Abstract
The term ‘visceral fascia’ is a general term used to describe the fascia lying immediately beneath the mesothelium of the serosa, together with that immediately surrounding the viscera, but there are many types of visceral fasciae. The aim of this paper was to identify the features they have in common and their specialisations. The visceral fascia of the abdomen (corresponding to the connective tissue lying immediately beneath the mesothelium of the parietal peritoneum), thorax (corresponding to the connective tissue lying immediately beneath the mesothelium of the parietal pleura), lung (corresponding to the connective tissue under the mesothelium of the visceral pleura), liver (corresponding to the connective tissue under the mesothelium of the visceral peritoneum), kidney (corresponding to the Gerota fascia), the oesophagus (corresponding to its adventitia) and heart (corresponding to the fibrous layer of the pericardial sac) from eight fresh cadavers were sampled and analysed with histological and immunohistochemical stains to evaluate collagen and elastic components and innervation. Although the visceral fasciae make up a well‐defined layer of connective tissue, the thickness, percentage of elastic fibres and innervation vary among the different viscera. In particular, the fascia of the lung has a mean thickness of 134 μm (± 21), that of heart 792 μm (± 132), oesophagus 105 μm (± 10), liver 131 μm (± 18), Gerota fascia 1009 μm (± 105) and the visceral fascia of the abdomen 987 μm (± 90). The greatest number of elastic fibres (9.79%) was found in the adventitia of the oesophagus. The connective layers lying immediately outside the mesothelium of the pleura and peritoneum also have many elastic fibres (4.98% and 4.52%, respectively), whereas the pericardium and Gerota fascia have few (0.27% and 1.38%). In the pleura, peritoneum and adventitia of the oesophagus, elastic fibres form a well‐defined layer, corresponding to the elastic lamina, while in the other cases they are thinner and scattered in the connective tissue. Collagen fibres also show precise spatial organisation, being arranged in several layers. In each layer, all the fibrous bundles are parallel with each other, but change direction among layers. Loose connective tissue rich in elastic fibres is found between contiguous fibrous layers. Unmyelinated nerve fibres were found in all samples, but myelinated fibres were only found in some fasciae, such as those of the liver and heart, and the visceral fascia of the abdomen. According to these findings, we propose distinguishing the visceral fasciae into two large groups. The first group includes all the fasciae closely related to the individual organ and giving shape to it, supporting the parenchyma; these are thin, elastic and very well innervated. The second group comprises all the fibrous sheets forming the compartments for the organs and also connecting the internal organs to the musculoskeletal system. These fasciae are thick, less elastic and less innervated, but they contain larger and myelinated nerves. We propose to call the first type of fasciae ‘investing fasciae’, and the second type ‘insertional fasciae’.
Keywords: elastic lamina, Gerota fascia, pericardium, peritoneum, serous membrane, visceral fascia, visceral manipulation
Introduction
The Terminologia Anatomica (1998) defines the visceral fasciae as “a generic term for the fascia which lies immediately outside the visceral layer of the serosae together with that which immediately surrounds the viscera” (Table 1). Willard (2012), reviewing the previous classification by Hollinshead (1961), distinguishes three different layers of visceral fasciae: (i) those forming neurovascular sheaths; (ii) those surrounding individual organs; and (iii) those underlying pleural and peritoneal linings. In the last few years, interest in them has been renewed, mainly in the field of surgery (Puntambekar et al. 2015), but also in physiotherapy and osteopathic medicine (Barral, 2005; Hedley, 2006; Paoletti, 2006; Bove & Chapelle, 2012; Stecco & Stecco, 2012). Accordingly, in the normal healthy state, the visceral fasciae are relaxed and can stretch and move without restriction, but physical trauma, scarring, infection or inflammation can alter their pliability and they may become tight, producing pain or restriction of motion of the organs. Manual therapies describe the elasticity of the visceral fasciae as regards their capacity to transmit forces and their possibility of causing pain. In fact, very little is known about them, and we still lack an overall view. Many authors have described an elastic lamina in the pleura (Gallagher & Urbanski, 1990; Michailova, 1996a,b, 1997; Mariassy & Wheeldon, 1983; Kagramanov et al. 1998), peritoneum (Knudsen, 1991) and pericardium (Ishihara et al. 1980; Kagramanov et al. 1998; Braga‐Vilela & De Campos Vidal, 2006). Kai et al. (2000) also described an elastic lamina in rat spleen, suggesting that it may play a role in the contraction of the whole spleen, but were unable to identify the same lamina in guinea‐pig, mouse or dog (Kai et al. 2000). Some elastic fibres have also been described in the hepatic capsule (Watanabe & Nishizono, 1994), arranged as thin threads in a dense mesh. Michailova (1996a,b) compared various visceral fasciae, and demonstrated that they show significant differences in the differing organs and regions. For example, the elastic membrane under the basal lamina is definitely an obligatory component of the visceral pleura and spleen capsule, whereas in the other visceral fasciae, elastic fibres are visible only singly. The first aim of this study was therefore to clarify whether the visceral fasciae are really elastic and whether differences occur in their elastin component.
