Abstract
Background
Cough is a common symptom of scleroderma-related interstitial lung disease (SSc-ILD), but its relationship to other characteristics of SSc-ILD, impact on cough-specific quality of life (QoL), and response to therapy for SSc-ILD have not been well studied.
Methods
We investigated frequent cough (FC) in patients with SSc-ILD (N = 142) enrolled in the Scleroderma Lung Study II, a randomized controlled trial comparing mycophenolate mofetil (MMF) and oral cyclophosphamide (CYC) as treatments for interstitial lung disease (ILD). We determined the impact of FC on QoL (Leicester Cough Questionnaire [LCQ]), evaluated the change in FC in response to treatment for SSc-ILD, and examined the relationship between gastroesophageal reflux disease (GERD) and cough during the trial.
Results
Study participants who reported FC at baseline (61.3%) reported significantly more dyspnea, exhibited more extensive ILD on high-resolution CT, had a lower diffusing capacity for carbon monoxide, and reported more GERD symptoms than did those without FC. Cough-specific QoL was modestly impaired in patients with FC (total LCQ score, 15.4 ± 3.7; normal range, 3-21 [higher scores indicate worse QoL]). The proportion of patients with FC at baseline declined by 44% and 41% over 2 years in the CYC and MMF treatment arms, respectively, and this decline was significantly related to changes in GERD and ILD severity.
Conclusions
FC occurs commonly in SSc-ILD, correlates with both the presence and severity of GERD and ILD at baseline, and declines in parallel with improvements in both ILD and GERD over a 2-year course of therapy. Frequent cough might serve as a useful surrogate marker of treatment response in SSc-ILD trials.
Trial Registry
ClinicalTrials.gov; No.: NCT00883129; URL: www.clinicaltrials.gov.
Key Words: cough, health-related quality of life, immunosuppressive therapy, interstitial lung disease, scleroderma
Abbreviations: BDI, baseline dyspnea index; CYC, cyclophosphamide; Dlco, diffusing capacity of lung for carbon monoxide; FC, frequent cough; GERD, gastroesophageal reflux disease; HAQ-DI, Health Assessment Questionnaire Disability Index; HRCT, high-resolution CT; ILD, interstitial lung disease; LCQ, Leicester Cough Questionnaire; LM, lobe of maximal involvement; MMF, mycophenolate mofetil; PPI, proton pump inhibitor; QILD, quantitative interstitial lung disease; QLF, quantitative lung fibrosis; QoL, quality of life; RCT, randomized controlled trial; SF-36, 36-item Short-Form Survey; SGRQ, St. George’s Respiratory Questionnaire; SLS, Scleroderma Lung Study; SSc-ILD, systemic sclerosis-related interstitial lung disease; TDI, transition dyspnea index; VAS, visual analog scale; WL, whole lung
Relatively little information exists regarding the characteristics of chronic cough in systemic sclerosis-related interstitial lung disease (SSc-ILD), its effect on quality of life (QoL), and its response to treatment. We previously reported that cough was present in most patients with SSc-ILD in the Scleroderma Lung Study (SLS) I, a clinical trial comparing cyclophosphamide (CYC) and placebo.1, 2, 3
In SLS I, patients with any cough at baseline had increased ILD severity and worse QoL compared with patients without cough.1 After 12 months of treatment, cough decreased in the CYC group compared with the placebo group.1 These findings suggested that the presence of cough might be an indicator of the extent of lung disease in SSc-ILD, and changes in cough might serve as a surrogate marker of the efficacy of disease-modifying therapy for SSc-ILD.
To further explore the prevalence and health burden of cough in SSc-ILD, the Leicester Cough Questionnaire (LCQ) was added to the design of SLS II, a randomized controlled trial (RCT) comparing mycophenolate mofetil (MMF) administered for 2 years vs CYC administered for 1 year followed by another year of placebo.4 We sought to determine the proportion of patients with SSc-ILD with frequent cough (FC), the impact of FC on cough-specific QoL, the correlation of FC with other features of SSc-ILD at baseline, the change in FC in response to treatment for SSc-ILD, and the impact of the presence and development of GERD on FC during the trial.
Methods
The design and eligibility criteria for SLS II have recently been reported.4 Briefly, adults with either limited or diffuse cutaneous systemic sclerosis (SSc)5 were enrolled if they had high-resolution CT (HRCT) evidence of interstitial lung disease (ILD), an FVC of ≤ 80% predicted, exertional dyspnea ≥ grade 2 on the magnitude of task domain of the Mahler baseline dyspnea index (BDI),6 and disease onset (first non-Raynaud symptom of SSc) within the previous 7 years. Eligible patients were randomized in a double-blind fashion to receive either MMF (n = 69) for 2 years or oral CYC (n = 73) for 1 year followed by a year of placebo treatment. The study was approved by the Office of Human Research Protection Program at University of California, Los Angeles (UCLA) (IRB No. 11-002659-CR-00005) and by the institutional review boards of all 14 participating centers.
