Abstract
Background
Lung transplantation (LTx) is frequently considered for patients with cystic fibrosis (CF) when the FEV1 reaches < 30%. This study estimated transplant-free survival for patients with CF and an FEV1 < 30% and identified predictors of death without LTx.
Methods
We conducted a retrospective cohort study using the CF Foundation Patient Registry from January 1, 2003 to December 31, 2013. Adult patients (≥ 18 years) with FEV1 < 30% prior to LTx were included. We performed Kaplan-Meier survival estimates censored at LTx. Multivariable Cox proportional hazard regression identified predictors of mortality.
Results
There were 3,340 patients with an FEV1 < 30%. Death without LTx occurred in 1,250 patients (37.4%); 951 patients (28.5%) underwent LTx; 918 patients (27.5%) remained alive without LTx at the end of follow-up; and 221 patients (6.6%) were lost to follow-up. Median transplant-free survival after FEV1 < 30% was 6.6 years (95% CI, 5.9-7.0). Adjusted predictors of death without LTx included supplemental oxygen use (hazard ratio [HR], 2.1; 95% CI, 1.7-2.6), Burkholderia cepacia infection (HR, 1.8; 95% CI, 1.3-2.6), BMI ≤ 18 (HR, 1.6; 95% CI, 1.3-1.9), female sex (HR, 1.6; 95% CI, 1.2-2.0), CF-related diabetes in patients receiving insulin (HR, 1.4; 95% CI, 1.2-1.8), and ≥ one exacerbation per year (HR, 1.7; 95% CI, 1.3-2.2 vs. 0 exacerbations).
Conclusions
Median survival was > 6.5 years for patients with CF and an FEV1 < 30%, exceeding prior survival estimates. There was substantial heterogeneity in survival, with some patients with CF dying soon after reaching this lung function threshold and others living for many years. For this reason, we conclude that FEV1 < 30% remains an important marker of disease severity for patients with CF. Patients with a supplemental oxygen requirement or frequent exacerbations should have prompt referral because of their increased risk of death.
Key Words: cystic fibrosis, FEV1, lung function, lung transplantation, survival
Abbreviations: CF, cystic fibrosis; CFF, Cystic Fibrosis Foundation; CFFPR, Cystic Fibrosis Foundation Patient Registry; HR, hazard ratio; LTx, lung transplantation; PH, proportional hazard
Cystic fibrosis (CF) is an autosomal recessive genetic disease that leads to dysfunction in multiple organ systems, including progressive respiratory failure, causing death in approximately 70% of patients.1, 2 Lung transplantation (LTx) is an option for treating end-stage lung disease in CF, and the International Society for Heart and Lung Transplantation (ISHLT) recommends referral for LTx evaluation when a patient has a 2-year predicted survival of < 50%.3 Referral for LTx evaluation is frequently considered in patients with CF once the FEV1 is < 30% of the predicted normal value.3 This guideline is based on data from a single-center CF cohort from Toronto (patients eligible 1977-1989) that documented 2-year survival falls to < 50% once the FEV1 reaches < 30%; they also documented high 2-year mortality for patients with CF and hypoxemia or hypercarbia, older patients, and women.4 In 1998, Milla et al5 documented a median survival of 3.8 years among patients with CF and with an FEV1 < 30% in a single-center cohort from Minneapolis that was eligible from 1975 to 1994.
Although LTx extends life for patients with CF and terminal lung disease, posttransplantation survival is limited by transplantation-related complications2; median survival after LTx is 8.9 years for patients with CF.6 Specifically focusing on patient survival prior to LTx (transplant-free survival) captures the natural history of CF-related lung disease and can inform decisions about the timing of referral and listing for LTx. A single-center study of 276 patients with cohort eligibility between 1990 and 2003 in London revealed that an FEV1 < 30% was associated with a median transplantation-free survival of 5.3 years.7 Our hypothesis was that among patients with CF with an FEV1 < 30% in the United States, median survival likely exceeds 2 years. Additionally, we sought to identify predictors of survival among patients with low lung function, which could potentially better identify those patients most suitable for referral for LTx evaluation and listing. We hypothesized that female sex,4, 8, 9, 10 pulmonary exacerbation frequency,9, 11, 12 low FEV1 % predicted,9, 12 low BMI,4, 9 supplemental oxygen use,4 and colonization with Burkholderia cepacia9, 13 would be associated with worsened survival, based on literature in patients with CF with all ranges of lung function.
