necessary, often for the remainder of the patient’s life. As such, BCa is the most expensive malignancy to treat, with lifetime cost per patient estimates ranging from $65 000 187 000 USD.3,4 Furthermore, bladder surveillance for tumour recurrence and treatment of eventual recurrences account for 60% of the total costs of managing BCa patients.4 Given these economic burdens in today’s fiscally challenged healthcare systems, additional cost-effective measures for surveillance and treatment are necessary.
In addition to tumour number and size, stage, grade, and presence of carcinoma in situ (CIS),2 an additional risk factor for recurrence is incomplete resection at initial transurethral resection of bladder tumour (TURBT).5 This can occur in the setting of multiple lesions where one is missed and/ or when there is difficulty identifying the exact extent and location of tumours, particularly CIS, using standard white light cystoscopy (WLC).5 To improve tumour visualization in these situations, hexaminolevulinate (HAL) hydrochloride (Photocure®, Oslo, Norway) has been used with blue light cystoscopy (BLC) for aiding detection of NMIBC. HAL has been commercially available in Europe since 2006 (marketed as Hexvix®), in the U.S. since 2010, and recently became available in Canada (2015). Several randomized, controlled trials (RCTs) have reported improvements in NMIBC recurrence rates; however, widespread adoption of the technique varies because of equipment availability and cost constraints.6
Previous cost-effectiveness studies have generally demonstrated potential cost savings using BLC with HAL compared to WLC-assisted TURBT for NMIBC, primarily due to an improvement in recurrence rates;7–12 however, these studies often use ‘best case scenario’ recurrence rate probabilities, thus decreasing generalizability of the findings. The objective of this study was to provide the first decision analysis using updated, meta-analyzed probabilities for risk of recurrence (BLC with HAL TURBT vs. WLC TURBT). We performed a cost-effectiveness analysis of BLC with HAL at initial TURBT compared to WLC assisted TURBT at the population level.
Methods
Evidence synthesis
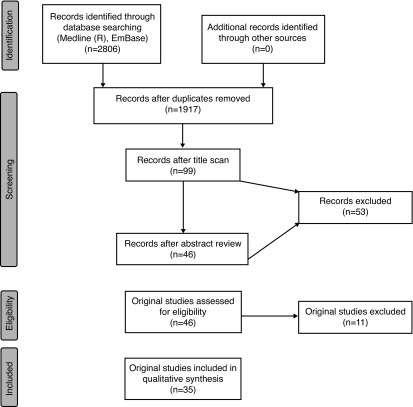
A systematic review was performed using the PRISMA guidelines to identify appropriate studies for inclusion (Fig. 1). Ovid Medline (R), Ovid Epub Ahead of Print, and Embase were queried, generating 2806 articles. After removing duplicates and unsuitable manuscripts, 33 studies were selected for inclusion in the decision-tree analysis. Among these 33 studies were 10 RCTs of BLC with HAL vs. WLC that were used to meta-analyze sensitivities and specificities.13–22 Burger et al recently used raw data of RCTs to meta-analyze recurrence relative risk (RR) for BLC with HAL vs. WLC-assisted TURBT, and this RR (0.761) was used in the baseline decision-tree analysis.23
Fig. 1.
PRISMA diagram for systematic review of the literature to identify appropriate studies to include for meta-analysis.
Decision model
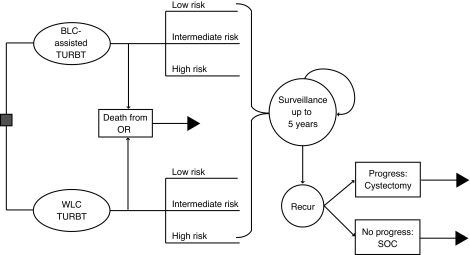
A decision model created and first used by the National Health Service (NHS, U.K.) was adapted to assess the cost-effectiveness of BLC with HAL-assisted vs. conventional WLC-assisted TURBT for patients with suspected new or recurrent NMIBC (Fig. 2).24 Specifically, we modified the model for BLC TURBT only (i.e., therapeutic) as opposed to BLC used for routine cystoscopy (i.e., diagnostic). Furthermore, we updated sensitivities, specificities, and recurrence rates with up-to-date published values (see below). The model was populated with provincial, population-based incident BCa cases per year (for 2013–2015 based on province) as follows: Ontario, n=4696; British Columbia, n=1204; Quebec, n=2680.25–27 We assumed a NMIBC rate of 75% based on the known distribution of incident BCa cases. The model was based on the Canadian universal (single-payer) healthcare system with a period of five years following initial BLC with HAL. Fig. 2 depicts the structure of the model tree for initial TURBT, recurrence monitoring, and ongoing surveillance. After initial TURBT, patients were classified as low-, intermediate-, or high-risk based on existing guidelines.28 Recurrence and progression probabilities at three months, 12 months, and then annually were incorporated to determine long-term risks of recurrence.
Fig. 2.
Structure of the decision model tree. BLC: blue light cystoscopy; OR: operating room; SOC: standard of care; TURBT: transurethral resection of bladder tumour; WLC: white light cystoscopy.
