Abstract
Background
Direct (pulmonary) and indirect (extrapulmonary) ARDS are distinct syndromes with important pathophysiologic differences. The goal of this study was to determine whether clinical characteristics and predictors of mortality differ between direct or indirect ARDS.
Methods
This retrospective observational cohort study included 417 patients with ARDS. Each patient was classified as having direct (pneumonia or aspiration, n = 250) or indirect (nonpulmonary sepsis or pancreatitis, n = 167) ARDS.
Results
Patients with direct ARDS had higher lung injury scores (3.0 vs 2.8; P < .001), lower Simplified Acute Physiology Score II scores (51 vs 62; P < .001), lower Acute Physiology and Chronic Health Evaluation II scores (27 vs 30; P < .001), and fewer nonpulmonary organ failures (1 vs 2; P < .001) compared with patients with indirect ARDS. Hospital mortality was similar (28% vs 31%). In patients with direct ARDS, age (OR, 1.29 per 10 years; P = .01; test for interaction, P = .03), lung injury scores (OR, 2.29 per point; P = .001; test for interaction, P = .058), and number of nonpulmonary organ failures (OR, 1.67; P = .01) were independent risk factors for increased hospital mortality. Preexisting diabetes mellitus was an independent risk factor for reduced hospital mortality (OR, 0.47; P = .04; test for interaction, P = .02). In indirect ARDS, only the number of organ failures was an independent predictor of mortality (OR, 2.08; P < .001).
Conclusions
Despite lower severity of illness and fewer organ failures, patients with direct ARDS had mortality rates similar to patients with indirect ARDS. Factors previously associated with mortality during ARDS were only associated with mortality in direct ARDS. These findings suggest that direct and indirect ARDS have distinct features that may differentially affect risk prediction and clinical outcomes.
Key words: ARDS, diabetes, direct lung injury, indirect lung injury, mortality
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; DM, diabetes mellitus; LIS, lung injury scores; SAPS, Simplified Acute Physiology Score
FOR EDITORIAL COMMENT SEE PAGE 731
ARDS remains associated with significant mortality during critical illness despite use of low tidal volume ventilation and conservative fluid strategies.1, 2, 3 Numerous studies have attempted to define contributors to mortality, with conflicting results,4, 5, 6, 7, 8, 9 which may be related to changes in clinical practice patterns and varying definitions of ARDS. There is also conflicting data regarding the importance of underlying comorbidities (eg, diabetes mellitus [DM]) to ARDS mortality, with multiple studies reaching different conclusions.10, 11, 12, 13, 14, 15, 16 One potential explanation for discrepant results in the literature is that different underlying etiologies of ARDS may have variable clinical phenotypes, with different risk and prognostic factors.13, 17, 18, 19, 20
Patients with ARDS are a heterogeneous group with significant variability in clinical presentation and outcomes. One approach to reducing this heterogeneity is to subclassify patients with ARDS as having direct (pulmonary) or indirect (extrapulmonary) ARDS.6 Such a classification is based on variability in the pathology, radiology, respiratory mechanics, and response to different management strategies between patients with direct and indirect ARDS.20, 21, 22, 23, 24, 25, 26 In addition, distinct plasma biomarker signatures have been identified in patients with direct and indirect ARDS27 that imply differences in underlying pathophysiology. For example, patients with direct ARDS had greater evidence of lung epithelial injury, whereas patients with indirect ARDS had greater evidence of endothelial injury.
The goal of the present study was to determine whether the clinical characteristics and predictors of hospital mortality differed between direct and indirect ARDS in a cohort of well-phenotyped patients with ARDS.
Methods
Study Population
This study used clinical data from the Validation of Biomarkers for Acute Lung Injury Diagnosis (VALID) study,28 a single-center, prospective, observational study of critically ill patients at risk for developing ARDS. The inclusion and exclusion criteria for the VALID study have been described previously. Informed consent was provided by the patient or their surrogate when possible; if unavailable, patients could be enrolled into this minimal-risk study with a waiver of consent. The study was approved by the Vanderbilt Institutional Review Board (institutional review board no. 051065).
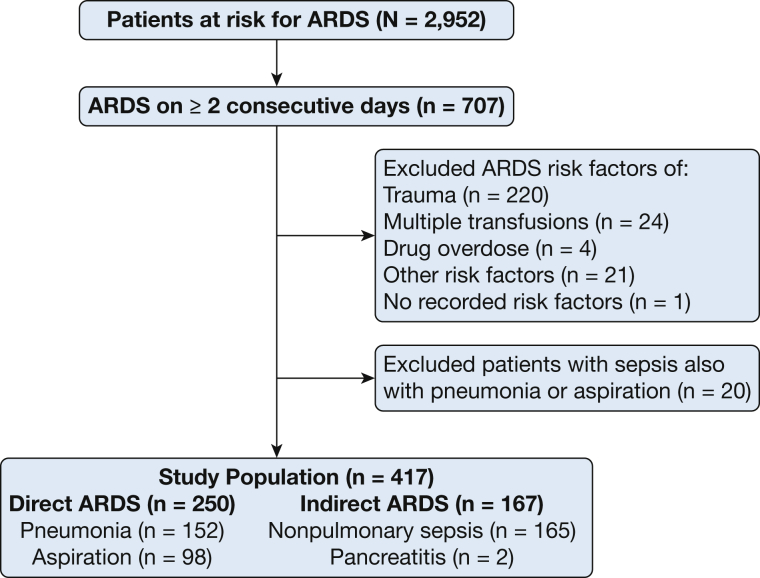
The present study included 2,952 patients enrolled between January 2006 and October 2014 (Fig 1). Of these, 707 patients were diagnosed with ARDS on at least 2 consecutive ICU days by using the American-European Consensus Conference definition of acute lung injury.6 Chest radiographs were scored by consensus between two physician investigators for ARDS diagnosis and for calculation of lung injury scores (LIS). When Pao2 was not available, the Spo2/Fio2 ratio was used to assess hypoxemia.29 Patients with ARDS were classified as having direct ARDS or indirect ARDS based on the underlying risk factor for ARDS recorded by study personnel. Patients with risk factors of pneumonia or aspiration of gastric contents were categorized as having direct ARDS (n = 250), whereas patients with risk factors of nonpulmonary sepsis or pancreatitis were categorized as having indirect ARDS (n = 167). For this study, patients who could not be classified as having uniquely direct or indirect ARDS were excluded. Patients with sepsis due to pneumonia (pulmonary sepsis) were classified as having direct ARDS. Patients with both pneumonia and nonpulmonary sepsis (n = 20) were excluded. A subgroup of 100 patients from the present study (44 with direct ARDS, 56 with indirect ARDS) were included in a previous biomarker study of molecular phenotypes in direct and indirect ARDS.27
Figure 1.