Methods
We performed a retrospective cohort study using the Cystic Fibrosis Foundation (CFF) Patient Registry (CFFPR), with data available between January 1, 2003 and December 31, 2013. The CFFPR captured demographic and encounter-based clinical data for approximately 81% to 84% of persons with CF in the United States during 2012.14 This project was approved by the University of Washington Institutional Review Board (Project No. 50298) and the CFF Patient Registry Committee (Bethesda, MD). Adult patients (aged ≥ 18 years) who had not yet undergone LTx were included in the analyses based on a lung function cutoff of FEV1 < 30% at cohort entry. Only lung function measurements recorded during “stable” encounters were considered for eligibility (e-Appendix 1). Patients were deemed lost to follow-up if their last encounter was more than 1 year prior to the end of the data set (December 31, 2013). Patient follow-up was censored at the encounter during which LTx was documented, at loss to follow-up, or at the end of the study. An indicator variable for LTx was created using data from two separate covariates in the CFFPR; date of LTx is assumed to be the encounter date when LTx status is updated (update to LTx status occurs at the patient’s “annual visit”). Referral status was also determined from the annual indicator variable.
Our primary analysis was determination of median transplant-free survival, with censoring at the time of LTx, using Kaplan-Meier estimates of the survival function. We also determined the incidence rate of death prior to LTx and the median time to LTx for patients with an FEV1 < 30%. Univariate Cox proportional hazard (PH) regression was first performed using a broad range of covariates that have a plausible association with mortality in CF (FEV1 % predicted, continuous and 5% intervals; change in FEV1 % predicted (absolute difference in maximum FEV1 % predicted in the calendar year prior to eligibility, and the FEV1 % predicted at cohort eligibility); calendar time (by year); age at entry; female sex; height; continuous BMI; BMI ≤ 18; F508del genotype; pancreatic insufficiency; number of pulmonary exacerbations in the year prior to eligibility; categorized pulmonary exacerbations (0 vs ≥ 1); supplemental oxygen requirement—continuous, nocturnal or exertional; any supplemental oxygen requirement; B cepacia infection; CF-related diabetes in a patient receiving insulin; end-stage renal disease in a patient receiving dialysis; pneumothorax requiring a chest tube during the year prior to eligibility; hemoptysis during the year prior to eligibility; cirrhosis; osteoporosis; depression; Medicaid insurance; high school education; white race; and marital status [married, living together]). Missing values for microbiological covariates were recoded to absence of infection at that time point and missing values for noninvasive mechanical ventilation were recoded to absence of ventilation at that time point. A multivariable model was then constructed, adding covariates to the model if their univariate association with mortality had a P value < .10. All covariates were then included as a block of covariates in the final multivariable model regardless of significance. Tests for violation of PH assumption were performed with Schoenfeld residuals.15 If the PH assumption failed, the covariate was either categorized (BMI modeled continuously violated the PH assumption and was therefore categorized to BMI ≤ 18) or was modeled as strata (FEV1 % predicted and calendar time) in the multivariate Cox PH regression. Time-varying effects were tested for FEV1 % predicted using the tvc option of stcox in Stata (StataCorp LP).
Sensitivity analyses were performed to obtain median survival among patients with FEV1 < 30% without censoring at LTx, FEV1 < 30% among those who never underwent LTx during the period of observation, and FEV1 < 30% among only those who subsequently underwent LTx during the period of data collection. Additional sensitivity analyses were performed using two more severe FEV1 cut points during stable clinical encounters (not marked as pulmonary exacerbation or hospitalization): (1) FEV1 < 30% during 2 consecutive years and (2) FEV1 < 25% once. Survival analysis using Kaplan-Meier estimates was performed to obtain transplant-free median survival in these additional cohorts with more severe lung disease. Additionally, median survival was estimated for patients stratified by covariates that were significant in the multivariate Cox regression model. Finally, a sensitivity analysis was performed to identify the number of pulmonary exacerbations per year during the year prior to reaching an FEV1 < 30%, which is associated with a clinically significant shortened median survival.
Results
The analysis included 3,340 patients with an FEV1 < 30% who had not yet undergone LTx. The average age of patients meeting eligibility was 33.2 years (Table 1). The cohort was predominantly white, with > 80% having graduated from high school and 38% being married. Thirty-five percent required supplemental oxygen (continuously or at night) at the time of eligibility.
Table 1.