Randomized patients were assessed for the presence of the following outcomes at baseline and every 3 months thereafter: FC (cough on several or most days of the week) based on positive responses to the first two questions in the St. George’s Respiratory Questionnaire (SGRQ)7; cough-specific QoL (LCQ, a validated 19-item QoL measure of chronic cough that is responsive to change)8; moderate to very severe GERD, defined by a score of ≥ 0.50 on a scale of 0 to 3 on the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract (GIT 2.0) questionnaire9, 10; breathlessness using the self-administered Mahler BDI/transition dyspnea index (TDI),6 and the visual analog scale (VAS) for breathing of the Health Assessment Questionnaire (HAQ)11, 12; other QoL measures (36-item Short-Form Survey [SF-36])13; and lung function (FVC, diffusing capacity for carbon monoxide [Dlco]). In addition, thoracic HRCT images were obtained at baseline and at 24 months, and the quantitative extent of lung fibrosis (QLF) and the quantitative ILD (QILD) in the lobe of maximal involvement (LM) and the whole lung (WL) were calculated.14, 15 The justification for defining FC based on the participants’ responses to the first two questions in the SGRQ is provided in e-Appendix 1.
Statistical Analysis
To assess differences between patients with FC and those with non-FC, two-sample t-tests and χ2 or Fisher exact tests were used. The Pearson correlation coefficient examined correlations between baseline measures and scores of change. Paired t tests were used to compare LCQ scores at baseline and at 24 months. χ2and logistic regression analyses were used to explore relationships between improvement in FC and improvements in other characteristics. The Cochran-Armitage test for trend was used to evaluate the association between the course of GERD and changes in LCQ scores. A mixed effects logistic model was used to examine the impact of treatment on FC over the course of study. No adjustments were made for multiple comparisons.
Results
Cough-Related Differences at Baseline
Of the 142 randomized subjects, 87 reported FC at baseline (61.3%), and 49 of them produced sputum (56.3%). Of those without FC, 26 reported cough on only a few days a month, seven reported cough only with acute chest illnesses, and 21 reported no cough at all. Baseline characteristics of the subjects stratified by the presence or absence of FC symptoms are shown in Table 1. Of the patients with FC, 77% (67 of 87) reported GERD at baseline, whereas GERD was present in only 59% of patients (32 of 54) without FC (P = .025). The average severity of GERD as measured by the GIT 2.0 (none to mild, 0.00-0.49; moderate, 0.50-1.00; severe/very severe, 1.01-3.00)9, 10 was moderate (0.69 ± 0.59) in those with FC and mild (0.38 ± 0.38) in those without FC (P < .001). Those with FC, compared with those without, experienced significantly more breathing difficulty (by VAS), and had a lower Dlco percent predicted and a greater extent of fibrosis (QLF) on HRCT in the WL and of total ILD (QILD) in both the WL and the LM (Table 1).
Table 1.