Study population. Risk factors for ARDS were recorded and each patient classified into direct ARDS or indirect ARDS as described. “Other” risk factors included alveolar hemorrhage (4), eosinophilic pneumonia (2), sickle cell crisis (1), acute myeloid leukemia (1), graft vs host disease (1), blast crisis (1), tumor lysis syndrome (2), drug toxicity (2), fat emboli (1), bronchiolitis obliterans organizing pneumonia (1), fat embolus (1), alveolar proteinosis (1), Wegener’s granulomatosis (1), primary graft dysfunction (1), and no known risk factors (1).
Data Collection
Demographic information, medical comorbidities, and clinical outcomes were determined based on the patient’s medical record. DM was characterized as type 1 or type 2 on the basis of patient history and medical documentation. Acute Physiology and Chronic Health Evaluation (APACHE) II and Simplified Acute Physiology Score (SAPS) II scores during the first 24 h after admission were calculated from the component variables.30, 31 LIS were calculated on the first day that each patient met ARDS criteria.32 The number of organ failures is the sum of nonpulmonary organ failures at enrollment, as defined by using the Brussels Organ Failure Scoring system.33
Statistical Analysis
Continuous variables are displayed by using medians (lower and upper quartiles) and categorical variables by using frequencies (percentage). Mann Whitney U tests (continuous variables) and Pearson χ2 tests (categorical variables) were used to compare patient groups.
To determine risk factors for hospital mortality, multivariable logistic regression analysis with testing for interactions was performed and included variables that were significantly different between survivors and nonsurvivors in the direct or indirect ARDS subgroups. A sensitivity analysis demonstrated that weight compared with BMI, and SAPS II compared with APACHE II, similarly predicted mortality (data not shown). Adjusted ORs with 95% CIs are reported. To further evaluate the observed differences in risk factors for mortality between direct and indirect ARDS, we utilized interaction terms between ARDS type and each risk factor. Statistical significance was considered at a two-sided 5% level. All analyses were conducted by using R version 3.1 (R Foundation for Statistical Computing).34
Results
Patients with direct and indirect ARDS had similar age, sex, race, weight, and BMI (Table 1). Chronic liver disease was more common in patients with indirect ARDS, whereas there were no significant differences in preexisting heart failure, renal disease, or DM between the ARDS subtypes. Patients with direct ARDS had higher LIS (P < .001), whereas patients with indirect ARDS had higher SAPS II scores, higher APACHE II scores, and more organ failures (P < .001 for each). Patients with direct ARDS had shorter lengths of stay in the ICU and in the hospital, despite having a similar duration of mechanical ventilation.
Table 1.
Demographic and Clinical Characteristics of Patients With Direct and Indirect ARDS
| Characteristic | Total ARDS (N = 417) |
Direct ARDS (n = 250) |
Indirect ARDS (n = 167) |
P Valuea |
|---|---|---|---|---|
| Age, y | 55 (45-66) | 55 (45-66) | 55 (45-66) | .78 |
| Male | 216 (52) | 122 (49) | 94 (56) | .13 |
| White race | 367 (88) | 220 (88) | 147 (88) | .99 |
| Weight, kg | 75 (64-95) | 74 (61-91) | 77 (66-95) | .16 |
| BMI, kg/m2 | 26 (23-31) | 26 (23-30) | 26 (23-31) | .64 |
| Current smoker | 137 (33) | 83 (33) | 54 (32) | .85 |
| Alcohol abuse | 71 (17) | 37 (15) | 34 (20) | .14 |
| Chronic liver disease | 40 (10) | 18 (7) | 20 (12) | .04 |
| Chronic renal disease | 69 (17) | 40 (10) | 29 (17) | .71 |
| Chronic heart failure | 44 (11) | 29 (12) | 15 (10) | .39 |
| MI/angina | 35 (8) | 25 (10) | 10 (6) | .15 |
| Diabetes mellitus | 122 (29) | 74 (30) | 48 (29) | .85 |
| Type 2 diabetes mellitus | 118 (28) | 71 (28) | 47 (28) | .96 |
| SAPS II score | 56 (41-69) | 51 (37-65) | 62 (49-74) | < .001 |
| APACHE II score | 29 (23-35) | 27 (21-34) | 30 (25-37) | < .001 |
| Lung injury score | 3.0 (2.3-3.3) | 3.0 (2.5-3.5) | 2.8 (2.3-3) | < .001 |
| Pao2/Fio2 | 120 (79-181) | 108 (73-159) | 154 (123-212) | < .001 |
| Shock | 294 (71) | 157 (63) | 137 (82) | < .001 |
| No. of organ failures | 1 (1-2) | 1 (1-2) | 2 (1-2) | < .001 |
| Mortality | 122 (29) | 70 (28) | 52 (31) | .49 |
| ICU LOS, d | 8 (5-14) | 7 (4-12) | 10 (6-17) | < .001 |
| Hospital LOS, d | 14 (9-24) | 13 (9-21) | 17 (10-27) | .001 |
| Ventilator-free days | 18 (2-24) | 21 (2-24) | 15 (2-23) | .054 |
Continuous variables are presented as median (interquartile range) and compared by using the Mann-Whitney U test. Categorical data are presented as No. (%) and compared by using Pearson χ2 test. Number of organ failures includes only nonpulmonary organ failures. APACHE = Acute Physiology and Chronic Health Evaluation; LOS = length of stay; MI = myocardial infarction; SAPS = Simplified Acute Physiology Score.
