Abstract
Malignant mesothelioma presenting with recurrent chylous effusion is rare. We describe the case of a 34‐year‐old female Macedonian immigrant who presented with central chest pain and subsequently a left‐sided chylous pleural effusion. The diagnosis was made on pleural biopsy via video‐assisted thoracoscopic surgery (VATS). Our case demonstrates the utility of thoracic magnetic resonance imaging (MRI) and the difficulties associated with pleural cytology and cervical lymph node biopsy in the establishment of a diagnosis of mesothelioma. It is a reminder that mesothelioma can metastasize to mediastinal and cervical lymph nodes, can occur in young people, and may present as a chylothorax.
Keywords: Chylothorax, malignant pleural mesothelioma
Introduction
Chyle is a fluid with a milky appearance and is composed of lymph and chylomicrons. Chylothorax refers to the accumulation of chyle in the pleural space due to obstruction or pathology of the thoracic duct or its tributaries. Chylothorax has numerous aetiologies, which may be divided into traumatic and non‐traumatic categories. Lymphoma is reported to be the most common cause of non‐traumatic chylothorax 1.
Case Report
A 34‐year‐old woman presented for evaluation of central chest pain followed by a large left‐sided pleural effusion causing pleurisy and dyspnoea. Her past medical history included autoimmune hepatitis, premature ovarian failure, and possible myelodysplastic disease with Trisomy 8 on bone marrow examination. She gave no history of trauma, no family history of lymphatic disorder, and there was no evidence of yellow nail syndrome. She was born in Macedonia, emigrated to Australia at the age of 20, and was a lifelong non‐smoker. The effusion was treated with therapeutic drainage with the temporary intercostal catheter and fluid with a milky appearance was visualized. Analysis of pleural fluid demonstrated chylomicrons, reactive mesothelial cells, and negative microbiology. Initial chest computed tomography (CT) scan demonstrated normal pulmonary parenchyma. There was no pleural thickening, mass, or nodules. Over the next year, she presented on three more occasions for drainage of left‐sided chylous pleural effusions. In order to further elucidate the cause of her pathology she underwent a number of imaging investigations. Lower limb lymphoscintogram demonstrated delayed movement on the right and accumulation of tracer in the abnormal soft tissue in the left superior mediastinal area. Positron emission tomography (PET)/CT scan demonstrated multiple enlarged superior mediastinal lymph nodes anterior to the great vessels of the neck and lateral to the aortic arc, measuring up to 15 mm in size with abnormal uptake in the mediastinal nodes and in the lower neck soft tissue masses. Mediastinal magnetic resonance imaging (MRI) demonstrated a complex multi‐lobulated high T2‐weighted structure extending from the anterior triangles of the neck down to the superior mediastinum where it surrounded the great vessels (Fig. 1). Initially, this appearance was thought to be consistent with a congenital lymphatic malformation and fine‐needle aspiration biopsy of a left‐sided cervical lymph node demonstrated benign mesothelial cells. Mediastinal inflow obstruction caused thrombosis of the proximal left subclavian vein and left internal jugular vein requiring therapeutic anticoagulation. Superior vena cava syndrome ensued, manifested as persistent facial oedema and dilated veins over the anterior chest wall. To obtain a diagnosis, she underwent excisional biopsy of the left supraclavicular lymph node which was complicated by the development of a lymphocoele. Histopathology of the node demonstrated atypical reactive mesothelial cells. Progress MRI scans of the chest demonstrated a large thoracic and neck complex lymphangioma with left‐sided chylothorax. Eighteen months after her initial presentation, she was admitted to hospital, with a persistent left‐sided pleural effusion. She had been compliant with a very low fat diet at home and subcutaneous octreotide to reduce chyle flow. On this occasion, the pleural fluid did not appear chylous, and there were no chylomicrons present. After drainage, the plain chest X‐ray demonstrated a pleural‐based soft tissue mass at the apex of the left thoracic cavity (Fig. 2). She then underwent a video‐assisted thoracoscopic (VAT) pleural biopsy and pleurodesis. Biopsies of the pleura demonstrated invasive epithelioid malignant mesothelioma.
Figure 1.