Model assumptions
The model assumes the following potential outcomes following initial TURBT: 1) recurrence monitoring for patients with NMIBC; 2) radical cystectomy for muscle-invasive disease and continued postoperative surveillance; and 3) palliative care for patients with metastatic disease. Patients with progressive disease incur the costs for radical cystectomy and possible downstream metastasis. Further general assumptions include: 1) all patients received a postoperative dose of mitomycin after TURBT; 2) after tumour recurrence, regardless of disease progression (accounting for downstream costs of progression), patients are removed from the model; 3) tumours that were initially missed by WLC were assumed to eventually become detectable within two years of followup; 4) after initial BLC with HAL TURBT, all future TURBTs are conducted with WLC assistance; 5) all patients with intermediate- and high-risk (including CIS) disease received six weeks of induction bacillus Calmette-Guerin (BCG) therapy; and 6) baseline relative risk for progression was 1.0 (equal between groups).
Cost estimates and probabilities
Micro-costing data were obtained from three large, academic teaching hospitals from Ontario, British Columbia, and Quebec, representing 75% of the entire Canadian population. Specifically, individual patient data at the University of Toronto (University Health Network), University of British Columbia (Vancouver General Hospital), and McGill University (McGill University Health Centre) were reviewed for costs associated with TURBT, surveillance, cystectomy, and palliative care. Every effort was made to obtain micro-cost data for each variable in every province; however, when a cost variable was not obtainable, the average cost for that variable between the other two provinces was included as a surrogate cost metric. Input probabilities were calculated from meta-analysis of the existing literature for sensitivities, specificities, and recurrence probabilities.13–22 As mentioned, the previous meta-analyzed RR for recurrence was used (0.761; 95% confidence interval [CI] 0.627–0.924).23 Provincial base outcomes were assessed, including: initial cost, followup cost, five-year amortized cost, cost-difference per case, bed day use, and recurrences.
Scenario and sensitivity analyses
To assess model robustness, one-way sensitivity and scenario analyses were performed with Ontario data, as the most complete cost data were from this jurisdiction. The specific scenario analyses assessed cost-effectiveness: 1) when the cost of additional BLC equipment was $0 (if the additional equipment were provided by the company); 2) without a postoperative dose of mitomycin; 3) when 20–50% improvement in progression RR was assumed; and 4) when BLC with HAL was only used at TURBT for in clinic cystoscopically appearing CIS and intermediate-/high-risk cases (not low-risk). These analyses allowed comparison of outcomes to the baseline model. Sensitivity analysis of selected exposure variables and outcome (five-year amortized cost) was performed for recurrence RR, progression RR, consumable costs (i.e., HAL), and additional equipment costs.
Results
Table 1 summarizes details of cost variables included in the model from across the three provinces, in addition to probabilities for recurrence stratified by bladder tumour risk.
Table 1.
Provincial cost estimates and probabilities
| Variable | Ontario | British Columbia | Quebec |
|---|---|---|---|
| Cost estimates | |||
|
| |||
| Cystoscopy (± 25%) | $394 ($295, $492) | $362 ($271, $452)a | $330 ($247, $412) |
| WLC-assisted TURBT (± 25%) | $3946 ($2959, $4933) | $3002 ($2252, $3753)a | $2059 ($1544, $2573) |
| BLC-assisted TURBT (± 25%) | $4890 ($3667, $6113) | $3930 ($2947, $4913) | $2990 ($2242, $3737) |
| CT scan (± 25%) | $263 ($197, $329) | $650 ($487, $812) | $400 ($300, $500) |
| Cystectomy (± 25%) | $24 486 ($18 364, $30 608) | $21 190 ($15 892, $26,487)a | $17 894 ($13 420, $22 367) |
| Palliative care (± 25%) | $55 215 ($41 411, $69,018) | $55 215 ($41 411, $69 018) | $55 215 ($41 411, $69 018) |
| Perioperative mitomycin intravesical therapy (± 25%) | $754 ($565, $943) | $739 ($554, $924)a | $725 ($543, $906) |
| BCG intravesical therapy** (± 25%) | $204 ($153, $256) | $134 ($100, $168)a | $64 ($48, $80) |
| BLC-extra nursing time (± 25%) | $25 ($18, $31) | $9 ($7, $11) | $10 ($7, $12) |
| BLC-extra staffing cost (± 25%) | $15 ($11, $18) | $9 ($7, $11) | $12 ($9, $15)b |
| BLC-consumables (± 25%) | $708 ($531, $885) | $712 ($534, $891) | $712 ($534, $891) |
| BLC-additional equipment (± 25%) | $195 ($146, $244) | $195 ($146, $244) | $195 ($146, $244) |