Relationship Between the Presence or Absence of Frequent Cough at Baseline and Other Baseline Characteristics
| Variable | Frequent Cough |
No Frequent Cough |
P Value | ||
|---|---|---|---|---|---|
| No. | Mean ± SD or Proportion | No. | Mean ± SD or Proportion | ||
| Age, y | 87 | 52.9 ± 9.23 | 54 | 51.0 ± 10.49 | .259 |
| Height, cm | 87 | 166.8 ± 10.0 | 54 | 164.8 ± 9.0 | .219 |
| Weight, kg | 87 | 77.1 ± 17.1 | 54 | 72.7 ± 17.1 | .139 |
| Raynaud syndromea, y | 87 | 4.5 ± 6.7 | 54 | 4.0 ± 4.8 | .638 |
| Non-Raynaud syndromea, y | 87 | 2.5 ± 1.7 | 54 | 2.7 ± 1.9 | .589 |
| Diffuse cutaneous disease, No. (%) | 87 | 49 (56) | 54 | 33 (61) | .579 |
| FVC, percent predicted | 87 | 65.6 ± 8.8 | 54 | 67.8 ± 9.4 | .163 |
| Dlcob, percent predicted | 87 | 51.1 ± 12.0 | 54 | 58.4 ± 12.3 | .001 |
| mRSS skin score | 87 | 13.6 ± 9.6 | 54 | 16.2 ± 11.7 | .170 |
| QLF-LM | 83 | 25.2 ± 19.5 | 53 | 19.0 ± 19.6 | .073 |
| QILD-LM | 83 | 54.1 ± 18.6 | 53 | 46.6 ± 22.3 | .046 |
| QLF-WL | 83 | 9.64 ± 6.7 | 53 | 7.0 ± 7.0 | .032 |
| QILD-WL | 83 | 31.7 ± 13.2 | 53 | 26.1 ± 14.6 | .025 |
| BDI | 80 | 6.9 ± 2.0 | 53 | 7.7 ± 2.4 | .063 |
| PCS, SF-36 | 87 | 35.0 ± 9.8 | 54 | 37.2 ± 9.9 | .211 |
| MCS, SF-36 | 87 | 48.8 ± 8.6 | 54 | 50.7 ± 9.6 | .233 |
| HAQ-DI | 87 | 0.66 ± 0.57 | 54 | 0.80 ± 0.81 | .273 |
| GERD score | 87 | 0.69 ± 0.59 | 54 | 0.38 ± 0.38 | .000 |
| GERD present | 87 | 67 (77) | 54 | 32 (59) | .025 |
| Breathing VAS | 87 | 5.5 ± 4.2 | 54 | 3.3 ± 3.6 | .001 |
| PPI use, No. (%) | 87 | 73 (84) | 54 | 39 (72) | .095 |
| ACE use, No. (%) | 87 | 12 (14) | 54 | 8 (15) | .866 |
| Th-To, No. (%) | 76 | 6 (8) | 50 | 20 (4) | .476 |
| Ro52, No. (%) | 76 | 16 (21) | 50 | 6 (12) | .235 |
| ANA, No. (%) | 81 | 4 (5) | 52 | 3 (6) | .999 |
| Centromere, No. (%) | 81 | 3 (4) | 52 | 0 (0) | .280 |
| Topoisomerase, No. (%) | 81 | 38 (47) | 52 | 23 (44) | .762 |
| RNA polymerase, No. (%) | 81 | 9 (11) | 52 | 8 (15) | .471 |
Scoring scales: BDI range = 0-12, with lower scores indicating worse dyspnea. Breathing VAS range = 1-100, with higher numbers indicating increasing difficulty breathing. PCS, SF-36 and MCS, SF-36 range = 0-100, with lower scores indicating worse health status. mRSS range = 0-51, with higher scores indicating more severe thickening. HAQ-DI range = 1-3, with higher numbers indicating greater disability.
ACE = angiotensin-converting enzyme inhibitor; ANA = antinuclear antibody; BDI = baseline dyspnea index; Dlco = diffusing capacity of the lung for carbon monoxide; GERD = gastroesophageal reflux disease; HAQ-DI = Health Assessment Questionnaire Disability Index; HRCT = high-resolution CT; LM = lobe of maximal involvement; MCS = mental component scale; mRSS = modified Rodnan skin score; PCS = physical component scale; PPI = proton pump inhibitor; QILD = quantitative interstitial lung disease; QLF = quantitative lung fibrosis; Ro52 = antibodies to Ro52 kilodalton; SF-36 = 36-Item Short Form Survey; SSc = systemic sclerosis; Th/To = antibodies to Th/To ribonucleoprotein; VAS = visual analog scale; WL = whole lung.
Years since first Raynaud or non-Raynaud symptom of SSc.
Adjusted for hemoglobin.
Cough-Specific Quality of Life
The LCQ was used to assess cough-specific QoL in those with FC (total score ranging from 3-21 and individual domain scores ranging from 1-7, with high scores reflecting better cough-specific QoL) and demonstrated modest impairment at baseline, as indicated by an average total LCQ score of 15.4 ± 3.7 (SD) and average domain LCQ scores of approximately 5 to 6 (Table 2). The cough-specific QoL scores (total and domain) were not significantly different between those with and those without GERD but were significantly lower (worse) in the 49 patients with cough and sputum (wet cough) than in the 38 subjects with dry cough (P < .01 for all comparisons).
Table 2.
