Abstract
Despite the long‐standing interest in the evolution of the brain, relatively little is known about variation in brain anatomy in frogs. Yet, frogs are ecologically diverse and, as such, variation in brain anatomy linked to differences in lifestyle or locomotor behavior can be expected. Here we present a comparative morphological study focusing on the macro‐ and micro‐anatomy of the six regions of the brain and its choroid plexus: the olfactory bulbs, the telencephalon, the diencephalon, the mesencephalon, the rhombencephalon, and the cerebellum. We also report on the comparative anatomy of the plexus brachialis responsible for the innervation of the forelimbs. It is commonly thought that amphibians have a simplified brain organization, associated with their supposedly limited behavioral complexity and reduced motor skills. We compare frogs with different ecologies that also use their limbs in different contexts and for other functions. Our results show that brain morphology is more complex and more variable than typically assumed. Moreover, variation in brain morphology among species appears related to locomotor behavior as suggested by our quantitative analyses. Thus we propose that brain morphology may be related to the locomotor mode, at least in the frogs included in our analysis.
Keywords: anurans, central nervous system morphology, locomotion
Introduction
The general organization and structure of the motor control system is thought to be evolutionarily conserved among vertebrates. Indeed, the general ‘bauplan’ of the vertebrate nervous system is similar from the forebrain to the spinal cord across many taxa (Grillner et al. 2007). Five vesicles develop in the areas corresponding to the telencephalon, the diencephalon, the mesencephalon, and the rhombencephalon in early embryos, in addition to the cerebellum and the brain stem. The transition from fish to amphibians marks the beginning of the origin of tetrapods and the major adaptive radiation in vertebrate evolution. This went hand in hand with striking changes in brain morphology (Northcutt & Royce, 1975; Ten Donkelaar, 1998). Several features characterize the first vertebrate group to pass from an aquatic to a terrestrial mode of life. Most amphibians, and particularly anurans, are characterized by a larval period and a metamorphosis that changes many of the body parts, allowing them to survive in a terrestrial environment. The central nervous system of anurans undergoes intrinsic changes such as the development of an acoustic vocal system, a binocular visual system, and specializations for tetrapod limb movement. These critical changes are reflected in the anatomy of the brain and spinal cord.
Almost no variation in the gross anatomy of the brain has been reported in anurans. However, most of the studies have focused on ranid frogs, some species of toads, and Xenopus laevis, as summarized in the works of Gaupp (1896), Llinás & Precht (1976), and Ten Donkelaar (1998). Moreover, studies on the functional morphology of the central nervous system in frogs have focused mainly on the prey‐capture visual system (Lettvin et al. 1968; Ewert, 1976, 1987; Borchers et al. 1978; Grobstein et al. 1983; Nishikawa et al. 1992; Gray & Nishikawa, 1995; Deban et al. 2001), and the vocalization and auditory systems (Edwards & Kelley, 2001). There are some experimental studies that suggest that in urodeles (salamanders) the brain does not differ in its anatomical or functional properties from that of anurans (Wicht & Himstedt, 1988). In contrast, a comparative anatomical study made by Taylor et al. (1995) in which the brain of frogs with four different modes of life was analyzed, proposed that a relationship between ecological habits and brain morphology exists. Taylor et al. (1995) specifically tested whether the six brain regions (the main olfactory bulbs, the accessory olfactory bulbs, the telencephalon, the optic tectum, the cerebellum, and the brain stem) were different in terrestrial, arboreal, fossorial, and aquatic frogs. Their results indicated that despite the overall similarity of anuran brains, some interesting differences and apparent adaptations to fossorial and arboreal modes of life do appear to exist. This is in accordance with the results of a recent study evaluating the effects of phylogeny and ecology on the evolution of brain size in frogs (Liao et al. 2015). These authors concluded that whereas ecology did not impact overall brain size, habitat and diet type did impact the size of the telencephalon (Liao et al. 2015).
