Abstract
Background
COPD is associated with reduced physical capacity. However, it is unclear whether pulmonary emphysema, which can occur without COPD, is associated with reduced physical activity in daily life, particularly among people without COPD and never smokers. We hypothesized that greater percentage of emphysema-like lung on CT scan is associated with reduced physical activity assessed by actigraphy and self-report.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) enrolled participants free of clinical cardiovascular disease from the general population. Percent emphysema was defined as percentage of voxels < −950 Hounsfield units on full-lung CT scans. Physical activity was measured by wrist actigraphy over 7 days and a questionnaire. Multivariable linear regression was used to adjust for age, sex, race/ethnicity, height, weight, education, smoking, pack-years, and lung function.
Results
Among 1,435 participants with actigraphy and lung measures, 47% had never smoked, and 8% had COPD. Percent emphysema was associated with lower activity levels on actigraphy (P = .001), corresponding to 1.5 hour less per week of moderately paced walking for the average participant in quintile 2 vs 4 of percent emphysema. This association was significant among participants without COPD (P = .004) and among ever (P = .01) and never smokers (P = .03). It was also independent of coronary artery calcium and left ventricular ejection fraction. There was no evidence that percent emphysema was associated with self-reported activity levels.
Conclusions
Percent emphysema was associated with decreased physical activity in daily life objectively assessed by actigraphy in the general population, among participants without COPD, and nonsmokers.
Key Words: actigraphy, computed tomography, emphysema, multicenter prospective cohort study, physical activity
Abbreviations: 6MWT, 6-minute walk test; LLN, lower limit of normal; LVEF, left ventricular ejection fraction; MESA, Multi-Ethnic Study of Atherosclerosis; MET, metabolic equivalent of task
COPD is the third leading cause of death in the United States.1 COPD is defined by accelerated, age-related loss in lung function, resulting in airway obstruction that is not completely reversible.2 Reduced functional status in COPD, characterized by reduced exercise tolerance, was described > 50 years ago by Filley et al3 and is well defined in more recent studies.4 Physical activity and exercise capacity are major predictors of mortality in COPD,5, 6 but activity limitation in COPD is still poorly understood.7, 8
Emphysema is defined as nonreversible air space enlargement with parenchymal destruction resulting in loss of lung architecture.9 The percentage of emphysema-like lung (percent emphysema) on CT scan provides in vivo assessment of pulmonary emphysema.10 Quantitative CT-based measurements of emphysema have previously been correlated with morphometric measurements.11 Parenchymal destruction in emphysema not just decreases the number of alveoli but is characterized by hyperinflation12 in addition to destruction of the vasculature.9, 13 These factors decrease maximal diffusion capacity and cardiac output,14 which lower peak O2 absorption and therefore limit exercise tolerance and reduce physical activity.15 Physical activity is reduced in patients with COPD with hyperinflation,16 and is possibly mediated by factors such as dyspnea, increased respiratory muscle work, and lower cardiac output through reduced right ventricular preload and increased afterload.17 Increased percent emphysema occurs not infrequently in the general population, in the setting of which it is associated with decreased cardiac output,14 subclinical vascular changes,18 decreased exercise capacity on cardiopulmonary exercise testing19 and the 6-minute walk test (6MWT),4 and increased dyspnea.20 However, exercise capacity and physical activity in daily life are different constructs, and exercise capacity does not necessarily translate into physical activity in daily life.21 Emphysema occurs not infrequently in the absence of spirometrically defined COPD22 and is also associated with increased dyspnea on exertion and higher all-cause mortality.23 Interestingly, the relationship of percent emphysema to dyspnea was not modified by current or former smoking,20 which may suggest a mechanism independent of smoking and possibly be reflective of the generally modest relationships of smoking to percent emphysema24 and some emphysema subtypes.25
Although a classic clinical correlate of emphysema is reduced activity levels, at times in absence of complaints of dyspnea, little is known about the correlation between actual daily activity and the extent of emphysema, particularly in patients who do not have spirometrically defined COPD. Available studies and guidelines focus on COPD independent of emphysema.26, 27, 28 Studies that objectively quantified emphysema on CT scan used measures of exercise capacity, such as the 6MWT4, 29, 30 or cardiopulmonary exercise testing,20, 31 which may not reflect general activity levels, did not adjust for lung function,32 focused on moderate to severe COPD,33 or comprised small sample sizes.
We therefore hypothesized that percent emphysema on CT scan would be associated with reduced physical activity levels in daily life, including among participants without COPD and among nonsmokers, in a general population sample. We assessed activity levels by actigraphy, which has proven to be superior to self-report,34 in addition to self-reported activity.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited 6,814 whites, African Americans, Hispanics, and Asian Americans ages 45 to 84 years from six US communities in 2000 to 2002. The major exclusion criteria were clinical cardiovascular disease and weight > 136 kg.35 There were 4,716 participants who attended the 10-year follow-up examination in 2010 through 2012, which included assessment of self-reported physical activity.
The MESA Lung Study enrolled 3,965 MESA participants who were sampled randomly among those who underwent baseline measurements of endothelial function, consented to genetic analyses, and attended an examination during the MESA Lung Study recruitment period in 2004 and 2006. We excluded participants who had a restrictive pattern on spirometry (FVC < lower limit of normal [LLN], with a ratio of FEV1 to FVC > 0.70).14, 24 The MESA Lung participants plus a random selection of 851 of the 1,543 MESA participants that were not selected for MESA Lung, but attended examination 5 were invited to undergo full-lung CT scans in 2010 through 2012, resulting in 3,131 technically acceptable scans. Of this group, 2,808 had spirometry measures after we excluded participants who had a restrictive pattern on spirometry (FVC < LLN, with a ratio of FEV1 to FVC > 0.70).14
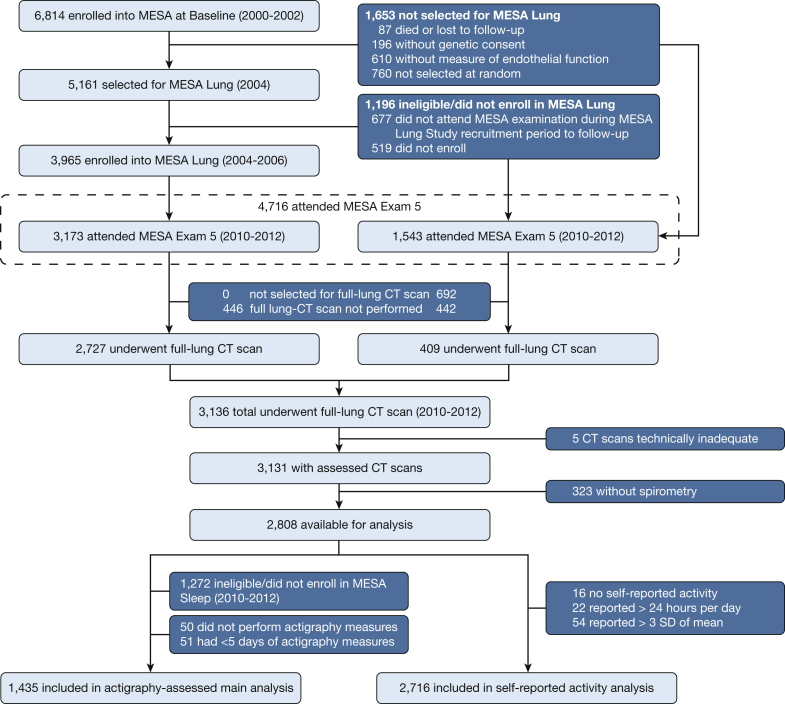
The MESA Sleep Study enrolled 2,230 participants in 2010 through 2012. Of the sample with full-lung CT scans, 1,435 had valid multiple day wrist actigraphy recording. Details of the recruitment of the present study sample are shown in Figure 1.
