Abstract
Background
People who inject drugs (PWID) who are highly connected within their injection drug networks may be important HIV transmission nodes if they frequently share syringes with other PWID and are not engaged in HIV care. In India, HIV transmission fueled by injection drug use is increasing, however little is known about the associations between injection network size and syringe sharing and viral suppression.
Methods
We recruited 14,481 PWID between October 2012 – December 2013 by respondent driven sampling across 15 sites in India. Interviewer-administered questionnaires assessed network characteristics, substance use, HIV testing experience, and access to health services. We used multilevel logistic regression modeling to evaluate the relationship between injection drug network size and 1) syringe sharing at last injection and 2) viral suppression among HIV-positive participants (<150 copies/ml).
Findings
The median injection network size was 3 [IQR: 1–5] and 7% of participants injected with >10 members in the past 30 days. PWID who had >10 members in their network were 1.65 times (95% CI: 1.12 – 2.42, p=0.0111) more likely to have shared a syringe at last injection compared to those in the 0–1 members in their drug networks. Additionally, individuals with the largest injection drug networks were also 31% (95% CI: 0.53 – 0.90, p=0.006) less likely to be virally suppressed compared to individuals in the smallest injection drug networks.
Discussion
Individuals with larger networks may be important in HIV transmission within injection drug networks since they were the most likely to engage in recent syringe sharing and least likely to be virally suppressed.
Keywords: viral suppression, injection drug use, network, India
Introduction
Injection drug use is increasingly accounting for new HIV infections in both low/middle income countries (LMICs) and countries that once saw notable declines in HIV incidence among people who inject drugs (PWID).1 Even in countries with notable declines of HIV incidence among PWID, such as the United States, the rise of prescription opioid use may result in a reemergence of injection drug use and associated HIV and hepatitis C virus (HCV). For HIV and HCV, the potential impact of treatment - not just for improving individual outcomes but also for achieving prevention of new infections at a population level - has resulted in a focus on Treatment as Prevention (TasP) interventions. Optimizing the potential impact of these interventions among PWID requires a more nuanced understanding of how network characteristics are associated with transmission risk. Given the limited resources particularly in LMICs, it may be prudent to initially identify and target interventions for high-risk injectors who are more likely to acquire/transmit HIV and HCV.2,3
PWID are highly inter-connected via their injection drug networks, but network size can vary. Network characteristics such as size 4 and density 5 have been associated with engaging in risky injection practices, such as sharing of syringes 6, and are key drivers of HIV transmission among PWID. However, as noted in a recent systematic review, little is known about how network characteristics are associated with outcomes along the HIV care continuum. 7 As a consequence, while several network based interventions have focused on HIV prevention via reducing risk behaviors 8,9, these types of interventions should also consider targeting adherence to antiretroviral therapy (ART) and achieving viral suppression.
In a recent study conducted across 15 Indian cities, we observed high HIV incidence (2.9 per 100 person-years) and prevalence (18%), and high HCV prevalence (37.2%)10 Some cities in our sample experienced rapidly escalating epidemics with HIV incidence up to 12.4 per 100 person-years.10 Injection drug use and HIV have been endemic in northeastern India, near the heroin producing regions of Myanmar, Thailand, and Laos (“golden triangle”) for decades11. However, recent evidence suggests that injection drug use is increasing in north and central India, primarily due to injection of buprenorphine and pharmaceutical opioids.12 As such, PWID have been identified as a key population to monitor trends and disease burden and the National AIDS Control Program of India has prioritized HIV prevention strategies in this group.13 Yet, injection drug network characteristics and their impact on risk behaviors and HIV clinical outcomes remain poorly understood. In one analysis from south India among 1,078 PWID, those who received material support (e.g., money) from one’s social network were 1.6 and 1.5 times more likely to be infected with HIV and hepatitis C virus (HCV), respectively.14 Another multi-site mixed methods study reported that syringe sharing was common with PWID outside their usual groups of partners, partly due to lack of clean syringes.15 However, these studies did not focus on the role of injecting networks in achieving critical HIV clinical benchmarks on the care continuum, such as viral suppression.
Accordingly, the objectives of this analysis were to characterize the variability in injection network size among PWID across India and to determine whether drug network characteristics were associated with risk behavior and optimal engagement in HIV care.
Methods
Study setting and recruitment
As part of a cluster-randomized trial, a baseline cross-sectional survey was administered across 15 cites in India – 7 cites in the northeast with historical drug use epidemics, 2 large cities (Delhi and Mumbai), and 6 cities in north and central India with emerging epidemics of injection drug use. 16 Recruitment at each site was coordinated with a local NGO partner that provided services to PWID. Approximately 1000 participants from each site were recruited by respondent-driven sampling (RDS). Briefly, RDS uses peer referral to recruit individuals through coupon disbursement 17 and in this study recruitment was initiated with 2–3 individuals who were well-connected to the PWID communities (“seeds”) at each site. Each participant was given two coupons to distribute randomly to other people drug users who they knew in their communities. Participants were compensated for both participation and recruitment of up to two eligible participants. Eligibility criteria were being at least 18 years of age, reporting injection drug use in previous 2 years, and presentation of an RDS coupon upon enrollment. To prevent duplicate enrollment, participants who consented provided a fingerprint image that was converted to a unique hexadecimal code. Overall, RDS worked efficiently in obtaining the desired sample size at each site within a median of 135 days over a median of 22 recruitment waves.10
Study procedures
Participants completed an interviewer-administered questionnaire that obtained data on sociodemographics, network characteristics, experience with HIV testing and treatment, substance use, and access to services. All participants were offered HIV and HCV testing, and pre- and post-test counseling.