Comparison between direct ARDS and indirect ARDS.
Patients with direct and indirect ARDS had similar hospital mortality (28% vs 31%, respectively; P = .49). However, because of the growing recognition that direct and indirect etiologies of ARDS have distinct pathogenetic mechanisms, we tested whether factors associated with mortality differed between the groups (Table 2). In the direct ARDS cohort, survivors were younger (P = .04) and had lower SAPS II scores (P = .002), lower APACHE II scores (P = .01), lower LIS (P = .04), and fewer organ failures (P = .001) compared with nonsurvivors. Survivors of direct ARDS were also more likely to have preexisting DM compared with nonsurvivors (P = .04). In the indirect ARDS cohort, survivors had fewer organ failures (P = .001), with no significant impact of age, SAPS II, APACHE, LIS, or preexisting DM. As expected, patients with chronic liver disease had increased mortality, and this result was similar regardless of ARDS subgroup, whereas there was no impact of underlying cardiac disease or renal disease on mortality in either the direct or indirect ARDS group.
Table 2.
Clinical Characteristics of Survivors Vs Nonsurvivors in Direct and Indirect ARDS
| Characteristic | Direct ARDS (n = 250) |
P Valuea | Indirect ARDS (n = 167) |
P Valueb | ||
|---|---|---|---|---|---|---|
| Survivors (n = 180) | Nonsurvivors (n = 70) | Survivors (n = 115) | Nonsurvivors (n = 52) | |||
| Age, y | 55 (43-65) | 58 (48-72) | .04 | 55 (46-67) | 56 (43-65) | .57 |
| Male | 87 (48) | 35 (50) | .81 | 62 (54) | 32 (62) | .36 |
| White race | 154 (86) | 66 (94) | .06 | 98 (85) | 49 (94) | .10 |
| Weight, kg | 75 (64-97) | 73 (59-83) | .10 | 77 (68-95) | 81 (65-98) | .88 |
| BMI, kg/m2 | 26 (23-32) | 25 (22-28) | .03 | 26 (23-31) | 26 (22-33) | .79 |
| Current smoker | 63 (35) | 20 (29) | .33 | 39 (34) | 15 (29) | .52 |
| Alcohol abuse | 26 (14) | 11 (16) | .80 | 24 (21) | 10 (19) | .81 |
| Chronic liver disease | 7 (4) | 11 (16) | .001 | 9 (8) | 13 (25) | .002 |
| Chronic renal disease | 32 (18) | 8 (11) | .22 | 16 (14) | 13 (25) | .08 |
| Congestive heart failure | 19 (11) | 10 (14) | .41 | 7 (6) | 8 (15) | .05 |
| MI/angina | 18 (10) | 7 (10) | .99 | 7 (6) | 3 (6) | .94 |
| Diabetes mellitus | 60 (33) | 14 (20) | .04 | 30 (26) | 18 (35) | .26 |
| Type 2 diabetes mellitus | 58 (32) | 13 (19) | .03 | 30 (26) | 17 (33) | .38 |
| SAPS II | 50 (36-61) | 61 (41-77) | .002 | 63 (49-74) | 61 (47-76) | .75 |
| APACHE II | 26 (20-33) | 31 (22-36) | .01 | 30 (25-37) | 31 (25-36) | .83 |
| Lung injury score | 3.0 (2.5-3.5) | 3.0 (2.8-3.5) | .04 | 2.7 (2.3-3.0) | 2.9 (2.3-3.3) | .64 |
| Pao2/Fio2 | 114 (82-192) | 116 (77-166) | .53 | 158 (109-215) | 156 (104-238) | .80 |
| Shock | 110 (61) | 47 (67) | .38 | 95 (83) | 42 (81) | .77 |
| No. of organ failures | 1 (0.3-1) | 1 (1-2) | < .001 | 2 (1-2) | 2 (2-3) | < .001 |
Continuous variables are presented as median (interquartile range) and compared by using the Mann-Whitney U test. Categorical data are presented as No. (%) and compared by using Pearson χ2 test. Number of organ failures includes only nonpulmonary organ failures. See Table 1 legend for expansion of abbreviations.
Comparison between survivors and nonsurvivors in direct ARDS.
Comparison between survivors and nonsurvivors in indirect ARDS.