Characteristics of 3,340 Patients With CF at the Time of Their First Stable Measurement of FEV1 < 30%, 2003-2013
| Age at eligibility, mean (SD), y | 33.2 (10.1) |
| Female sex | 1,444 (43.2) |
| Race, white vs nonwhite | 3,220 (96.4) |
| FEV1 at eligibility, mean, SD (minimum-maximum), % predicted | 25.6, 3.9 (4.3-29.9) |
| No. acute exacerbations/y, mean, SD (minimum-maximum)a | 1.8, 1.7 (0-11) |
| Sputum culture positive | |
| Pseudomonas aeruginosab | 2,681 (80.3) |
| Burkholderia cepacia complexc | 205 (6.1) |
| Nontuberculous mycobacteriumc | 41 (1.2) |
| Supplemental oxygend | 1,154 (34.6) |
| Noninvasive mechanical ventilatione | 167 (5.0) |
| BMI at eligibility, mean (SD), kg/m2 | 20.4 (3.7) |
| BMI ≤ 18 | 721 (21.6) |
| CF-related diabetes in patients receiving insulinc | 879 (26.3) |
| F508del mutation status | |
| Homozygous F508del | 1,427 (42.7) |
| Heterozygous F508del | 1,106 (33.1) |
| Non-F508del | 245 (7.3) |
| Unknownf | 562 (16.8) |
| Pancreatic insufficiencyg | 3,046 (91.2) |
| CF-related liver cirrhosis | 91 (2.7) |
| Renal failure requiring dialysis | 24 (0.7) |
| Osteoporosis | 325 (9.7) |
| Pneumothorax requiring chest tubeh | 74 (2.2) |
| Hemoptysish | 100 (3.0) |
| Depression | 769 (23.0) |
| Marital status | |
| Married | 1,253 (37.5) |
| Living together | 228 (6.8) |
| High school graduate | 2,719 (81.4) |
| Medicaid insurance | 1,304 (39.0) |
Data are presented as No. (%) unless indicated otherwise. Missing < 1% unless indicated otherwise. Covariate values are observed at the time of eligibility for the FEV1 < 30% cohort unless indicated otherwise. CF = cystic fibrosis.
Number of pulmonary exacerbations requiring IV antibiotics during the year prior to FEV1 < 30%.
Pseudomonas culture data missing for 172 (5.2%).
Positive if documented as present, otherwise recoded to negative.
Supplemental oxygen continuous or nocturnal.
Noninvasive mechanical ventilation data missing for 1,923 (57.6%).
More than 75% of nongenotyped patients entered the cohort by 2007.
Pancreatic insufficiency if patient was documented to take pancreatic enzymes.
During the year prior to eligibility.
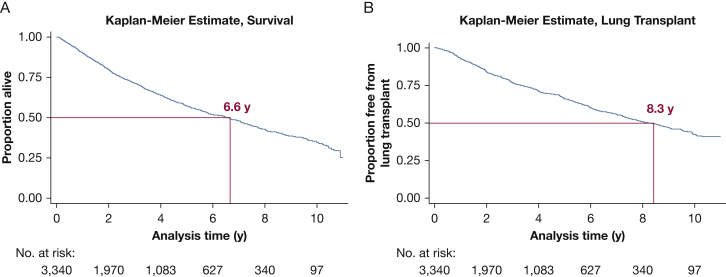
Of the 3,340 patients in the analysis, 1,250 (37.4%) later died without having undergone LTx, 951 (28.5%) underwent LTx, 918 (27.5%) remained alive without LTx at the end of follow-up, and 221 (6.6%) were lost to follow-up (e-Fig 1). Median transplant-free survival after an FEV1 < 30% was 6.6 years (95% CI, 5.9-7.0), and median time to transplantation for patients who did not die was 8.3 years (95% CI, 7.6-8.9) after an FEV1 < 30% (Figure 1, Figure 2). The incidence rate of death among patients with FEV1 < 30% predicted was 109.5 per 1,000 person-years. Of the 1,810 patients referred for LTx evaluation, 485 (26.8%) died without having undergone LTx (e-Fig 2). Of the 1,250 patients who died, 765 (61.2%) were not referred for LTx evaluation according to CFFPR records.
Figure 1.
Kaplan Meier estimates for survival after (A) FEV1 < 30% predicted and (B) time to lung transplantation after FEV1 < 30% among patients who did not die.