Baseline Mean (± SD) Leicester Cough Questionnaire Total and Domain Scores in Patients With Frequent Cougha
| Variable | Patients With Frequent Cough |
||||||
|---|---|---|---|---|---|---|---|
| All |
GERD Present |
GERD Absent |
P Value | Dry Cough |
Wet Cough |
P Value | |
| (N = 87) | (n = 67) | (n = 20) | (n = 38) | (n = 49) | |||
| LCQ domain | |||||||
| Total scoreb | 15.4 ± 3.7 | 15.3 ± 3.6 | 15.7 ± 3.9 | .65 | 16.8 ± 3.6 | 14.3 ± 3.4 | .001 |
| Physical domainc | 4.9 ± 5.3 | 4.8 ± 1.1 | 5.2 ± 1.1 | .18 | 5.34 ± 1.05 | 4.58 ± .96 | .001 |
| Psychological domainc | 5.2 ± 1.5 | 5.2 ± 1.5 | 5.2 ± 1.6 | .85 | 5.66 ± 1.35 | 4.80 ± 1.46 | .006 |
| Social domainc | 5.3 ± 1.4 | 5.3 ± 1.4 | 5.4 ± 1.6 | .79 | 5.86 ± 1.32 | 4.89 ± 1.34 | .001 |
LCQ = Leicester Cough Questionnaire; QoL = quality of life. See Table 1 legend for expansion of other abbreviations.
Stratified by the presence or absence of gastrointestinal reflux and the presence or absence of sputum (wet and dry cough, respectively) at baseline.
Scale = 3-21: 3 equals worst cough-specific QoL and 21 equals best.
Scale = 1-3: 1 equals worst cough-specific QoL and 3 equals best.
Significant correlations were found between the LCQ scores and the degree of breathlessness as measured by the VAS for breathing and the BDI, as well as the physical composite summary, a non-cough specific QoL measure derived from the SF-36 (e-Table 1). Other significant correlations were found between the LCQ scores and each of the following: the duration of SSc (number of years from the onset of the first non-Raynaud symptom of SSc), the severity of reflux (GIT 2.0), the baseline FVC and Dlco percent predicted, and the QLF-WL, QILD-WL, and QILD-LM. No significant associations were noted between cough-specific QoL measures and demographic or anthropomorphic features.
Cough Improves Over the Course of Treatment for ILD
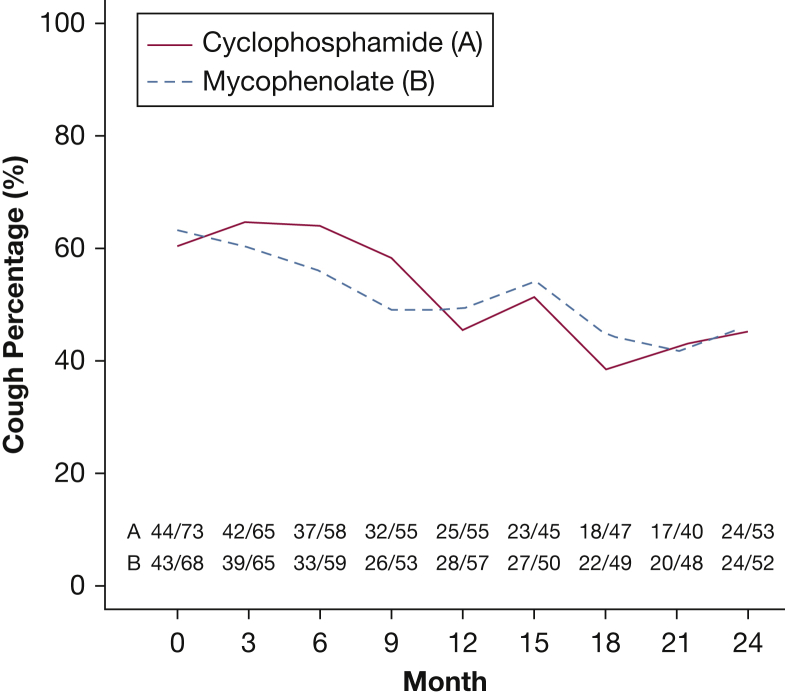
The reporting of FC decreased over the 24-month trial in both arms (Fig 1). In the CYC arm, 44 of 73 patients (60.3%) reported FC at baseline, whereas only 24 of the 53 patients who completed their 24-month visit (45.3%) reported FC at trial end. Moreover, 13 of the 32 patients receiving CYC with FC at baseline (40.6%) no longer reported FC at 24 months. In the MMF arm, 43 of the 68 patients (63.2%) reported FC at baseline, whereas only 24 of the 52 patients with data at 24 months (46.2%) continued to report FC. Additionally, 15 of the 34 patients who received MMF and had FC at baseline (44.1%) no longer reported FC at 24 months. In contrast, 10 patients (five in each treatment group) of a total of 39 patients (25.6%) who did not start the study with FC reported that they had acquired FC at 24 months. However, the frequency of FC was significantly improved at 24 months for all patients with 24-month cough data (P = .0051), with more subjects having lost (n = 28) than having acquired FC (n = 10) and no difference between the two treatment arms.