Figure 1.
Flow diagram of the study sample. MESA = Multi-Ethnic Study of Atherosclerosis.
All data assessed were obtained during 10-year follow-up (examination 5) of the MESA cohort. The median time between the CT scan examination and the beginning of the actigraphy assessment was 249 (interquartile range, 125-386) days.
Study procedures were approved by the institutional review boards of all participating institutions (e-Appendix 1) and by the National Heart, Lung, and Blood Institute. All participants provided informed consent.
Imaging
CT scans were acquired after coaching to full inspiration using 64-slice multidetector row CT scanners (Siemens Sensation 64 or General Electric V64). The CT scan acquisition followed the SPIROMICS/MESA-Lung full-inspiration protocol.36
Trained technologists at a single center and blinded to patient information measured attenuation levels of the volumetric units (voxels) using APOLLO software (VIDA Diagnostics). Percent emphysema was defined as the percentage of lung voxels with an attenuation level < −950 Hounsfield units, adjusted for attenuation of air outside the chest.37 Emphysema on CT scan was defined as percent emphysema greater than the upper limit of normal, based on reference equations derived from healthy participants of this cohort. The reference values are a function of age, sex, race/ethnicity, height, current smoking, scanner manufacturer, and milliampere seconds.38
For secondary analyses, hyperinflation was assessed as percentage of the total pulmonary air volume on CT scan divided by predicted pulmonary air volume based on similarly derived CT scan reference equations.38 Cardiac CT scans gated to the cardiac cycle were acquired in a subset at the same visit, and Agatston-adjusted coronary artery calcium score was measured.39 Left ventricular ejection fraction (LVEF) was assessed by cardiac MRI in a subset at the same examination, as previously described.40
Pulmonary Function Testing and COPD Case Status
Spirometry was assessed according to the American Thoracic Society/European Respiratory Society recommendations as previously described, and using predicted values and LLN based on the Hankinson reference equations. COPD was defined as a postbronchodilator FEV1/FVC ratio < 0.7, and airflow limitation was defined as prebronchodilator FEV1/FVC ratio < 0.7 and secondary FEV1/FVC ratio < LLN.2
Actigraphy
Participants were asked to wear a uniaxial actigraphy device (Actiwatch Spectrum; Philips Respironics) on the nondominant wrist for 7 consecutive days. Epoch length was set to 30 seconds, and activity count was then processed using the Actiware-Sleep version 5.59 analysis software (Mini Mitter Co., Inc.), a validated weighting algorithm,41 and scored at a central reading center. To assess motor activity in daily life, the mean daily activity count was assessed by analyzing the sum of the recorded accelerations per 24-hour period from 12 pm to 12 pm, which we then averaged over the measurement days (abbreviated as mean activity count). To account for possible increased need of sleep or rest and to avoid a bias toward underestimating inactivity or periods of low activity, these periods were included in the analysis.42 Time that the device was not worn, as indicated by the capacitive sensor, was excluded (time per day in percent: median, 0; interquartile range, 0-6.9). Based on current recommendations for healthy subjects and patients with COPD, a minimum of 4 weekdays and 1 weekend day were required for analysis,33 resulting in the exclusion of 51 participants.
The uniaxial Actiwatch Spectrum has been shown to have a good correlation (r = 0.68, P < .001; regression equation: y[activity counts] = 166.07 x[kcal] + 68,579) to active energy expenditure as measured by the gold standard of doubly labeled water.43
Self-Reported Physical Activity
The MESA Typical Week Physical Activity Survey was used as described previously, with moderate and vigorous physical activity levels as a summary measure (abbreviated as self-reported activity level).44
Covariates
Anthropometry, height and weight, and other covariates were measured following the MESA protocol (http://www.mesa-nhlbi.org). Race/ethnicity was self-reported according to the 2000 US Census criteria.
Statistical Analysis
Multivariable linear regression was used to test the relationship and statistical significance between percent emphysema (independent variable) and physical activity (dependent variable) as continuous variables. Activity breakdowns by quintile of percent emphysema are shown for descriptive purposes. Percent emphysema was logarithmically transformed to account for the skewed data distribution. Additional analysis of untransformed percent emphysema using generalized additive models and linear regression with robust estimators is also provided. Covariates were chosen based on known associations with physical activity and with percent emphysema. SAS 9.3 (SAS Institute) was used for analyses.
For additional information on the methods, please refer to e-Appendix 1.
Results
The 1,435 participants with valid measures of percent emphysema and activity count on actigraphy had a mean age of 68 ± 9 years, 48% were male, and the median percent emphysema was 1.4% (interquartile range, 0.5%-2.9%). Of the participants, 47% were never smokers, and the prevalence of COPD was 8%. Participants wore their actigraphy device for an average of 7.1 ± 0.8 days, with an average of 180 (interquartile range, 138-229) accelerations per 24-hour period. This translates to an average active energy expenditure of 2,807 kJ (interquartile range, 1,735-4,045 kJ).