Table 1.
Definitions of various visceral fasciae mentioned in this paper and corresponding terms according to Terminologia Anatomica (1998)
| Terms used in the paper | Terminologia Anatomica (1998) | Definition |
|---|---|---|
| Visceral fasciae | Internal fascia | “A generic term for the fascia which lies immediately outside the visceral layer of the serosae together with that which immediately surrounds the viscera” (from the Terminologia Anatomica, 1998) |
| Visceral fascia of abdomen | Visceral abdominal fascia, subserosal layer | Connective tissue lying immediately beneath the mesotelium of the parietal peritoneum |
| Visceral fascia of thorax | Parietal subserosal layer | Connective tissue lying immediately beneath the mesotelium of the parietal pleura |
| Lung fascia | Visceral subserosal layer | Connective tissue lying immediately beneath the mesotelium of the visceral pleura |
| Liver fascia | Subserosa of liver | Connective tissue lying immediately beneath the mesotelium of the visceral peritoneum |
| Kidney fascia | Fascia renalis, Gerota fascia | Zone of dense endoabdominal fascia |
| Oesophagus fascia | Tunica adventitia of oesophagus | It is the outermost connective tissue layer covering the oesophagus |
| Heart fascia | Pericardium fibrosus | Corresponding to the fibrous layer of the pericardial sac |
A second point regards the organisation of collagen fibre in the visceral fasciae. Watanabe & Nishizono (1994), describing the hepatic capsule, stated that collagen fibres were arranged in thick bundles extending in various directions to form rough meshes, whereas Kai et al. (2000), examining the splenic capsule, described two different fibrous layers, in which the components were arranged more densely in the external than in the internal layer.
The third element to clarify concerns innervation. Although the fasciae are considered to give rise to pain, it is unknown whether they are all innervated and, if so, what type of innervation they have (Pintelon et al. 2007). The parietal peritoneum is known to have the same somatic nerve supply as the region of the abdominal wall that it lines, so that pain from the parietal peritoneum is clearly localised and sensitive to pressure, pain, laceration and temperature. Instead, pain from the visceral peritoneum is carried in visceral afferent fibres that course along with autonomic fibres, and it is consequently poorly localised and sensitive only to stretching and chemical irritation (Standring et al. 2009; Tanaka et al. 2011). In several cases, the exact type of innervation of many visceral fasciae is unknown.
The aim of this study was therefore to understand the common features and specialisations of these fasciae.
Materials and methods
Macroscopic study
Anatomical studies (approved by the local ethical committee) were performed on eight fresh cadavers (four men, four women; age range at death: 47–87 years), managed by the ‘Body Donation Program’ at the Institute of Anatomy, University of Padova (de Caro et al. 2009; Macchi et al. 2011; Porzionato et al. 2012).
From each cadaver, the following samples of visceral fasciae were removed:
the heart fascia (corresponding to the fibrous layer of the pericardial sac; Fig. 1A);
the liver fascia (= the connective tissue lying immediately outside the mesothelium of the visceral peritoneum; Fig. 1B);
the visceral fascia of the abdomen (= the connective tissue lying immediately outside the mesothelium of the parietal peritoneum; Fig. 1C);
the oesophagus fascia (corresponding to its adventitia; Fig. 1D);
the visceral fascia of the thorax (= the connective tissue lying immediately outside the mesothelium of the parietal pleura; Fig. 1E);
the lung fascia (= the connective tissue lying immediately outside the mesothelium of the visceral pleura; Fig. 1F);
the kidney fascia (= the Gerota fascia; Fig. 1G).
Figure 1.