To determine whether the independent contributors to hospital mortality differed between direct and indirect ARDS, multivariable logistic regression models for hospital mortality were applied in the overall study cohort and then in the direct and indirect ARDS groups (Table 3). In the combined ARDS cohort, age, LIS, and number of organ failures were independently associated with hospital mortality; preexisting DM was not. In the direct ARDS group, age, LIS, and number of organ failures were independently associated with increased mortality, whereas preexisting DM was independently associated with reduced mortality. In the indirect ARDS group, the only factor independently associated with mortality was number of organ failures.
Table 3.
Direct and Indirect ARDS Have Distinct Independent Predictors of Mortality
| Characteristic | Total ARDS (N = 417) |
Direct ARDS (n = 250) |
Indirect ARDS (n = 167) |
P Value for Interaction With ARDS Type | |||
|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | ||
| Age (per 10 y) | 1.17 (1.00-1.36) | .04 | 1.29 (1.06-1.58) | .01 | 1.00 (0.78-1.28) | .99 | .03 |
| Male | 1.06 (0.66-1.69) | .82 | 1.06 (0.57-1.99) | .85 | 1.06 (0.50-2.23) | .88 | |
| Weight (per 5 kg) | 0.97 (0.93-1.02) | .26 | 0.95 (0.88-1.02) | .13 | 0.99 (0.93-1.06) | .79 | |
| SAPS II (per 15) | 0.97 (0.80-1.19) | .80 | 1.21 (0.91-1.60) | .19 | 0.77 (0.56-1.06) | .11 | |
| Lung injury score | 1.63 (1.14-2.31) | .007 | 2.29 (1.39-3.80) | .001 | 1.07 (0.62-1.87) | .81 | .058 |
| No. of organ failures | 1.90 (1.45-2.48) | < .001 | 1.67 (1.12-2.49) | .01 | 2.08 (1.39-3.12) | < .001 | .87 |
| Diabetes mellitus | 0.79 (0.47-1.33) | .38 | 0.47 (0.22-0.99) | .04 | 1.41 (0.64-3.09) | .39 | .02 |
Multivariable logistic regression was performed in the total ARDS cohort and then in the direct ARDS and indirect ARDS populations separately. The interactions of ARDS type (direct or indirect) with age, lung injury score, number of organ failures, and diabetes mellitus were included in the regression analysis. See Table 1 legend for expansion of abbreviations.
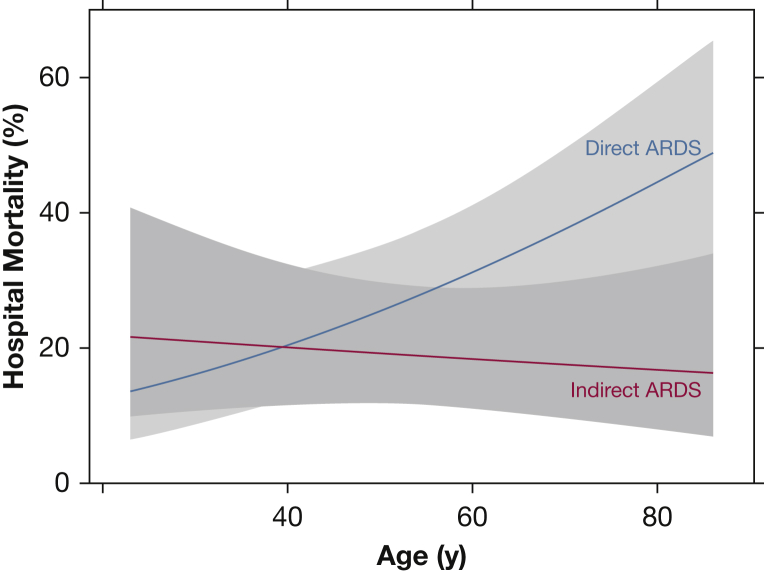
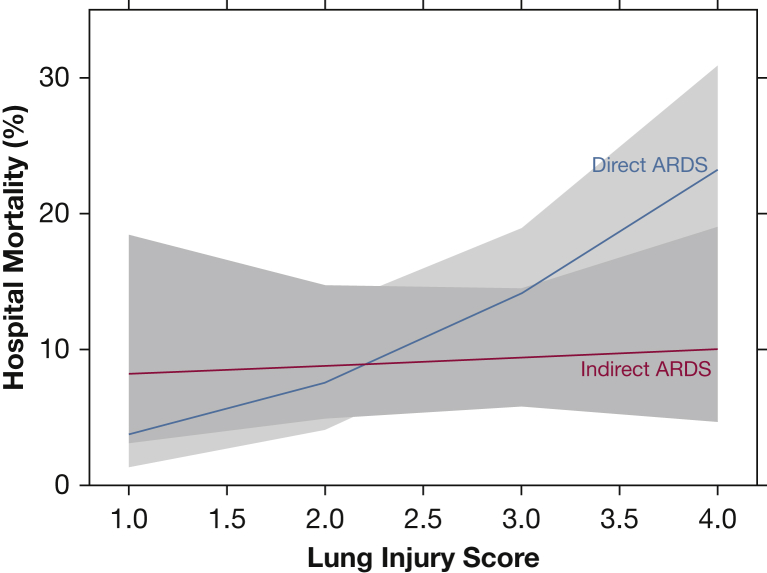
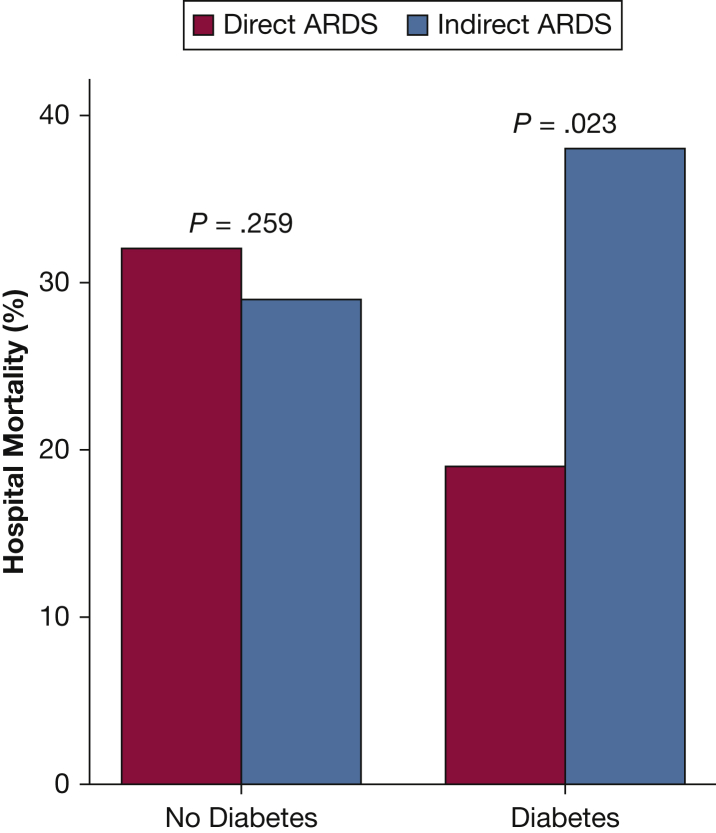
Figure 2 graphically depicts the association between hospital mortality and age according to ARDS subgroup, adjusted for confounders included in the multivariable analysis. Hospital mortality increased significantly with age in direct ARDS but not in indirect ARDS (test for interaction, P = .03). Figure 3 graphically depicts the association between hospital mortality and LIS according to ARDS subgroup (test for interaction, P = .058). Figure 4 shows that hospital mortality of direct ARDS in the presence of preexisting DM was significantly lower than that of indirect ARDS with preexisting DM (19% vs 38%; P = .02); there was no significant difference in hospital mortality between direct ARDS and indirect ARDS in the absence of preexisting DM (32% vs 29%; P = .26). The P value for interaction between ARDS cohort (direct vs indirect) and DM equaled .02.
Figure 2.
Hospital mortality increased with age only with direct ARDS. Mortality in direct ARDS (blue line) rose with increasing age, whereas age had no association with mortality in indirect ARDS (red line). Data are adjusted for sex, weight, Simplified Acute Physiology Score II, lung injury score, number of organ failures, and diabetes mellitus.