Table 1 summarizes the characteristics of the study sample, stratified by quintile of percent emphysema. Participants with greater percent emphysema were older, more likely to be men or white, and to have smoked cigarettes. Participants with greater percent emphysema also had lower BMI, were less likely to have diabetes, and more likely to have reduced LVEF and more coronary artery calcification.
Table 1.
Characteristics of Study Participants Stratified by Quintiles of Percent Emphysema (N = 1,435)
| Characteristic | Quintile of Percent Emphysema |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| No. of participants | 287 | 287 | 287 | 287 | 287 |
| Percent emphysema | 0.3 (0.2-0.5) | 0.7 (0.5-0.8) | 1.4 (1.1-1.6) | 2.5 (2.1-2.9) | 5.5 (4.2-7.5) |
| Participants with percent emphysema > upper limit of normal, % | 0 | 0 | 2 | 9 | 30 |
| Age, y | 66 ± 9 | 68 ± 9 | 69 ± 9 | 69 ± 9 | 70 ± 9 |
| Male sex, % | 23 | 34 | 46 | 60 | 76 |
| Race or ethnic group, % | |||||
| Caucasian | 23 | 28 | 35 | 37 | 52 |
| African American | 29 | 29 | 29 | 31 | 22 |
| Hispanic | 40 | 31 | 21 | 17 | 11 |
| Chinese | 8 | 12 | 15 | 15 | 15 |
| Cigarette smoking, % | |||||
| Never smoker | 54 | 52 | 49 | 43 | 37 |
| Former smoker | 33 | 37 | 43 | 49 | 54 |
| Current smoker | 13 | 11 | 8 | 8 | 9 |
| Pack-years of smoking in never smokers | 11 (2-32) | 14 (2-30) | 14 (2-31) | 14 (3-29) | 18 (5-35) |
| Height, cm | 161 ± 9 | 164 ± 10 | 166 ± 10 | 168 ± 9 | 171 ± 9 |
| Weight, kg | 79 ± 16 | 79 ± 19 | 78 ± 18 | 80 ± 18 | 80 ± 17 |
| BMI, kg/m2 | 30.4 ± 5.6 | 29.5 ± 5.8 | 28.4 ± 5.3 | 28.2 ± 5.3 | 27.4 ± 5.0 |
| Diabetes, % | 24 | 19 | 18 | 14 | 12 |
| COPD,a % (assessment available in n = 1,241) | 2 | 4 | 6 | 9 | 23 |
| Hypertension, % | 57 | 61 | 58 | 60 | 51 |
| Ejection fraction < 55%, % (assessment available in n = 1,074) | 12 | 17 | 19 | 18 | 24 |
| Coronary calcification (any), % (assessment available in n = 1,279) | 57 | 64 | 69 | 68 | 71 |
| Beta-blocker, % | 20 | 17 | 16 | 16 | 14 |
| FEV1, % predicted | 90 ± 18 | 95 ± 18 | 95 ± 18 | 99 ± 18 | 95 ± 22 |
| FVC, % predicted | 89 ± 16 | 94 ± 17 | 97 ± 16 | 100 ± 16 | 101 ± 18 |
| FEV1/FVC ratio | 0.78 ± 0.07 | 0.77 ± 0.07 | 0.75 ± 0.08 | 0.74 ± 0.08 | 0.70 ± 0.10 |
Values are presented as median (interquartile range), mean ± SD, or as otherwise indicated. Race or ethnic group, smoking status, and pack-year history were self-reported.
Defined by postbronchodilator FEV1/FVC ratio of < 0.7.
Percent Emphysema and Mean Activity Count on Actigraphy
Greater percent emphysema was significantly associated with reduced activity levels assessed by actigraphy. This inverse association was significant in the unadjusted model (P = .02), minimally adjusted model (P = .003), and fully adjusted model (and P = .001) (Table 2), with an effect estimate of percent emphysema in the full model of −7,043 activity counts. The difference between the mean absolute activity counts of quintiles 2 and 4 of percent emphysema (which avoids the impact of extreme values on either end of the range and happens to correspond roughly to 1 SD) (e-Appendix 2) in the fully adjusted model (Table 2) is approximately equivalent to a 237 kJ change in mean energy expenditure per day or 1,660 kJ per week.43 This translates into 1.5 hours of moderately fast walking per week for an average weight participant (detailed calculations in e-Appendix 2, e-Table 1).45
Table 2.
Mean Activity Counts by Quintile of Percent Emphysema and Mean Differences Per 1 Log-Unit Change in Percent Emphysema
| Model | Mean Absolute Activity Counts in Thousands According to Quintile of Percent Emphysema | Mean Difference Actigraphy (95% CI) Per 1 Log-Unit Increase of Percent Emphysema | P Value | ||||
|---|---|---|---|---|---|---|---|
| Quintile No. | 1 | 2 | 3 | 4 | 5 | ||
| Measured mean activity count | 192 | 177 | 178 | 175 | 174 | … | … |
| Estimated active energy expenditure,a kJ/d | 3,102 | 2,727 | 2,754 | 2,668 | 2,660 | … | … |
| Estimated h/wk walking at 3.3 METSb | 19.8 | 17.4 | 17.6 | 17.1 | 17.0 | … | … |
| Minimally adjusted modelc (n = 1,435) | 193 | 187 | 183 | 180 | 175 | −6 (−10 to −2) | .003 |
| Estimated active energy expenditure,a kJ/d | 3,135 | 2,984 | 2,883 | 2,807 | 2,681 | … | … |
| Estimated h/wk walking at 3.3 METSb | 20.0 | 19.0 | 18.4 | 17.9 | 17.1 | … | … |
| Fully adjusted modeld (n = 1,316) | 196 | 189 | 184 | 180 | 174 | −7 (−11 to −3) | .001 |
| Estimated active energy expenditure,a kJ/d | 3,218 | 3,043 | 2,916 | 2,806 | 2,650 | … | … |
| Estimated h/wk walking at 3.3 METSb | 20.5 | 19.4 | 18.6 | 17.9 | 17.0 | … | … |
Mean activity counts according to quintile of percent emphysema are adjusted as indicated by the model where applicable. CI = confidence interval.