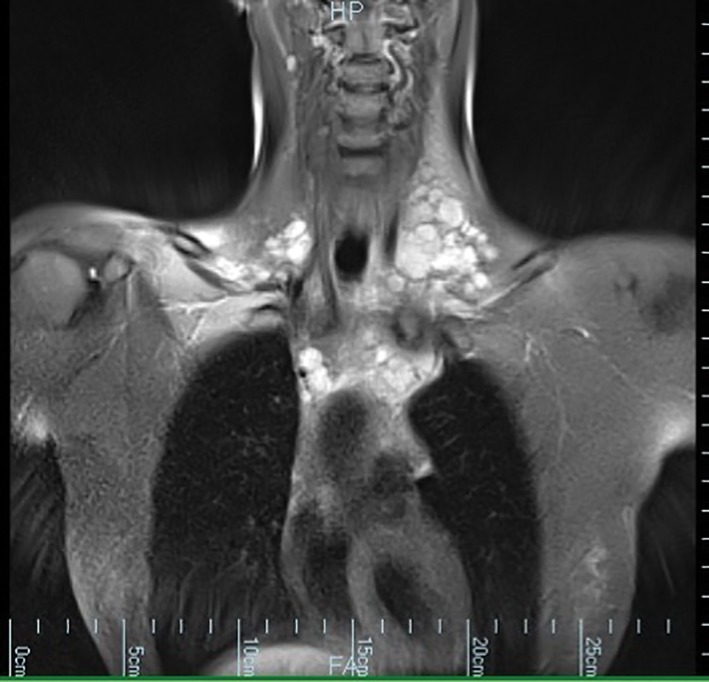
Magnetic resonance imaging (MRI) of the mediastinum demonstrated a complex multi‐lobulated high T2‐weighted structure extending from the anterior triangles of the neck down to the superior mediastinum where it surrounded the great vessels.
Figure 2.
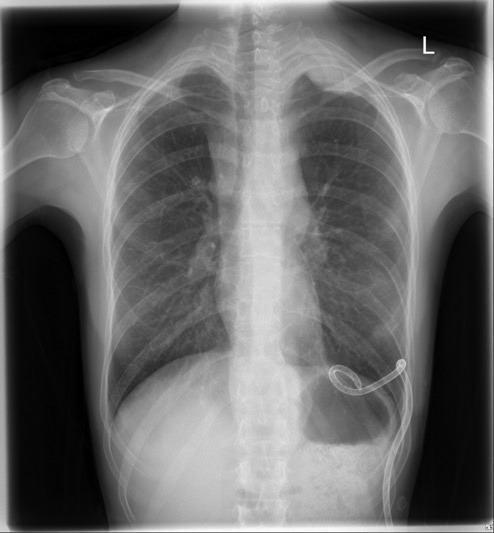
Plain posterior–anterior (PA) chest X‐ray demonstrated a pleural‐based soft tissue mass at the apex of the left thoracic cavity.
Discussion
Malignant pleural mesothelioma (MPM) is rare and our case demonstrates the challenges in establishing a diagnosis. Recurrent chylothorax and an absence of pleural involvement at initial imaging were unusual. The large complex thoracic lymphatic structure on imaging was a distraction but likely represented metastatic disease. Chylothorax as a first presentation of malignant mesothelioma is rare and there have been only four published case reports to our knowledge 2, 3, 4, 5. Bilateral chylothorax was the initial manifestation in two of these cases. Chylothorax is, however, a recognized post‐operative complication following extrapleural pneumonectomy for MPM with a rate of 2.9–7.9% 6, 7. Presumably, the malignancy spread through the mediastinal lymphatic structures and caused mechanical obstruction to lymphatic flow at a range of anatomical sites. The lymphatic structures distal to these sites of obstruction became swollen and enlarged due to accumulation of lymph. This gave the MRI appearance of extensive, ill‐defined, low attenuating soft tissue lesions within the mediastinum. The rate of lymphatic leak into the pleural space overwhelmed the clearance mechanisms of this anatomical site. The left subclavian vein thrombosis and superior vena cava syndrome is another potential mechanism of chylothorax. The diagnosis of chylothorax in this case, however, was made one year prior to the development of the clinical signs of superior vena cava syndrome. In our opinion, the left subclavian vein thrombosis may have contributed to the persistence of chylothorax but was not the primary aetiologic factor. This case is a reminder that MPM should be considered in the differential diagnosis of chylothorax.