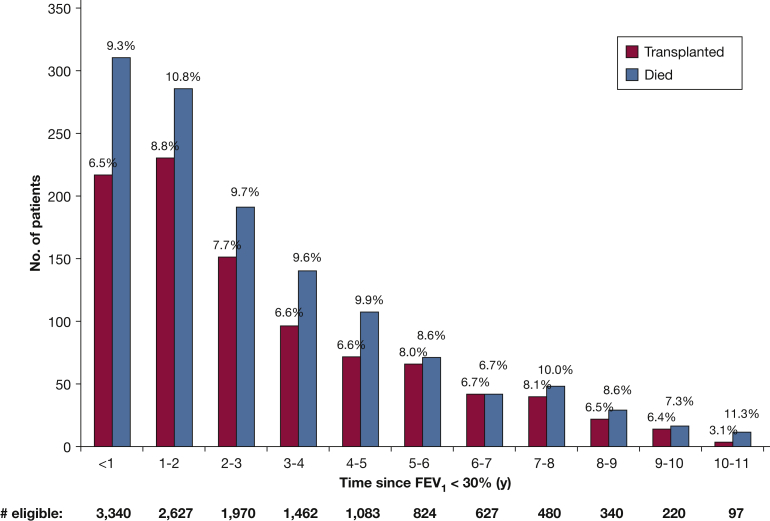
Figure 2.
Number of lung transplantations and deaths each year after FEV1 < 30%. The Figure represents the proportion of eligible patients remaining each year who died or underwent lung transplantation.
Sensitivity analyses revealed a median survival of 6.7 years (95% CI, 6.4-7.1 years) for patients with an FEV1 < 30% when there was no censoring at LTx, which is almost exactly the same as the estimate from the model with censoring at LTx (Table 2). When patients were excluded if they eventually underwent LTx, median transplant-free survival decreased and when patients were considered only if they eventually underwent LTx, median transplant-free survival increased markedly (Table 2). Further sensitivity analyses showed a median survival of > 5 years for patients with 2 consecutive years of an FEV1 < 30% predicted and a median survival of nearly 5 years for patients with an FEV1 < 25% predicted at cohort entry.
Table 2.
Sensitivity Analyses of Median Survival for Patients With Different Lung Disease Severity and Differing Survival Analysis Methods
| FEV1 Cutoff | No. of Subjects | Median Survival, y (95% CI) | No. of Deaths |
|---|---|---|---|
| < 30% once | 3,340 | 6.6 (5.9-7.0) | 1,250 |
| < 30% once, not censored at transplantation | 3,421 | 6.7 (6.4-7.1) | 1,530 |
| < 30% once, excluded if ever transplanted | 2,389 | 4.5 (4.3-4.9) | 1,250 |
| < 30% once, only if ever transplanteda | 1,032 | . . . | 280 |
| < 30% in 2 consecutive y | 1,818 | 5.2 (4.8-5.6) | 693 |
| < 25% once | 2,214 | 4.8 (4.4-5.1) | 876 |
At the end of study follow-up, only 39.6% of eligible patients had died; analysis is not censored at transplantation; 5-year survival is 82.3% (79.6%-84.8%), and 10-year survival is 60.4% (56.2%-64.3%).
Univariate Cox PH analysis revealed a significant association of nearly all the tested covariates with transplant-free survival (Table 3). There was evidence of a time-varying effect of FEV1 % predicted on survival (P < .001), and calendar time also violated the PH assumption; therefore, multivariable analyses were stratified by FEV1 (in 5% increments) and calendar time. After adjustment for confounding using multivariable Cox PH regression stratified by calendar time and FEV1, several predictors of death without LTx were identified (Table 4). The strongest adjusted predictors of death included supplemental oxygen use (HR, 2.1; 95% CI, 1.7-2.6), and B cepacia infection (HR, 1.8; 95% CI, 1.3-2.6). Importantly, patients with a BMI ≤ 18, female sex, and CF-related diabetes receiving insulin were also at an increased risk of death. Genotype information is likely an indirect proxy for calendar time in this cohort, because > 75% of nongenotyped patients entered the cohort by 2007. The number of pulmonary exacerbations per year in the year prior to reaching an FEV1 < 30% was also associated with the risk of death; patients with one or more exacerbations per year were at a 70% increased risk of death (HR, 1.7; 95% CI, 1.3-2.2) when compared with patients with no pulmonary exacerbations. In a sensitivity analysis, we evaluated the adjusted risk of death and median survival for an increasing number of pulmonary exacerbations and identified five exacerbations or more per year (compared with 0-4 exacerbations) to be associated with 2-year median survival (e-Table 1).