Figure 1.
Changes in the proportion of patients with frequent cough over the 24-month course of the trial by treatment arm based on logistic regression.
The percentages of patients with FC at baseline and at trial end stratified by the presence or absence of GERD are shown in e-Table 2. Although very infrequent, a few patients experienced new GERD by month 24, and a few had resolution of their GERD. All the patients (n = 7) who acquired GERD at 24 months continued to have FC. Conversely, among those whose GERD resolved at 24 months (n = 8), most (63%) no longer reported FC (P = .032). Proton pump inhibitor (PPI) use was not associated with the presence or absence of GERD or of FC at baseline nor were changes in PPI use associated with changes in GERD or FC (P = .162 and P = .391, respectively).
Univariate logistic regression was carried out to identify significant relationships between the resolution of FC and other measures of change over time in response to treatment (Table 3). Significant correlations were identified for an improving TDI score (P = .037), a decrease in breathlessness assessed by VAS (P = .021), and a reduction in the quantitative extent of ILD in the WL by HRCT (P = .032). There were also trends (P = .05 to .10) toward a relationship of the resolution of FC with improvement in FVC percent and loss of GERD (P = .077 for both). In contrast, acquisition of GERD showed a trend toward a negative association with loss of FC (P = .078). However, multiple logistic regression failed to show a significant independent association of any of these factors with loss of cough (Table 3).
Table 3.
Logistic Model to Predict Loss of Cough at 24 Months in Those With Cough at Baseline
| Variable | Univariate OR (95% CI) | P Value | Multivariate OR (95% CI) | P Value |
|---|---|---|---|---|
| 24-mo change in FVC | 1.064 (0.993-1.140) | .077 | 0.970 (0.865-1.088) | .6012 |
| 24-mo change in QILD-WL | 0.943 (0.892-0.998) | .041 | 0.928 (0.842-1.023) | .1352 |
| 24-mo TDI | 1.164 (1.001-1.355) | .0491 | 1.141 (0.943-1.380) | .1758 |
| 24-mo change in breathing | 0.858 (0.748-0.984) | .0281 | 0.907 (0.771-1.066) | .2342 |
| 24-mo change in GERD, new onset of GERD | 0.081 (0.004-1.812) | .078 | 0.090 (0.004-2.009) | .1482 |
| 24-mo change in GERD, resolution of GERD | 1.906 (0.415-8.755) | .077 | 2.190 (0.214-22.384) | .0895 |
TDI = transition dyspnea index. See Table 1 legend for expansion of other abbreviations.
Five of the 30 patients with dry cough at baseline (16.7%) experienced cough with sputum (wet cough) by 24 months, whereas four of the 36 patients with wet cough at baseline (11.1%) continued to cough at 24 months but without sputum (Table 4). Twelve of the 30 patients (40%) with dry cough at baseline and 16 of the 36 patients (44%) with wet cough at baseline had resolution of their coughs completely by 24 months, although five of the latter 16 patients (31.2%) continued to produce sputum in the absence of cough. Although the numbers are small, there did not appear to be a preferential effect of ILD-directed treatment on FC based on the presence or absence of sputum.
Table 4.
Changes in Cough Stratified by Cough With and Without Sputum (Wet and Dry, Respectively)
| Baseline |
24 Months |
||
|---|---|---|---|
| No. | No. | ||
| Dry cough | 30 | No cough | 12 (0 wet) |
| Dry cough | 13 | ||
| Wet cough | 5 | ||
| Wet cough | 36 | No cough | 16 (5 wet) |
| Dry cough | 4 | ||
| Wet cough | 16 | ||
Changes in Cough-Specific QoL
The LCQ scores (total and domain) in patients with FC at baseline and at 24 months and the mean differences between the two time points are shown in e-Table 3 by treatment group and by both treatments combined. By 24 months, in both the CYC and MMF arms combined, LCQ scores improved slightly with treatment, but in paired analysis for both treatment arms combined, the degree of improvement was not significant (P = .083 for total LCQ and P = .079, P = .124, and P = .205 for the physical, psychological, and social LCQ domain scores, respectively). No difference in treatment response between the two treatment groups was observed.
The total and domain LCQ scores numerically increased (improved) in those in whom moderate to severe GERD resolved and, to a lesser extent, in those in whom the presence or absence of GERD did not change (Table 5). In contrast, the scores numerically increased (worsened) in those who experienced moderate to severe GERD. The pattern of these changes differed significantly across the different subgroups of patients for both the total and domain LCQ scores (P < .01). Similar findings were observed for each treatment group separately.