Yet, anurans are capable of engaging in different complex behaviors independent of their mode of life, often reflected in the use of the hands and feet. Indeed, frogs can perform skilled movements using their wrists and hands (Gray et al. 1997; Manzano et al. 2008; Sustaita et al. 2013). Limb movement implies complex interactions of the sensory and central nervous systems, muscular mechanics, and the interaction with the environment. Iwaniuk & Whishaw (2000) stressed that skilled forelimb movements are common among tetrapod taxa and probably share a common origin. They considered that skilled movements of the limbs are homologous among tetrapods and likely derived from an elaborated food‐handling behavior; however, this remains to be tested. The use of the hands in anurans has been studied, focusing on the diverse types of movements possible. For example, skilled wrist movements have been shown to be present in arboreal frogs using their hands to reach and grab prey and pushing it into, or pulling it out of, the mouth (Gray et al. 1997). Moreover, some frogs can perform other kinds of complex movements using their hands, for example when spreading lipid substances over their body, a behavior called ‘wiping’ by Blaylock et al. (1976). Also frogs use complex forelimb movements when building ‘leaf nests’ to deposit eggs (Lillywhite et al. 1998). Finally, Manzano et al. (2008) and Herrel et al. (2013) observed differences in the use of the hand to grasp narrow substrates during locomotion in arboreal frogs.
Based on the presence of skilled limb movement in arboreal frogs we hypothesize that the central nervous system may show variation in gross anatomy in taxa capable of these movements. We here provide a comparative morphological study focusing on the macro‐ and micro‐anatomy of the six regions of the brain and its choroid plexus: the olfactory bulbs, the telencephalon, the diencephalon, the mesencephalon, the rhombencephalon, and the cerebellum. We compare frogs with different ecologies that accordingly use their limbs in different contexts, specifically focusing on the difference between arboreal frogs and those with other locomotor modes. We also report on the comparative anatomy of the plexus brachialis responsible for the innervation of the forelimbs. Our main objectives were thus to investigate: (i) the diversity in brain morphology across a range of frogs exhibiting different locomotor modes, and (ii) provide a preliminary analysis of the relationship between morphology and locomotor mode.
Methods
Gross anatomy
The brain and spinal cord of formalin‐fixed specimens of adult frogs of three suprageneric taxa proposed by Frost et al. (2006) and Duellman et al. (2016) were dissected. They belong to Microhylidae: Elachistocleis bicolor (n = 4 – Gastrophryninae); Phyllomedusidae: Phyllomedusa sauvagii (n = 3), Phyllomedusa hypochondrialis (n = 1), Phyllomedusa boliviana (n = 2); Hylidae: Pseudis minuta (n = 4–Pseudinae), Hypsiboas pulchellus (n = 4 – Hylinae); and S. fuscovarius (n = 4 – Scinaxinae); Leptodactylidae: Leptodactylus chaquensis (n = 1), Leptodactylus latrans (n = 4); Bufonidae: Rhinella fernandezae (n = 4). Specimens are listed in Appendix 1 and belong to the collections of CICyTTP‐CONICET. Note that formaldehyde may induce some shrinkage; however, this should be similar for all specimens (but see Liao et al. 2015). Although this does not affect our comparative study, it may need to be considered in future studies aiming to integrate the data presented here.
Each of the six brain regions of all specimens were measured using an ICO digital caliper (0.15 mm). They were measured by three times each to minimize the error. More than one specimen was used (a minimum of three) for most of the analyzed species; in these cases an average and its standard deviation (±) were calculated (Table 1).The following morphological measurements were taken for every specimen (Table 1): length, width, height, and volumes of each region of the brain: olfactory bulbs and cerebral hemisphere (telencephalon), diencephalon, optic bulbs (mesencephalon), cerebellum, and rhombencephalon (ventriculus quartus and recessus lateralis). The length and width were measured across the extremes of each structure and the height across the taller part of each (Supporting Information Fig. S2). Estimated volumes of the brain and its six parts were calculated assuming that the frog brain is an ellipsoid: (4/3)π.r1r2.r3 (Table 2; Ullmann et al. 2010; Yopak & Lisney, 2012).
Table 1.