Figure 3.
Hospital mortality increased with lung injury score only with direct ARDS. Mortality in direct ARDS (blue line) increased with increasing lung injury score, whereas lung injury score had no association with mortality in indirect ARDS (red line). Data are adjusted for age, sex, weight, Simplified Acute Physiology Score II, number of organ failures, and diabetes mellitus.
Figure 4.
Preexisting DM was associated with reduced mortality only in direct ARDS. Patients with preexisting DM had reduced mortality from direct ARDS compared with indirect ARDS, whereas there was no impact of ARDS subtype on mortality in the absence of DM. The adjusted OR for the effect of DM on hospital mortality in ARDS is 0.45 (95% CI, 0.22-0.96 [P = .04]; test for interaction, P = .02). P values shown were determined by using Pearson χ2 test. DM = diabetes mellitus.
Discussion
The main goal of the present study was to determine whether the clinical characteristics and predictors of hospital mortality differ between direct and indirect ARDS. In this cohort, patients with direct ARDS had higher LIS, whereas those with indirect ARDS had more organ failures. In addition, notable differences were seen between direct and indirect ARDS in terms of predictors of hospital mortality. Age and LIS were independent risk factors for hospital mortality only in the direct ARDS cohort, whereas the number of organ failures was independently associated with hospital mortality only in the indirect ARDS cohort. Preexisting DM was independently associated with reduced mortality in the direct ARDS cohort but not in the indirect ARDS or the overall heterogeneous ARDS cohort. To our knowledge, this study is the first description of distinct associations among clinical characteristics and hospital mortality in patients with direct vs indirect ARDS.
Advanced age is a well-recognized independent risk factor for mortality in patients with ARDS.7, 13, 35, 36, 37, 38, 39, 40, 41 In the present study, the independent association of increased hospital mortality with age was limited to direct ARDS. In animal models of acute lung injury, increasing age is associated with excessive inflammatory responses, greater changes in lung permeability, and increased mortality.42, 43, 44, 45, 46 These experimental studies include both direct and indirect models of lung injury. Interestingly, age has not been associated with death in patients with indirect ARDS, despite age being a known risk factor for mortality in the general population with sepsis.47, 48, 49, 50, 51 This finding may, in part, be due to a relatively small effect of ARDS on mortality in the setting of sepsis.
LIS have been widely used for the assessment of ARDS severity.32, 52 In a previous analysis of the VALID study cohort, a significant association between LIS and hospital mortality in ARDS was reported, and the investigators proposed that LIS may be more suited to discriminate pulmonary-specific outcomes.52 Our results confirm this hypothesis and extend these findings in a subanalysis cohort by showing that the relationship between LIS and hospital mortality is limited to patients with direct ARDS and absent in those with indirect ARDS.
