Abstract
Cystic fibrosis (CF) is a life-shortening autosomal recessive disorder caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is an anion channel that conducts bicarbonate and chloride across cell membranes. Although defective anion transport across epithelial cells is accepted as the basic defect in CF, many of the features observed in people with CF and organs affected by CF are modulated by the nervous system. This is of interest because CFTR expression has been reported in both the peripheral and central nervous systems, and it is well known that the transport of anions, such as chloride, greatly modulates neuronal excitability. Thus it is predicted that in CF, lack of CFTR in the nervous system affects neuronal function. Consistent with this prediction, several nervous system abnormalities and nervous system disorders have been described in people with CF and in animal models of CF. The goal of this special feature article is to highlight the expression and function of CFTR in the nervous system. Special emphasis is placed on nervous system abnormalities described in people with CF and in animal models of CF. Finally, features of CF that may be modulated by or attributed to faulty nervous system function are discussed.
Key Words: CFTR, cough, cystic fibrosis, glucose regulation, mucus secretion, nervous system
Abbreviations: ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CF, cystic fibrosis; CFRD, cystic-fibrosis-related diabetes; CFTR, cystic fibrosis transmembrane conductance regulator
Cystic fibrosis (CF) is one of the most common lethal autosomal recessive genetic disorders seen in the United States.1 Approximately 1,000 new cases of CF are diagnosed every year.2 The expectation of life for newborns in the United States born with CF is approximately 40 years.1
Mutations in the cystic fibrosis conductance regulator gene (CFTR) are responsible for CF.3, 4, 5 CFTR is a member of the adenosine triphosphate (ATP) binding cassette transporters.6 The channel uses energy from ATP hydrolysis to actively transport anions, such as bicarbonate and chloride, down their concentration gradients. Its structure consists of two membrane-spanning domains, two nucleotide-binding domains, and an unstructured R domain, with a total weight of approximately 170 kDa.7 The channel activity is regulated by cyclic adenosine monophosphate (cAMP)-dependent phosphorylation of the R domain and hydrolysis of intracellular nucleotides at the nucleotide-binding domains.
Currently, > 1,800 mutations in the CFTR gene are known.1, 8 All mutations do not produce the same degree of disease nor do all mutations affect channel function equally.9 The most common mutation is a three-base pair deletion, resulting in a loss of a phenylalanine at position 508. This mutation is responsible for approximately 70% of CF alleles.5 The ΔF508 mutation impairs CFTR’s ability to exit the endoplasmic reticulum, reducing expression at the cell surface.10 Channels that do make it to the surface open less frequently. A person who is homozygous for the ΔF508 mutation has very little channel activity and subsequently a severe disease phenotype.11
Distinguishing features of CF is complex and include salty skin, poor growth, intestinal obstruction and malabsorption, infertility, pancreatic insufficiency, frequent airway infection and inflammation, persistent coughing, adherent and viscous mucus, and difficulty breathing.1, 12 Respiratory disease is the major cause of mortality and morbidity in people with CF.13 Decades of work suggest that a lack of bicarbonate and chloride transport across epithelia is the cause of CF pathogenesis.14, 15, 16, 17, 18, 19, 20 Those studies are reviewed elsewhere and will not be reviewed here.12, 21, 22, 23, 24, 25
Managing CF often requires individualized treatment and care. Current treatments targeting the CF airway include mucolytic agents, chest physiotherapy, antiviral agents, antibiotics, antiinflammatory drugs, bronchodilators, and lung transplantation.26, 27 Small-molecule therapies that target CFTR mutations have also become available recently and have shown great success.27, 28, 29 Several institutions continue to pursue gene therapy to correct and restore normal CFTR function30, 31, 32, 33
CFTR Expression and Function in the Nervous System
Expression of CFTR is developmentally regulated and widespread.34, 35, 36, 37 Although expression in epithelial cells has been studied most, immune cells,38 fibroblasts,39 heart cells,40, 41 kidney cells,42 liver cells,43 skeletal44 and smooth muscle cells,45 chondrocytes,46 osteoclasts,47 and neural cells40, 48 all express CFTR. Nonepithelial expression is consistent with work demonstrating that CFTR does not require epithelial cell-specific machinery to form a functional channel.49
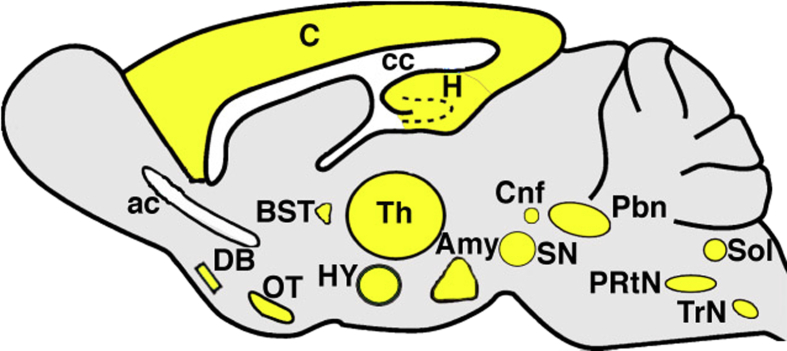
Expression of CFTR in the nervous system is particularly intriguing because chloride transport is a well-known modulator of neuronal activity.50 In addition, as highlighted further on, many of the features of CF could manifest due to nervous system dysfunction. McGrath et al40 were the first to describe CFTR in the nervous system. Subsequent studies by Mulberg et al51, 52 detailed CFTR expression in the rat brain (Fig 1). The expression pattern of CFTR suggested a possible role in regulation of mood and memory, energy homeostasis, olfaction, motor function, respiration, and autonomic control of visceral organs.51, 52 Additional studies found CFTR expression or activity (or both) in rodent dorsal root ganglion neurons,53 hypothalamic neurons,54 and motor neurons.37 In pigs, CFTR is expressed in both the peripheral and central nervous systems.55, 56 In humans, CFTR has been identified in parvocellular ganglion,57 hypothalamic neurons,58 spinal cord neurons,59 ganglion cells of the heart,60 and sympathetic ganglion.61 Both neuronal and glial cell CFTR expression has been reported in multiple species,55, 62, 63, 64 with the exception of humans, in whom only neuronal CFTR has been reported.48, 54
Figure 1.