| WLC sensitivity (95% CI) | 0.65 (0.55–0.74) | 0.65 (0.55–0.74) | 0.65 (0.55–0.74) |
| BLC sensitivity (95% CI) | 0.93 (0.90–0.96) | 0.93 (0.90–0.96) | 0.93 (0.90–0.96) |
| *RR, BLC vs. WLC (95% CI) | 0.761 (0.627–0.924) | 0.761 (0.627–0.924) | 0.761 (0.627–0.924) |
|
| |||
| Probabilities*** | |||
|
| |||
| 3-month recurrence, low-risk | 0.02 | ||
| 3-month recurrence, int-risk | 0.04 | ||
| 3-month recurrence, high-risk | 0.094 | ||
| 12-month recurrence, low-risk | 0.15 | ||
| 12-month recurrence, int-risk | 0.26 | ||
| 12-month recurrence, high-risk | 0.39 | ||
| 2-year recurrence, low-risk | 0.10 | ||
| 2-year recurrence, int-risk | 0.13 | ||
| 2-year recurrence, high-risk | 0.11 | ||
| 3-year recurrence, low-risk | 0.05 | ||
| 3-year recurrence, int-risk | 0.06 | ||
| 3-year recurrence, high-risk | 0.06 | ||
| 4-year recurrence, low-risk | 0.08 | ||
| 4-year recurrence, int-risk | 0.05 | ||
| 4-year recurrence, high-risk | 0.02 | ||
| 5-year recurrence, low-risk | 0.07 | ||
| 5-year recurrence, int-risk | 0.03 | ||
| 5-year recurrence, high-risk | 0.03 | ||
Average of Ontario and Quebec values;
average of Ontario and British Columbia values;
relative risk for recurrence;23
cost per weekly instillation;
BCG: bacillus Calmette-Guerin; BLC: blue light cystoscopy; CI: confidence interval; CT: computed tomography; PDD: photodynamic diagnosis; RR: relative risk; TURBT: transurethral resection of bladder tumour; WLC: white light cystoscopy.
Table 2 shows the base case estimates for each province, comparing initial TURBT using BLC with HAL vs. WLC. The initial cost of establishing a BLC with HAL provincial program ranged from $2 064 033–9 620 422 at the population level for a single year of incident NMIBC tumours. After five years, the amortized cost of using BLC with HAL on every patient compared to WLC assisted TURBT dropped to $1 168 968–4 832 908 annually across the provinces, as additional recurrences in the WLC cohort added to overall costs. Cost per patient ranged from $1236–1372, resulting in 87–338 fewer recurrences and 268–1045 saved bed days. In terms of cost-effectiveness (initial difference in cost between BLC with HAL and WLC, divided by recurrences prevented), this corresponds to $28 463/recurrence saved in Ontario, and $23 724 and $19 354/recurrence saved in British Columbia and Quebec, respectively.
Table 2.
Baseline provincial decision analysis
| Ontario (n=3522) | British Columbia (n=903) | Quebec (n=2010) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| BLC | WLC | Net | BLC | WLC | Net | BLC | WLC | Net | |
| Initial costs | $26 165 385 | $16 544 963 | $9 620 422 | $5 374 301 | $3 310 268 | 2 064 033 | $9 029 938 | $5 294 536 | $3 735 401 |
| Followup costs | |||||||||
| No recurrence | $6 480 842 | $5 804 944 | $675 898 | $1 526 481 | $1 367 282 | $159 199 | $3 097 022 | $2 774 029 | $322 994 |
| Recurrences | $8 049 965 | $13 513 376 | −$5 463 411 | $1 606 304 | $2 660 568 | −$1 054 264 | $2 558 788 | $4 132 311 | −$1 573 523 |
| 5-year costs (total) | $40 696 192 | $35 863 283 | $4 832 908 | $8 507 086 | $7 338 118 | $1 168 968 | $14 685 748 | 12 200 876 | $2 484 872 |
| Cost-difference/case | $1372 | $1295 | $1236 | ||||||
| Events | |||||||||
| Bed days (n) | 7167 | 8212 | −1045 | 1837 | 2105 | −268 | 4,090 | 4687 | −597 |
| Bed days/patient | 2.03 | 2.33 | −0.30 | 2.03 | 2.33 | −0.30 | 2.03 | 2.33 | −0.30 |
| Recurrences (n) | 1351 | 1689 | −338 | 346 | 433 | −87 | 771 | 964 | −193 |
| Recurrences/patient | 0.38 | 0.48 | −0.10 | 0.38 | 0.48 | −0.10 | 0.38 | 0.48 | −0.10 |
BLC: blue light cystoscopy; WLC: white light cystoscopy.
Using the Ontario cost and probability data, several scenario analyses were performed to assess for cost differences compared to the baseline model (Table 3). If BLC with HAL equipment were provided to the province at no charge, the five-year amortized cost would be $4 158 814 and $1181 per patient. If postoperative mitomycin were excluded, the five-year amortized cost would be $4 200 398 and $1193 per patient. If BLC with HAL were only used in patients deemed on preoperative cystoscopy to be at risk of CIS, the five-year amortized cost would be $484 327, with a higher price per case of $1528 secondary to more upfront use of BCG. Similarly, if BLC with HAL were only used for cystoscopically appearing, aggressive tumours (intermediate- and high-risk), the five-year amortized cost would be $3 874 098, with a cost per patient of $1222. If there was a 20% or 50% improvement in progression rates with BLC plus HAL, the five-year amortized cost would be $2 660 529 ($755 per patient) and −$598,039 (−$170 per patient; cost saving), respectively.