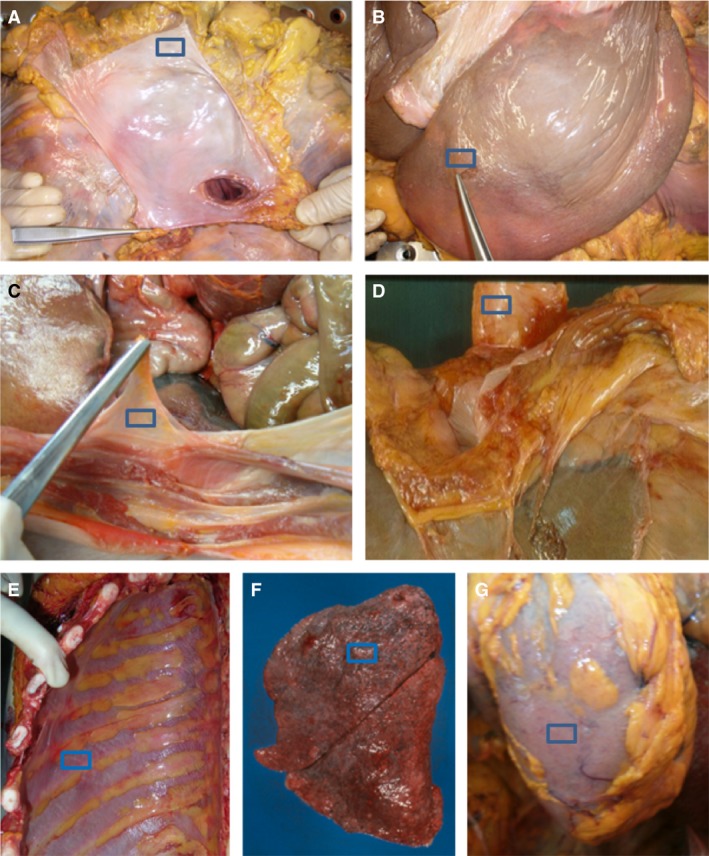
Dissection of pericardial sac (A), liver fascia (B), visceral fascia of abdomen (C), esophagus fascia (D), visceral fascia of thorax (E), lung fascia (F) and kidney fascia (G).
Histological analyses
In each of the eight fresh cadavers, samples of visceral fasciae of approximately 3 × 2 cm2 were taken. Each specimen was carefully oriented, mounted on cardboard to avoid deformation artefacts, and stored in neutral 10% formalin for 24 h. Then, after fixation, each specimen was divided into two: one part was embedded in paraffin wax, with the entire surface carefully oriented parallel to the plane of the deep fascia; the second was oriented perpendicularly. Sections 5 μm thick were obtained from each sample. Haematoxylin and eosin, azan‐Mallory and Weigert‐van Gieson histological stainings were carried out.
Immunohistochemistry
Immunohistochemical tests were carried out with the rabbit poly‐clonal anti‐βIII tubulin antibody (Couvance, Milan, Italy), dilution 1 : 5000, to identify neurofilaments, and with the rabbit poly‐clonal anti‐S100 (Dako, Milan, Italy), dilution 1 : 5000, to identify nerve structures in the fascia. The sections had previously been unmasked with high pH pre‐treatment and incubated with the DAKO Autostainer System. Sections incubated without primary antibodies showed no immunoreactivity, confirming the specificity of the immunostaining.
Morphometric analyses
Random images of 15 sections per sample, oriented parallel or perpendicular to the fascial plane, were analysed to measure the total thickness of each layer, the percentage of fascial elastic component (violet‐stained with Weigert‐van Gieson) and percentage of S100 and βIII‐tubulin immunopositivity, with image j 1.6.0 software (National Institutes of Health, USA), freely available at http://rsb.info.nih.gov/ij/, and image analysis procedures previously described by our group (Porzionato et al. 2005; Guidolin et al. 2014).
The content of elastic fibres was evaluated in terms of percentual areas stained in violet. The characteristics of the images, in terms of hue, brightness and saturation, were evaluated in histograms. The violet‐coloured intervals corresponding to positive reactions were identified and used to convert the colour images into eight‐bit black‐and‐white images. The percentage of the elastic component was then automatically measured by the software. Similarly, the contents of S100‐ and βIII‐tubulin‐positive cells were evaluated in terms of percentual areas stained in brown, respectively. Immunopositive cells were also counted. The same procedure was used to count the immunonegative cells for blue colour intervals. The percentage of immunopositive cells was then measured, the number of brown cells being divided by the number of blue cells.
Lastly, random images of 15 sections of all samples oriented parallel to the plane of the fascia were analysed, to evaluate the angle of incidence between adjacent fascial sheets.