Estimations of active energy expenditure (kJ/d) are based on the following formulas: y[activity counts] = 166.07 x[kcal] + 68,579, where 1 kcal = 4.184 kJ (ISO31-4 standard).43
Assuming no other physical activity. Estimated active energy expenditure is calculated for the average participant with 79.4 kg, walking on level ground for 1 h with a 3 mi/h(= 4.8 km/h) pace (= 3.3 METs) = 79.4 kg × 3.3 MET = 262 kcal/h = 1,097.00 kJ/h.45
Minimally adjusted multivariable linear regression model includes age, sex, race/ethnicity, education, and scanner manufacturer as covariates.
Fully adjusted model also adds height, weight, BMI category, FEV1, cotinine level, and pack-years (differentiating between current, former, and never smokers).
Additional adjustment for coronary artery calcium score and LVEF yielded highly consistent results that were also statistically significant (coefficient in thousands, −6; 95% CI, −11 to −1; P = .03). Consistent results were also obtained for the full model additionally adjusted for hyperinflation (coefficient in thousands, −7; 95% CI, −13 to −2; P = 0.01). Hyperinflation on CT scan was also associated with lower activity levels on actigraphy in unadjusted and fully adjusted models that did not include percent emphysema (P = .049 and P = .035, respectively). However, addition of percent emphysema rendered the association with hyperinflation null (P = .90), whereas the association of percent emphysema and actigraphy was largely unchanged.
Male sex was also significantly associated with activity counts (P = .03) in the full model, whereas other covariates were not. Neither coronary artery calcium score nor LVEF were significantly associated with activity counts in the extended model.
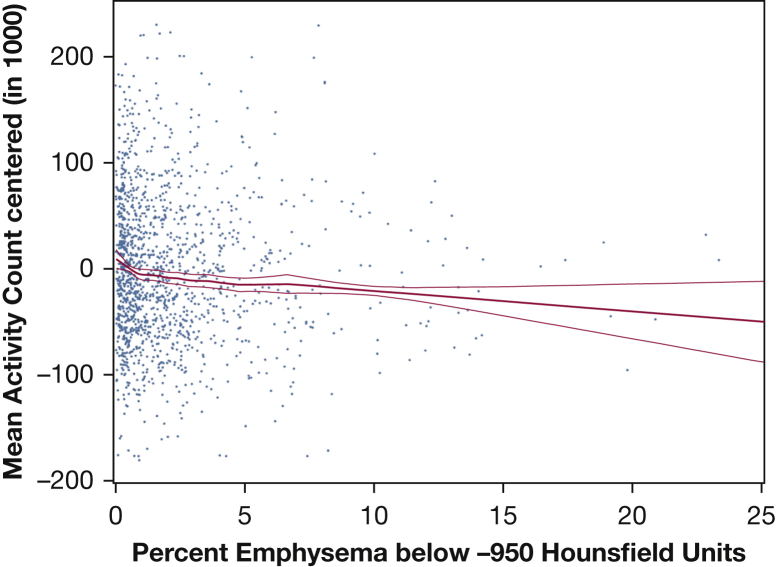
The relationship of untransformed percent emphysema and mean activity count was approximately linear across the spectrum of percent emphysema (Fig 2), without evidence of a threshold (P value for nonlinearity = .32). The findings were not driven by a small number of individuals with severe emphysema because exclusion of percent emphysema values above the 95th percentile showed similar results (P = .002). Further, the decrement in activity levels in the 121 participants with percent emphysema greater than the upper limit of normal was not statistically significant (P = .50).
Figure 2.
Multivariate relationship between percent emphysema and mean activity count in a centered scatterplot and generalized additive model with local regression smoothing (LOESS) plot. Centered scatterplot and LOESS plot with 95% confidence interval and smoothing parameter of 0.6. Fully adjusted model includes age, sex, race/ethnicity, education, scanner manufacturer height, weight, BMI category, FEV1, cotinine level, and pack-years (n = 1,316). The P value for the main association = .003. The deviation for nonlinearity was nonsignificant (P= .32). Excluding high percent emphysema values above the 95th percentile shows similar results (main association coefficient, −4.4; P = .002; nonlinearity P = .28; graph not shown). In a second generalized cross-validation model using the prediction of the sum of squares (df = 0.97), the P value for the main association was .001, and the deviation for nonlinearity stayed nonsignificant (P = .34; graph not shown).
Examination of untransformed percent emphysema with robust estimator led to similar results in the unadjusted and full model (P = .09 and P = .04, respectively) (e-Figure 1).
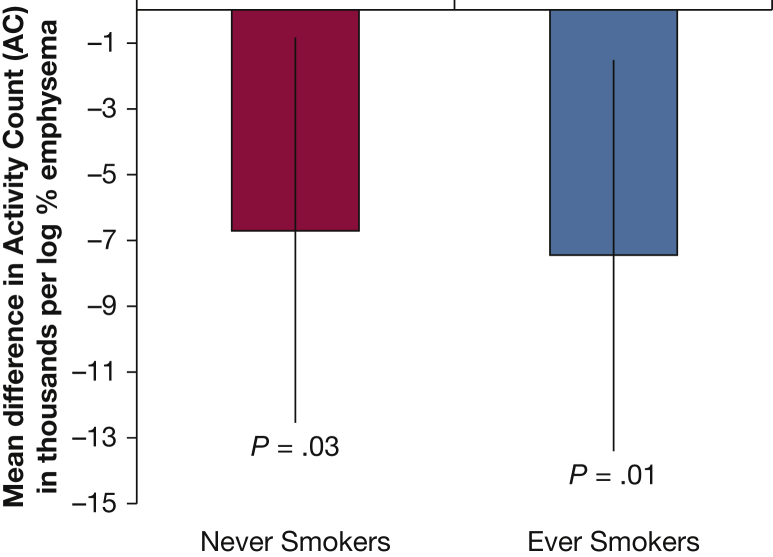
The association of percent emphysema on CT scan and mean activity count on actigraphy was consistent and statistically significant among ever smokers (coefficient in thousands, −7.5; 95% CI, −13.4 to −1.5; P = .01) and also among never smokers (coefficient in thousands, −6.7; 95% CI, −12.5 to −8.4; P = .03), without evidence for interaction by smoking status (interaction P = .90) (Fig 3).
Figure 3.
Percent emphysema on CT scan and mean activity count by actigraphy stratified by smoking status. Activity counts in the thousands. Fully adjusted model includes age, sex, race/ethnicity, education, scanner manufacturer height, weight, BMI category, FEV1, cotinine level, and pack-years. There was no significant interaction (P = .90). Never smokers: n = 671; ever smokers: n = 645.