Table 3.
Ontario data scenario analyses
| BLC | WLC | Net | Difference* | |
|---|---|---|---|---|
| Without BLC-additional equipment | ||||
| Initial costs | $25 524 410 | $16 544 963 | $8 976 447 | |
| Followup costs | ||||
| No recurrence | $6 480 842 | $5 804 944 | $675 898 | |
| Recurrences | $8 016 845 | $13 513 376 | −$5 496 531 | |
| 5-year costs (total) | $40 022 097 | $35 863 283 | $4 158 814 | −$674 095 |
| Cost-difference/case | $1181 | −$191 | ||
| Recurrences | 1351 | 1689 | −338 | 0 |
| Without postoperative mitomycin | ||||
| Initial costs | $23 706 733 | $14 826 550 | $8 880 182 | |
| Followup costs | ||||
| No recurrence | $6 480 842 | $5 804 944 | $675 898 | |
| Recurrences | $7 519 289 | $12 874 972 | −$5 355 683 | |
| 5-year costs (total) | $37 706 864 | $33 506 466 | $4 200 398 | −$632 511 |
| Cost-difference/case | $1193 | −$179 | ||
| Recurrences | 1351 | 1689 | −338 | 0 |
| 20% improvement in progression | ||||
| Initial costs | $26 165 385 | $16 544 963 | $9 620 422 | |
| Followup costs | ||||
| No recurrence | $6 571 009 | $5 804 944 | $766 065 | |
| Recurrences | $8 049 965 | $13 513 376 | −$5 463 411 | |
| Progression | $9 901 680 | $12 164 226 | −$2 262 546 | |
| 5-year costs (total) | $50 688 039 | $48 027 509 | $2 660 529 | −$2 172 380 |
| Cost-difference/case | $755 | −$617 | ||
| Recurrences | 1351 | 1689 | −338 | 0 |
| 50% improvement in progression | ||||
| Initial costs | $26 165 385 | $16 544 963 | $9 620 422 | |
| Followup costs | ||||
| No recurrence | $6 706 259 | $5 804 944 | $901 316 | |
| Recurrences | $8 049 965 | $13 513 376 | −$5 463 411 | |
| Progression | $6 507 861 | $12 164 226 | −$5 656 365 | |
| 5-year costs (total) | $47 429 470 | $48 027 509 | −$598 039 | −$5 430 948 |
| Cost-difference/case | −$170 | −$1542 | ||
| Recurrences | 1351 | 1689 | −338 | 0 |
| Only CIS** (n=317)# | ||||
| Initial costs | $2 968 779 | $1 918 103 | $1 050 676 | |
| Followup costs | ||||
| No recurrence | $536 891 | $478 850 | $58 041 | |
| Recurrences | $852 863 | $1 476 374 | −$623 511 | |
| 5-year costs (total) | $4 358 233 | $3 873 327 | $484 327 | |
| Cost-difference/case | $1528 | $156 | ||
| Recurrences | 136 | 169 | −33 | |
| Only intermediate-/high-grade (n=3170)# | ||||
| Initial costs | $22 096 531 | $13 875 308 | $8 221 222 | |
| Followup costs | ||||
| No recurrence | $5 773 887 | $5 168 103 | $605 784 | |
| Recurrences | $6 997 063 | $11 949 972 | −$4 952 909 | |
| 5-year costs (total) | $34 867 481 | $30 993 383 | $3 874 098 | |
| Cost-difference/case | $1222 | −$150 | ||
| Recurrences | 1246 | 1560 | −314 | |
Compared to baseline;
assumption – CIS is 20% of high-risk disease;
adjusted for no perioperative mitomycin instillation.
BLC: blue light cystoscopy; CIS: carcinoma in situ; WLC: white light cystoscopy.
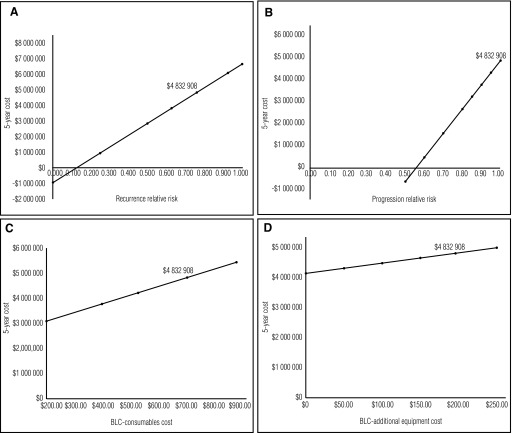
Sensitivity analyses provide further insight into changes in the model that may impact cost-effectiveness (Figs. 3A–D). Although no study to date has definitively demonstrated an improvement in NMIBC progression rates, scenario (Table 3) and sensitivity analyses (Fig. 3B) show that even a modest improvement in progression RR in favour of BLC with HAL would provide substantial economic benefit over a five-year time period. Additionally, BLC consumables (Fig. 3C), which contribute to the main cost of HAL, are a big driver of the overall cost.
Fig. 3.