Summary of measurements of the different brain areas
| Sp | Lm | Olfactory bulbs | Telencephalon | Diencephalon | Optic bulbs | Cerebellum | Rhomboencephalon | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | Width | Length | Height | Width | Length | Height | Width | Length | Height | Width | Length | Height | Width | Length | Height | Width | Length | ||
| Eb | TB | 0.43 ± 0.04 | 0.85 ± 0.13 | 0.44 ± 0.1 | 0.56 ± 0.07 | 1.53 ± 0.18 | 1.01 ± 0.18 | 0.57 ± 0.04 | 0.61 ± 0.11 | 0.40 ± 0.05 | 0.50 ± 0.05 | 0.82 ± 0.07 | 0.47 ± 0.01 | 0.48 ± 0.08 | 0.64 ± 0.01 | 0.19 ± 0.02 | 0.34 ± 0.005 | 0.61 ± 0.04 | 0.74 ± 0.04 |
| Hp | AJ | 0.37 ± 0.08 | 0.72 ± 0.10 | 0.32 ± 0.03 | 0.92 ± 0.05 | 1.40 ± 0.06 | 1.60 ± 0.14 | 0.85 ± 0.04 | 1.14 ± 0.04 | 0.67 ± 0.06 | 0.92 ± 0.04 | 1.83 ± 0.08 | 0.91 ± 0.21 | 0.79 ± 0.02 | 1.06 ± 0.10 | 0.37 ± 0.04 | 0.47 ± 0.01 | 0.98 ± 0.03 | 1.30 ± 0.26 |
| Ll | TJ | 0.39 ± 0.02 | 0.62 ± 0.02 | 0.38 ± 0.04 | 0.80 ± 0.09 | 0.85 ± 0.03 | 1.36 ± 0.14 | 0.75 ± 0.16 | 0.71 ± 0.06 | 0.69 ± 0.02 | 0.65 ± 0.11 | 1.52 ± 0.10 | 0.73 ± 0.10 | 0.67 ± 0.02 | 0.87 ± 0.07 | 0.13 ± 0.06 | 0.46 ± 0.05 | 0.85 ± 0.09 | 1.15 ± 0.11 |
| Pb | AW | 0.56 | 0.43 | 1.09 | 0.87 | 1.92 | 1.89 | 0.66 | 1.26 | 0.77 | 0.94 | 1.74 | 0.80 | 0.87 | 1.08 | 0.44 | 0.54 | 1.12 | 1.41 |
| Ph | AW | 0.41 | 0.71 | 0.36 | 0.81 | 1.15 | 1.15 | 0.73 | 0.75 | 0.68 | 0.73 | 1.18 | 0.54 | 0.77 | 0.71 | 0.31 | 0.44 | 0.73 | 0.73 |
| Pm | SW | 0.50 ± 0.08 | 0.60 ± 0.10 | 0.44 ± 0.03 | 1.03 ± 0.11 | 1.28 ± 0.20 | 1.56 ± 0.02 | 0.84 ± 0.10 | 1.04 ± 0.14 | 0.51 ± 0.06 | 0.85 ± 0.10 | 1.85 ± 0.03 | 0.78 ± 0.02 | 0.68 ± 0.03 | 1.06 ± 0.11 | 0.13 ± 0.02 | 0.42 ± 0.01 | 1.01 ± 0.03 | 1.21 ± 0.18 |
| Ps | AW | 0.70 ± 0.19 | 1.38 ± 0.37 | 0.44 ± 0.02 | 1.34 ± 0.30 | 1.92 ± 0.12 | 1.66 ± 0.05 | 1.23 ± 0.09 | 1.28 ± 0.22 | 0.80 ± 0.15 | 1.29 ± 0.28 | 1.88 ± 0.30 | 0.90 ± 0.003 | 0.95 ± 0.10 | 1.19 ± 0.13 | 0.35 ± 0.06 | 0.59 ± 0.03 | 1.26 ± 0.30 | 1.28 ± 0.14 |
| Rf | TH | 0.50 ± 0.13 | 0.83 ± 0.08 | 0.27 ± 0.07 | 0.98 ± 0.15 | 1.69 ± 0.23 | 1.36 ± 0.12 | 0.81 ± 0.07 | 0.91 ± 0.19 | 0.53 ± 0.05 | 0.81 ± 0.10 | 1.55 ± 0.07 | 0.64 ± 0.08 | 0.79 ± 0.11 | 0.90 ± 0.05 | 0.23 ± 0.05 | 0.56 ± 0.01 | 0.97 ± 0.08 | 0.98 ± 0.13 |