Multiple studies have shown that underlying DM is associated with a reduced risk of developing ARDS,10, 11, 12, 13 with one meta-analysis reporting that preexisting DM has an OR for ARDS development of 0.66.14 However, there are conflicting data about whether DM affects clinical outcomes of ARDS, with some studies reporting higher mortality of patients with ARDS and DM,16 lower mortality of patients with septic shock and DM,11 and some showing no association between DM and ARDS.10, 15, 53 Our study found no significant association of DM with mortality in the overall ARDS cohort (mortality 26% with DM vs 31% without DM; P = .38). However, to our knowledge, our study is the first to assess differences in mortality related to DM in ARDS by direct or indirect etiology. We found that the independent association of DM with mortality risk in ARDS is modified by the underlying ARDS risk factor, with a protective association of DM with hospital mortality limited to patients with direct ARDS. The protective association of DM for hospital mortality in direct ARDS persisted after adjustment for weight and BMI.54
The potential mechanisms by which DM might lead to better outcomes in direct ARDS are unclear and may be related to glycemic control or nonglycemic mechanisms.12 Studies of acute lung injury in rats have shown that the presence of DM and hyperglycemia were associated with less lung injury in direct lung injury models but with greater lung injury in indirect models of injury.55, 56, 57, 58 In addition, in patients with direct ARDS, higher levels of adiponectin (a hormone that affects glycemic control and fatty acid oxidation) were associated with increased mortality.59 Patients with type 2 DM have reduced levels of adiponectin,60, 61, 62 which may explain the association of DM with improved survival during direct ARDS.
Differences in predictors of mortality between direct and indirect ARDS support the growing body of literature suggesting that there are subphenotypes of ARDS that affect clinical outcomes.21, 27, 63, 64, 65, 66, 67 One previous study comparing sepsis-associated ARDS vs nonsepsis-associated ARDS reported that patients with sepsis-associated ARDS had increased severity of illness, lower Pao2/Fio2 ratios, and increased mortality, primarily due to overall disease severity and comorbidities.67 In the present study, preexisting DM, age, and LIS had different associations with mortality in patients with direct or indirect ARDS. These findings suggest that subgroup analysis of previous ARDS studies may reveal new insights into the pathogenesis and clinical course of ARDS by identifying factors associated with disease progression and clinical outcomes that are relevant in specific subpopulations of patients with ARDS. In addition, this approach also identified several variables associated with mortality in the overall study population that are common across ARDS subtypes, including chronic liver disease and number of organ failures. We found no differences in the associations of sex, ethnicity, alcohol abuse, or current smoking with mortality between direct and indirect ARDS; however, each of these variables has been associated with mortality in other ARDS studies that did not assess underlying etiology.68, 69, 70, 71, 72, 73, 74 Retrospective analysis of previous clinical trials for ARDS may yield additional mechanistic clues about how the underlying etiology of ARDS might affect clinical outcomes and could identify new potential therapeutic targets.
The present study has some limitations. First, to avoid overfitting, only a limited number of clinical variables were entered into the logistic regression models, and it is possible that potentially relevant variables were not evaluated. However, our focus was on those study variables that had been previously associated with poor outcomes in ARDS and other critical illnesses. In addition, the smaller sample size of patients with indirect ARDS may limit identification of prognostic factors in this subgroup. We attempted to account for this factor by specifically testing for interactions between age, LIS, DM, and mortality between ARDS subgroups. Our findings suggest that the magnitude of the effect rather than the sample size drives the statistical significance. Because of the retrospective nature of this study, we were unable to establish causality between DM and improved mortality in direct ARDS. Future studies will be necessary to determine the mechanisms that explain our observations. Because of the low numbers of patients with type 1 DM in our study, whether the impact of DM also differs according to etiology of DM remains unknown. Finally, because we excluded patients with trauma, drug overdose, and other less common risk factors for ARDS, the findings cannot be generalized to these patients.
Conclusions
The present study reports significant differences in predictors of mortality between patients with direct ARDS and those with indirect ARDS. Despite having lower severity of illness scores and fewer organ failures, patients with direct ARDS had similar mortality compared with patients with indirect ARDS. Factors that have previously been associated with increased mortality (eg, age, LIS) or reduced mortality (eg, DM) were only associated with mortality in direct ARDS in this analysis. Overall, this study provides new support for the focused analysis of subpopulations of patients with ARDS to reduce heterogeneity and target new therapies to the most appropriate subgroups of patients.
Acknowledgments
Author contributions: L. L., C. M. S., and L. B. W. had full access to all the data in the study and are responsible for the integrity of the data and accuracy of data analysis. L. L., C. M. S., J. A. B., C. S. C., and L. B. W. contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript. Z. Z. and T. K. provided statistical analysis.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. S. C. has served on the medical advisory boards for GlaxoSmithKline and Boehringer Ingelheim. None declared (L. L., C. M. S., Z. Z., T. K., J. A. B., L. B. W.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [grants HL103836 (L. B. W.), HL126671 (J. A. B.), HL087738 (C. M. S.), and HL131621 (C. S. C.)], a Vanderbilt Faculty Research Scholars Award (C. M. S.), and a Jiangsu Government Scholarship for Overseas Studies (JS-2013-270 [L. L.]).