Sensitivity analyses for Ontario data including (A) recurrence relative risk; (B) progression relative risk; (C) blue light cystoscopy (BLC) consumable cost; and (D) BLC additional equipment cost.
Discussion
There have been several RCTs comparing BLC with HAL to WLC-assisted TURBT for patients with NMIBC.13–22 Systematic reviews and meta-analyses have concluded that BLC with HAL improves recurrence rates, particularly in patients with high-risk bladder tumours.6,23–24,28–32 The current study is the first analysis conducted in the setting of a universal, public-access healthcare system using updated meta-analyzed sensitivities, specificities, and relative risk for recurrence based on prior RCTs. We were also able to incorporate a meta-analyzed progression RR based on recent data as an exploratory, scenario analysis.33 We demonstrate that despite an initial cost for implementing a comprehensive BLC with HAL program, it decreases bladder tumour recurrences and saves bed days. At five years after implementation, the jurisdictional differential cost for BLC with HAL is approximately $1–5 million (based on populations of 4–13 million people). This initial cost leads to improved tumour identification, which supports better disease management through decreased recurrence rates. The reduced recurrence rates would lead to more time between procedures, potentially contributing to a patient’s state of well-being.
Previous cost-effectiveness studies have been conducted in Germany,7,8 Sweden,10 France,12 the U.K.,9 and the U.S.11 An early study from Germany8 assessing use of BLC with HAL for newly diagnosed cases of BCa reported a cost of €584 per patient, although this study did not assess cost of followup and ongoing management. A more detailed study from Germany in 2009 reported cost savings for BLC with HAL of €140 per patient considering HAL instillation, equipment amortized over 10 years, staffing, pathology, and repeat resection after 3–6 months for the WLC-assisted group;7 however, this study assumed a 20% chance of second TURBT after 3–6 months, cost of €458 for WLC TURBT, and an overly optimistic €0 (no recurrence cost) for BLC with HAL. A Swedish study looked at newly diagnosed BCa, reporting a cost savings of €73 per patient over the first year, accounting for cost of cystoscopy, TURBT, post-TURBT treatment (i.e., BCG for NMIBC, cystectomy for muscle-invasive disease, etc.), and annual monitoring.10 Although this study delineated patients based on European Association of Urology (EAU) risk guidelines, the authors presumed a very optimistic 40% reduction in recurrence rates using BLC with HAL compared to WLC. In a U.S. study of new NMIBC cases, Garfield et al looked at all costs in the management of these patients (cystoscopy, TURBT, radical or partial cystectomy, Hexvix®, biopsy and pathology, BCG therapy, chemotherapy, imaging studies, ongoing surveillance of muscle-invasive disease) demonstrating a cost saving using BLC with HAL of $4660 per patient over five year compared to patients initially receiving WLC;11 however, the generalizability of these results is concerning since the probabilities used were primarily from best case scenario prior analyses. Taken together, these studies are heterogeneous with regards to use of HAL, cost variation between countries, variables implemented in the decision analysis, length and rigor of followup, and complexity of the decision analysis models.
As demonstrated in this study, there is an initial cost to establish a BLC with HAL program. Additional drivers of five-year expenditure include the cost of the HAL medication, as well as the equipment required to perform BLC-assisted TURBT; however, despite these additional costs, optimization of patient care is reliant on a complete TUR resection. The Canadian Urological Association (CUA) guidelines are well-established that for the management of NMIBC “TURBT is the first and gold standard treatment option. The quality of the initial TURBT is of utmost importance. Complete resection of the tumour, including focal areas of suspected CIS and abnormal areas in the prostatic urethra and bladder neck, should be performed.”34 Similarly, the American Urologic Association/Society of Urologic Oncology (AUA/SUO) guidelines recommend, “in a patient with NMIBC, a clinician should offer blue light cystoscopy at the time of TURBT, if available, to increase detection and decrease recurrence (moderate recommendation; evidence strength: Grade B).”35 BLC with HAL supports the most complete resection possible by enabling the visualization and complete resection of bladder tumours.
Undoubtedly, BLC with HAL decreases NMIBC recurrence rates6 and thus improves patient care, as recognized by several urology associations worldwide. Furthermore, particularly in settings where patients are admitted to the ward for overnight postoperative observation, BLC with HAL decreases the number of bed days associated with TURBTs. In Ontario, this may save an estimated $842 per bed/day,36 and importantly allocate beds to other types of patients with acuity requiring admission. In a healthcare system reliant on reducing/limiting cost, urologists likely need to be judicious with which NMIBC patients may receive maximal benefit from a BLC with HAL TURBT (e.g., CIS). Although the cost-per-patient in those with CIS is increased compared to the base case scenario (secondary to usage of BCG induction), improved visualization of grossly resected but perhaps microscopically unresected disease at the time of BLC with HAL TURBT will have the greatest impact for improving patient care and outcomes. Furthermore, this may obviate the need for postoperative mitomycin instillation in these cases, which has been advocated in the recent literature28,37 and would also contribute to cost savings.