CFTR in the rat brain. Summary of work by Mulberg et al51, 52 showing CFTR expression (yellow) in the rat brain. Location suggests involvement in energy homeostasis, limbic function, olfaction, motor function, respiration, and autonomic regulation of numerous effector organs, including the airways, GI tract, and heart. Amy = amygdala; ac = anterior commissure; BST = bed nucleus of stria terminalis; C = cortex; cc = corpus callosum; Cnf = cuneiform nucleus; DB = diagonal band of Brocha; H = hippocampus; HY = hypothalamus; OT = olfactory tubercle; Pbn = parabrachial nucleus, PRtN = parvocellular reticular nucleus; Sol = nucleus of the solitary tract; SN = substantia nigra; Th = thalamus; TrN = trigeminal nucleus.
The function of CFTR in the nervous system is uncertain. Reports of CFTR in the hypothalamus sparked speculations that neural CFTR may be important in maintenance of the neuroendocrine axis.51, 52, 58 Indeed, decreasing CFTR expression in a rat hypothalamic cell line reduced the release of gonadotropin-releasing hormone.54 In an additional study, the responsivity of glucose-sensitive neurons in the rat hypothalamus decreased with pharmacologic inhibition of CFTR.65 Our studies in newborn pigs revealed CFTR mRNA in the pituitary and hypothalamus and demonstrated that release of growth hormone is reduced in pituitary slice cultures isolated from CF pigs compared with non-CF littermates.56 These studies are all consistent with a function of CFTR in modulating the neuroendocrine axis.
A nonneuroendocrine function of CFTR has also been proposed. Ostroumov et al37 used CFTR inhibitors and computer modeling to demonstrate that CFTR modifies the excitability of motor neurons in the rat spinal cord. The authors went on to speculate that exaggerated neuronal excitability in individuals with CF might contribute to an increased incidence of seizures following lung transplantation.66 A role for CFTR in modulating ATP release from dorsal root ganglion neurons was reported.53 Given the important role of ATP in pain responses, it is possible that CFTR in the dorsal root ganglia might modify nociception.67, 68
An additional set of experiments by Liu et al63 suggested that microglia isolated from CF mice release less ATP.63 The implications from this study were that lack of CFTR decreases propagation of calcium waves in microglia, which in turn affects the extension and retraction of microglial processes. The extension and retraction of microglial processes plays an important role in the scavenging of damaged brain tissue.69 Another study found no obvious role for CFTR in modulating glial cell activity.64
Neural Abnormalities in Cystic Fibrosis
Given that CFTR is expressed and functional in the nervous system, it is not surprising that several nervous system abnormalities have been reported in CF. Although it is not known whether these abnormalities are primary or secondary or the extent to which they are manifested in all people with CF, their presence may be a component of, or may contribute to, pathogenesis, or both. Following is a brief summary of selected anomalies.
Innervation
Alterations in neural innervation have been repeatedly reported in CF (Table 1). Heinz-Erian et al70 were first to describe reduced innervation of the sweat gland in humans with CF. The authors suggested that the reduced innervation of the sweat gland might contribute to the high salt content of sweat in people with CF. A subsequent study also found that cutaneous innervation was decreased in humans with CF.71 Pan et al72 described decreased innervation of the CF mouse airway in utero that persisted postnatally. The decreased innervation was associated with decreased smooth muscle mass and decreased pulmonary neuroendocrine cells. Interestingly, in humans with CF, the number of pulmonary neuroendocrine cells is elevated.73, 74 Our studies in the pig suggest that innervation of the sinuses55 and trachea75 is also decreased. The pattern of innervation in the CF pig pancreas is also qualitatively different and is decreased as assessed by the neural markers β-tubulin III and PGP9.5.76 No information is available regarding innervation of the intestinal tract in animal models of CF, but hyperplasia of the intestinal nervous system77 and more prominent nerves78 has been reported in children with CF. Information regarding innervation of the heart, gallbladder, and reproductive tract is either not known or unavailable. However, abnormalities in neurally controlled functions of the heart,79, 80 gallbladder,81 and reproductive tract82 have been reported.
Table 1.
Summary of Neural Innervation of Organs Affected in CF and Broadly Related Functions
| Major Organ | Innervation Affected in CF | Species | Bodily Function Controlled by Nervous System | Affected in CF | Species |
|---|---|---|---|---|---|
| Airways (trachea, bronchus, lungs) | + Pan et al72 Reznikov et al75 | Mouse, pig | Smooth muscle contraction Cough Submucosal gland secretion Respiration |
+ Cook et al45 Reznikov et al75 + Smith96 Hamutcu et al97 Smith et al98 Chang et al99 + Sun et al104 Ianowski et al107 Joo et al108 Salinas et al109 + Bonora et al83 Bureau et al84 Bongers et al85 |
Pig, mouse Human Mouse, pig, ferret, human Mouse, human |
| Intestines | + Wildhaber et al77 Collins et al78 | Human | Gastrointestinal motility | + Rogers et al103 Sun et al104 Zhou et al105 van der Doef et al106 | Human, mouse, pig, ferret |
| Sweat gland | + Heinz-Erian et al70 | Human | Sweating | + Cystic Fibrosis Foundation1 Wine25 | Human |
| Heart | Unknown | . . . | Heart rate | + Florencio et al79 Szollosi et al80 | Human |
| Pancreas | + Uc et al76 | Pig | Glucose regulation | + Moran et al111 Marshall et al112 Kerem et al113 Moran et al114 Yi et al115 Olivier et al116 | Human, pig, ferret |
| Gallbladder | Unknown | . . . | Gallbladder emptying | + Debray et al81 | Mouse |
| Reproductive tract | Unknown | . . . | Ovulation | + Johannesson et al82 | Human |
| Eye | Unknown | . . . | Pupillary constriction/dilatation | + Davis and Kaliner87 | Human |
| Skin | + Savage et al71 | Human | Skin wrinkling | + Grasemann et al91 Park et al92 Wilder-Smith93 | Human |
“+” means there are data to support the finding that the innervation or function is affected in CF. References listed directly support the finding that either innervation or bodily function controlled by the nervous system is affected in CF. CF = cystic fibrosis.