Laboratory measures
Three rapid HIV testing kits (Alere Determine HIV-1/2 [Alere Medical]; First Response HIV card test 1–2.0 [PMC Medical India]; and Signal Flow Through HIV 1 = 2 Spot/Immunodot Test kit [Span Diagnostics]) were used for on-site rapid HIV testing. The RealTime HIV-1 assay was used to measure HIV-1 RNA levels, with a lower limit of quantification of 150 copies/ml (Abbott Laboratories). HCV antibody testing was performed using the Genedia HCV ELISA 3.0 (Green Cross Medical Science, Chungbuk, Korea).
Outcomes
Outcomes of interest included injection-related risk behavior and viral suppression. Injection risk behavior was defined as sharing (distributive or receptive) a needle/syringe with someone else at last injection. Viral suppression was defined as HIV RNA <150 copies/ml.
Explanatory variables
Our explanatory variables of interest included different parameterizations of participants’ injection drug networks. The primary variable was the number of persons an individual reported injecting with in the prior 30 days. Due to imprecision in the self-reported network size 18,19, we categorized injection drug network size into 4 categories selected to measure an ordinal level of risk: 0–1, 2–5, 6–10, and >10 individuals. Of the 14,481 participants enrolled across the 15 sites, 103 participants (0.7%) did not remember or did not know how many people they had injected with in the previous 30 days and were excluded from all analyses. Other quantitative network-level variables of interest included: the number of PWID the participant knew and saw at least once in the past 30 days, the frequency of injecting with multiple persons in the past 30 days, the number of persons the participant passed a syringe after using it, and the number of persons who used a syringe prior to the respondent. We also included shooting gallery attendance as a “qualitative” measure of injection network size and social support as a measure of the function of one’s broader social network that the injection risk network is embedded in. Social support consisted of five items that measured frequency (5-point Likert scale from none of the time to all of the time) of 1) feeling wanted, 2) receiving help with daily chores, 3) buying medicine, 4) help with transportation, and 5) money when the participant needed it. The responses were summed and categorized into low (<10), medium (11–19), and high (20–25) social support.
Statistical analyses
As reported previously, all RDS process measures were satisfied (homophily, number of waves, and equilibrium).20 In prior analyses, we used the Volz-Heckathorn estimator to weight population estimates using the overall network size (number of PWID seen in past 30 days). However, since injection drug network size was a primary explanatory variable of interest, we present unweighted estimates because injection drug network size and overall network size were positively and significantly correlated (r=0.44, p<0.0001). RDS-II weighted analyses (with the exclusion of the estimate of overall network size) can be found in the Supplementary Digital Content (Tables S1–S3).
We used multilevel logistic regression models with random intercepts for each site to assess the association between network factors and syringe sharing at last injection and viral suppression. All models controlled for age, sex, study site, while other sociodemographic (educational level, marital status) and substance use variables (drugs injected within the past 6 months, alcohol use [hazardous use and dependence defined according to validated cutoffs from the alcohol use disorders identification test 21], syringe exchange program, and opioid substitution therapy utilization) were retained if they were significantly associated with the outcome in the univariable model (p<0.10) and retained significance in multivariable models (p<0.05). We ran separate models for each of the explanatory variables. Sensitivity analyses excluded individuals who did not report actively injecting in the prior six months (n=1,882). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Ethical approval
This study was approved by the Y.R. Gaitonde Centre for AIDS Research and Education (YRGCARE) and the Johns Hopkins Medical Institutions (JHMI) Institutional Review Boards. All participants provided verbal informed consent.