To date, prior studies have not demonstrated a discernible improvement in BCa progression rates using BLC with HAL compared to WLC-assisted TURBT. A recent study analyzing long-term followup of a controlled, phase 3 study15 demonstrated a trend toward improved progression rates in patients treated with BLC with HAL, although the findings were not statistically significant due to lack of power.38 This study had 255 patients in the BLC with HAL arm and 261 patients in the WLC cystoscopy arm. In the original analysis, after a median followup of 4.5 years, eight HAL patients and 16 WLC patients had progressed to muscle-invasive disease (T2–T4) (p=0.066).15 Using a new definition for progression proposed by the International Bladder Cancer Group (IBCG)39 (change in T-stage, change to T2 or higher, or change from low- to high-grade disease), additional patients were deemed as having progressed: 21 (12.2%) HAL patients compared to 46 (17.6%) WLC patients (p=0.085).38 This included four HAL patients and 11 WLC patients progressing from Ta to CIS. A recent systematic review and meta-analysis of 14 RCTs assessing outcomes of HAL and 5-aminolevulinic acid (5-ALA) with BLC demonstrated no overall improvement in progression (RR 0.74; 95% CI 0.52–1.03);33 however, when performing a subanalysis of four HAL trials, there was a significant improvement in progression (RR 0.51; 95% CI 0.28–0.96), albeit based on only 14 events in the treatment arm and 28 events in the control arm. These findings must be interpreted with caution, as actual event rates are low and the meta-analyzed RR for progression is from subgroup analyses, which may be prone to bias. Overall, these results are encouraging and hopefully BLC with HAL will lead to significant improvements in progression rates for NMIBC patients with longer followup. As we have demonstrated in the scenario analyses (20% and 50% improvement in progression RR), an improvement in progression rates for BLC with HAL would have important economic implications for implementing this procedure for NMIBC patients.
The strength of the current decision/cost-effectiveness analysis for BLC with HAL is that this is the first model to use meta-analyzed probabilities for recurrence rates from previous RCTs. Earlier cost-effectiveness analyses used data from single RCTs or ‘best case scenario’ probabilities, thus the results from the current analysis are likely more generalizable. Second, the current manuscript provides scenario and sensitivity analyses that guide where future cost savings may be attained. Third, our study uses patient level, micro-costing data. Limitations to this study are as follows. First, the costs were derived from a universal healthcare model, which may not be generalizable to other private insurance-based or two-tiered (private insurance and public sector) health-care systems. Second, the model design does not allow for cost-analyses to be performed beyond five years after initial TURBT; thus, potential cost-effectiveness beyond five years is not ascertainable and we cannot delineate the time point when BLC with HAL generates cost savings. Third, the model does not account for the fact that, in WLC, an early recurrence secondary to incomplete resection may lead to repeat induction BCG in the WLC group, thus underestimating cost savings possible in the base case. Finally, the decision model did not allow for consideration of utilities (measures of global health-related quality of life) or quality-adjusted life years (QALY), thus preventing reporting of quality-adjusted outcome data or incremental cost-effectiveness ratios.
Conclusion
BLC with HAL decreases disease recurrence in patients with NMIBC, with a five-year cost of approximately $1–5 million for jurisdictions of 4–13 million people. Of interest to healthcare administrators, this also reduces the bed-day requirement of patients undergoing TURBT, allowing redistribution of hospital resources, including the treatment of more patients. If BLC with HAL truly improves progression rates, this would considerably improve cost-effectiveness and may even yield cost savings.
Acknowledgement
We would like to thank Rouhi Fazelzad for her assistance with performing the systematic review.
Footnotes
Competing interests: This study was funded by BioSyent Pharma Inc. Dr. Kassouf has received grants/honoraria from Amgen, Astellas, and Janseen. Dr. Black has been an advisor for Abbvie, Amgen, Astellas, Biocancell, Cubist, Janssen, Novartis, and Sitka; a speaker for Abbvie, Janssen, Ferring, Novartis, and Red Leaf Medical; has received grants/honoraria from Pendopharm; has participated in clinical trials supported by Amgen, Astellas, Ferring, Janssen, and Roche; and has received research funding from GenomeDx, iProgen, Lilly, and New B Innovation. The remaining authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. https://doi.org/10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der Meijden AP, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–75. doi: 10.1016/j.eururo.2005.12.031. https://doi.org/10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. https://doi.org/10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 4.Avritscher EB, Cooksley CD, Grossman HB, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68:549–53. doi: 10.1016/j.urology.2006.03.062. https://doi.org/10.1016/j.urology.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW, Donat SM. Quality control in transurethral resection of bladder tumours. BJU Int. 2008;102:1242–6. doi: 10.1111/j.1464-410X.2008.07966.x. https://doi.org/10.1111/j.1464-410X.2008.07966.x. [DOI] [PubMed] [Google Scholar]