| Sf | AJ | 0.70 ± 0.11 | 0.84 ± 0.11 | 0.24 ± 0.06 | 1.06 ± 0.07 | 1.38 ± 0.26 | 1.75 ± 0.10 | 1.03 ± 0.04 | 1.06 ± 0.08 | 0.64 ± 0.14 | 1.05 ± 0.07 | 2.12 ± 0.10 | 0.70 ± 0.04 | 0.84 ± 0.05 | 1.04 ± 0.02 | 0.34 ± 0.07 | 0.57 ± 0.06 | 1.03 ± 0.01 | 1.23 ± 0.21 |
Table entries are means (in mm) ± standard deviations. Locomotor modes (Lm): AJ, arboreal jumper; AW, arboreal walker; SW, swimmer; TB, terrestrial burrower; TH, terrestrial hopper; TJ, terrestrial jumper. Species abbreviations: Eb, Elachistocleis bicolor; Hp, Hypsiboas pulchellus; Ll, Leptodactylus latrans; Pb, Phyllomedusa boliviana; Ph, Phyllomedusa hypochondrialis; Pm, Pseudis minutus; Ps, Phyllomedusa sauvagii; Rf, Rhinella fernandezae; Sf, Scinax fuscovarius.
Table 2.
Estimated volumes of the brain areas in mm3 calculated as an ellipsoid: (4/3)π.r1r2.r3
| Species | Olfactory bulbs | Telencephalon | Diencephalon | Optic bulbs | Cerebellum | Rhomboencephalon | Head width (mm) | SVL (mm) |
|---|---|---|---|---|---|---|---|---|
| Elachistocleis bicolor | 0.71 ± 0.27 | 3.73 ± 1.25 | 0.59 ± 0.19 | 0.80 ± 0.14 | 0.25 ± 0.05 | 0.63 ± 0.06 | 6.8 ± 0.3 | 31.9 ± 1.3 |
| Hypsiboas pulchellus | 0.35 ± 0.07 | 8.57 ± 1.24 | 2.72 ± 0.37 | 6.43 ± 1.91 | 1.29 ± 0.16 | 2.51 ± 0.50 | 12.1 ± 1.1 | 46.4 ± 4.7 |
| Leptodactylus latrans | 0.38 ± 0.04 | 3.91 ± 0.93 | 1.53 ± 0.27 | 3.10 ± 1.09 | 0.30 ± 0.14 | 1.87 ± 0.19 | 10.4 ± 1.1 | 31.1 ± 3.1 |
| Phyllomedusa boliviana | 1.09 | 13.08 | 2.66 | 5.45 | 1.72 | 3.54 | 16.5 | 57.9 |
| Phyllomedusa hypochondrialis | 0.44 | 4.49 | 1.54 | 1.92 | 0.69 | 0.97 | 10.7 | 31.6 |
| Pseudis minutus | 0.55 ± 0.15 | 8.50 ± 0.26 | 1.85 ± 0.26 | 5.08 ± 0.56 | 0.39 ± 0.09 | 2.14 ± 0.24 | 12.3 ± 1.7 | 32.2 ± 2.5 |
| Phyllomedusa sauvagii | 1.88 ± 1.09 | 18.02 ± 5.82 | 5.39 ± 2.37 | 9.35 ± 3.54 | 1.63 ± 0.06 | 4.04 ± 1.64 | 22.2 ± 0.3 | 64.3 ± 1.8 |
| Rhinella fernandezae | 0.48 ± 0.25 | 9.55 ± 3.02 | 1.63 ± 0.40 | 3.41 ± 1.07 | 0.69 ± 0.26 | 2.22 ± 0.34 | 14.4 ± 3.84 | 55.7 ± 7.18 |
| Scinax fuscovarius | 0.56 ± 0.09 | 10.86 ± 3.12 | 2.98 ± 0.93 | 6.55 ± 0.46 | 1.24 ± 0.30 | 3.02 ± 0.38 | 12.9 ± 1.5 | 40.2 ± 2.7 |
SVL, snout‐vent length. Table entries are means ± standard deviation.