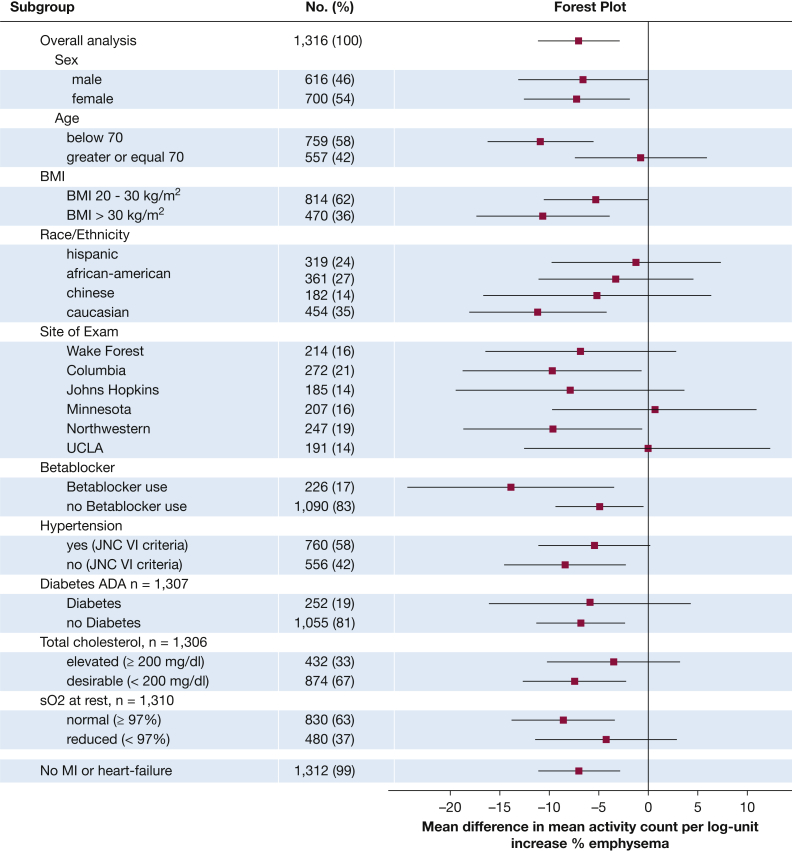
There was a significant effect modification by age and site but not by sex, race/ethnicity, obesity, presence of cardiac risk factors, and resting oxygen saturation (Fig 4), with a stronger association among younger participants and consistent results at 4 of 6 sites. Findings were similar and statistically significant among participants without a history of myocardial infarction or heart failure (Fig 4).
Figure 4.
Sensitivity analysis for association of percent emphysema on CT scan and mean activity count in actigraphy. Activity counts in the thousands (n = 1,316). Analysis for the fully adjusted model includes age, sex, race/ethnicity, education, scanner manufacturer, height, weight, BMI category, cotinine, pack-years, and FEV1. No interactions besides age (P = .0413) and site (P < .0001) were significant (all other P values > .05). ADA = American Diabetes Association; JNC VI = Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; sO2 = oxygen saturation; MI = myocardial infarction.
The inverse association between percent emphysema and mean activity count persisted among participants without COPD or without airflow limitation, regardless of its definition (Table 3). Effect estimates among these groups were highly similar to those in the full sample. Effect estimates restricted to participants with airflow limitation (prebronchodilator FEV1/FVC ratio < 70%; n = 352) showed highly similar effect estimates in the unadjusted, minimally adjusted, and fully adjusted model (coefficient in thousands, −4; 95% CI, −11 to 2; coefficient in thousands, −4; 95% CI, −11 to 3; and coefficient in thousands, −5; 95% CI, −13 to 3, respectively), but failed to reach significance.
Table 3.
Association of Mean Activity Counts by Quintile of Percent Emphysema and Mean Differences Per 1 Log-Unit Change in Percent Emphysema Restricted to Participants Without COPD or Airflow Limitation
| Model | Mean Absolute Activity Counts in Thousands According to Quintile of Percent Emphysema | Mean Difference Actigraphy (95% CI) Per 1 Log-Unit Increase of Percent Emphysema | P Value | ||||
|---|---|---|---|---|---|---|---|
| Without COPD (n = 1,140) | |||||||
| Minimally adjusteda | 195 | 188 | 184 | 179 | 174 | −7 (−12 to −2) | .003 |
| Fully adjustedb | 200 | 193 | 188 | 183 | 177 | −7 (−12 to −2) | .004 |
| Without airflow limitation (prebronchodilator FEV1/FVC ratio ≥ 70%) (n = 1,083) | |||||||
| Minimally adjusteda | 194 | 188 | 184 | 180 | 175 | −6 (−11 to −1) | .01 |
| Fully adjustedb | 198 | 192 | 188 | 183 | 178 | −7 (−12 to −2) | .01 |
| Without airflow limitation (prebronchodilator FEV1/FVC ratio ≥ LLN) (n = 1,273) | |||||||
| Minimally adjusteda | 194 | 188 | 183 | 179 | 174 | −7 (−11 to −2) | .003 |
| Fully adjustedb | 198 | 191 | 186 | 182 | 176 | −7 (−12 to −3) | .002 |
LLN = lower limit of normal. See Table 2 legend for expansion of other abbreviations.
Minimally adjusted model includes age, sex, race/ethnicity, education, and scanner manufacturer as covariates.
Fully adjusted model also adds height, weight, BMI category, FEV1, cotinine level, and pack-years.
The variance in mean activity count explained by percent emphysema was modest at 0.8%; however, it accounted for 54% of the variance explained by the full model. The other significant predictor was male sex (P = .03).
Percent Emphysema and Self-Reported Activity Levels
e-Appendix 3, e-Table 2 summarize the characteristics of the 2,716 participants, with measures of percent emphysema and self-reported activity level stratified by quintile of percent emphysema.
Although there was an inverse association of percent emphysema with self-reported moderate and vigorous activity levels, results did not attain statistical significance (e-Appendix 3, e-Table 3). There was no association in the sample restricted to those with additionally available actigraphy measures (e-Table 4). Stratified results demonstrated a generally inverse association (e-Fig 2).
Discussion
This study demonstrates that greater percent emphysema on CT scan was associated with reduced physical activity levels in daily life measured by actigraphy in a general population sample. The difference in physical activity between the average participant in quintile 2 vs 4 of percent emphysema was approximately equivalent to 1.5 hours of moderately fast walking per week. This relationship was independent of FEV1, cardiac function, and hyperinflation; present among participants without COPD; and evident among never smokers.