- 6.Witjes JA, Babjuk M, Gontero P, et al. Clinical and cost-effectiveness of hexaminolevulinate-guided blue-light cystoscopy: Evidence review and updated expert recommendations. Eur Urol. 2014;66:863–71. doi: 10.1016/j.eururo.2014.06.037. https://doi.org/10.1016/j.eururo.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: What are the benefits and costs? World J Urol. 2009;27:295–300. doi: 10.1007/s00345-009-0395-z. https://doi.org/10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burger M, Petshcl S, Volkmer BG. [Kalkulation einer neuen Unter-suchungs- and Behandlungsmethode (NUB): Analyse am Beispiel der PDD-gestutzten TUR-B mit Hexaminolavulinsaure]. Urologe A. 2008;47:1239–44. doi: 10.1007/s00120-008-1810-6. https://doi.org/10.1007/s00120-008-1810-6. [DOI] [PubMed] [Google Scholar]
- 9.Dindyal S, Nitkunan T, Bunce CJ. The economic benefit of photodynamic diagnosis in non-muscle-invasive bladder cancer. Photodiagnosis Photodyn Ther. 2008;5:153–8. doi: 10.1016/j.pdpdt.2008.05.001. https://doi.org/10.1016/j.pdpdt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Malmstrom PU, Hedelin H, Thomas YK, et al. Fluorescence-guided transurethral resection of bladder cancer using hexaminolevulinate: Analysis of health economic impact in Sweden. Scand J Urol Nephrol. 2009;43:192–8. doi: 10.1080/00365590902808541. https://doi.org/10.1080/00365590902808541. [DOI] [PubMed] [Google Scholar]
- 11.Garfield SS, Gavaghan MB, Armstrong SO, et al. The cost-effectiveness of blue light cystoscopy in bladder cancer detection: U.S. projections based on clinical data showing 4.5 years of followup after a single hexaminolevulinate hydrochloride instillation. Can J Urol. 2013;20:6682–9. [PubMed] [Google Scholar]
- 12.Roupret M, Malavaud B, Molinier L, et al. Cost-effectiveness of transurethral resection of bladder with blue light in patients with non-muscle-invasive bladder cancer in France. Prog Urol. 2015;25:256–64. doi: 10.1016/j.purol.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Hermann GG, Mogensen K, Carlsson S, et al. Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients: A randomized, two-centre study. BJU Int. 2011;108:E297–303. doi: 10.1111/j.1464-410X.2011.10090.x. https://doi.org/10.1111/j.1464-410X.2011.10090.x. [DOI] [PubMed] [Google Scholar]
- 14.Geavlete B, Multescu R, Georgescu D, et al. Treatment changes in long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: Does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU Int. 2012;109:549–56. doi: 10.1111/j.1464-410X.2011.10374.x. https://doi.org/10.1111/j.1464-410X.2011.10374.x. [DOI] [PubMed] [Google Scholar]
- 15.Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided-fluorescence cystoscopy reduces recurrence in patients with non-muscle-invasive bladder cancer. J Urol. 2010;184:1907–13. doi: 10.1016/j.juro.2010.06.148. https://doi.org/10.1016/j.juro.2010.06.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karaolides T, Skolarikos A, Bourdoumis A, et al. Hexaminolevulinate-induces fluorescence vs. white light during transurethral resection of non-invasive bladder tumour: Does it reduce recurrences? Urology. 2012;80:354–60. doi: 10.1016/j.urology.2012.03.067. https://doi.org/10.1016/j.urology.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 17.Lapini A, Minervini A, Masala A, et al. A comparison of hexaminolevulinate (Hexvix®) fluorescence cystoscopy and white-light cystoscopy for detection of bladder cancer: Results of the HeRo observational study. Surg Endosc. 2012;26:3634–41. doi: 10.1007/s00464-012-2387-0. https://doi.org/10.1007/s00464-012-2387-0. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien T, Ray E, Chatterton K, et al. Prospective, randomized trial of hexylaminolevulinate photodynamic-assisted transurethral resection of bladder tumour (TURBT) plus single-shot intravesical mitomycin C vs. conventional white light TURBT plus mitomycin C in newly presenting non-muscle-invasive bladder cancer. BJU Int. 2013;112:1096–1104. doi: 10.1111/bju.12355. https://doi.org/10.1111/bju.12355. [DOI] [PubMed] [Google Scholar]
- 19.Gkritsios P, Hatzimouratidis K, Kazantzidis S, et al. Hexaminolevulinate-guided transurethral resection of non-muscle-invasive bladder cancer does not reduce the recurrence rates after a two-year followup: A prospective, randomized trial. Int Urol Nephrol. 2014;46:927–33. doi: 10.1007/s11255-013-0603-z. https://doi.org/10.1007/s11255-013-0603-z. [DOI] [PubMed] [Google Scholar]
- 20.Mariappan P, Rai B, El-Mokadem I, et al. Real-life experience: Early recurrence with Hexvix® photodynamic diagnosis-assisted transurethral resection of bladder tumour vs. good-quality white light TURBT in new non-muscle-invasive bladder cancer. Urology. 2015;86:327–31. doi: 10.1016/j.urology.2015.04.015. https://doi.org/10.1016/j.urology.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Palou J, Hernandez C, Solsona E, et al. Effectiveness of hexaminolevulinate fluorescence cystoscopy for the diagnosis of non-muscle-invasive bladder cancer in daily clinical practice: A Spanish multicentre observational study. BJU Int. 2015;116:37–43. doi: 10.1111/bju.13020. https://doi.org/10.1111/bju.13020. [DOI] [PubMed] [Google Scholar]
- 22.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality, and recurrence. J Urol. 2000;164:680–4. doi: 10.1016/s0022-5347(05)67280-1. https://doi.org/10.1016/S0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 23.Burger M, Grossman HB, Droller M, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis of detection and recurrence based on raw data. Eur Urol. 2013;64:846–54. doi: 10.1016/j.eururo.2013.03.059. https://doi.org/10.1016/j.eururo.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 24.Mowatt G, Zhu S, Kilonzo M, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and followup of bladder cancer. Health Technol Assess. 2010;14:1–331. doi: 10.3310/hta14040. https://doi.org/10.3310/hta14040. [DOI] [PubMed] [Google Scholar]
- 25.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Accessed May 18, 2017]. Available at http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2014-EN.pdf. [Google Scholar]
- 26.BC Cancer Agency – Statistics by cancer type. [Accessed December 29, 2016]. Available at: www.bccancer.bc.ca/statistics-and-reports-site/Documents/Cancer_Type_Bladder_2013.pdf.