Specimens were selected because of their different locomotor modes (Manzano et al. 2008) defined as follows:
Climber‐walkers (AW) such as Phyllomedusa frogs. These arboreal frogs move across narrow substrates using their arms independently from one another for walking and are able to close their hands around the substrate to climb. They move slowly across branches, very occasionally jumping to escape (Manzano et al. 2008; Herrel et al. 2013; Sustaita et al. 2013). They have long limbs and their fingers move independently in both hands and feet. Phyllomedusa species have an opposable first digit at the hands and feet that allows them to execute complex movements with a fine motor control of their fingers (Blaylock et al. 1976; Manzano et al. 2008; Sustaita et al. 2013).
Climber‐jumpers (AJ), such as Hypsiboas and Scinax, move among surfaces like branches mainly by jumping, typically using bilaterally simultaneous movements of the arms and legs. Their hands and feet, usually webbed, have independent movements but without opposable fingers, and no fine motor control of the fingers was recorded (Manzano et al. 2008). They also have long extremities.
Swimmers‐jumpers (SW), such as Pseudis minuta, are predominantly aquatic frogs that swim with bilaterally simultaneous movements of their legs; flexing the tibia‐fibula‐tarsal‐metatarsal articulation, and propelling themselves by a kick of both legs (Manzano & Barg, 2005). The hind limbs have a strongly developed musculature and webbed feet; the forelimbs are short and remain largely immobile. The hands have opposable first fingers, but these are not functional. The hands are used for flotation during rest. These frogs are very good jumpers and show explosive escape responses (Manzano & Barg, 2005).
Burrowers (TB), such as Elachistocleis bicolor, are terrestrial frogs that live under grass, organic debris or in ant nests; the knowledge of their legs movements is insufficient, but both forelimbs and hind limbs are short, robust, and not webbed. They usually remain in their refuges, not moving much.
Hopper‐walkers (TW), such as Rhinella fernandezae, are frogs that can walk on terrestrial surfaces but their main locomotion mode is hopping. They can migrate by hopping long distances.
Jumpers (TJ), such as Leptodactylus species, have forelimbs that are typically short and that provide body support during sitting or help absorb impact forces during landing (Nauwelaerts & Aerts, 2006). The hind limbs are the principal limb pair generating propulsion for locomotion during jumping or swimming. They can jump long distances and have webbed feet. They spend most of their time on land near the border of water.
Histological procedures
Histological transverse sections of 5 μm were made of specimens of all above described locomotor modes: the climber‐jumper Hypsiboas pulchellus (n = 2); the hopper‐walker Rhinella fernandezae (n = 1), the burrower Elachistocleis bicolor (n = 1), the swimmer‐jumper Pseudis minuta (n = 2), and two climber‐walkers Phyllomedusa boliviana (n = 1) and P. sauvagii (n = 2). Standard procedures for paraffin sections were followed and sections were stained using a hematoxylin‐eosin stain (H‐E), a Nissl stain (García del Moral, 1993; Bancroft & Gamble, 2001), a pricosirius‐hematoxylin (P‐H) differential stain (Junqueira et al. 1995) and a cresyl violet stain (García del Moral, 1993). Parasagittal sections of 10 μm were made of the brain of P. boliviana, (n = 1), P. sauvagii (n = 1), S. fuscovarius (n = 1), and Leptodactylus latrans (n = 1). For descriptions of the brain parts we follow Llinás & Precht (1976) and Ten Donkelaar (1998); for the brachial plexus we follow Gaupp (1896). For histological orientations and descriptions we follow the terminology of Llinás & Precht (1976), Ten Donkelaar (1998) and Kemali & Braitenberg (1969). For the telencephalon terminology we follow Northcutt & Kicliter (1980), and Neary (1990).