Results
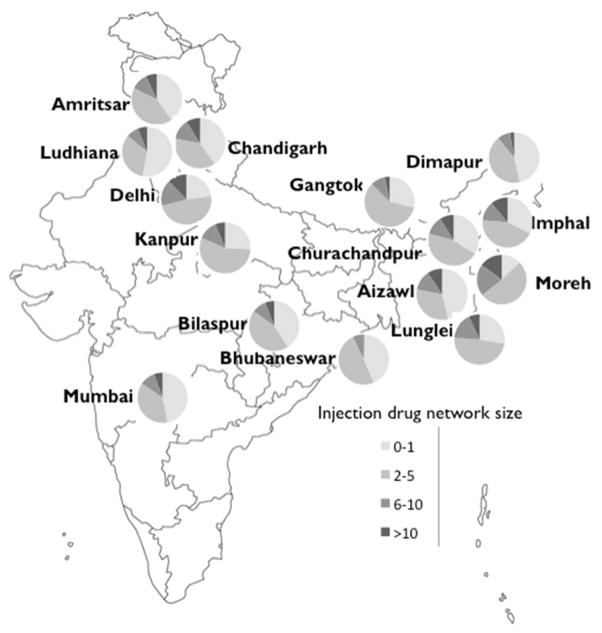
The median age of the entire sample (N=14,378) was 30 [IQR: 24–36] and the vast majority (N=13,542, 94%) were male. The median drug network size was 3 (IQR: 1–5) and 7.1% reported injecting with more than 10 other people in the past 30 days. Distribution of injection drug network size varied considerably across the 15 sites (Figure 1). In Moreh, located in the northeast, more than 35% of participants reported injecting with >5 individuals in the past 30 days, while in Dimapur, also in the northeast, approximately 10% of participants reported injecting with more than 5 individuals in the past 30 days.
Figure 1.
Distribution of injection drug network size across 15 sites in India
Demographic and risk behavior variables differed significantly by size of the injection drug network (Table 1). Persons with larger drug using networks tended to have lower educational levels and be unmarried and were more likely to report sharing a syringe at their last injection. Persons with > 10 injection network members had significantly higher prevalence of HIV (26%), HCV (60%) and HIV/HCV (23.5%) coinfection than those with 2–5 and 6–10 injection network members (see Figure S1, Supplementary Digital Content). Those with 0–1 network members in their network had slightly higher HIV and HCV prevalence than those with 2–5 injection network members.
Table 1.
Characteristics by injection drug network size among 14,378 persons recruited across 15 cities in India
| 0–1 (37.0%) | 2–5 (44.4%) | 6–10 (11.6%) | >10 (7.1%) | p-value | |
|---|---|---|---|---|---|
|
| |||||
| Age | 30 (25 – 37) | 29 (24 – 35) | 29 (24 – 35) | 29 (25 – 35) | 0.0019 |
| Sex | <.0001 | ||||
| Male | 4911 (92.5) | 6052 (94.9) | 1591 (95.6) | 988 (97.2) | |
| Female | 401 (7.6) | 324 (5.1) | 74 (4.4) | 29 (2.9) | |
|
| |||||
| Education | <.0001 | ||||
| Primary school or less | 1706 (32.1) | 2363 (37.0) | 587 (35.2) | 343 (33.7) | |
| Secondary school | 2536 (47.8) | 2789 (43.7) | 756 (45.4) | 505 (49.6) | |
| High school and above | 1069 (20.1) | 1229 (19.3) | 324 (19.4) | 170 (16.7) | |
|
| |||||
| Married | <.0001 | ||||
| Yes | 2403 (45.2) | 2504 (39.2) | 618 (37.1) | 319 (31.3) | |
| No | 2909 (54.8) | 3877 (60.8) | 1048 (62.9) | 699 (68.7) | |
|
| |||||
| Alcohol use* | <.0001 | ||||
| No alcohol use | 2356 (44.4) | 2562 (40.2) | 667 (40.0) | 404 (39.7) | |
| Low/moderate use | 961 (18.1) | 1197 (18.8) | 273 (16.4) | 151 (14.8) | |
| Harmful/hazardous | 875 (16.5) | 1205 (18.9) | 307 (18.4) | 194 (19.1) | |
| Dependence | 1120 (21.1) | 1416 (22.2) | 419 (25.2) | 269 (26.4) | |
|
| |||||
| Number of years injecting | <.0001 | ||||
| 0 – 3 | 1154 (21.9) | 1521 (23.9) | 331 (19.9) | 153 (15.1) | |
| 4 – 6 | 1387 (26.3) | 1878 (29.5) | 472 (28.4) | 268 (26.4) | |
| 7 – 12 | 1345 (25.5) | 1589 (25.0) | 471 (28.4) | 323 (31.8) | |
| >12 | 1396 (26.4) | 1378 (21.7) | 387 (23.3) | 271 (26.7) | |
|
| |||||
| Drug injected in past 6 months | |||||
| Heroin | 1701 (32.0) | 2527 (39.6) | 742 (44.5) | 547 (53.7) | <.0001 |
| Buprenorphine | 1463 (27.5) | 2106 (33.0) | 558 (33.5) | 394 (38.7) | 0.0055 |
| Painkillers | 1135 (21.4) | 2268 (35.5) | 573 (34.4) | 284 (27.9) | 0.0001 |
|
| |||||
| Shared needle/syringe at last injection | <.0001 | ||||
| Yes | 1148 (21.6) | 1778 (27.9) | 522 (31.3) | 328 (32.2) | |
| No | 4164 (78.4) | 4603 (72.1) | 1145 (68.7) | 690 (67.8) | |
|
| |||||
| Syringe exchange program (SEP) utilization | <.0001 | ||||
| Never used | 3119 (59.2) | 3819 (60.1) | 808 (48.7) | 442 (43.6) | |
| More than 30 days ago | 1050 (19.9) | 745 (11.7) | 279 (16.9) | 152 (15.0) | |
| Within past 30 days | 1103 (20.9) | 1788 (28.2) | 572 (34.5) | 421 (41.5) | |
|
| |||||
| Opioid substitution therapy (OST) utilization | <.0001 | ||||