References
- 1.Rubenfeld G.D., Caldwell E., Peabody E. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Goss C.H., Brower R.G., Hudson L.D. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31(6):1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Ware L.B. Prognostic factors in the acute respiratory distress syndrome. Clin Transl Med. 2015;4(1):65. doi: 10.1186/s40169-015-0065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caser E.B., Zandonade E., Pereira E. Impact of distinct definitions of acute lung injury on its incidence and outcomes in Brazilian ICUs: prospective evaluation of 7,133 patients. Crit Care Med. 2014;42(3):574–582. doi: 10.1097/01.ccm.0000435676.68435.56. [DOI] [PubMed] [Google Scholar]
- 5.Ranieri V.M., Rubenfeld G.D., Thompson B.T. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Bernard G.R., Artigas A., Brigham K.L. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 7.Seeley E., McAuley D.F., Eisner M. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63(11):994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villar J., Blanco J., Anon J.M. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37(12):1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 9.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Yu S., Christiani D.C., Thompson B.T. Role of diabetes in the development of acute respiratory distress syndrome. Crit Care Med. 2013;41(12):2720–2732. doi: 10.1097/CCM.0b013e318298a2eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss M., Guidot D.M., Steinberg K.P. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28(7):2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Honiden S., Gong M.N. Diabetes, insulin, and development of acute lung injury. Crit Care Med. 2009;37(8):2455–2464. doi: 10.1097/CCM.0b013e3181a0fea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong M.N., Thompson B.T., Williams P. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 14.Gu W.J., Wan Y.D., Tie H.T. Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: a meta-analysis. PLoS One. 2014;9(2):e90426. doi: 10.1371/journal.pone.0090426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singla A., Turner P., Pendurthi M.K. Effect of type II diabetes mellitus on outcomes in patients with acute respiratory distress syndrome. J Crit Care. 2014;29(1):66–69. doi: 10.1016/j.jcrc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Koh G.C., Vlaar A.P., Hofstra J.J. In the critically ill patient, diabetes predicts mortality independent of statin therapy but is not associated with acute lung injury: a cohort study. Crit Care Med. 2012;40(6):1835–1843. doi: 10.1097/CCM.0b013e31824e1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R., Srinivas R., Nath A. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS? A meta analysis. Chest. 2008;133(6):1463–1473. doi: 10.1378/chest.07-2182. [DOI] [PubMed] [Google Scholar]
- 18.Sevransky J.E., Martin G.S., Mendez-Tellez P. Pulmonary vs nonpulmonary sepsis and mortality in acute lung injury. Chest. 2008;134(3):534–538. doi: 10.1378/chest.08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal R., Aggarwal A.N., Gupta D. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in north India. Chest. 2006;130(3):724–729. doi: 10.1378/chest.130.3.724. [DOI] [PubMed] [Google Scholar]
- 20.Callister M.E., Evans T.W. Pulmonary versus extrapulmonary acute respiratory distress syndrome: different diseases or just a useful concept? Curr Opin Crit Care. 2002;8(1):21–25. doi: 10.1097/00075198-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Shaver C.M., Bastarache J.A. Clinical and biological heterogeneity in acute respiratory distress syndrome: direct versus indirect lung injury. Clin Chest Med. 2014;35(4):639–653. doi: 10.1016/j.ccm.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelosi P., D'Onofrio D., Chiumello D. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003;42:48s–56s. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 23.Perl M., Lomas-Neira J., Venet F. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med. 2011;5(1):115–126. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong M.N., Wei Z., Xu L.L. Polymorphism in the surfactant protein-B gene, gender, and the risk of direct pulmonary injury and ARDS. Chest. 2004;125(1):203–211. doi: 10.1378/chest.125.1.203. [DOI] [PubMed] [Google Scholar]
- 25.Rocco P.R., Zin W.A. Pulmonary and extrapulmonary acute respiratory distress syndrome: are they different? Curr Opin Crit Care. 2005;11(1):10–17. doi: 10.1097/00075198-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Gattinoni L., Pelosi P., Suter P.M. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. 1998;158(1):3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 27.Calfee C.S., Janz D.R., Bernard G.R. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6):1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siew E.D., Ware L.B., Gebretsadik T. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–1832. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice T.W., Wheeler A.P., Bernard G.R. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 30.Knaus W.A., Draper E.A., Wagner D.P. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 31.Le Gall J.R., Lemeshow S., Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 32.Murray J.F., Matthay M.A., Luce J.M. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 33.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 34.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. http://www.R-project.org/. Accessed July 14, 2016.