Mesothelioma remains a problematic pathologic diagnosis. Cytology of pleural fluid was negative on multiple occasions in our patient and is reported to provide a diagnosis of MPM in up to only 32% of cases 8. Excisional cervical lymph node biopsy demonstrated atypical mesothelial cells which increased our clinical suspicion but was not diagnostic for mesothelioma. MPM has rarely been diagnosed on cervical lymph node biopsies. Chylous leakage is a common complication after neck dissection as in our case. In this case, the presence of distal dilated lymphatic structures in the mediastinum may have been a predictor of an increased risk of the development of a more proximal lymphocoele. Therefore, an individualized and robust risk–benefit analysis should be conducted prior to excisional lymph node biopsy.
Asbestos is the predominant aetiologic factor in MPM. There is significant regional variability in the incidence of mesothelioma related to the prevalence of asbestos utilization 9. Development of MPM in our patient was most likely related to domestic rather than occupational asbestos exposure. She had emigrated from Macedonia where she lived in the town of Ohrid in a fairly new apartment. She did visit relatives who lived in smaller white‐washed buildings in nearby towns. High concentrations of chrysotile and tremolite asbestos fibres were found in the “white stones” which were used to white‐wash houses in central Macedonia, Northern Greece 10. Asbestos incorporation as a component of insulation in schools and in building materials was previously common in Macedonia. The lead time to the development of mesothelioma was unusually short in our patient and this serves as a reminder that the disease can occur in young people.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Darley, D.R. , Granger, E. and Glanville, A.R. (2017) Chest pain and recurrent chylothorax: an unusual presentation of malignant pleural mesothelioma. Respirology Case Reports, 5 (5), e00250. doi: 10.1002/rcr2.250.
Associate Editor: Sjaak Burgers
References
- 1. Doerr CH, Allen MS, and Nichols FC. 2005. Etiology of chylothorax is 203 patients. Mayo Clin. Proc. 80(7):867. [DOI] [PubMed] [Google Scholar]
- 2. Kostiainen S, Meurala H, Mattila S, et al. 1983. Chylothorax: clinical experience in nine cases. Scand. J. Thorac. Cardiovasc. Surg. 17(1):79–83. [DOI] [PubMed] [Google Scholar]
- 3. Ito S, Takashima Y, Sano T, et al. 2001. Chylothorax as the initial manifestation of malignant pleural mesothelioma—a case report. Nihon Kokyuki Gakkai Zasshi 39(10):775–780. [PubMed] [Google Scholar]
- 4. Shimizudani N, Morisako T, Miyao Y, et al. 2002. A case of diffuse malignant mesothelioma of the pleura with bilateral chylothorax. Nihon Kokyuki Gakkai Zasshi 40(10):832–836. [PubMed] [Google Scholar]
- 5. Kurtipek E, Eren Karanis Mİ, Düzgün N, et al. 2015. A cause of bilateral chylothorax: a case of mesothelioma without pleural involvement during initial diagnosis. Case Rep. Pulmonol. 2015:962504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Opitz I, Kestenholz P, Lardinois D, et al. 2006. Incidence and management of complications after neoadjuvant chemotherapy followed by extrapleural pneumonectomy for malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 29(4):579–584. [DOI] [PubMed] [Google Scholar]
- 7. Lauk O, Hoda MA, de Perrot M, et al. 2014. Extrapleural pneumonectomy after induction chemotherapy: perioperative outcome in 251 mesothelioma patients from three high‐volume institutions. Ann. Thorac. Surg. 98(5):1748–1754. [DOI] [PubMed] [Google Scholar]
- 8. Renshaw AA, Dean BR, Antman KH, et al. 1997. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 111:106–109. [DOI] [PubMed] [Google Scholar]
- 9. Robinson BM. 2012. Malignant pleural mesothelioma: an epidemiological perspective. Ann. Cardiothorac. Surg. 1:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sichletidis L, Daskalopoulou E, Chloros D, et al. 1992. Pleural plaques in a rural population in central Macedonia, Greece. Med. Lav. 83(3):259–265. [PubMed] [Google Scholar]