| Never used | 3946 (74.9) | 5018 (79.1) | 1270 (76.6) | 715 (70.6) | |
| More than 30 days ago | 597 (11.3) | 706 (11.1) | 238 (14.4) | 194 (19.2) | |
| Within past 30 days | 722 (13.7) | 619 (9.8) | 150 (9.1) | 104 (10.3) | |
|
| |||||
| Injection location in past 6 months**§ | <.0001 | ||||
| Home | 1788 (33.7) | 2584 (40.5) | 723 (43.4) | 491 (48.2) | |
| Friend’s house | 840 (15.8) | 2195 (34.4) | 612 (36.7) | 357 (35.1) | |
| Dealer’s house | 647 (12.2) | 1400 (21.9) | 483 (29.0) | 386 (37.9) | |
| Public park | 1618 (30.5) | 2853 (44.7) | 772 (46.3) | 474 (46.6) | |
| Public toilet | 696 (13.1) | 1741 (27.3) | 451 (27.1) | 365 (35.9) | |
| Shooting gallery | 928 (17.5) | 1728 (27.1) | 468 (28.1) | 309 (30.4) | |
| Graveyard/cemetery | 719 (13.5) | 1598 (25.0) | 529 (31.7) | 350 (34.4) | |
|
| |||||
| Number of PWID seen in past 30 days | <.0001 | ||||
| 1–5 | 1842 (34.7) | 2392 (37.5) | 0 (0.0) | 0 (0.0) | |
| 6–10 | 1111 (20.9) | 1621 (25.4) | 607 (36.4) | 0 (0.0) | |
| 11–20 | 1285 (24.2) | 1301 (20.4) | 469 (28.1) | 309 (30.4) | |
| >20 | 1074 (20.2) | 1067 (16.7) | 591 (35.5) | 709 (69.6) | |
|
| |||||
| Number of persons respondent passed needle/syringe to in past 30 days | <.0001 | ||||
| 0 | 5009 (94.4) | 5192 (81.6) | 1291 (77.5) | 765 (75.3) | |
| 1–3 | 263 (5.0) | 887 (13.9) | 209 (12.6) | 149 (14.7) | |
| >3 | 35 (0.7) | 287 (4.5) | 165 (9.9) | 102 (10.0) | |
|
| |||||
| Number of persons that used needle/syringe before respondent in past 30 days | <.0001 | ||||
| 0 | 5050 (95.2) | 5323 (83.8) | 1330 (80.6) | 798 (78.9) | |
| 1–3 | 235 (4.4) | 812 (12.8) | 200 (12.1) | 142 (14.1) | |
| >3 | 19 (0.4) | 221 (3.5) | 121 (7.3) | 71 (7.0) | |
|
| |||||
| Frequency injected with multiple other persons | |||||
| Never | 3478 (65.7) | 1546 (24.3) | 325 (19.5) | 174 (17.2) | <.0001 |
| Less than half/half of the time | 1120 (21.1) | 2807 (44.1) | 712 (42.8) | 446 (44.0) | |
| More than half of the time | 699 (13.2) | 2014 (31.6) | 627 (37.7) | 393 (38.8) | |
|
| |||||
| Social support | <.0001 | ||||
| Low (<10) | 1220 (23.0) | 1601 (25.1) | 397 (23.8) | 249 (24.5) | |
| Medium (10–19) | 2504 (47.2) | 3422 (53.6) | 882 (52.9) | 521 (51.2) | |
| High (20–25) | 1585 (29.9) | 1357 (21.3) | 387 (23.2) | 248 (24.4) | |
|
| |||||
| Suppressed viral load (among HIV positives only, N=2915) | 399 (34.5) | 271 (24.1) | 80 (23.3) | 49 (18.4) | <.0001 |
classified according to the Alcohol Use Disorder Identification Test (AUDIT) standard cutoffs
multiple responses allowed
only among those who had injected in the past 6 months
Sharing syringe at last injection
Table 2 presents the results from the univariable and multivariable logistic regression of sharing a syringe at last injection. In univariable analysis, compared to individuals who reported 0–1 PWID in their injection drug network in the past 30 days, those with >10 injection drug network members were 1.91 (95% CI: 1.28 –2.85) times more likely to have shared a syringe at last injection. After adjusting for age, sex, region, educational level, marital status, number of years injecting, injected heroin in past 6 months, alcohol use, and utilization of syringe exchange program, this association attenuated slightly but retained statistical significance (aOR: 1.65 (95% CI: 1.12 – 2.42) and was similar for those with 6–10 members in their network (aOR: 1.69, 95% CI: 1.17 – 2.45). Additionally, in separate multivariable models, there were statistically significant associations with injecting with multiple people more than half the time in the past 6 months (aOR: 1.79, 95% CI: 1.21 – 2.66) and injecting in a shooting gallery in the past 6 months (aOR: 1.83; 95% CI: 1.12 – 3.00). In sensitivity analyses that were limited to only PWID who injected in the past 6 months, the associations remained consistent (see Tables S4–S6 Supplementary Digital Content).