This report, to our knowledge, presents the first large-scale assessment of objectively assessed percent emphysema and activity levels measured in real-world settings of a general population outside of the clinic. By assessing activity levels in the real-world setting, we extend findings from previous, much smaller (n ≤ 100) studies, which measured exercise capacity tests, such as the 6MWT46 or incremental treadmill testing, instead of activity levels.19, 31 Although the absolute change in activity associated with percent emphysema was modest, the aforementioned difference of 90 minutes of moderately fast walking per week was associated, in a different study, with 14% lower death rates over a follow-up of 8 years,47 suggesting a difference of this magnitude might have an impact.
In contrast with previous studies, this study was sampled from the general population, without oversampling based on spirometrically defined COPD; had a large sample of participants without COPD; had more extensive control for potential confounders; and measured daily activity levels at home by actigraphy over 1 week.
Prior studies that oversampled patients with COPD among smokers demonstrated that percent emphysema was associated with lower 6MWT.4, 33 Differences between the prior studies and ours include the case-control design of the prior studies, which required stratification of analyses into relatively small sample sizes; their over selection of more severe COPD, in which activity limitation is more likely to result from airflow obstruction than cardiac limitation; and the assessment of activity by 6MWT, which assesses provoked functional capacity at only one time point and which is known to be insensitive in mild disease.46
In comparison, the current population-based study demonstrated a consistent inverse association between percent emphysema and week-long assessment of activity by means of actigraphy in unadjusted and extensively adjusted linear and nonlinear models, including among smokers and nonsmokers without COPD.
The mechanisms of reduced activity are not directly addressed in this report, and there are likely multiple factors at play. We hypothesize that emphysema is characterized by destruction of the vasculature,9, 13 decreasing maximal diffusion capacity and cardiac output,14 which lower peak O2 absorption and therefore limit exercise tolerance and reduce physical activity in the general population.15 However, a systematic review of studies in patients with COPD implicated prior exacerbations, systemic inflammation, and hyperinflation with reduced physical activity (but not FEV1, FVC, BMI, and comorbidities),7 all of which are also related to emphysema.48, 49, 50
Systemic inflammation has been shown to be associated with right ventricular impairment in MESA,51 and low to moderate percent emphysema has been shown to have a strong inverse association with function of the left and right ventricle,14, 52 pulmonary blood flow,53 and all-cause mortality in persons without airflow obstruction.23 Therefore, systemic inflammation is a possible link between physical activity and emphysema. Unfortunately, systemic inflammation markers were not available at the examination of the current analysis. Also, the number of exacerbations among the analyzed subjects was too small to find a correlation in our sample.
Hyperinflation assessed on CT scan, however, was inversely associated with activity. Although this measure has not yet been validated against total lung capacity on plethysmography and does not reflect gas trapping, we found that the association of percent emphysema with activity was independent of hyperinflation, rather than the other way around, suggesting a role of emphysema independent of hyperinflation.
Interestingly, a recent publication showed that fluticasone/vilanterol—a combination that has anti-inflammatory, bronchodilating, and vasodilatory properties—significantly improved cardiac function in patients with COPD with hyperinflation,13 suggesting that one or several mechanisms may be at work.
We found significant effect modification of the association of emphysema and activity counts by age and site. Aging generally lowers physical activity because of additional limiting factors besides those in the pulmonary system. However, the median age of the participants in our study is 67 years, and National Health and Nutrition Examination Survey data showed decreasing sex differences in total activity with ageing, with men and woman aged ≥ 60 years having similar activity counts on actigraphy.54 Minor modification by site effect is not unusual and may be because of different socioeconomic composition of the participants, walkability of the areas of recruitment, and other factors. Stratification by resting O2 saturation showed a more pronounced and significant result in participants with normal O2 saturation. This might be explained with activity being associated more with percent emphysema than gas exchange in our population with a low to moderate degree of emphysema. In this manner, emphysema may contribute to cardiopulmonary failure,55 likely producing a heart failure-like phenotype absent marked fluid overload, with reduced activity levels and increased dyspnea, even in the absence of airflow limitation.
The significant inverse association between percent emphysema and objectively assessed physical activity in daily life persisted among never smokers. This is particularly notable because this finding suggests that increasing percent emphysema may have a negative impact on physical activity in daily life independent of an individual’s direct exposure to cigarette smoke and related airway obstruction. Possible mechanisms for development of emphysema independent of direct proinflammatory stimuli of cigarette smoke on airways are thought to be mediated by inflammatory factors causing endothelial dysfunction, such as tumor necrosis factor-α, lipopolysaccharides, ionizing radiation, vascular endothelial growth factor receptor inhibition, or excess ceramides.56 A hypothesized genetic cause of emphysema is the increase of α1-antitrypsin degradation through α-mannosidase. Gene variants coding for α-mannosidase have been identified as a possible genetic cause of emphysema in Hispanics, African Americans, and Chinese, according to a genome-wide association study in MESA. In the same study, additional gene variants have been identified as a possible cause of emphysema in the general population.57
In contrast, percent emphysema was not significantly associated with self-reported physical activity level. Self-reported activity questionnaires are the most widely used method to assess physical activity in epidemiologic research.42 However, in COPD, questionnaires have performed poorly in assessing physical activity.34 This could be because of biased response in questionnaires, possibly with overreporting of self-reported activity level among those less active.58 Also, low- or moderate-intensity activities for a longer duration may not be equivalent to strenuous activity for a shorter duration, despite equal resulting metabolic equivalent of task (MET) sums. Further inaccuracies include using MET hours through definition of association of MET to different tasks and ignoring both body weight and mechanical efficiency.43 Furthermore, self-reported activity questionnaires typically perform worse in multiethnic samples such as the present.59 Nonetheless, the overall observation is consistent with the clinically perceived and classic phenotype of activity-limited patients with emphysema who deny symptoms.3
Our study has several limitations. First, actigraphy recording used a uniaxial and not a triaxial actigraphy, and there is no literature describing a minimally clinical important difference for actigraphy scores. However, our device has been shown to have a good correlation to the gold standard of the measurement of active energy expenditure, doubly labeled water.43, 58 Second, CT scans were performed on different scanners across the study. Although the protocol was highly standardized with tight quality control and phantom data showing no substantial differences across the limited number of sites, there were differences in percent emphysema measurements by scanner manufacturer.38 We addressed this problem by including scanner model as a covariate in our multivariable analyses. Nonetheless, scanner effects might account for the lack of association at two sites, as might chance. Third, the study was cross-sectional, and we infer that greater percent emphysema may contribute to less activity; however, we cannot rule out that the reverse may be true. Finally, residual confounding, particularly by socioeconomic status and smoking cannot be entirely excluded, as cannot unmeasured confounding. Nonetheless, precise measures were available on major confounders, including cardiovascular function.