Ventilatory Responses
Abnormalities in ventilation have also been reported in CF. In mice, the ventilatory response to hypoxia is decreased.83 In people with CF, ventilatory responses to hypoxia are also reduced,84 whereas exercise causes an exaggerated ventilatory response.85 Since CF mice do not exhibit lung infections like humans, it seems less likely that a decreased ventilatory response is explained by airway infection or inflammation. Because ventilatory responses involve neural control of both pulmonary and cardiac systems, one interpretation of these findings is that people with CF have dysregulation of the neural cardiopulmonary reflexes. Other factors, such as reduced lung compliance, might also contribute.86
Autonomic Function
Several studies suggest that autonomic function is altered in people with CF. Davis and Kaliner87 found increased pupillary reflexes to cholinergic agonists and α-adrenergic agonists in people with CF. Other studies suggest that people with CF have decreased leukocytic generation of cAMP88 and impaired cardiac and peripheral hemodynamic responses89 to β-adrenergic stimulation, as well as exaggerated sweat and saliva secretion in response to cholinergic agonists.90 Aquagenic wrinkling of the skin is a sympathetic nervous system-mediated response and is also exaggerated in people with CF.91, 92, 93 Alterations in smooth muscle contractile responses to cholinergic agonists have also been described,45, 94 as has abnormal heart rate variability.79, 80 Altered autonomic function could contribute to manifestations of CF, including cough, GI motility, and mucus secretion, topics explored in greater detail further on.
Possible Neural Contributions to CF Pathogenesis
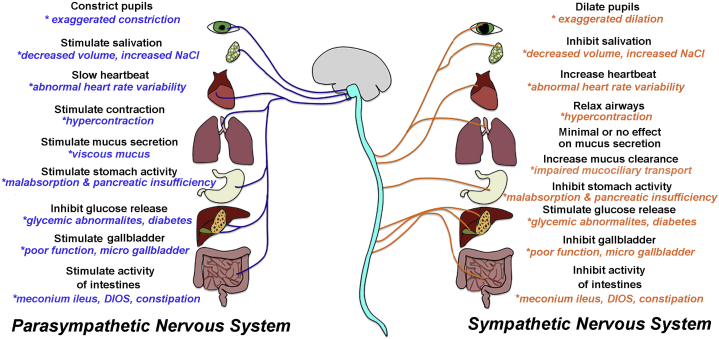
The nervous system exerts widespread control of multiple organs, including those affected in CF (Fig 2). Thus it is possible that nervous system defects could contribute to CF pathogenesis (Fig 3). Select categories of CF features that might be modified by or attributed to (or both) nervous system dysfunction are discussed in the following sections. Additional features are highlighted in Table 1. The following sections are speculative due to limited literature and a lack of clinical studies focused on the nervous system. They are also intentionally brief to minimize diluting relevant points and concepts.
Figure 2.
Organ dysfunction and the potential impact of neural dysfunction within that organ. Many of the organs affected in cystic fibrosis (CF) are controlled by the autonomic nervous system. Some hallmark features of CF could be modified by, or attributed to, defective neural regulation. Examples of known features and organ functions that are affected in CF are shown with asterisks in blue and orange text. It is important to note that there has been no direct cause and effect relationship established between organ dysfunction and neural dysfunction in CF. DIOS = distal intestinal obstruction syndrome.
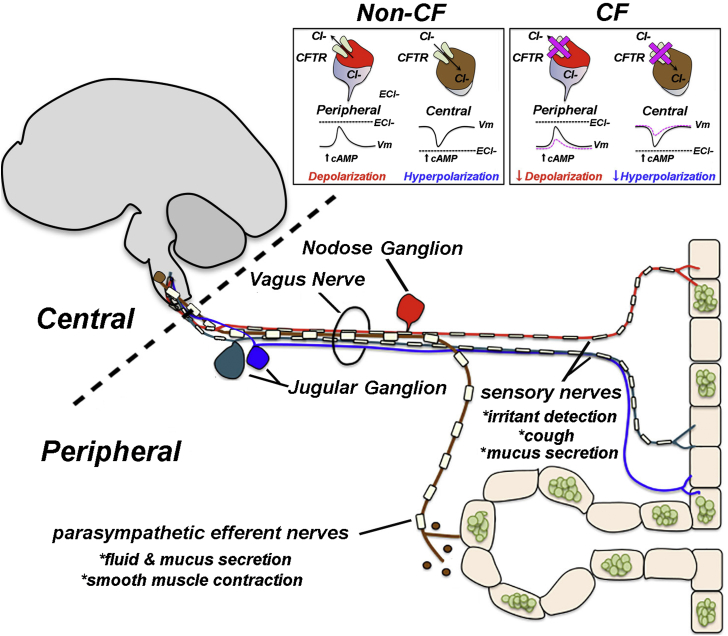
Figure 3.
Potential mechanism for how loss of CFTR might affect neuronal function and contribute to pathogenesis. Peripheral neurons participate in the detection of irritants and generation of protective reflexes, such as cough. The information is sent to central neurons in the brainstem to cause efferent release of acetylcholine, causing smooth muscle contraction and mucus secretion. In peripheral neurons, chloride (Cl–) currents are excitatory; therefore, loss of CFTR might decrease peripheral neuron activity. In central neurons, chloride currents are generally inhibitory, and therefore loss of CFTR might enhance central neuron activity. Inset shows hypothetical activation of CFTR by agents that raise intracellular cyclic adenosine monophosphate (cAMP) and predicted outcome on neuronal activity in the absence of CFTR. The asterisks (*) and text identify functions carried out by specific nerves innervating the airway. The nodose and jugular ganglia house sensory neurons that innervate the airway. The dashed line distinguishes central vs peripheral nervous system. It should be noted that the vagus nerve innervates many organs, including the GI tract and pancreas.