Table 5.
Mean (± SD) Leicester Cough Questionnaire Total and Domain Scores (Both Treatment Groups Combined) at Baseline and 24 Monthsa
| Score | GERD Present at Baseline, Absent at 24 Months | GERD Absent at Baseline, Present at 24 Months | GERD Absent at Baseline and at 24 Months | GERD Present at Baseline and at 24 Months | P Valueb | |
|---|---|---|---|---|---|---|
| Total LCQ scores | Baseline | n = 8 | n = 6 | n = 7 | n = 44 | .0016 |
| 14.34 (2.27) | 17.86 (1.85) | 14.51 (5.37) | 16.11 (3.22) | |||
| 24 mo | n = 8 | n = 7 | n = 7 | n = 44 | ||
| 17.99 (3.48) | 14.55 (5.07) | 15.84 (5.44) | 16.94 (3.70) | |||
| Physical LCQ scores | Baseline | n = 8 | n = 7 | n = 7 | n = 43 | .0007 |
| 4.43 (0.93) | 5.57 (0.87) | 5.26 (1.21) | 5.06 (0.91) | |||
| 24 mo | n = 8 | n = 7 | n = 7 | n = 44 | ||
| 5.59 (1.27) | 4.68 (1.55) | 5.48 (1.57) | 5.35 (1.09) | |||
| Psychological LCQ scores | Baseline | n = 8 | n = 7 | n = 7 | n = 44 | .0004 |
| 4.95 (0.79) | 6.20 (0.47) | 4.47 (2.33) | 5.47 (1.27) | |||
| 24 mo | n = 8 | n = 7 | n = 7 | n = 44 | ||
| 6.27 (1.06) | 4.94 (1.83) | 5.14 (2.04) | 5.74 (1.40) | |||
| Social LCQ scores | Baseline | n = 8 | n = 6 | n = 7 | n = 44 | .0079 |
| 4.97 (1.03) | 6.17 (0.94) | 4.79 (2.10) | 5.57 (1.29) | |||
| 24 mo | n = 8 | n = 7 | n = 7 | n = 43 | ||
| 6.13 (1.30) | 4.93 (1.80) | 5.21 (2.02) | 5.80 (1.40) | |||
Stratified by change or no change in the presence of moderate to severe GERD.
P values refer to the trend test comparing three ordinal categories of change or no change in GERD (the two no-change categories were merged for the Cochran-Armitage trend analysis).
Similar to the correlations at baseline, changes in cough-specific QoL were significantly associated with changes in breathlessness and non-cough specific health status assessments, and these changes were all in a favorable direction (Table 6). However, no correlation was found between changes in LCQ scores and changes in the physiological or radiographic variables, with the exception of a modestly significant association between a reduction (improvement) in the physical LCQ domain score and a decrease in the Dlco.
Table 6.
Correlations Between Changes at 24 Months From Baseline in LCQ Scores (Total and Domains) and Changes in Physiological, HRCT, Clinical, and Non-Cough Specific Quality of Life Measures
| Variable | Total_LCQ | Physical_LCQ | Psychological_LCQ | Social_LCQ |
|---|---|---|---|---|
| FVC, % predicted | 0.005 | 0.073 | –0.084 | –0.025 |
| Dlco, % predicted | 0.204 | 0.26a | 0.162 | 0.149 |
| mRSS skin score | 0.15 | –0.218 | –0.087 | –0.091 |
| QLF-LM | 0.102 | 0.012 | 0.109 | 0.159 |
| QILD-LM | –0.169 | –0.19 | –0.155 | –0.099 |
| QLF-WL | –0.027 | –0.155 | 0.007 | 0.055 |
| QILD-WL | –0.156 | –0.245 | –0.141 | –0.066 |
| BDI/TDI | 0.369b | 0.266 | 0.39b | 0.292a |
| PCS, SF-36 | 0.535c | 0.563c | 0.418c | 0.401c |
| MCS, SF-36 | 0.449c | 0.352b | 0.311a | 0.47c |
| HAQ-DI | –0.033b | –0.399c | –0.28a | –0.247a |
| Breathing VAS | –0.462c | –0.286a | –0.271a | –0.475c |
Discussion
Using data from the first RCT of MMF vs CYC in symptomatic patients with SSc-ILD, we found that most patients had FC at trial onset and that slightly more than half of these patients produced sputum on several to most days of the week. Since current smoking was an SLS II exclusionary criterion, productive cough could not be attributed to active smoking. Consistent with the results of SLS I, patients with FC in SLS II had significantly more dyspnea, a lower Dlco, and a greater quantitative extent of fibrosis and total ILD on HRCT than did those without FC. The distributions of the associations of FC with these baseline measures of ILD severity are shown in e-Figure 1; inspection of these histograms makes it unlikely that the significance of these associations is attributable to only a few outliers with FC (e-Fig 1). These findings support the concept that FC in SSc-ILD is associated with fibrotic/inflammatory changes in the lung, although an independent influence of GERD is also likely. The mechanisms underlying the association between FC and parenchymal scarring/inflammation in the lung are unclear but could involve stimulation of the cough reflex through excitation of stretch receptors in the lung and airway irritant receptors with sensitization of C-fiber afferent nerves.16, 17 In addition, it is possible that patients are more aware of their cough because of their reduced pulmonary reserve.