In conclusion, greater percent emphysema on CT scan was associated with reduced physical activity levels in daily life assessed objectively, independent of lung function, among smokers and never smokers. Furthermore, greater percent emphysema on CT scan was also associated with reduced physical activity levels in daily life assessed objectively among participants without COPD on spirometry. Emphysema may contribute to reduced physical activity levels in daily life among patients with and without COPD.
Acknowledgments
Author contributions: C. M. L. delineated the hypotheses, analyzed the data, and wrote the manuscript. M. Q. and S. R. provided and assessed actigraphy data and contributed data interpretation and the revision of the manuscript. E. A. H. provided CT scan acquisition and assessment and contributed to the revision of the manuscript. A. G. B. contributed to data interpretation and the revision of the manuscript. C. P. A. contributed to data analysis, interpretation, writing, and revision of the manuscript. J. E. S. contributed to data analysis, interpretation, and revision of the manuscript. M. V. A., V. S. F., G. S. L. and S. M. K. contributed to data interpretation and revision of the manuscript. J. H. M. A. provided CT scan assessment and contributed to the writing of the manuscript. R. G. B. was involved in study conception, contributed to data acquisition, hypothesis delineation, data analysis, interpretation, and manuscript writing. S. R. and R. G. B. obtained funding.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: E. A. H. is the founder and co-owner of VIDA Diagnostics. None declared (C. M. L., M. Q., A. G. B., C. P. A., J. E. S., M. V. A., V. S. F., G. S. L., S. M. K., J. H. M. A., S. R., R. G. B.).
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Other contributions: We thank Megha A. Parikh, MS, (Columbia University Medical Center, New York, NY) for her support with SAS, Roberto A. Rabinovich, MD, PhD, (University of Edinburgh, Edinburgh, UK) for sharing the regression equation between Actiwatch Spectrum activity counts and doubly labeled water energy expenditure, and the participants of the MESA studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Additional information: The e-Appendixes, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute [Grants R01-HL077612, R01-HL093081, R01-HL098433, N01-HC95159-HC95169, UL1-TR000040]; and the Environmental Protection Agency [Grant RD83169701].
Supplementary Data
References
- 1.National Center for Health Statistics . US Government Printing Office; Hyattsville, MD: 2013. Health, United States, 2012: with special feature on emergency care. [PubMed] [Google Scholar]
- 2.Celli B.R., MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 3.Filley G.F., Beckwitt H.J., Reeves J.T. Chronic obstructive bronchopulmonary disease. II. Oxygen transport in two clinical types. Am J Med. 1968;44(1):26–38. doi: 10.1016/0002-9343(68)90234-9. [DOI] [PubMed] [Google Scholar]
- 4.Rambod M., Porszasz J., Make B.J. Six-minute walk distance predictors, including CT scan measures, in the COPDGene cohort. Chest. 2012;141(4):867–875. doi: 10.1378/chest.11-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waschki B., Kirsten A., Holz O. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 6.Celli B.R., Cote C.G., Marin J.M. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 7.Gimeno-Santos E., Frei A., Steurer-Stey C. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014;69(8):731–739. doi: 10.1136/thoraxjnl-2013-204763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agusti A.G., Noguera A., Sauleda J. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 9.The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132(1):182–185. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- 10.Bergin C., Müller N., Nichols D.M. The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis. 1986;133(4):541–546. doi: 10.1164/arrd.1986.133.4.541. [DOI] [PubMed] [Google Scholar]
- 11.Gould G.A., Macnee W., Mclean A. CT measurements of lung density in life can quantitate distal airspace enlargement - an essential defining feature of human emphysema. Am Rev Respir Dis. 1988;137(2):380–392. doi: 10.1164/ajrccm/137.2.380. [DOI] [PubMed] [Google Scholar]
- 12.Bancalari E., Clausen J. Pathophysiology of changes in absolute lung volumes. Eur Respir J. 1998;12(1):248–258. doi: 10.1183/09031936.98.12010248. [DOI] [PubMed] [Google Scholar]
- 13.Stone I.S., Barnes N.C., James W.Y. Lung deflation and cardiovascular structure and function in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med. 2016;193(7):717–726. doi: 10.1164/rccm.201508-1647OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr R.G., Bluemke D.A., Ahmed F.S. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Aymerich J., Serra I., Gomez F.P. Physical activity and clinical and functional status in COPD. Chest. 2009;136(1):62–70. doi: 10.1378/chest.08-2532. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Rio F., Lores V., Mediano O. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506–512. doi: 10.1164/rccm.200812-1873OC. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell D.E., Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225–236. doi: 10.1080/15412550701480455. [DOI] [PubMed] [Google Scholar]
- 18.Barr R.G., Ahmed F.S., Carr J.J. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J. 2012;39(4):846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paoletti P., De Filippis F., Fraioli F. Cardiopulmonary exercise testing (CPET) in pulmonary emphysema. Respir Physiol Neurobiol. 2011;179(2-3):167–173. doi: 10.1016/j.resp.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Oelsner E.C., Lima J.A., Kawut S.M. Non-invasive tests for the diagnostic evaluation of dyspnea among outpatients: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Am J Med. 2014;128(2):171–180. doi: 10.1016/j.amjmed.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spruit M.A., Pitta F., McAuley E. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):924–933. doi: 10.1164/rccm.201505-0929CI. [DOI] [PubMed] [Google Scholar]
- 22.Mets O.M., Buckens C.F., Zanen P. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011;306(16):1775–1781. doi: 10.1001/jama.2011.1531. [DOI] [PubMed] [Google Scholar]
- 23.Oelsner E.C., Hoffman E.A., Folsom A.R. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161(12):863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez J., Jiang R., Johnson W.C. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152(4):201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson A.E., Jr., Hernandez J.A., Eckert P. Emphysema in lung macrosections correlated with smoking habits. Science. 1964;144(3621):1025–1026. doi: 10.1126/science.144.3621.1025. [DOI] [PubMed] [Google Scholar]
- 26.Donaire-Gonzalez D., Gimeno-Santos E., Balcells E. Benefits of physical activity on COPD hospitalisation depend on intensity. Eur Respir J. 2015;46(5):1281–1289. doi: 10.1183/13993003.01699-2014. [DOI] [PubMed] [Google Scholar]