Cough
Cough is a protective behavior mediated by the nerves innervating the airway.95 The presumed triggers for cough are viscous and adherent mucus, impaired mucociliary transport, and chronic airway infection and inflammation.96 IV antibiotics in children with CF do not decrease cough frequency,97 whereas they do in adults with CF.98 Therefore, the mechanisms that mediate or the triggers that initiate cough may be different in children with CF vs adults with CF and not solely explained by infection. A different mechanism mediating cough is supported by the finding that children with CF have a decreased cough response to sensory nerve irritants compared with healthy and diseased control populations.99 Of note, CFTR mRNA has been found in the nucleus of the solitary tract,51, 52 a brain region that regulates cough.95, 100 Thus, the neural mechanisms orchestrating cough may be different in CF.
Intestinal Obstruction and Motility
The GI tract is highly innervated. Recently, CFTR mRNA and protein have been found in the neuronal ganglia of the GI tract of humans.101 Interestingly, the pattern and extent of neural innervation of the GI tract might be altered in some people with CF.77, 78 In Hirschsprung’s disease, innervation of the intestine is absent, and intestinal blockage ensues.102 These observations might suggest that some GI features of CF, such as meconium ileus,103, 104, 105 intestinal obstruction, and constipation,106 have a neural component.
Mucus
CF mice display defective submucosal gland secretion in response to sensory nerve stimulation,107 whereas cholinergic stimulation reveals minor to moderate secretion defects.104, 108, 109 Sensory nerves innervating the airway terminate in the nucleus of the solitary tract,51, 52 a key brain region that mediates submucosal gland stimulation and secretion.110 Thus, it is possible that in CF, the neural circuits mediating submucosal gland secretion are abnormal.
Glucose Regulation
Cystic fibrosis-related diabetes (CFRD) is one of the most common comorbidities in people with CF.111 CFRD is associated with more frequent pulmonary exacerbations,112 more severe lung disease,113 and greater mortality.114 Prior to the development of overt diabetes, people with CF display a spectrum of glucose tolerance abnormalities.115 Similar observations have been made in CF animal models.76, 116 Over time, insulin deficiency and insulin resistance develop. CFTR has been found in key neural regions, such as the hypothalamus and sympathetic nervous system,56, 58, 61 that exert control over the allocation of glucose and endocrine pancreatic function.117 In the hypothalamus, diminished CFTR activity impairs neuronal glucose-sensing properties.65 Decreased neuronal glucose-sensing in specific subregions of the hypothalamus induces glucose intolerance and deficient insulin secretion.118 Thus, it is possible that defects in the neural mechanisms governing endocrine pancreatic function and glucose regulation contribute to CFRD.
Conclusions
CF is a complex disease that affects many organs. Although the nervous system is not traditionally viewed as an organ affected in CF, limited evidence suggests that it is impacted and might contribute to CF pathogenesis. It is well accepted that chloride transport greatly modifies neuronal activity.50 Thus it is predicted that the presence or absence of CFTR within the nervous system might influence neuronal function. Yet little is known regarding the role of CFTR in the nervous system or how the nervous system might contribute to CF. Further investigations that focus on neural CFTR in clinically relevant phenotypes, such as cough, GI obstruction, mucus abnormalities, and glucose regulation, are of considerable interest. The use of animal models with conditional CFTR expression might be of particular value in this regard. Alternatively, selective rescue of CFTR to distinct neural subcompartments might also yield significant information. Clinical assessment of the effects of CFTR potentiators and correctors on neural function in people with CF might also be highly interesting. Such studies might embrace noninvasive measurements of neural function, including auditory brainstem-evoked potentials, pupillary reflex, and aquagenic wrinkling. Carrying out any one of these lines of work might reveal new knowledge and unanticipated insight into CF pathogenesis.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The author thanks Donald Bolser, PhD, Mahmoud Abou Alaiwa, MD, and Lawrence Reagan, PhD for helpful comments and suggestions.
Footnotes
FUNDING/SUPPORT: The author is supported by National Heart, Lung, and Blood Institute [Grant R00HL119560-03].
References
- 1.Cystic Fibrosis Foundation. About cystic fibrosis. 2016. https://www.cff.org/What-is-CF/About-Cystic-Fibrosis/. Accessed July 29, 2016.
- 2.O'Sullivan A.K., Sullivan J., Higuchi K., Montgomery A.B. Health care utilization & costs for cystic fibrosis patients with pulmonary infections. Manag Care. 2011;20(2):37–44. [PubMed] [Google Scholar]
- 3.Riordan J.R., Rommens J.M., Kerem B. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 4.Rommens J.M., Iannuzzi M.C., Kerem B. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 5.Kerem B., Rommens J.M., Buchanan J.A. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 6.Hyde S.C., Emsley P., Hartshorn M.J. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- 7.Ostedgaard L.S., Baldursson O., Vermeer D.W., Welsh M.J., Robertson A.D. A functional R domain from cystic fibrosis transmembrane conductance regulator is predominantly unstructured in solution. Proc Natl Acad Sci U S A. 2000;97(10):5657–5662. doi: 10.1073/pnas.100588797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spicuzza L., Sciuto C., Di Dio L. Mild cystic fibrosis in patients with the rare P5L CFTR mutation. J Cyst Fibros. 2012;11(1):30–33. doi: 10.1016/j.jcf.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Davies J.C. The future of CFTR modulating therapies for cystic fibrosis. Curr Opin Pulm Med. 2015;21(6):579–584. doi: 10.1097/MCP.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 10.Ostedgaard L.S., Rogers C.S., Dong Q. Processing and function of CFTR-DeltaF508 are species-dependent. Proc Natl Acad Sci U S A. 2007;104(39):15370–15375. doi: 10.1073/pnas.0706974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogan M.P., Stoltz D.A., Hornick D.B. Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. Chest. 2011;139(6):1480–1490. doi: 10.1378/chest.10-2077. [DOI] [PubMed] [Google Scholar]