Statistical analysis
We log10‐transformed all measurements to fulfill assumptions of normality and homoscedasticity. Next, we performed a mancova with the brain measures as dependent variables and head width or snout‐vent length (SVL) as a covariate to test whether frogs with different locomotor modes differ in brain proportions.
Because species cannot be considered independent data points or disconnected from their evolutionary history (Felsenstein, 1985), comparative analysis has been advocated to take into account shared ancestry in explaining patterns of phenotypic diversity (see also Liao et al. 2015). However, such approaches require a minimum number of species for the analysis to be robust. Given the time‐consuming nature of our anatomical analyses involving histological sections in addition to dissections, our dataset remains restricted. Thus, rather than doing explicit comparative analyses we decided to map the phylogeny onto the morphological space, allowing us to evaluate whether structuring is driven by phylogeny. To do so we ran a principal component analysis on the log10‐transformed brain volume variables (Table 3). Next we plotted the phylogeny in the morphospace using the ‘phylomorphospace’ function in r (R Core Team, 2016) implemented in the ‘phytools’ library (Revell, 2012). We used a consensus phylogeny based on Frost et al. (2006), Roelants et al. (2007), and Bruschi et al. (2013) (Fig. 1). Branch lengths were computed using the Grafen method (Grafen, 1989) with the ‘compute.brlen’ function of the ‘Ape’ library (Paradis et al. 2004) in r (R Core Team, 2016).
Table 3.
Loadings of the brain volume variables on the principal component axes
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Proportion of variance | 80.56 | 10.94 | 6.02 |
| Olfactory bulb volume | −0.19 | 0.87 | 0.31 |
| Telencephalon volume | −0.37 | 0.21 | 0.09 |
| Diencephalon volume | −0.42 | −0.10 | 0.03 |
| Optic bulb volume | −0.51 | −0.39 | 0.32 |
| Cerebellum volume | −0.47 | 0.14 | −0.84 |
| Rhomboencephalon volume | −0.41 | −0.18 | 0.30 |
Values in bold indicate loadings greater than 0.7.
Figure 1.
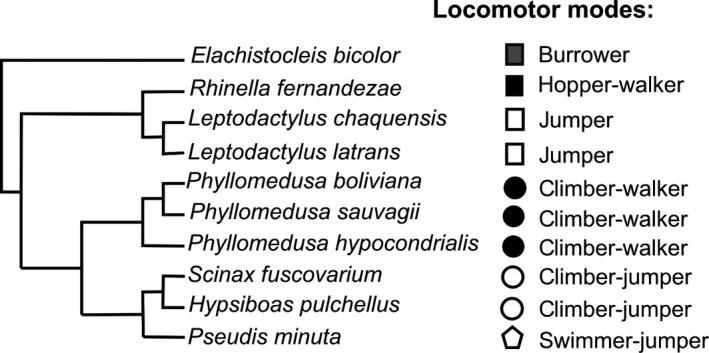
Phylogenetic tree representing the relationships among the species included in our study. The tree is a composite tree based on Frost et al. (2006), Roelants et al. (2007), and Bruschi et al. (2013). To the right of the tree are indicated the different locomotor modes and lifestyles of the species.