Table 2.
Correlates of sharing syringe at last injection among 14,378 persons recruited across 15 cities in India
| Univariable OR (95% CI) | p-value | Multivariable aOR* (95% CI) | p-value | |
|---|---|---|---|---|
|
| ||||
| Number of PWID injected with in past 30 days | ||||
| 0–1 | Ref | Ref | ||
| 2–5 | 1.41 (1.06 – 1.86) | 0.0179 | 1.36 (1.05 – 1.77) | 0.0194 |
| 6–10 | 1.86 (1.26 – 2.73) | 0.0016 | 1.69 (1.17 – 2.45) | 0.0054 |
| >10 | 1.91 (1.28 – 2.85) | 0.0016 | 1.65 (1.12 – 2.42) | 0.0111 |
|
| ||||
| Number of PWID known and seen in past 30 days | ||||
| 1–5 | Ref | Ref | ||
| 6–10 | 1.02 (0.79 – 1.32) | 0.8613 | 0.99 (0.78 – 1.26) | 0.9293 |
| 11 – 20 | 1.03 (0.85 – 1.24) | 0.7860 | 0.95 (0.81 – 1.12) | 0.5388 |
| >20 | 1.18 (0.93 – 1.51) | 0.1771 | 1.06 (0.84 – 1.34) | 0.6100 |
|
| ||||
| Frequency injected with multiple people | ||||
| Never | Ref | Ref | ||
| Less than half the time/half | 1.54 (1.11 – 2.14) | 0.0091 | 1.46 (1.07 −2.00) | 0.0139 |
| More than half | 1.82 (1.19 – 2.78) | 0.0057 | 1.79 (1.21 – 2.66) | 0.0037 |
|
| ||||
| Shooting gallery in past 6 months | ||||
| No | Ref | Ref | ||
| Yes | 1.96 (1.22 – 3.15) | 0.0053 | 1.83 (1.12 – 3.00) | 0.0163 |
Adjusted for age, sex, region, educational level, marital status, number of years injecting, injected heroin in past 6 months, alcohol use (AUDIT), utilization of syringe exchange program,
Network size and the HIV care continuum
Among HIV infected individuals (N=2,915), those with the largest (>10) drug networks were least likely to be linked to care, on ART and have undetectable viral load (see Figure S2, Supplementary Digital Content). Many of the network variables that were positively and significantly associated with recent syringe sharing were also negatively associated with viral suppression (Table 3). Individuals who reported injecting with >10 individuals had lower odds of viral suppression (aOR: 0.69, 95% CI: 0.53 – 0.90) than those with 0–1 members in their injection drug network.
Table 3.
Correlates of HIV viral suppression among 2,915 persons infected with HIV recruited across 15 cities in India
| Univariable OR (95% CI) | p-value | Multivariable aOR* (95% CI) | p-value | |
|---|---|---|---|---|
|
| ||||
| Number PWID injected with in past 30 days | ||||
| 0–1 | Ref | Ref | ||
| 2–5 | 0.68 (0.52 − 0.89) | 0.0047 | 0.78 (0.63 – 0.98) | 0.0290 |
| 6–10 | 0.71 (0.55 – 0.91) | 0.0075 | 0.91 (0.69 – 1.19) | 0.4834 |
| >10 | 0.55 (0.40 – 0.75) | 0.0002 | 0.69 (0.53 – 0.90) | 0.0063 |
|
| ||||
| Number of PWID known and seen in past 30 days | ||||
| 1–5 | Ref | Ref | ||
| 6–10 | 1.11 (0.90 – 1.37) | 0.3356 | 1.17 (0.95 – 1.44) | 0.1377 |
| 11 – 20 | 1.22 (1.01 – 1.49) | 0.0434 | 1.20 (0.99 – 1.47) | 0.0696 |
| >20 | 1.10 (0.81 – 1.48) | 0.5454 | 1.15 (0.86 – 1.55) | 0.3464 |
|
| ||||
| Frequency injected with multiple people | ||||
| Never | Ref | Ref | ||
| Less than half the time/half | 0.73 (0.59 – 0.89) | 0.0025 | 0.85 (0.70 – 1.03) | 0.0974 |
| More than half | 0.70 (0.52 – 0.94) | 0.0162 | 0.89 (0.69 – 1.15) | 0.3762 |
|
| ||||
| Number of persons respondent passed needle/syringe to in past 30 days | ||||
| 0 | Ref | Ref | ||
| 1–3 | 0.71 (0.53 – 0.95) | 0.0220 | 0.88 (0.68 – 1.13) | 0.3083 |
| >3 | 0.41 (0.27 – 0.61) | <.0001 | 0.56 (0.40 – 0.79) | 0.0008 |
|
| ||||
| Number of persons that used needle/syringe prior to respondent in past 30 days | ||||
| 0 | Ref | Ref | ||
| 1–3 | 0.71 (0.50 – 1.00) | 0.0525 | 0.87 (0.64 – 1.19) | 0.3849 |
| >3 | 0.55 (0.30 – 1.01) | 0.0543 | 0.69 (0.35 – 1.33) | 0.2631 |
|
| ||||
| Shooting gallery in past 6 months | ||||
| No | Ref | Ref | ||
| Yes | 0.56 (0.44 – 0.71) | <.0001 | 0.68 (0.56 – 0.83) | 0.0068 |
Adjusted for age, sex, region, educational level, number of years injecting, injected buprenorphine in past 6 months, alcohol use (AUDIT), and social support
Additional multivariable models showed that participants who reported passing a used needle/syringe to more than 3 individuals in the past 30 days had 44% (95% CI: 0.40 – 0.79) lower odds of being virally suppressed than those who passed a syringe to no one. No statistically significant association was found between those who reported using a syringe after more than 3 individuals had used it and viral suppression (aOR: 0.69, 95%CI: 0.35 – 1.33). PWID who reported injecting in a shooting gallery had lower odds of viral suppression (aOR: 0.56, 95% CI: 0.44 – 0.71) than those who did not inject in a shooting gallery, which remained significant in the multivariable model (aOR: 0.68 95%CI: 0.56 – 0.83). Lastly, the highest level of social support was associated with an increased odds of viral suppression (OR: 1.46, 95% CI: 1.09 – 1.97).