- 12.Quinton P.M. Role of epithelial HCO3(-) transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010;299(6):C1222–C1233. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenfeld M., Emerson J., Williams-Warren J. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139(3):359–365. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- 14.Frizzell R.A., Rechkemmer G., Shoemaker R.L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- 15.Widdicombe J.H., Welsh M.J., Finkbeiner W.E. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci U S A. 1985;82(18):6167–6171. doi: 10.1073/pnas.82.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsh M.J., Liedtke C.M. Chloride and potassium channels in cystic fibrosis airway epithelia. Nature. 1986;322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- 17.Yankaskas J.R., Knowles M.R., Gatzy J.T., Boucher R.C. Persistence of abnormal chloride ion permeability in cystic fibrosis nasal epithelial cells in heterologous culture. Lancet. 1985;1(8435):954–956. doi: 10.1016/s0140-6736(85)91728-3. [DOI] [PubMed] [Google Scholar]
- 18.Quinton P.M. Cystic fibrosis. Righting the wrong protein. Nature. 1990;347(6290):226. doi: 10.1038/347226a0. [DOI] [PubMed] [Google Scholar]
- 19.Knowles M.R., Stutts M.J., Spock A., Fischer N., Gatzy J.T., Boucher R.C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 20.Boucher R.C., Cotton C.U., Gatzy J.T., Knowles M.R., Yankaskas J.R. Evidence for reduced Cl- and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol. 1988;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoltz D.A., Meyerholz D.K., Welsh M.J. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372(4):351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinton P.M. Too much salt, too little soda: cystic fibrosis. Sheng Li Xue Bao. 2007;59(4):397–415. [PubMed] [Google Scholar]
- 23.Verkman A.S., Song Y., Thiagarajah J.R. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol Cell Physiol. 2003;284(1):C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- 24.Rowe S.M., Miller S., Sorscher E.J. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 25.Wine J.J. The genesis of cystic fibrosis lung disease. J Clin Invest. 1999;103(3):309–312. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flume P.A., Van Devanter D.R. State of progress in treating cystic fibrosis respiratory disease. BMC Med. 2012;10:88. doi: 10.1186/1741-7015-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong T., Ramsey B.W. Modifying disease in cystic fibrosis: current and future therapies on the horizon. Curr Opin Pulm Med. 2013;19(6):645–651. doi: 10.1097/MCP.0b013e328365ab5f. [DOI] [PubMed] [Google Scholar]
- 28.Deeks E.D. Lumacaftor/Ivacaftor: a review in cystic fibrosis. Drugs. 2016;76(12):1191–1201. doi: 10.1007/s40265-016-0611-2. [DOI] [PubMed] [Google Scholar]
- 29.Quon B.S., Rowe S.M. New and emerging targeted therapies for cystic fibrosis. BMJ. 2016;352:i859. doi: 10.1136/bmj.i859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alton E.W.F.W., Armstrong D.K., Ashby D. A randomised, double-blind, placebo-controlled trial of repeated nebulisation of non-viral cystic fibrosis transmembrane conductance regulator (CFTR) gene therapy in patients with cystic fibrosis. Southampton (UK) NIHR J Library. 2016 [PubMed] [Google Scholar]
- 31.Karda R., Buckley S.M., Waddington S.N. Gene Therapy with adeno-associated virus for cystic fibrosis. Am J Respir Crit Care Med. 2016;193(3):234–236. doi: 10.1164/rccm.201510-2024ED. [DOI] [PubMed] [Google Scholar]
- 32.Cooney A.L., Singh B.K., Sinn P.L. Hybrid nonviral/viral vector systems for improved piggyBac DNA transposon in vivo delivery. Mol Ther. 2015;23(4):667–674. doi: 10.1038/mt.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang E.H., Zabner J. Precision genomic medicine in cystic fibrosis. Clin Transl Sci. 2015;8(5):606–610. doi: 10.1111/cts.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sehgal A., Presente A., Engelhardt J.F. Developmental expression patterns of CFTR in ferret tracheal surface airway and submucosal gland epithelia. Am J Respir Cell Mol Biol. 1996;15(1):122–131. doi: 10.1165/ajrcmb.15.1.8679216. [DOI] [PubMed] [Google Scholar]
- 35.Devuyst O., Burrow C.R., Schwiebert E.M., Guggino W.B., Wilson P.D. Developmental regulation of CFTR expression during human nephrogenesis. Am J Physiol. 1996;271(3 Pt 2):F723–F735. doi: 10.1152/ajprenal.1996.271.3.F723. [DOI] [PubMed] [Google Scholar]
- 36.Mouchel N., Broackes-Carter F., Harris A. Alternative 5' exons of the CFTR gene show developmental regulation. Hum Mol Genet. 2003;12(7):759–769. doi: 10.1093/hmg/ddg079. [DOI] [PubMed] [Google Scholar]
- 37.Ostroumov A., Simonetti M., Nistri A. Cystic fibrosis transmembrane conductance regulator modulates synaptic chloride homeostasis in motoneurons of the rat spinal cord during neonatal development. Dev Neurobiol. 2011;71(3):253–268. doi: 10.1002/dneu.20855. [DOI] [PubMed] [Google Scholar]
- 38.McDonald T.V., Nghiem P.T., Gardner P., Martens C.L. Human lymphocytes transcribe the cystic fibrosis transmembrane conductance regulator gene and exhibit CF-defective cAMP-regulated chloride current. J Biol Chem. 1992;267(5):3242–3248. [PubMed] [Google Scholar]