Results
Macroscopic observations
Macroscopically, the visceral fasciae appeared to be whitish, shiny and translucent, forming a protective layer on the surface of various organs, but some specialisations were already evident. The visceral fascia of the abdomen, corresponding to the connective tissue lying immediately beneath the mesothelium of the parietal peritoneum, can easily be separated from the muscular fascia of the transversus abdominis muscle, thanks to the presence of loose connective tissue between the fasciae, whereas the visceral fascia of the thorax cannot be isolated from the muscular fascia of the intercostal muscle, but the two are fused, forming the endothoracic fascia. Consequently, by ‘visceral fascia of the thorax’, we mean all the connective tissue lying immediately beneath the mesothelium of the parietal pleura as far as the internal surface of the rib cage. The Gerota fascia appears as a thick fibrous layer, easily identifiable thanks to the surrounding fat tissue, whereas the oesophagus and liver fasciae appear as thin layers of connective tissue, adhering completely to the underlying organs.
Microscopic and morphometric evaluation
All the visceral fasciae are well‐defined layers of connective tissue arranged in bundles of collagen fibres with a certain number of elastic fibres. Fascial thickness varies: in lung, it has a mean thickness of 134 μm (± 21), in heart 792 μm (± 132), oesophagus 105 μm (± 10), liver 131 μm (± 18), kidney 1009 μm (± 105) and the visceral fascia of the abdomen 987 μm (± 90). The mean thicknesses of all the fasciae are listed in Fig. 2, except for the parietal pleura, because its borders were not clear, due to its fusion with the muscular fascia of the intercostal muscles.
Figure 2.
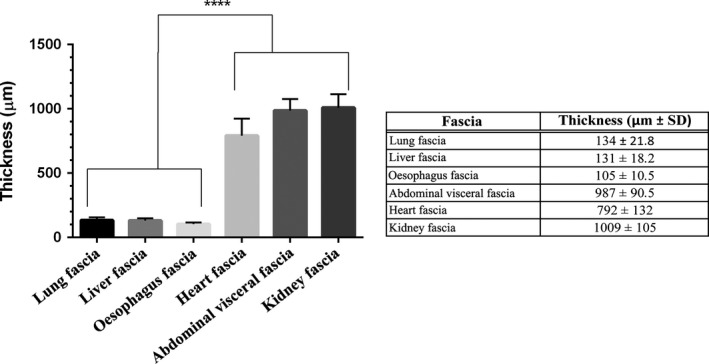
Full wall thickness of various visceral fasciae. Graph shows statistically significant differences (****P < 0.0001); table lists mean thickness of various fasciae in μm.
In all the sections of visceral fasciae, Weigert‐van Gieson staining identified a network of elastic fibres (Fig. 3). The highest numbers of such fibres (9.79%) were found in the oesophagus fascia. The connective tissue lying immediately outside the mesothelium of the serosa also have many elastic fibres (4.98% and 4.52%, respectively), although the heart and kidney fasciae have few (0.27% and 1.38%). The elastic fibres form a well‐defined layer in the visceral fasciae of abdomen and thorax, and in those of liver, lung and oesophagus, corresponding to the elastic lamina already described in the literature (Fig. 4). In the other cases, the lamina is not so easy to identify, the fibres being thinner and interspersed in the connective tissue.
Figure 3.

Percentage of elastic fibres in human visceral fasciae. Graph shows statistically significant difference among fasciae (****P < 0.0001); table lists mean percentage of elastic fibres in various visceral fasciae.
Figure 4.
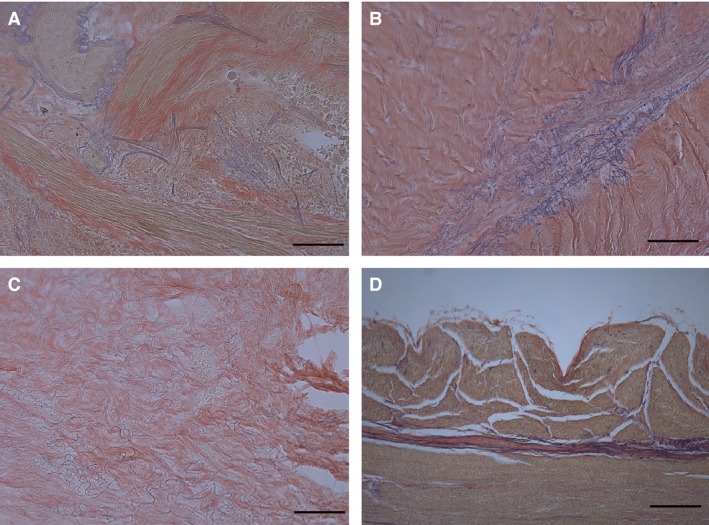
Weigert‐van Gieson stains of various fasciae to show arrangement of elastic fibres: lung fascia (A), visceral fascia of abdomen (B), liver fascia (C), oesophagus fascia (D). Scale bars: 150 μm (A–D). In visceral fascia of abdomen, elastic fibres are mainly found in loose connective tissue between fibrous sublayers.