- 27.Cancer Care Ontario – Incidence and mortality. [Accessed December 29, 2016]. Available at: https://www.cancercare.on.ca/cms/one.aspx.
- 28.Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur Urol. 2017;71:447–61. doi: 10.1016/j.eururo.2016.05.041. https://doi.org/10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Kausch I, Sommerauer M, Montorsi F, et al. Photodynamic diagnosis in non-muscle-invasive bladder cancer: A systematic review and cumulative analysis of perspective studies. Eur Urol. 2010;57:595–606. doi: 10.1016/j.eururo.2009.11.041. https://doi.org/10.1016/j.eururo.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 30.Rink M, Babjuk M, Catto JWF, et al. Hexyl Aminolevulinate-guided fluorescence cystoscopy in the diagnosis and followup of patients with non-muscle-invasive bladder cancer: A critical review of the current literature. Eur Urol. 2013;64:624–38. doi: 10.1016/j.eururo.2013.07.007. https://doi.org/10.1016/j.eururo.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Shen P, Yang J, Wei W, et al. Effects of fluorescent light-guided transurethral resection on non-muscle-invasive bladder cancer: A systematic review and meta-analysis. BJU Int. 2012;110:E209–15. doi: 10.1111/j.1464-410X.2011.10892.x. https://doi.org/10.1111/j.1464-410X.2011.10892.x. [DOI] [PubMed] [Google Scholar]
- 32.Yuan H, Qiu J, Liu L, et al. Therapeutic outcome of fluorescence cystoscopy transurethral resection in patients with non-muscle-invasive bladder cancer: A meta-analysis of randomized, controlled trials. PloS ONE. 2013;8:e74142. doi: 10.1371/journal.pone.0074142. https://doi.org/10.1371/journal.pone.0074142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou R, Selph S, Buckley DI, et al. Comparative effectiveness of fluorescent vs. white light cystoscopy for initial diagnosis or surveillance of bladder cancer on clinical outcomes: Systematic review and meta-analysis. J Urol. 2017;197:548–58. doi: 10.1016/j.juro.2016.10.061. https://doi.org/10.1016/j.juro.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 34.Kassouf W, Traboulsi SL, Kulkarni GS, et al. CUA guidelines on the management of non-muscle-invasive bladder cancer. Can Urol Assoc J. 2015;9:E690–704. doi: 10.5489/cuaj.3320. https://doi.org/10.5489/cuaj.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle-invasive bladder bancer: AUA/SUO guideline. J Urol. 2016;196:1021–9. doi: 10.1016/j.juro.2016.06.049. https://doi.org/10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 36.Canadian Life and Health Insurance Association. Improving the accessibility, quality, and sustainability of long-term care in Canada. [Accessed December 23, 2016]. Available at: https://www.clhia.ca/domino/html/clhia/CLHIA_LP4W_LND_Webstation.nsf/resources/Content_PDFs/$file/LTC_Policy_Paper.pdf.
- 37.Sylvester RJ, Oosterlinck W, Holmang S, et al. Systematic review and individual patient data meta-analysis of randomized trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa–pT1 urothelial carcinoma of the bladder: Which patients benefit from instillation? Eur Urol. 2016;69:231–44. doi: 10.1016/j.eururo.2015.05.050. https://doi.org/10.1016/j.eururo.2015.05.050. [DOI] [PubMed] [Google Scholar]
- 38.Kamat AM, Cookson M, Witjes JA, et al. The impact of blue light cystoscopy with hexaminolevulinate (HAL) on progression of bladder cancer – A new analysis. Bladder Cancer. 2016;2:273–8. doi: 10.3233/BLC-160048. https://doi.org/10.3233/BLC-160048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamm D, Persad R, Brausi M, et al. Defining progression in non-muscle-invasive bladder cancer: It is time for a new standard definition. J Urol. 2014;191:20–7. doi: 10.1016/j.juro.2013.07.102. https://doi.org/10.1016/j.juro.2013.07.102. [DOI] [PubMed] [Google Scholar]