- 39.Yoshimura K., Nakamura H., Trapnell B.C. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991;19(19):5417–5423. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGrath S.A., Basu A., Zeitlin P.L. Cystic fibrosis gene and protein expression during fetal lung development. Am J Respir Cell Mol Biol. 1993;8(2):201–208. doi: 10.1165/ajrcmb/8.2.201. [DOI] [PubMed] [Google Scholar]
- 41.Wong K.R., Trezise A.E., Vandenberg J.I. Developmental regulation of the gradient of cftr expression in the rabbit heart. Mech Dev. 2000;94(1-2):195–197. doi: 10.1016/s0925-4773(00)00312-9. [DOI] [PubMed] [Google Scholar]
- 42.Todd-Turla K.M., Rusvai E., Naray-Fejes-Toth A., Fejes-Toth G. CFTR expression in cortical collecting duct cells. Am J Physiol. 1996;270(1 Pt 2):F237–F244. doi: 10.1152/ajprenal.1996.270.1.F237. [DOI] [PubMed] [Google Scholar]
- 43.Tran-Paterson R., Davin D., Krauss R.D., Rado T.A., Miller D.M. Expression and regulation of the cystic fibrosis gene during rat liver regeneration. Am J Physiol. 1992;263(1 Pt 1):C55–C60. doi: 10.1152/ajpcell.1992.263.1.C55. [DOI] [PubMed] [Google Scholar]
- 44.Tu J., Le G., Ballard H.J. Involvement of the cystic fibrosis transmembrane conductance regulator in the acidosis-induced efflux of ATP from rat skeletal muscle. J Physiol. 2010;588(Pt 22):4563–4578. doi: 10.1113/jphysiol.2010.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook D.P., Rector M.V., Bouzek D.C. CFTR in sarcoplasmic reticulum of airway smooth muscle: implications for airway contractility. Am J Respir Crit Care Med. 2016;193(4):417–426. doi: 10.1164/rccm.201508-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang H., Yang L., Ma T., Zhao Y. Functional expression of cystic fibrosis transmembrane conductance regulator in mouse chondrocytes. Clin Exp Pharmacol Physiol. 2010;37(4):506–508. doi: 10.1111/j.1440-1681.2009.05319.x. [DOI] [PubMed] [Google Scholar]
- 47.Stalvey M.S., Clines K.L., Havasi V. Osteoblast CFTR inactivation reduces differentiation and osteoprotegerin expression in a mouse model of cystic fibrosis-related bone disease. PLoS One. 2013;8(11):e80098. doi: 10.1371/journal.pone.0080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcorelles P., Friocourt G., Uguen A., Lede F., Ferec C., Laquerriere A. Cystic fibrosis transmembrane conductance regulator protein (CFTR) expression in the developing human brain: comparative immunohistochemical study between patients with normal and mutated CFTR. J Histochem Cytochem. 2014;62(11):791–801. doi: 10.1369/0022155414546190. [DOI] [PubMed] [Google Scholar]
- 49.Kartner N., Hanrahan J.W., Jensen T.J. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- 50.Kahle K.T., Staley K.J., Nahed B.V. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4(9):490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- 51.Mulberg A.E., Wiedner E.B., Bao X., Marshall J., Jefferson D.M., Altschuler S.M. Cystic fibrosis transmembrane conductance regulator protein expression in brain. Neuroreport. 1994;5(13):1684–1688. doi: 10.1097/00001756-199408150-00035. [DOI] [PubMed] [Google Scholar]
- 52.Mulberg A.E., Resta L.P., Wiedner E.B., Altschuler S.M., Jefferson D.M., Broussard D.L. Expression and localization of the cystic fibrosis transmembrane conductance regulator mRNA and its protein in rat brain. J Clin Invest. 1995;96(1):646–652. doi: 10.1172/JCI118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanno T., Nishizaki T. CFTR mediates noradrenaline-induced ATP efflux from DRG neurons. Mol Pain. 2011;7:72. doi: 10.1186/1744-8069-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weyler R.T., Yurko-Mauro K.A., Rubenstein R. CFTR is functionally active in GnRH-expressing GT1-7 hypothalamic neurons. Am J Physiol. 1999;277(3 Pt 1):C563–C571. doi: 10.1152/ajpcell.1999.277.3.C563. [DOI] [PubMed] [Google Scholar]
- 55.Reznikov L.R., Dong Q., Chen J.H. CFTR-deficient pigs display peripheral nervous system defects at birth. Proc Natl Acad Sci U S A. 2013;110(8):3083–3088. doi: 10.1073/pnas.1222729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogan M.P., Reznikov L.R., Pezzulo A.A. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci U S A. 2010;107(47):20571–20575. doi: 10.1073/pnas.1015281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su M., Guo Y., Zhao Y., Korteweg C., Gu J. Expression of cystic fibrosis transmembrane conductance regulator in paracervical ganglia. Biochem Cell Biol. 2010;88(4):747–755. doi: 10.1139/O10-016. [DOI] [PubMed] [Google Scholar]
- 58.Mulberg A.E., Weyler R.T., Altschuler S.M., Hyde T.M. Cystic fibrosis transmembrane conductance regulator expression in human hypothalamus. Neuroreport. 1998;9(1):141–144. doi: 10.1097/00001756-199801050-00028. [DOI] [PubMed] [Google Scholar]
- 59.Guo Y., Su M., Su M., McNutt M.A., Gu J. Expression and distribution of cystic fibrosis transmembrane conductance regulator in neurons of the spinal cord. J Neurosci Res. 2009;87(16):3611–3619. doi: 10.1002/jnr.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan P., Guo Y., Gu J. Expression of cystic fibrosis transmembrane conductance regulator in ganglion cells of the hearts. Neurosci Lett. 2008;441(1):35–38. doi: 10.1016/j.neulet.2008.05.087. [DOI] [PubMed] [Google Scholar]
- 61.Niu N., Zhang J., Guo Y., Yang C., Gu J. Cystic fibrosis transmembrane conductance regulator expression in human spinal and sympathetic ganglia. Lab Invest. 2009;89(6):636–644. doi: 10.1038/labinvest.2009.28. [DOI] [PubMed] [Google Scholar]
- 62.Ballerini P., Di Iorio P., Ciccarelli R. Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. Neuroreport. 2002;13(14):1789–1792. doi: 10.1097/00001756-200210070-00019. [DOI] [PubMed] [Google Scholar]
- 63.Liu G.J., Kalous A., Werry E.L., Bennett M.R. Purine release from spinal cord microglia after elevation of calcium by glutamate. Mol Pharmacol. 2006;70(3):851–859. doi: 10.1124/mol.105.021436. [DOI] [PubMed] [Google Scholar]