Collagen fibres also have precise spatial organisation. Figure 5 shows that the bundles of collagen fibres are organised in several layers, all parallel with each other (Fig. 5D), whereas their direction changes among layers, generally forming an angle of about 54 ° (Fig. 5C). Loose connective tissue rich in elastic fibres is found between contiguous fibrous layers. In the heart and kidney fasciae, elastic fibres appear above all in this loose connective tissue; they are almost absent in the fibrous layers.
Figure 5.
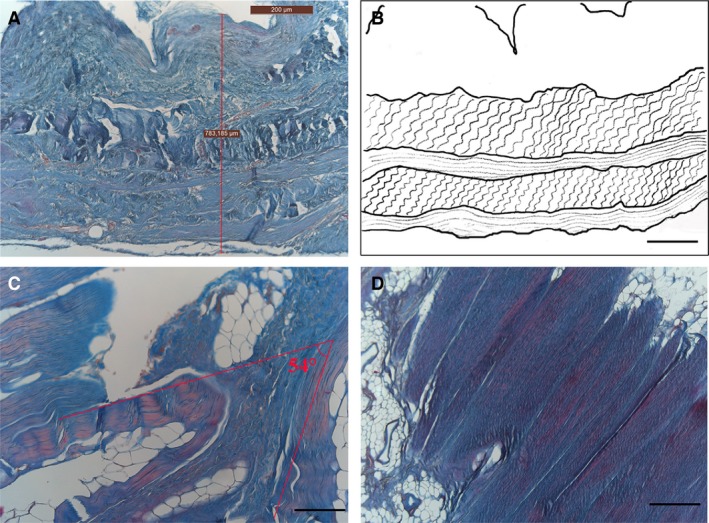
Arrangement of collagen fibres in visceral fasciae, azan‐Mallory stain (A, C, D). (A) Full wall thickness of pericardial sac: note several fibrous sublayers. (B) Diagram of collagen arrangement. (C) Visceral fascia of thorax: note angle of approximately 54 ° between adjacent fibrous sublayers forming (C). Section of a sublayer of visceral fascia of abdomen, showing how collagen fibres are all arranged in the same parallel direction (D). Scale bars: 300 μm (A); 150 μm (B–D).
In all samples, immunohistochemical analysis showed a positive reaction to βIII‐tubulin antibody, which is the major component of neuronal microtubules, indicating the existence of neurofilaments inside all the visceral fasciae. These data were supported by morphometric analyses, which revealed a mean percentage of positive cells of 16.8%. Instead, S100 immunohistochemical analysis gave a positive reaction only in some specimens, such as liver and heart fasciae and the visceral fascia of the abdomen (Fig. 6). Morphometric analyses indicated a mean percentage of 2.3% of positive cells.
Figure 6.
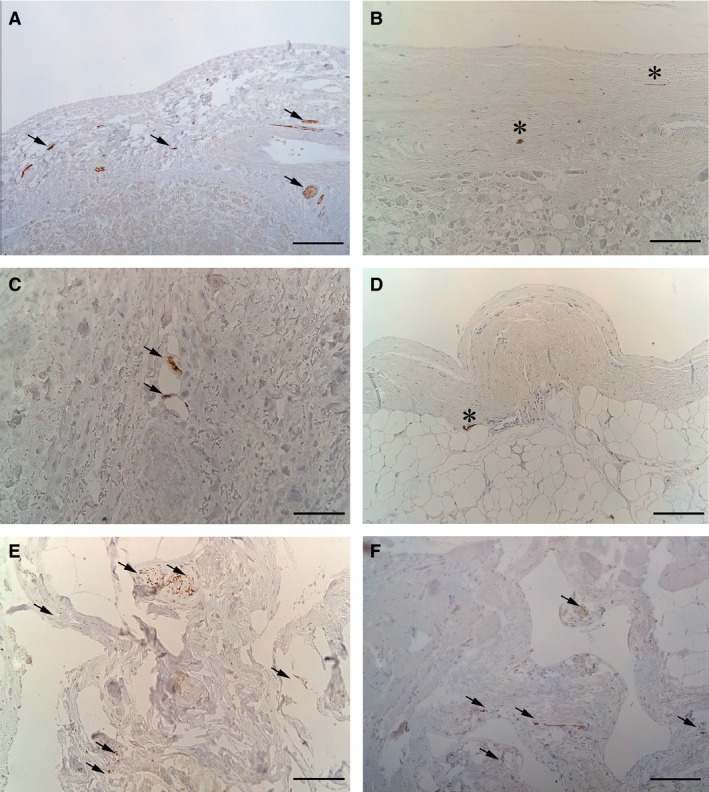
Immunohistochemical anti‐βIII‐tubulin reactions (arrows: A, C, E, F) and anti‐S100 (stars: B, D) in human visceral fasciae: liver fascia (A,B), heart fascia (C,D), renal fascia (E), visceral fascia of thorax (F). Scale bars: 150 μm (A, C, E, F); 75 μm (B, D).