Our study also found that GERD may be an important contributor to cough, as more patients with FC reported symptoms of GERD compared with patients without FC. Moreover, severity of GERD was correlated with cough-specific QoL scores.
Largely consistent with the observations in the CYC arm of SLS I, the presence of FC declined similarly in both treatment arms in SLS II (Fig 1), and this decline paralleled the improvement in FVC percent predicted in both the CYC and MMF treatment arms over the 24-month trial.4 Moreover, although the FVC percent predicted improved to a modest degree (about 3%), we observed a substantial decline in the proportion of patients with FC during treatment with either CYC or MMF (40.6%-44.1%). These findings suggest that a reduction in cough might serve as a useful secondary marker of the therapeutic response in SSc-ILD. A limitation of our study, however, is that it did not include a placebo arm, raising the possibility of a placebo effect on the patients’ subjective perception of cough. To minimize this possibility, the use of objective cough frequency monitors should be considered in future studies.
Although the proportion of patients with FC declined significantly during treatment, cough-specific QoL measures improved only slightly and not significantly. The reason for this disparity is not clear but may be due to the relatively modest impairment in LCQ scores at baseline, suggesting limited room for improvement. Interestingly, the improvements in the LCQ scores, while relatively modest, were largely confined to those patients with FC who had symptoms of moderate to severe GERD at baseline, whereas those patients with FC and no GERD symptoms at baseline tended to experience slightly worse cough-specific QoL, although not to a significant degree. These findings might have been influenced by the resolution of reflux symptoms in five of eight patients with baseline GERD in contrast to the development of reflux in all seven patients who did not have baseline GERD.
Improvements in cough-specific QoL over the 24-month course of the trial, although relatively small, correlated significantly with improvements in breathing as well as in generic (SF-36) and scleroderma-related (Health Assessment Questionnaire Disability Index [HAQ-DI]) QoL measures, suggesting that the improvements in cough-specific measures may have contributed to improvements in overall health status. However, improvements in cough-specific QoL were generally not correlated with improvements in lung function or in extent of disease on HRCT. This lack of correlation might be due to the relatively small changes in cough-specific health status as well as to the variability in the generally modest changes in objective physiological and radiographic measures of ILD severity with treatment.
Our overall findings suggest that persistence or resolution of FC over the 2-year trial was probably influenced, in part, by the trajectory of GERD symptoms. In addition, the significantly greater severity of ILD in patients with FC at baseline suggests that the underlying ILD was at least partially responsible for the presence of cough. A trend toward an association between loss of FC and resolution of GERD independent of the changes in FVC and extent of disease on HRCT was also observed. The aforementioned findings suggest that the resolution of FC might be related to improvements in both the inflammatory and fibrotic components of the underlying lung disease with ILD-directed therapy and a reduction in GERD.
Conclusions
The present findings indicate that FC is common in patients with SSc-ILD, is probably influenced by the presence of both GERD and ILD, and declines substantially in parallel with improvements in measures of ILD severity over 2 years of treatment for SSc-ILD. Therefore, reduction in cough might serve as a useful surrogate patient-centered marker of response to immunosuppressive therapy in patients with SSc-ILD.
Acknowledgments
Author contributions: All authors provided final approval and have agreed to be accountable for all aspects of the work. D. P. T., E. V., C-H. T., M. D. R., D. K., D. E. F., P. C., G. H. K., J. G., and R. M. E. contributed to the conception and design of the study. D. P. T., E. V., M. D. R., D. K., D. E. F., P. C., and A. T. acquired the data and all authors contributed to the analysis and/or interpretation of the data. D. P. T. wrote the initial draft and all the other authors revised it critically.