- 27.Watz H., Pitta F., Rochester C.L. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi: 10.1183/09031936.00046814. [DOI] [PubMed] [Google Scholar]
- 28.Watz H., Waschki B., Meyer T. Physical activity in patients with COPD. T Eur Respir J. 2009;33(2):262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 29.Mair G., Miller J.J., McAllister D. Computed tomographic emphysema distribution: relationship to clinical features in a cohort of smokers. Eur Respir J. 2009;33(3):536–542. doi: 10.1183/09031936.00111808. [DOI] [PubMed] [Google Scholar]
- 30.Marquez-Martin E., Ramos P.C., Lopez-Campos J.L. Components of physical capacity in patients with chronic obstructive pulmonary disease: relationship with phenotypic expression. Int J Chron Obstruct Pulmon Dis. 2011;6:105–112. doi: 10.2147/COPD.S16646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakayama K., Kurihara N., Fujimoto S. Relationship between exercise capacity and the severity of emphysema as determined by high resolution CT. Eur Respir J. 1993;6(9):1362–1367. [PubMed] [Google Scholar]
- 32.Vestbo J., Anderson W., Coxson H.O. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31(4):869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 33.Waschki B., Spruit M.A., Watz H. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106(4):522–530. doi: 10.1016/j.rmed.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Pitta F., Troosters T., Spruit M.A. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2005;86(10):1979–1985. doi: 10.1016/j.apmr.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Bild D.E., Bluemke D.A., Burke G.L. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 36.Sieren J.P., Newell J.D., Jr., Barr R.G. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith B.M., Austin J.H., Newell J.D., Jr. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014;127(1) doi: 10.1016/j.amjmed.2013.09.020. 94.e97-e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman E.A., Ahmed F.S., Baumhauer H. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc. 2014;11(6):898–907. doi: 10.1513/AnnalsATS.201310-364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Detrano R., Guerci A.D., Carr J.J. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 40.Donekal S., Venkatesh B.A., Liu Y.C. Interstitial fibrosis, left ventricular remodeling, and myocardial mechanical behavior in a population-based multiethnic cohort: the Multi-Ethnic Study of Atherosclerosis (MESA) study. Circ Cardiovasc Imaging. 2014;7(2):292–302. doi: 10.1161/CIRCIMAGING.113.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakley NR. Validation with Polysomnography of the Sleepwatch Sleep/Wake Scoring Algorithm Used by the Actiwatch Activity Monitoring System. Technical Report to Mini Mitter Co., Inc. 1997.
- 42.Warren J.M., Ekelund U., Besson H. Assessment of physical activity - a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17(2):127–139. doi: 10.1097/HJR.0b013e32832ed875. [DOI] [PubMed] [Google Scholar]
- 43.Rabinovich R.A., Louvaris Z., Raste Y. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J. 2013;42(5):1205–1215. doi: 10.1183/09031936.00134312. [DOI] [PubMed] [Google Scholar]
- 44.Aaron C.P., Tandri H., Barr R.G. Physical activity and right ventricular structure and function. The MESA-Right Ventricle Study. Am J Respir Crit Care Med. 2011;183(3):396–404. doi: 10.1164/rccm.201003-0469OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ainsworth B.E., Haskell W.L., Whitt M.C. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 46.Diaz A.A., Morales A., Diaz J.C. CT and physiologic determinants of dyspnea and exercise capacity during the six-minute walk test in mild COPD. Respir Med. 2013;107(4):570–579. doi: 10.1016/j.rmed.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Wen C.P., Wai J.P., Tsai M.K. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 48.McAllister D.A., Ahmed F.S., Austin J.H. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PloS One. 2014;9(4):e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith B.M., Hoffman E.A., Basner R.C. Not all measures of hyperinflation are created equal: lung structure and clinical correlates of gas-trapping and hyper-expansion in COPD: The Multi-Ethnic Study of Atherosclerosis Study (MESA) COPD Study. Chest. 2014;145(6):1305–1315. doi: 10.1378/chest.13-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharafkhaneh A., Hanania N.A., Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc. 2008;5(4):475–477. doi: 10.1513/pats.200708-126ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawut S.M., Barr R.G., Johnson W.C. Matrix metalloproteinase-9 and plasminogen activator inhibitor-1 are associated with right ventricular structure and function: the MESA-RV Study. Biomarkers. 2010;15(8):731–738. doi: 10.3109/1354750X.2010.516455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grau M., Barr R.G., Lima J.A. Percent emphysema and right ventricular structure and function: the multi-ethnic study of atherosclerosis-lung and multi-ethnic study of atherosclerosis-right ventricle studies. Chest. 2013;144(1):136–144. doi: 10.1378/chest.12-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hueper K., Vogel-Claussen J., Parikh M.A. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD Study. Am J Respir Crit Care Med. 2015;192(5):570–580. doi: 10.1164/rccm.201411-2120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin K.R., Koster A., Murphy R.A. Changes in daily activity patterns with age in U.S. men and women: National Health and Nutrition Examination Survey 2003-04 and 2005-06. J Am Geriatr Soc. 2014;62(7):1263–1271. doi: 10.1111/jgs.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Come C.E., Divo M.J., San Jose Estepar R. Lung deflation and oxygen pulse in COPD: results from the NETT randomized trial. Respir Med. 2012;106(1):109–119. doi: 10.1016/j.rmed.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrache I., Petrusca D.N., Bowler R.P. Involvement of ceramide in cell death responses in the pulmonary circulation. Proc Am Thorac Soc. 2011;8(6):492–496. doi: 10.1513/pats.201104-034MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manichaikul A., Hoffman E.A., Smolonska J. Genome-wide study of percent emphysema on computed tomography in the general population: the Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189(4):408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pitta F., Troosters T., Probst V.S. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006;27(5):1040–1055. doi: 10.1183/09031936.06.00064105. [DOI] [PubMed] [Google Scholar]
- 59.Loprinzi P.D. Factors influencing the disconnect between self-perceived health status and actual health profile: implications for improving self-awareness of health status. Prev Med. 2015;73:37–39. doi: 10.1016/j.ypmed.2015.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.