Discussion
Dissections revealed that two different types of fasciae may be identified in each organ: the first is a thin fascia surrounding the organ and closely adhering to it, as in the liver, lung and oesophagus fasciae; the second is composed of a thicker fascia, not usually adhering to the organ, which acts as the compartment for the various organs and which is connected in various ways to the parietal wall. The fibrous layers of the pericardial sac, renal fascia, and the visceral fasciae of the abdomen and thorax belong to this group. Morphometric analyses revealed a statistically significant difference in the thicknesses between the fasciae of the groups, in that those of the second group are thicker (929.3 ± 145.2 μm vs. 123.3 ± 21.56 μm, P < 0.0001; Fig. 7). These two groups were also distinguished by their elastic component: elastic fibres were more abundant in the fasciae of the first group (5.768 ± 1.88%) than in the second (1.371 ± 0.98%, P < 0.0001; Fig. 8). This distribution of fibres is probably due to the functional and mechanical features of the fasciae: those adhering to organs and viscera have to support their physiological compliance and movements, whereas the other visceral fasciae define specific compartments and maintain vital space round the organs. Differences among the visceral fasciae in terms of elastic properties and composition have already been suggested in experimental animal models, such as the visceral pleura of mice (Nomura et al. 1998), sheep (Mariassy & Wheeldon 1983) and cats (Michailova, 1996a,b). Ozdogan et al. (2006) also demonstrated that the percentage of elastic and collagen fibres in the transversalis fascia and peritoneum changes in patients with inguinal hernia, in comparison with a control group.
Figure 7.
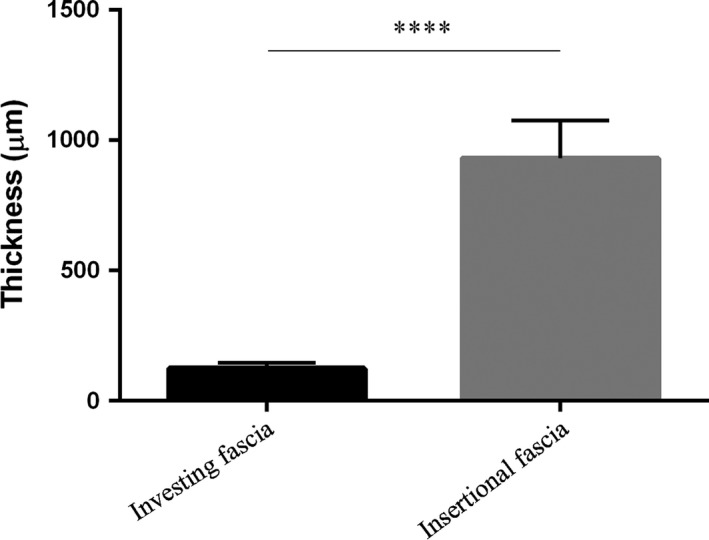
Comparison of total wall thickness of investing and insertional fasciae, showing statistically significant difference between groups (****P < 0.0001).
Figure 8.
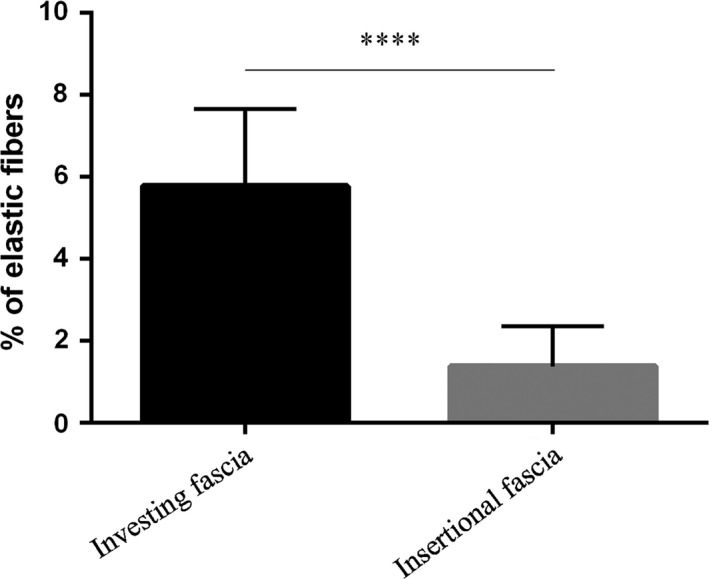
Comparison of percentage of elastic fibres in investing and insertional fasciae, showing statistically significant difference (****P < 0.0001).