Table 3.
Relative Transplant-Free Survival in Patients With CF and FEV1 < 30% Predicted: Univariate Cox Proportional Hazards Regression Results
| Covariate | HR (95% CI) |
|---|---|
| FEV1 % predicted, per 1% increasea | 0.94 (0.92-0.95)b |
| FEV1 % predicted, 5% intervalsa | 0.73 (0.68-0.78)b |
| Change in FEV1 % predicted, per 1% differencea,c | 1.00 (0.99-1.00) |
| Calendar year of first FEV1 < 30%a | 0.91 (0.89-0.93)b |
| Entry age, y | 1.00 (1.00-1.01) |
| Female sex | 1.39 (1.24-1.55)b |
| Height, in. | 0.95 (0.94-0.96)b |
| BMI ≤ 18 | 1.68 (1.49-1.90)b |
| F508del mutation status | |
| Homozygousd | 1.30 (1.03-1.65)e |
| Heterozygousd | 1.20 (0.95-1.53) |
| Unknownd | 2.06 (1.60-2.64)b |
| Pancreatic insufficiencyf | 1.05 (0.86-1.27) |
| No. pulmonary exacerbations a,g | 1.28 (1.24-1.31)b |
| 1 or more exacerbations (vs none)a | 1.99 (1.73-2.29)b |
| Supplemental oxygena,h | 2.96 (2.64-3.32)b |
| Any supplemental oxygena,i | 2.90 (2.59-3.25)b |
| Burkholderia cepaciaj | 1.99 (1.65-2.41)b |
| CFRD, on insulin | 1.70 (1.50-1.91)b |
| ESRD, on dialysis | 4.04 (2.53-6.45)b |
| Pneumothoraxk | 1.75 (1.22-2.49)e |
| Hemoptysis | 1.44 (1.04-1.98)e |
| Cirrhosis | 2.08 (1.59-2.74)b |
| Osteoporosis | 1.50 (1.26-1.80)b |
| Depression | 1.71 (1.53-1.92)b |
| Medicaid Insurance | 1.67 (1.50-1.87)b |
| High school graduatel | 0.78 (0.63-0.98)e |
| Whitem | 0.95 (0.72-1.26) |
| Marital status | |
| Marriedn | 0.74 (0.65-0.84)b |
| Living Togethern | 1.08 (0.87-1.34) |
| Noninvasive ventilationo | 3.14 (2.49-3.96)b |
| Smokingo | 1.41 (0.89-2.24) |
Covariates included in the multivariable analysis if P < .10.
CFRD = cystic fibrosis-related diabetes; ESRD = end-stage renal disease. See Table 1 legend for expansion of other abbreviation.
Violation of proportional hazards assumption.
P < .001.
Absolute difference in maximum FEV1 % predicted in the year prior to eligibility and the FEV1 % predicted at cohort entry.
Reference group has non-F508del mutations.
P < .10.
Pancreatic insufficiency defined as documentation of pancreatic enzyme use.
Number of exacerbations requiring IV antibiotics in the year prior to FEV1 <30%.
Continuous or nocturnal use of oxygen.
Any use of oxygen, including continuous, nocturnal, with exacerbations or PRN.
Sputum infection with Burkholderia cepacia complex.
Pneumothorax that required a chest tube.
High school graduate education or higher.
Race includes white, compared to non-white race.
Reference group is not married and not living together with a partner.
Noninvasive mechanical ventilation and smoking status were missing in > 50% of observations; missing values were imputed with “0,” which assumes a lack of ventilation and a lack of smoking, respectively, if it is not documented; these variables were not included in multivariate analyses due to high proportion missing data.
Table 4.