Discussion
In this large sample of PWID from across India, most PWID had relatively small injection networks with a median of three persons in the prior month. While only a minority had networks of more than 10 individuals, these individuals exhibited high levels of risk behavior, HIV and HCV prevalence, and poor engagement in care including low levels of viral suppression. Collectively, our findings suggest that these individuals may play a disproportionate role in HIV and HCV transmission and may be a strategic population to target first when implementing interventions to reduce HIV and HCV transmission while maximizing cost-effectiveness.
Our findings regarding the association between syringe sharing and network size are consistent with prior studies in the United States and India.15,22–24 Further, in our study, the overall prevalence of syringe sharing at last injection was higher than in a previous multisite bio-behavioral study conducted in India in 2008.24 We found that injection drug network size was a more robust correlate of syringe sharing than the number of PWID the participant knew and had seen in the past 30 days, which is used in RDS analyses as a proxy for network size. Several other network factors including visiting a shooting gallery, injecting with multiple people, the number of people the respondent passed a needle/syringe to, and the number of people who used the needle/syringe prior to the respondent were also found to be significantly associated with sharing a syringe at last injection. The minority of participants who had the largest injection drug networks in terms of these characteristics may have also been the individuals least likely to be in care, possibly due to more severe addiction-related problems.25
Consequently, we also observed a negative relationship between having larger injection drug networks and viral suppression using both quantitative (number of PWID in injection drug network) and qualitative (injecting in shooting gallery) measures. The implications of these findings are important since shooting galleries are locations where many PWID congregate, often anonymously, to inject drugs. As reported in a simulation study, HIV can diffuse rapidly into PWID networks from shooting galleries and may act as transmission hubs by linking well-connected individuals to others on the peripheries of the network.26 Within the framework of TasP ensuring that HIV-infected individuals who have multiple PWID in their injection drug network are diagnosed, engaged in care, receiving ART, and are virally suppressed could reduce transmission to the members of the injection drug network who are HIV uninfected. This may be especially relevant in settings where ART access may be limited due to cost.
These analyses reinforce the importance of considering network characteristics in designing HIV prevention and treatment interventions.7,27,28 Indeed, network-based or peer-interventions targeting a reduction in risk behaviors, such as syringe sharing have been shown to be feasible and efficacious. For example, peer-intervention studies conducted in Baltimore found lower engagement in HIV risk behavior among active PWID who were trained to be peer educators within their networks compared to those who did not receive training.9,29 A study conducted in Canada reported that peer-driven interventions could increase ART adherence among female sex workers who use drugs.30 Another recent peer-led intervention in Ukraine showed that PWID who had received trainings to instruct their network members on HIV risk reduction were significantly less likely to seroconvert 8 Peer-led interventions that specifically target PWID with high network centrality or bridging to serve as peer educators are lacking, however among MSM, highly connected individuals were more likely to be “innovative” or willing to accept new ideas.31 A more thorough understanding of sociometric network structure of PWID and whether similar characteristics exist and willingness to serve in this capacity is warranted.