In all the visceral fasciae, we identified the same sublayer organisation described for the muscular fasciae (Purslow, 2010; Benetazzo et al. 2011; Tesarz et al. 2011). That is, they are formed of two or three layers of fibrous collagen bundles. The fibres are oriented in the same direction in each layer, but the direction changes in the adjacent layer, forming a mean angle of 54 °. The layers are separated by loose connective tissue, permitting gliding and autonomy among the various sublayers.
Nerves were found in all samples, but were more numerous in the βIII‐tubulin‐positive cells (16.8% vs. 2.3%) and mainly in fasciae adhering to organs and viscera; S100‐positive nerve cells were found more frequently in the thicker fasciae. βIII‐tubulin highlights unmyelinated nervous fibres and S100 myelinated ones. This finding is probably due to the fact that it is known (Standring et al. 2009) that fasciae close to organs have only autonomic innervation, whereas the visceral fasciae of abdomen and thorax also have sensitive, somatic innervation. Further studies will be necessary to evaluate the autonomic and somatic innervation of the visceral fasciae.
Conclusions
According to the above findings, we propose distinguishing the visceral fasciae into two large groups. The first comprises all the fasciae closely related to organs and giving form to them, supporting the parenchyma. They are thin, elastic and contain many nerve fibres, probably from the autonomic nervous system. The second group comprises all the fibrous sheets forming organ compartments and also connecting internal organs to the musculoskeletal system. These fasciae are thick, less elastic and contain fewer nerve fibres, but they are larger and myelinated. We propose to call the first type of fasciae ‘investing fasciae’ and the second type ‘insertional fasciae’.
Authors’ contributions
Carla Stecco: dissections and histology, data interpretation, writing of text, revision. Sfriso MM: histology, writing of text. Porzionato A: data analysis. Macchi V: data interpretation. Albertin G: evaluation of histological samples. Rambaldo A: histological staining. De Caro R: critical revision and approval of manuscript.
References
- Barral JP (2005) Visceral Manipulation. Vista, CA: Eastland Press. [Google Scholar]
- Benetazzo L, Bizzego A, de Caro R, et al. (2011) 3D reconstruction of the crural and thoracolumbar fasciae. Surg Radiol Anat 33, 855–862. [DOI] [PubMed] [Google Scholar]
- Bove GM, Chapelle SL (2012) Visceral mobilization can lyse and prevent peritoneal adhesions in a rat model. J Bodyw Mov Ther 1, 76–82. [DOI] [PubMed] [Google Scholar]
- Braga‐Vilela AS, De Campos Vidal B (2006) Identification of elastic fibers and lamellae in porcine pericardium and aorta by confocal, fluorescence and polarized light microscopy. Acta Histochem 108, 125–132. [DOI] [PubMed] [Google Scholar]
- de Caro R, Macchi V, Porzionato A (2009) Promotion of body donation and use of cadavers in anatomical education at the University of Padova. Anat Sci Educ 2, 91–92. [DOI] [PubMed] [Google Scholar]
- Federative Committee on Anatomical Terminology (1998) Terminologia Anatomica. New York: Thieme Stuttgart. [Google Scholar]
- Gallagher B, Urbanski SJ (1990) The significance of pleural elastica invasion by lung carcinomas. Hum Pathol 21, 512–517. [DOI] [PubMed] [Google Scholar]
- Guidolin D, Porzionato A, Tortorella C, et al. (2014) Fractal analysis of the structural complexity of the connective tissue in human carotid bodies. Front Physiol 5, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley G (2006) The Integral Anatomy Series, Vol. 3: Cranial and Visceral Fasciae. Integral Anatomy Productions. Available from: http://integralanatomy.com/
- Hollinshead WH (1961) Anatomy for Surgeons: the Thorax, Abdomen and Pelvis, Vol. 2 New York: Paul B. Hoeber. [Google Scholar]
- Ishihara T, Ferrans VJ, Jones M, et al. (1980) Histologic and ultrastructural features of normal human parietal pericardium. Am J Cardiol 46, 744–753. [DOI] [PubMed] [Google Scholar]