- 64.Liu H.T., Toychiev A.H., Takahashi N., Sabirov R.Z., Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res. 2008;18(5):558–565. doi: 10.1038/cr.2008.49. [DOI] [PubMed] [Google Scholar]
- 65.Murphy B.A., Fakira K.A., Song Z., Beuve A., Routh V.H. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol. 2009;297(3):C750–C758. doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaughn B.V., Ali I.I., Olivier K.N. Seizures in lung transplant recipients. Epilepsia. 1996;37(12):1175–1179. doi: 10.1111/j.1528-1157.1996.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 67.Jahr C.E., Jessell T.M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- 68.Gu J.G., MacDermott A.B. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389(6652):749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 69.Davalos D., Grutzendler J., Yang G. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 70.Heinz-Erian P., Dey R.D., Flux M., Said S.I. Deficient vasoactive intestinal peptide innervation in the sweat glands of cystic fibrosis patients. Science. 1985;229(4720):1407–1408. doi: 10.1126/science.4035357. [DOI] [PubMed] [Google Scholar]
- 71.Savage M.V., Brengelmann G.L., Buchan A.M., Freund P.R. Cystic fibrosis, vasoactive intestinal polypeptide, and active cutaneous vasodilation. J Appl Physiol (1985) 1990;69(6):2149–2154. doi: 10.1152/jappl.1990.69.6.2149. [DOI] [PubMed] [Google Scholar]
- 72.Pan J., Luk C., Kent G., Cutz E., Yeger H. Pulmonary neuroendocrine cells, airway innervation, and smooth muscle are altered in Cftr null mice. Am J Respir Cell Mol Biol. 2006;35(3):320–326. doi: 10.1165/rcmb.2005-0468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aguayo S.M., Miller Y.E., Waldron J.A., Jr. Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med. 1992;327(18):1285–1288. doi: 10.1056/NEJM199210293271806. [DOI] [PubMed] [Google Scholar]
- 74.Johnson D.E., Wobken J.D., Landrum B.G. Changes in bombesin, calcitonin, and serotonin immunoreactive pulmonary neuroendocrine cells in cystic fibrosis and after prolonged mechanical ventilation. Am Rev Respir Dis. 1988;137(1):123–131. doi: 10.1164/ajrccm/137.1.123. [DOI] [PubMed] [Google Scholar]
- 75.Reznikov L.R., Michalski A.S., Rector M.V., Stoltz D.A., Welsh M.J. Neural involvement in airway smooth muscle defects in cystic fibrosis. Pediatr Pulmonol. 2014;49(suppl S38):270. [Google Scholar]
- 76.Uc A., Olivier A.K., Griffin M.A. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128(2):131–142. doi: 10.1042/CS20140059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wildhaber J., Seelentag W.K., Spiegel R., Schoni M.H. Cystic fibrosis associated with neuronal intestinal dysplasia type B: a case report. J Pediatr Surg. 1996;31(7):951–954. doi: 10.1016/s0022-3468(96)90419-4. [DOI] [PubMed] [Google Scholar]
- 78.Collins M.H., Azzarelli B., West K.W., Chong S.K., Maguiness K.M., Stevens J.C. Neuropathy and vasculopathy in colonic strictures from children with cystic fibrosis. J Pediatr Surg. 1996;31(7):945–950. doi: 10.1016/s0022-3468(96)90418-2. [DOI] [PubMed] [Google Scholar]
- 79.Florencio R., Fregonezi G., Brilhante S., Borghi-Silva A., Dias F., Resqueti V. Heart rate variability at rest and after the 6-minute walk test (6MWT) in children with cystic fibrosis. Braz J Phys Ther. 2013;17(5):419–426. [PubMed] [Google Scholar]
- 80.Szollosi I., King S.J., Wilson J.W., Naughton M.T. Tachycardia in adults with cystic fibrosis is associated with normal autonomic function. Intern Med J. 2011;41(6):455–461. doi: 10.1111/j.1445-5994.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 81.Debray D., Rainteau D., Barbu V. Defects in gallbladder emptying and bile acid homeostasis in mice with cystic fibrosis transmembrane conductance regulator deficiencies. Gastroenterology. 2012;142(7):1581–1591.e1586. doi: 10.1053/j.gastro.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johannesson M., Landgren B.M., Csemiczky G., Hjelte L., Gottlieb C. Female patients with cystic fibrosis suffer from reproductive endocrinological disorders despite good clinical status. Hum Reprod. 1998;13(8):2092–2097. doi: 10.1093/humrep/13.8.2092. [DOI] [PubMed] [Google Scholar]
- 83.Bonora M., Bernaudin J.F., Guernier C., Brahimi-Horn M.C. Ventilatory responses to hypercapnia and hypoxia in conscious cystic fibrosis knockout mice Cftr. Pediatr Res. 2004;55(5):738–746. doi: 10.1203/01.PDR.0000117841.81730.2B. [DOI] [PubMed] [Google Scholar]
- 84.Bureau M.A., Lupien L., Begin R. Neural drive and ventilatory strategy of breathing in normal children, and in patients with cystic fibrosis and asthma. Pediatrics. 1981;68(2):187–194. [PubMed] [Google Scholar]
- 85.Bongers B.C., Werkman M.S., Takken T., Hulzebos E.H. Ventilatory response to exercise in adolescents with cystic fibrosis and mild-to-moderate airway obstruction. Springerplus. 2014;3:696. doi: 10.1186/2193-1801-3-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Darrah RJ, Mitchell AL, Campanaro CK, et al. Early pulmonary disease manifestations in cystic fibrosis mice [published online ahead of print May 23, 2016]. J Cyst Fibros. http://dx.doi.org/10.1016/j.jcf.2016.05.002. [DOI] [PMC free article] [PubMed]
- 87.Davis P.B., Kaliner M. Autonomic nervous system abnormalities in cystic fibrosis. J Chronic Dis. 1983;36(3):269–278. doi: 10.1016/0021-9681(83)90062-0. [DOI] [PubMed] [Google Scholar]
- 88.Davis P.B., Braunstein M., Jay C. Decreased adenosine 3':5'-monophosphate response to isoproterenol in cystic fibrosis leukocytes. Pediatr Res. 1978;12(6):703–707. doi: 10.1203/00006450-197806000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Van Iterson E.H., Karpen S.R., Baker S.E., Wheatley C.M., Morgan W.J., Snyder E.M. Impaired cardiac and peripheral hemodynamic responses to inhaled beta(2)-agonist in cystic fibrosis. Respir Res. 2015;16:103. doi: 10.1186/s12931-015-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis P.B., Shelhamer J.R., Kaliner M. Abnormal adrenergic and cholinergic sensitivity in cystic fibrosis. N Engl J Med. 1980;302(26):1453–1456. doi: 10.1056/NEJM198006263022605. [DOI] [PubMed] [Google Scholar]