Multivariable Cox Proportional Hazards Regression Analysis of Predictors of Transplant-Free Survival in Patients With CF and FEV1 < 30% Predicteda
| Covariate | HR (95% CI) | P Value |
|---|---|---|
| Female sex | 1.55 (1.21-2.01) | .001 |
| Height, in. | 1.00 (0.97-1.04) | .796 |
| BMI ≤ 18 | 1.57 (1.28-1.94) | < .001 |
| F508del mutation status | ||
| Homozygousb | 1.25 (0.84-1.85) | .273 |
| Heterozygousb | 1.19 (0.80-1.78) | .394 |
| Unknownb | 1.86 (1.21-2.84) | .004 |
| One or more pulmonary exacerbationsc | 1.71 (1.34-2.18) | < .001 |
| Supplemental oxygend | 2.08 (1.68-2.57) | < .001 |
| Burkholderia cepaciae | 1.81 (1.29-2.55) | .001 |
| CFRD, receiving insulin | 1.44 (1.17-1.79) | .001 |
| ESRD, receiving dialysis | 2.24 (0.76-6.56) | .141 |
| Pneumothoraxf | 0.98 (0.54-1.78) | .941 |
| Hemoptysis | 0.80 (0.45-1.42) | .445 |
| Cirrhosis | 1.10 (0.67-1.82) | .702 |
| Osteoporosis | 1.07 (0.78-1.46) | .671 |
| Depression | 1.15 (0.92-1.43) | .210 |
| Medicaid insurance | 1.16 (0.95-1.41) | .154 |
| High school graduateg | 1.05 (0.73-1.51) | .784 |
| Marital status | ||
| Marriedh | 0.71 (0.57-0.88) | .002 |
| Living togetherh | 0.86 (0.58-1.28) | .458 |
| Global proportional hazards test | .8900 |
Statistically significant P values are shown in bold.
Analysis adjusted for all covariates listed in the table; analysis stratified by calendar time and FEV1 at baseline (in 5% increments) due to proportional hazards assumption violation.
Reference group has no F508del mutations.
Number of exacerbations requiring IV antibiotics in the year prior to FEV1 < 30%, modeled as 1 or more exacerbations/y vs no exacerbations.
Continuous or nocturnal use of oxygen.
Sputum infection with Burkholderia cepacia complex.
Pneumothorax that required a chest tube.
High school graduate education or higher.
Reference group is not married and not living with a partner.
Median survival estimates for patients stratified by sex revealed a significant survival gap among patients with an FEV1 < 30% predicted (Table 5); stratification by other significant covariates also demonstrated differences among patients with low BMI, supplemental oxygen requirement, B cepacia complex in sputum culture, and CF-related diabetes in patients receiving insulin.
Table 5.
Median Survival Estimates for Patients With CF and FEV1 < 30% Predicted, Stratified by Covariates of Interest
| Variable | Median survival (95% CI) | P Value for Log-Rank Test |
|---|---|---|
| Female sex, y | 5.1 (4.6-5.8) | < .001 |
| Male sex, y | 7.2 (6.7-8.0) | |
| BMI ≤ 18, y | 4.2 (3.7-4.5) | < .001 |
| BMI > 18, y | 7.4 (6.9-7.9) | |
| Supplemental oxygen,a y | 3.1 (2.8-3.4) | < .001 |
| No supplemental oxygen, y | 8.3 (7.8-9.1) | |
| Burkholderia cepacia complex, y | 2.8 (2.4-3.9) | < .001 |
| No B cepacia complex, y | 6.9 (6.4-7.3) | |
| CFRD, on insulin, y | 4.3 (3.7-4.7) | < .001 |
| No CFRD, y | 7.4 (6.9-7.9) |
Discussion
In this nationwide US cohort of patients with CF during 2003 to 2013, median transplant-free survival after the development of advanced lung disease with an FEV1 < 30% was 6.6 years. Although this level of survival is greater than that seen in earlier years in similar persons with CF, there remains about a 10% per year probability of death once the FEV1 has fallen to < 30%. As shown in Figure 2, the annual risk of death does not seem to decline much over time. Although lung transplantation in these patients can provide the potential for improved survival, this procedure entails its own risks. Furthermore, patients and families may experience emotional and financial stress during LTx evaluation or listing for transplantation. The evaluation for LTx involves assessing a patient’s indication for transplantation, identifying potential contraindications or barriers to transplantation (through procedures that carry the risk of complications), and providing the patient/family with information about the LTx process.16 Certain contraindications or barriers to LTx (eg, low BMI or poor social support) may be modifiable if a patient is referred early enough in the disease course.
Referral for LTx does not always lead to immediate listing for LTx. We have identified several important baseline predictors of death among patients with CF with low lung function, which may prompt earlier listing for LTx. These risk factors include both static and variable patient characteristics, including female sex, BMI ≤ 18, supplemental oxygen requirement, the number of pulmonary exacerbations in the prior year, and the presence of B cepacia infection and CF-related diabetes requiring insulin.