- Kagramanov II, Kokshenev IV, Dobrova NB, et al. (1998) Comparative assessment of hepatic Glisson's capsule and bovine pericardium in heart valve bioprostheses. J Heart Valve Dis 7, 273–277. [PubMed] [Google Scholar]
- Kai T, Nishizono H, Kimura K (2000) Fiber arrangement of the rat splenic capsule with special reference to elastic fibers. Okajimas Folia Anat Jpn 77, 167–179. [DOI] [PubMed] [Google Scholar]
- Knudsen PJ (1991) The peritoneal elastic lamina. J Anat 177, 41–46. [PMC free article] [PubMed] [Google Scholar]
- Macchi V, Porzionato A, Stecco C, et al. (2011) Body parts removed during surgery: a useful training source. Anat Sci Educ 4, 151–156. [DOI] [PubMed] [Google Scholar]
- Mariassy AT, Wheeldon EB (1983) The pleura: a combined light microscopic, scanning, and transmission electron microscopic study in the sheep. I. Normal pleura. Exp Lung Res 4, 293–314. [DOI] [PubMed] [Google Scholar]
- Michailova KN (1996a) Development of the human fetal visceral pleura. An ultrastructural study. Ann Anat 178, 91–99. [DOI] [PubMed] [Google Scholar]
- Michailova KN (1996b) The serous membranes in the cat. Electron microscopic observations. Ann Anat 178, 413–424. [DOI] [PubMed] [Google Scholar]
- Michailova KN (1997) Ultrastructural observations on the human visceral pleura. Eur J Morphol 35, 125–135. [DOI] [PubMed] [Google Scholar]
- Nomura K, Kida K, Kudoh S (1998) A morphological study to elucidate the differences in visceral pleura in young and old mice. Nihon Ika Daigaku Zasshi 65, 227–235. [DOI] [PubMed] [Google Scholar]
- Ozdogan M, Yildiz F, Gurer A, et al. (2006) Changes in collagen and elastic fiber contents of the skin, rectus sheath, transversalis fascia and peritoneum in primary inguinal hernia patients. Bratisl Lek Listy 107, 235–238. [PubMed] [Google Scholar]
- Paoletti S (2006) The Fasciae: Anatomy, Dysfunction & Treatment, p. 146 Seattle, WA: Eastland Press. [Google Scholar]
- Pintelon I, Brouns I, de Proost I, et al. (2007) Sensory receptors in the visceral pleura: neurochemical coding and live staining in whole mounts. Am J Respir Cell Mol Biol 36, 541–551. [DOI] [PubMed] [Google Scholar]
- Porzionato A, Macchi V, Guidolin D, et al. (2005) Histopathology of carotid body in heroin addiction. Possible chemosensitive impairment. Histopathology 46, 296–306. [DOI] [PubMed] [Google Scholar]
- Porzionato A, Macchi V, Stecco C, et al. (2012) Quality management of Body Donation Program at the University of Padova. Anat Sci Educ 5, 264–272. [DOI] [PubMed] [Google Scholar]
- Puntambekar SP, Puntambekar SP, Gadkari Y, et al. (2015) Fascial anatomy and its relevance in safe laparoscopic hysterectomy. J Minim Invasive Gynecol 22, 1137. [DOI] [PubMed] [Google Scholar]
- Purslow PP (2010) Muscle fascia and force transmission. J Bodyw Mov Ther 14, 411–417. [DOI] [PubMed] [Google Scholar]
- Standring S, et al. (2009) Gray's Anatomy: the Anatomical Basis of Clinical Practice, 40th edn, 2, p. 1101 Edimburgh: Churchill Livingstone. [Google Scholar]
- Stecco C, Stecco L (2012) Fascial Manipulation for Internal Dysfunction. Padova: Piccin. [Google Scholar]
- Tanaka K, Hayakawa T, Maeda S, et al. (2011) Distribution and ultrastructure of afferent fibers in the parietal peritoneum of the rat. Anat Rec 294, 1736–1742. [DOI] [PubMed] [Google Scholar]
- Tesarz J, Hoheisel U, Wiedenhöfer B, et al. (2011) Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience 27, 302–308. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Nishizono H (1994) A scanning and transmission electron microscopic study of fiber arrangement in the hepatic capsule. Okajimas Folia Anat Jpn 71, 279–295. [DOI] [PubMed] [Google Scholar]
- Willard FH (2012) Visceral fascia Cap. 1.8. In: Fascia – the Tensional Network of the Human Body. (eds Schleip R, Findley TW, Chaitow L, Huijing P.), pp. 53–56. Edimburgh: Elsevier. [Google Scholar]