Further evaluation of peer-interventions on ART adherence is also needed among PWID, who are more likely to be male and engage in different HIV risk behaviors than female sex workers. Initiation and adherence to ART may be especially challenging in settings with HIV epidemics fueled by injection drug use and where drug use is criminalized and HIV infection highly stigmatized. Thus, peer interventions could focus on achieving viral suppression among the minority of individuals with the largest networks and leverage their connections as a way to optimize prevention among HIV-uninfected PWID within their networks. Similar interventions would also be applicable for HCV given the possibility of reinfection. Treating individuals with the largest networks alongside their network partners may help to prevent onward transmission of HCV.
Limitations
We were limited by the lack of egocentric and sociometric data since we did not ascertain names of other network members, risk behaviors of these network members, nor strength of the ties among members in the drug network. While homophily (the tendency of participants to recruit participants with similar characteristics) was low (between −0.2 and 0.2 for most sites32), because we did not obtain any sociometric data, we could not verify whether individuals in the largest and riskiest networks overlapped and were in the same network. Understanding various measures of centrality in the network structure (i.e. degree [egocentric network size], eigenvector [extent of being connected to many influential people], betweenness [extent of being a bridge within a network]) would have strengthened our assertion that individuals who were in the largest self-reported injection network groups were also important transmission nodes in the network. Indeed, a study among PWID in Lithuania showed that high betweenness centrality was associated with increased HIV prevalence.2 Generalizability may also be limited since we could not obtain a random sample of the underlying population and recruiting a random sample of a population with no sampling frame is challenging. Lastly, these data were cross-sectional and we did not have data on number of injection partners over their lifetime.. Additional longitudinal research is needed on elucidating the stability of these networks over time. It is possible that high-risk injectors may reduce their risk behaviors and network size after becoming aware of their HIV diagnosis. It is also possible that network structure and size changes over the natural history of drug use. For example, people may have larger networks and more frequently switch networks when they have newly initiated drug use but over time they may tend to have smaller more stable networks.
In sum, our analysis highlights the importance of understanding the role of network characteristics that could improve targeted HIV and HCV prevention interventions. Scale-up of ART and HCV treatment may have more impact on averting onward transmission if targeted interventions focus on individuals with larger networks who are more likely to transmit and less likely to be virally suppressed.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (T32AI102623, R25TW009343, T32DA023356, R01MH89266, R01DA032059, R01AI095068, K24DA035684, DP2DA040244) and the Johns Hopkins Center for AIDS Research (P30AI094189). We thank the National AIDS Control Organization (India) and all of our partner non-governmental organizations throughout India who assisted with the recruitment of the study sample, and especially our participants, without whom this research would not have been possible.
Footnotes
Conflicts of interest: None
References
- 1.Joint United Nations Programme on HIV/AIDS. Do No Harm - Health, Human Rights, and People who Use Drugs. Geneva: UNAIDS; 2016. [Google Scholar]
- 2.Gyarmathy VA, Caplinskiene I, Caplinskas S, Latkin CA. Social network structure and HIV infection among injecting drug users in Lithuania: gatekeepers as bridges of infection. AIDS and behavior. 2014;18(3):505–510. doi: 10.1007/s10461-014-0702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young AM, Jonas AB, Mullins UL, Halgin DS, Havens JR. Network structure and the risk for HIV transmission among rural drug users. AIDS and behavior. 2013;17(7):2341–2351. doi: 10.1007/s10461-012-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh T, Mandell W, Latkin C, Kim J. Social network characteristics and injecting HIV-risk behaviors among street injection drug users. Drug and alcohol dependence. 1997;47(2):137–143. doi: 10.1016/s0376-8716(97)00082-3. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related mechanisms may help explain long-term HIV-1 seroprevalence levels that remain high but do not approach population-group saturation. American journal of epidemiology. 2000;152(10):913–922. doi: 10.1093/aje/152.10.913. [DOI] [PubMed] [Google Scholar]
- 6.De P, Cox J, Boivin JF, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102(11):1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh D, Krishnan A, Gibson B, Brown SE, Latkin CA, Altice FL. Social Network Strategies to Address HIV Prevention and Treatment Continuum of Care Among At-risk and HIV-infected Substance Users: A Systematic Scoping Review. AIDS and behavior. 2016 doi: 10.1007/s10461-016-1413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth RE, Davis JM, Dvoryak S, et al. HIV incidence among people who inject drugs (PWIDs) in Ukraine: results from a clustered randomised trial. The lancet. HIV. 2016;3(10):e482–489. doi: 10.1016/S2352-3018(16)30040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychology. 2003;22(4):332. doi: 10.1037/0278-6133.22.4.332. [DOI] [PubMed] [Google Scholar]
- 10.Lucas GM, Solomon SS, Srikrishnan AK, et al. High HIV burden among people who inject drugs in 15 Indian cities. Aids. 2015;29(5):619–628. doi: 10.1097/QAD.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medhi GK, Mahanta J, Akoijam BS, Adhikary R. Size estimation of injecting drug users (IDU) using multiplier method in five districts of India. Substance abuse treatment, prevention, and policy. 2012;7:9. doi: 10.1186/1747-597X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambekar A, Tripathi BM. Size estimation of injecting drug use in Punjab and Haryana. UNAIDS; India, New Delhi: 2008. [Google Scholar]
- 13.Department of AIDS Control MoHaFW, Government of India. [Accessed 16 MAY, 2016];National AIDS Control Programme Phase-IV (2012–2017) 2013 http://www.naco.gov.in/upload/NACP%20-%20IV/NACP-IV%20Strategy%20Document%20.pdf.