Prior studies evaluating survival in patients with CF have documented increased risk of death among patients with lower FEV1 % predicted,9, 12 lower BMI,4, 9 reduced Pao2,4 an increased number of pulmonary exacerbations,9, 11, 12 B cepacia infection,9, 13 and female sex,4, 8, 9, 10, 17 and our study confirms these earlier findings in a careful examination of the subgroup with very severe lung disease. Our study did not identify a change in FEV1 % predicted as a significant predictor of transplant-free survival (as has been previously documented).5 It is possible that among this group of patients with very severely reduced FEV1, the absolute change in FEV1 % predicted during the 1 year prior to reaching an FEV1 < 30% may not be the right tool for identifying the patients who are most likely to die without transplantation. Although predicting death for an individual patient is notoriously difficult to do,9, 12 our sensitivity analyses evaluating median survival among specific cohorts with low lung function highlight the persistent sex gap in survival in more recent years, the high mortality associated with a supplemental oxygen requirement and BMI ≤ 18, and the dismal prognosis for patients with B cepacia infection; importantly, despite the increased risk of death, the lower limit of the 95% CI for median survival is > 2 years for all cohorts. Additionally, we show that patients who have reached the threshold of FEV1 < 30% and have five or more pulmonary exacerbations per year have a median survival of 2 years, which is the current recommended life expectancy threshold for referral for LTx evaluation.3 Describing median survival for these cohorts allows clinicians to have a practical interpretation of the relative risk of mortality for their patients and emphasizes certain traits that should increase the concern for potential deterioration and prompt timely referral for LTx evaluation. These factors should also be considered in the timing of listing for LTx.
We performed a variety of sensitivity analyses to address different approaches to survival analysis in patients with CF who will potentially undergo LTx. To answer the question regarding the timing of LTx referral, transplant-free survival with censoring at the time of LTx most closely estimates the amount of time a patient is expected to live with advanced lung disease without LTx. In analyses not censored at LTx, survival estimates cannot help with decisions about the timing of LTx referral; analyses that include post-LTx survival data add to our understanding of overall life expectancy when LTx is included with all other available treatment options.18 Excluding from analysis all patients who will eventually undergo LTx7 induces selection bias, because there is an inherent difference in patients who do not undergo transplantation when compared with the general population of patients with CF. Additionally, when making clinical decisions for an individual patient at the time when their FEV1 reaches < 30% predicted, there is no way to know if the individual will never undergo transplantation unless an absolute contraindication exists or they do not desire transplantation. To illustrate the bias of excluding individuals who will undergo LTx, we included only the patients who eventually underwent LTx (those not included in the prior analysis) and demonstrated a longer survival time because of the immortal time bias that exists, because these patients necessarily live long enough to get to LTx and must be good candidates to undergo LTx; the immortal time bias is also present in the estimate of time to LTx for patients who did not die.
Limitations
This study has important limitations. First, although the CFFPR captures data on a large number of variables for most patients with CF in the United States, our study is limited to the variables included in the database. Unfortunately, certain variables of clinical and scientific interest (eg, the presence of pulmonary hypertension, Pao2/Paco2, or an indicator for nonadherence) are not captured in the registry. B cenocepacia is the only known genomovar associated with increased mortality,13 but the species were not differentiated in the CFFPR until 2010, and our study necessarily combines all species in the B cepacia complex. Additionally, there is a risk of misclassification of patients if physicians do not assess disease severity or comorbidities (eg, supplemental oxygen requirement or the presence of diabetes) or if data are not accurately entered into the database. Also, there is some degree of missingness in the database, as documented in Table 1 (specifically for sputum culture results and the noninvasive mechanical ventilation covariate; noninvasive ventilation data were missing for almost 60% of patients and could not be included in analyses), which could also lead to misclassification. Second, we assessed covariates only at cohort entry, which limits their interpretation in a population with extended survival. Third, there is some difficulty with capturing death in the CFFPR if a patient is post-LTx. Loss to follow-up is approximately 2% per year for all patients in the CFFPR but approaches 7% for patients who have undergone LTx. Such losses to follow-up likely represent informative censoring. Additionally, time-to-failure models (including Kaplan-Meier estimates of survival) are based on the assumption of uninformative censoring at the time of LTx, but it is clear that patients who undergo LTx are not similar to those who remain in the cohort. Fourth, there could be an element of selection bias in our cohort, because the exclusion of patients who reach an FEV1 < 30% only during exacerbations could lead to the exclusion of patients who died or underwent LTx prior to a “stable” FEV1 < 30%; such patients likely represent a minority but are important to acknowledge when considering generalizability of these results. Finally, the current model applies only to those who had not yet undergone transplantation at the time of cohort entry, and it should be noted that LTx and death are competing risks in this population. The HRs presented represent the hazard of death if a patient has not undergone transplantation.