Financial/nonfinancial disclosures: The authors reported to CHEST the following: D. P. T. served as a consultant to EMD Serono. M. D. R. received a contract for study drug supply from Roche in support of SLS II. D. K. is funded by NIH/NIAMS (K24AR063120); received grant support from Bayer, Bristol Myers-Squibb, Roche/Genentech, and Pfizer; and served as a consultant to Actelion, Bristol Myers-Squibb, Corbus, Roche/Genentech, GlaxoSmithKline, Sanofi-Aventis, and EMD Serono. D. E. F. received grant support from Amgen, Bristol Myers-Squibb, Novartis, Pfizer, and Roche/Genentech and served as a consultant to AbbVie, Amgen, Bristol Myers-Squibb, Cytori, Janssen, Novartis, Pfizer, Roche/Genentech, and UCB Pharma. None declared (C-H. T., P. C., A. T., S. K., G. H. K., J. G., R. M. E.).
Additional contributions: Mycophenolate was kindly supplied by Hoffmann-La Roche/Genentech, which had no role in the design or conduct of the study or the preparation of this paper.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The Scleroderma Lung Study II was funded by the National Heart, Lung, and Blood Institute [R01 HL089758 and R01 HL 089901].
Supplementary Data
References
- 1.Theodore A.C., Tseng C.-H., Li N., Elashoff R.M., Tashkin D.P. Correlation of cough with disease activity and treatment with cyclophosphamide in scleroderma interstitial lung disease. Chest. 2012;142(3):614–621. doi: 10.1378/chest.11-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tashkin D.P., Elashoff R., Clements F.J., Scleroderma Lung Study Research Group Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin D.P., Elashoff R., Clements P.J. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176(10):1026–1034. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tashkin D.P., Roth M.D., Clements P.J. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease: Scleroderma Lung Study II (SLS-II), a double-blind, parallel group, randomized controlled trial. Lancet Respir Med. 2016;4(9):708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheumatol. 1980;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 6.Mahler D.A., Ward J., Fierro-Carrion G. Development of self-administered versions of modified baseline and transition dyspnea indexes in COPD. COPD. 2004;1(2):1–8. doi: 10.1081/copd-120030829. [DOI] [PubMed] [Google Scholar]
- 7.Beretta L., Santaniello A., Lemos A., Masciocchi M., Scorza R. Validity of the Saint George’s Respiratory Questionnaire in the evaluation of the health-related QoL in patients with interstitial lung disease secondary to systemic sclerosis. Rheumatology (Oxford) 2007;46(2):296–301. doi: 10.1093/rheumatology/kel221. [DOI] [PubMed] [Google Scholar]
- 8.Birring S.S., Prudon B., Carr A.J. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna D., Hays R.D., Maranian P., Seibold Reliability and validity of the University of California, Los Angeles scleroderma clinical trial consortium gastrointestinal tract instrument. Arthritis Rheum. 2009;61(9):1257–1263. doi: 10.1002/art.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae S., Allanore Y., Furst D.E. Associations between a scleroderma-specific gastrointestinal instrument and objective tests of upper gastrointestinal involvements in systemic sclerosis. Clin Exp Rheumatol. 2013;31(2 suppl 76):57–63. [PubMed] [Google Scholar]
- 11.McCloskey D.A., Patella S.J., Seibold J.R. Health Assessment Questionnaire (HAQ) in systemic sclerosis (SSc) Arthritis Care Res. 1990;3:S12. [Google Scholar]
- 12.Steen V.D., Medsger T.A., Jr. Health assessment questionnaire (HAQ) in demonstrating disability and organ system change in systemic sclerosis (SSc) Arthritis Rheum. 1995;38:S176. doi: 10.1002/art.1780401110. [DOI] [PubMed] [Google Scholar]
- 13.McHorney C.A., Ware J.E., Raczek A.E. The MOS 36-item sort form health survey (SF-36): psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kim H.J., Brown M.S., Elashoff R. Quantitative texture-based assessment of one-year changes in fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosphamide. Eur Radiol. 2011;21(12):2455–2465. doi: 10.1007/s00330-011-2223-2. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.G., Tashkin D.P., Clements P.J. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol. 2010;28(5 suppl 62):S26–S35. [PMC free article] [PubMed] [Google Scholar]
- 16.Hanacek J., Davies A., Widdicombe J.G. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration. 1984;45(3):161–168. doi: 10.1159/000194614. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson J.A. The role of capsaicin-sensitive C-fibre afferent nerves in the cough reflex. Pulm Pharmacol. 1996;9(5-6):315–321. doi: 10.1006/pulp.1996.0041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.