- 91.Grasemann H., Ratjen F., Solomon M. Aquagenic wrinkling of the palms in a patient with cystic fibrosis. N Engl J Med. 2013;369(24):2362–2363. doi: 10.1056/NEJMc1308349. [DOI] [PubMed] [Google Scholar]
- 92.Park L., Khani C., Tamburro J. Aquagenic wrinkling of the palms and the potential role for genetic testing. Pediatr Dermatol. 2012;29(3):237–242. doi: 10.1111/j.1525-1470.2011.01609.x. [DOI] [PubMed] [Google Scholar]
- 93.Wilder-Smith E.P. Stimulated skin wrinkling as an indicator of limb sympathetic function. Clin Neurophysiol. 2015;126(1):10–16. doi: 10.1016/j.clinph.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 94.De Lisle R.C., Meldi L., Mueller R. Intestinal smooth muscle dysfunction develops postnatally in cystic fibrosis mice. J Pediatr Gastroenterol Nutr. 2012;55(6):689–694. doi: 10.1097/MPG.0b013e3182638bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canning B.J., Chang A.B., Bolser D.C. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest. 2014;146(6):1633–1648. doi: 10.1378/chest.14-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith J.A. Cough frequency and patterns of cough in cystic fibrosis. J R Soc Med. 2006;99(Suppl 46):17–22. [PubMed] [Google Scholar]
- 97.Hamutcu R., Francis J., Karakoc F., Bush A. Objective monitoring of cough in children with cystic fibrosis. Pediatr Pulmonol. 2002;34(5):331–335. doi: 10.1002/ppul.10174. [DOI] [PubMed] [Google Scholar]
- 98.Smith J.A., Owen E.C., Jones A.M., Dodd M.E., Webb A.K., Woodcock A. Objective measurement of cough during pulmonary exacerbations in adults with cystic fibrosis. Thorax. 2006;61(5):425–429. doi: 10.1136/thx.2005.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang A.B., Phelan P.D., Sawyer S.M., Del Brocco S., Robertson C.F. Cough sensitivity in children with asthma, recurrent cough, and cystic fibrosis. Arch Dis Child. 1997;77(4):331–334. doi: 10.1136/adc.77.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Canning B.J. Central regulation of the cough reflex: therapeutic implications. Pulm Pharmacol Ther. 2009;22(2):75–81. doi: 10.1016/j.pupt.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xue R., Gu H., Qiu Y. Expression of cystic fibrosis transmembrane conductance regulator in ganglia of human gastrointestinal tract. Sci Rep. 2016;6:30926. doi: 10.1038/srep30926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schriemer D., Sribudiani Y., IJpma A. Regulators of gene expression in Enteric Neural Crest Cells are putative Hirschsprung disease genes. Dev Biol. 2016;416(1):255–265. doi: 10.1016/j.ydbio.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 103.Rogers C.S., Stoltz D.A., Meyerholz D.K. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun X., Sui H., Fisher J.T. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou L., Dey C.R., Wert S.E., DuVall M.D., Frizzell R.A., Whitsett J.A. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266(5191):1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 106.van der Doef H.P., Kokke F.T., van der Ent C.K., Houwen R.H. Intestinal obstruction syndromes in cystic fibrosis: meconium ileus, distal intestinal obstruction syndrome, and constipation. Curr Gastroenterol Rep. 2011;13(3):265–270. doi: 10.1007/s11894-011-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ianowski J.P., Choi J.Y., Wine J.J., Hanrahan J.W. Mucus secretion by single tracheal submucosal glands from normal and cystic fibrosis transmembrane conductance regulator knockout mice. J Physiol. 2007;580(Pt 1):301–314. doi: 10.1113/jphysiol.2006.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joo N.S., Cho H.J., Khansaheb M., Wine J.J. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest. 2010;120(9):3161–3166. doi: 10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salinas D., Haggie P.M., Thiagarajah J.R. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J. 2005;19(3):431–433. doi: 10.1096/fj.04-2879fje. [DOI] [PubMed] [Google Scholar]
- 110.Wine J.J. Parasympathetic control of airway submucosal glands: central reflexes and the airway intrinsic nervous system. Auton Neurosci. 2007;133(1):35–54. doi: 10.1016/j.autneu.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moran A., Brunzell C., Cohen R.C. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–2708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marshall B.C., Butler S.M., Stoddard M., Moran A.M., Liou T.G., Morgan W.J. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005;146(5):681–687. doi: 10.1016/j.jpeds.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 113.Kerem E., Viviani L., Zolin A. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS patient registry. Eur Respir J. 2014;43(1):125–133. doi: 10.1183/09031936.00166412. [DOI] [PubMed] [Google Scholar]
- 114.Moran A., Dunitz J., Nathan B., Saeed A., Holme B., Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yi Y., Norris A.W., Wang K. Abnormal glucose tolerance in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2016;194(8):974–980. doi: 10.1164/rccm.201512-2518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olivier A.K., Yi Y., Sun X. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122(10):3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peters A., Schweiger U., Fruhwald-Schultes B., Born J., Fehm H.L. The neuroendocrine control of glucose allocation. Exp Clin Endocrinol Diabetes. 2002;110(5):199–211. doi: 10.1055/s-2002-33068. [DOI] [PubMed] [Google Scholar]
- 118.Rosario W., Singh I., Wautlet A. The brain-to-pancreatic islet neuronal map reveals differential glucose regulation from distinct hypothalamic regions. Diabetes. 2016;65(9):2711–2723. doi: 10.2337/db15-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]