- 14.Latkin C, Yang C, Srikrishnan AK, et al. The relationship between social network factors, HIV, and Hepatitis C among injection drug users in Chennai, India. Drug and alcohol dependence. 2011;117(1):50–54. doi: 10.1016/j.drugalcdep.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medhi GK, Mahanta J, Adhikary R, et al. Spatial distribution and characteristics of injecting drug users (IDU) in five Northeastern states of India. BMC public health. 2011;11:64. doi: 10.1186/1471-2458-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon SS, Mehta SH, Srikirishnan AK. High burden of HCV disease and poor access to HCV services among people who inject drugs in India: A crosssectional study among 14,481 drug users across India. Lancet Infect Dis. 2014:71054–71050. [Google Scholar]
- 17.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Social problems. 1997:174–199. [Google Scholar]
- 18.Bell DC, Belli-McQueen B, Haider A. Partner naming and forgetting: recall of network members. Social networks. 2007;29(2):279–299. doi: 10.1016/j.socnet.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewer DD, Garrett SB, Kulasingam S. Forgetting as a cause of incomplete reporting of sexual and drug injection partners. Sexually transmitted diseases. 1999;26(3):166–176. doi: 10.1097/00007435-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Mehta SH, Lucas GM, Solomon S, et al. HIV Care Continuum Among Men Who Have Sex With Men and Persons Who Inject Drugs in India: Barriers to Successful Engagement. Clinical Infectious Diseases. 2015:civ669. doi: 10.1093/cid/civ669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption - II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 22.Mandell W, Kim J, Latkin C, Suh T. Depressive symptoms, drug network, and their synergistic effect on needle-sharing behavior among street injection drug users. The American journal of drug and alcohol abuse. 1999;25(1):117–127. doi: 10.1081/ada-100101849. [DOI] [PubMed] [Google Scholar]
- 23.Latkin C, Mandell W, Vlahov D, Oziemkowska M, Celentano D. People and places: behavioral settings and personal network characteristics as correlates of needle sharing. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1996;13(3):273–280. doi: 10.1097/00042560-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 24.Mahanta J, Medhi GK, Paranjape RS, et al. Injecting and sexual risk behaviours, sexually transmitted infections and HIV prevalence in injecting drug users in three states in India. Aids. 2008;22:S59–S68. doi: 10.1097/01.aids.0000343764.62455.9e. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Latkin C, Davey-Rothwell M, Agarwal M. Bidirectional Influence: A Longitudinal Analysis of Size of Drug Network and Depression Among Inner-City Residents in Baltimore, Maryland. Substance use & misuse. 2015;50(12):1544–1551. doi: 10.3109/10826084.2015.1023452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson L, Grund T. Modeling the impact of supra-structural network nodes: The case of anonymous syringe sharing and HIV among people who inject drugs. Social science research. 2012;41(3):624–636. doi: 10.1016/j.ssresearch.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Tsang MA, Schneider JA, Sypsa V, et al. Network Characteristics of People Who Inject Drugs Within a New HIV Epidemic Following Austerity in Athens, Greece. Journal of acquired immune deficiency syndromes. 2015;69(4):499–508. doi: 10.1097/QAI.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman SR, Bolyard M, Mateu-Gelabert P, et al. Some data-driven reflections on priorities in AIDS network research. AIDS and behavior. 2007;11(5):641–651. doi: 10.1007/s10461-006-9166-7. [DOI] [PubMed] [Google Scholar]
- 29.Tobin KE, Kuramoto SJ, Davey-Rothwell MA, Latkin CA. The STEP into Action study: A peer - based, personal risk network - focused HIV prevention intervention with injection drug users in Baltimore, Maryland. Addiction. 2011;106(2):366–375. doi: 10.1111/j.1360-0443.2010.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deering KN, Shannon K, Sinclair H, Parsad D, Gilbert E, Tyndall MW. Piloting a peer-driven intervention model to increase access and adherence to antiretroviral therapy and HIV care among street-entrenched HIV-positive women in Vancouver. AIDS patient care and STDs. 2009;23(8):603–609. doi: 10.1089/apc.2009.0022. [DOI] [PubMed] [Google Scholar]
- 31.Schneider JA, Zhou AN, Laumann EO. A new HIV prevention network approach: sociometric peer change agent selection. Social science & medicine. 2015;125:192–202. doi: 10.1016/j.socscimed.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon SS, Mehta SH, McFall AM, et al. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: a cross-sectional, comparative study. The lancet. HIV. 2016;3(4):e183–190. doi: 10.1016/S2352-3018(16)00019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.