Conclusions
The current study demonstrates a median survival > 6.5 years for patients with CF and an FEV1 < 30%, exceeding prior survival estimates. There is a substantial proportion of patients with CF and an FEV1 < 30% who die without LTx, a majority of whom die without referral for evaluation. The strongest predictors of death in this cohort with low lung function included supplemental oxygen use, the presence of B cepacia complex infection, increased frequency of exacerbations, a BMI ≤ 18, and female sex. This study highlights the heterogeneity among patients with an FEV1 < 30%, with some patients dying soon after reaching this threshold and others living many years. For this reason, we conclude that an FEV1 < 30% remains an important marker of disease severity for patients with CF and is a reasonable time to consider referral for LTx, and patients with identified risk factors for death should have prompt referral for LTx evaluation with serious consideration for listing.
Acknowledgments
Author contributions: C. H. G. is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. K. J. R., B. S. Q., and C. H. G. conceived the study question and design. K. J. R. performed the data analyses, wrote the initial draft of the manuscript, and coordinated revisions. B. S. Q. participated in data acquisition from the CFFPR and edited the manuscript. N. M. H. and S. L. H. were involved in refinement of statistical methods and data interpretation and edited the manuscript. E. D. L. provided clinical lung transplantation expertise, refined the study question, and edited the manuscript. M. L. A. provided clinical cystic fibrosis expertise, refined the interpretation of the results, and edited the manuscript. N. S. W. participated in the study design and refinement of epidemiologic methods and edited the manuscript. All authors approved of the final version of the submitted manuscript.
Financial/nonfinancial disclosures: None declared.
Other contributions: The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: C. H. G. received support from the Cystic Fibrosis Foundation, the National Institutes of Health [R01HL103965, R01 AI101307, P30 DK089507], and the US Food and Drug Administration [R01 FD003704]. K. J. R. received support from the Cystic Fibrosis Foundation Third Year Clinical Fellowship Award [RAMOS16A0] and the National Institutes of Health [F32 HL131246-01].
Supplementary Data
References
- 1.O'Sullivan B.P., Freedman S.D. Cystic fibrosis. Lancet. 2009;373(9678):1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation Patient Registry, 2014 Annual Data Report. Bethesda, Maryland, Cystic Fibrosis Foundation, 2015.
- 3.Weill D., Benden C., Corris P.A. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Kerem E., Reisman J., Corey M., Canny G.J., Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326(18):1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 5.Milla C.E., Warwick W.J. Risk of death in cystic fibrosis patients with severely compromised lung function. Chest. 1998;113(5):1230–1234. doi: 10.1378/chest.113.5.1230. [DOI] [PubMed] [Google Scholar]
- 6.Yusen R.D., Edwards L.B., Kucheryavaya A.Y. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplantation report—2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35(10):1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 7.George P.M., Banya W., Pareek N. Improved survival at low lung function in cystic fibrosis: cohort study from 1990 to 2007. BMJ. 2011;342:d1008. doi: 10.1136/bmj.d1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld M., Davis R., FitzSimmons S., Pepe M., Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol. 1997;145(9):794–803. doi: 10.1093/oxfordjournals.aje.a009172. [DOI] [PubMed] [Google Scholar]
- 9.Liou T.G., Adler F.R., FitzSimmons S.C., Cahill B.C., Hibbs J.R., Marshall B.C. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor G.T., Quinton H.B., Kahn R. Case-mix adjustment for evaluation of mortality in cystic fibrosis. Pediatr Pulmonol. 2002;33(2):99–105. doi: 10.1002/ppul.10042. [DOI] [PubMed] [Google Scholar]
- 11.Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 12.Mayer-Hamblett N., Rosenfeld M., Emerson J., Goss C.H., Aitken M.L. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166(12):1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 13.Jones A., Dodd M., Govan J. Burkholderia cenocepacia and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax. 2004;59(11):948–951. doi: 10.1136/thx.2003.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapp E.A., Fink A.K., Goss C.H. The Cystic Fibrosis Foundation Patient Registry: design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13(7):1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 15.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 16.Orens J.B., Estenne M., Arcasoy S. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie T., Gifford A.H., Sabadosa K.A. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med. 2014;161(4):233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephenson A.L., Tom M., Berthiaume Y. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J. 2015;45(3):670–679. doi: 10.1183/09031936.00119714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.