- 35.Sigurdsson M.I., Sigvaldason K., Gunnarsson T.S. Acute respiratory distress syndrome: nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand. 2013;57(1):37–45. doi: 10.1111/aas.12001. [DOI] [PubMed] [Google Scholar]
- 36.Zilberberg M.D., Epstein S.K. Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998;157(4 pt 1):1159–1164. doi: 10.1164/ajrccm.157.4.9704088. [DOI] [PubMed] [Google Scholar]
- 37.Suchyta M.R., Clemmer T.P., Elliott C.G. The adult respiratory distress syndrome. A report of survival and modifying factors. Chest. 1992;101(4):1074–1079. doi: 10.1378/chest.101.4.1074. [DOI] [PubMed] [Google Scholar]
- 38.Fowler A.A., Hamman R.F., Good J.T. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98(5 pt 1):593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 39.Schmickl C.N., Biehl M., Wilson G.A. Comparison of hospital mortality and long-term survival in patients with acute lung injury/ARDS vs cardiogenic pulmonary edema. Chest. 2015;147(3):618–625. doi: 10.1378/chest.14-1371. [DOI] [PubMed] [Google Scholar]
- 40.Ware L.B., Koyama T., Billheimer D.D. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137(2):288–296. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luecke T., Muench E., Roth H. Predictors of mortality in ARDS patients referred to a tertiary care centre: a pilot study. Eur J Anaesthesiol. 2006;23(5):403–410. doi: 10.1017/S0265021505001870. [DOI] [PubMed] [Google Scholar]
- 42.Prows D.R., Gibbons W.J., Jr., Smith J.J. Age and sex of mice markedly affect survival times associated with hyperoxic acute lung injury. PLoS One. 2015;10(6):e0130936. doi: 10.1371/journal.pone.0130936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schouten L.R., Schultz M.J., van Kaam A.H. Association between maturation and aging and pulmonary responses in animal models of lung injury: a systematic review. Anesthesiology. 2015;123(2):389–408. doi: 10.1097/ALN.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 44.Gomez C.R., Hirano S., Cutro B.T. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35(1):246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 45.Redente E.F., Jacobsen K.M., Solomon J.J. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301(4):L510–L518. doi: 10.1152/ajplung.00122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linge H.M., Lee J.Y., Ochani K. Age influences inflammatory responses, hemodynamics, and cardiac proteasome activation during acute lung injury. Exp Lung Res. 2015;41(4):216–227. doi: 10.3109/01902148.2014.999174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnston J.A. Determinants of mortality in patients with severe sepsis. Med Decis Making. 2005;25(4):374–386. doi: 10.1177/0272989X05278933. [DOI] [PubMed] [Google Scholar]
- 48.Pavon A., Binquet C., Kara F. Profile of the risk of death after septic shock in the present era: an epidemiologic study. Crit Care Med. 2013;41(11):2600–2609. doi: 10.1097/CCM.0b013e31829a6e89. [DOI] [PubMed] [Google Scholar]
- 49.Bernard G.R., Vincent J.L., Laterre P.F. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 50.Annane D., Sebille V., Charpentier C. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 51.Ranieri V.M., Thompson B.T., Barie P.S. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 52.Kangelaris K.N., Calfee C.S., May A.K. Is there still a role for the lung injury score in the era of the Berlin definition ARDS? Ann Intensive Care. 2014;4(1):4. doi: 10.1186/2110-5820-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soubani A.O., Chen W., Jang H. The outcome of acute respiratory distress syndrome in relation to body mass index and diabetes mellitus. Heart Lung. 2015;44(5):441–447. doi: 10.1016/j.hrtlng.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 54.O'Brien J.M., Jr., Phillips G.S., Ali N.A. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34(3):738–744. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boichot E., Sannomiya P., Escofier N. Endotoxin-induced acute lung injury in rats. Role of insulin. Pulm Pharmacol Ther. 1999;12(5):285–290. doi: 10.1006/pupt.1999.0212. [DOI] [PubMed] [Google Scholar]
- 56.de Oliveira Martins J., Meyer-Pflug A.R., Alba-Loureiro T.C. Modulation of lipopolysaccharide-induced acute lung inflammation: role of insulin. Shock. 2006;25(3):260–266. doi: 10.1097/01.shk.0000194042.18699.b4. [DOI] [PubMed] [Google Scholar]
- 57.Wright J.K., Nwariaku F.N., Clark J. Effect of diabetes mellitus on endotoxin-induced lung injury. Arch Surg. 1999;134(12):1354–1358. doi: 10.1001/archsurg.134.12.1354. discussion 1358-1359. [DOI] [PubMed] [Google Scholar]
- 58.Hagiwara S., Iwasaka H., Hasegawa A. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model. Crit Care Med. 2008;36(8):2407–2413. doi: 10.1097/CCM.0b013e318180b3ba. [DOI] [PubMed] [Google Scholar]
- 59.Langouche L., Vander Perre S., Frystyk J. Adiponectin, retinol-binding protein 4, and leptin in protracted critical illness of pulmonary origin. Crit Care. 2009;13(4):R112. doi: 10.1186/cc7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hotta K., Funahashi T., Arita Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 61.Weyer C., Funahashi T., Tanaka S. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 62.Diez J.J., Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148(3):293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 63.Jabaudon M., Blondonnet R., Lutz J. Net alveolar fluid clearance is associated with lung morphology phenotypes in acute respiratory distress syndrome. Anaesth Crit Care Pain Med. 2016;35(2):81–86. doi: 10.1016/j.accpm.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Kim S.J., Oh B.J., Lee J.S. Recovery from lung injury in survivors of acute respiratory distress syndrome: difference between pulmonary and extrapulmonary subtypes. Intensive Care Med. 2004;30(10):1960–1963. doi: 10.1007/s00134-004-2374-6. [DOI] [PubMed] [Google Scholar]
- 65.Calfee C.S., Delucchi K., Parsons P.E. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reilly J.P., Bellamy S., Shashaty M.G. Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma. Ann Am Thorac Soc. 2014;11(5):728–736. doi: 10.1513/AnnalsATS.201308-280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheu C.C., Gong M.N., Zhai R. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest. 2010;138(3):559–567. doi: 10.1378/chest.09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carey M.A., Card J.W., Voltz J.W. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L272–L278. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 69.Moss M., Mannino D.M. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979-1996) Crit Care Med. 2002;30(8):1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Perelman R.H., Palta M., Kirby R. Discordance between male and female deaths due to the respiratory distress syndrome. Pediatrics. 1986;78(2):238–244. [PubMed] [Google Scholar]
- 71.Lingappan K., Srinivasan C., Jiang W. Analysis of the transcriptome in hyperoxic lung injury and sex-specific alterations in gene expression. PLoS One. 2014;9(7):e101581. doi: 10.1371/journal.pone.0101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moss M., Bucher B., Moore F.A. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275(1):50–54. [PubMed] [Google Scholar]
- 73.Moss M., Parsons P.E., Steinberg K.P. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 74.Calfee C.S., Matthay M.A., Kangelaris K.N. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med. 2015;43(9):1790–1797. doi: 10.1097/CCM.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]