Results
Gross anatomy
Telencephalon
The frog telencephalon consists of a pair of evaginated cerebral hemispheres and the olfactory bulbs are fused at the midline (Fig. 2). There is a medial sulcus that divides the hemispheres into two portions (Fig. 3). Although the medial sulcus continues superficially to the midline of the olfactory bulbs, the olfactory bulbs themselves remain transversally undivided. Among the species of Hylinae the gross anatomy of olfactory bulbs is variable. The bulbs are cylindrical, short, and separated from the hemispheres by a deep constriction in the arboreal jumpers of the genera Hypsiboas and Scinax (Figs 3A,S1A), whereas in the aquatic Pseudis, they are cylindrical, long, and undivided (Fig. 2E). In all species of Phyllomedusa the olfactory bulbs are short, undivided, and with no constriction between the hemispheres (Figs 2A,B,3B and S1B). In the terrestrial borrower Elachistocleis the bulbs are large and divided into two regions separated by a superficial medial sulcus (Figs S1C,D). In this case, the bulbs are separated from the hemispheres by a V‐shaped superficial constriction (Figs 2C,3C and S1C). In the terrestrial hopper‐walker Rhinella, the bulbs are large, as in Elachistocleis, and divided into two regions (Fig. 3E,F and S1E,F). The rostral region is undivided. A bigger posterior region is separated in two halves by a superficial medial sulcus that continues posteriorly to the hemispheres. There are also variations in the diameter of the olfactory nerves, being in some species such as Rhinella fernandezae extremely thick and composed of several fibers (Figs 2D,3E,Fand S1E,F), and Phyllomedusa sp. (Figs 3B and S1B). In the terrestrial jumper Leptodactylus, the bulbs are quarter sphere‐shaped and thinner than the hemispheres divided by the medial sulcus. There is a superficial constriction between the hemispheres (Figs 2F and 3D).
Figure 2.
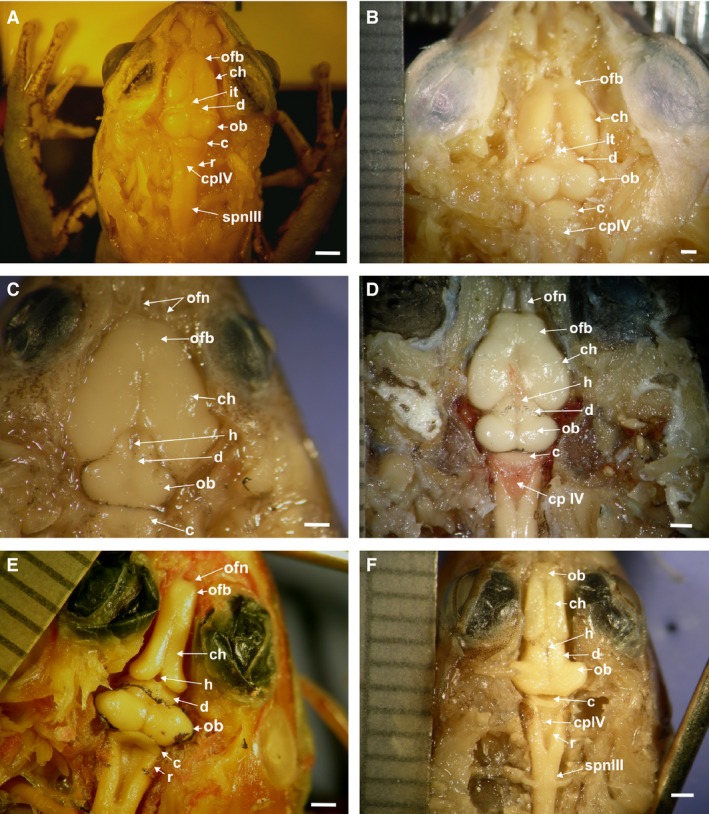
Dorsal view of the brain morphotypes: (A) Phyllomedusa hypochondrialis; (B) Phyllomedusa sauvagii; (C) Elachistocleis bicolor; (D) Rhinella fernandezae; (E) Pseudis minuta; (F) Leptodactylus latrans. c, cerebellum; ch, cerebral hemisphere; cpIV, choroid plexus of the 4th ventricle; d, diencephalon; h, habenular sulcus; it, iridescent tissue covering the habenular sulcus of the epiphyseal complex; ob, optic bulbs; ofb, olfactory bulb; Ofn, olfactory nerve; r, rhombencephalon; spn III, pre‐ganglion branch of the spinal nerve III (brachial). Scale bar: 1 mm.
Figure 3.
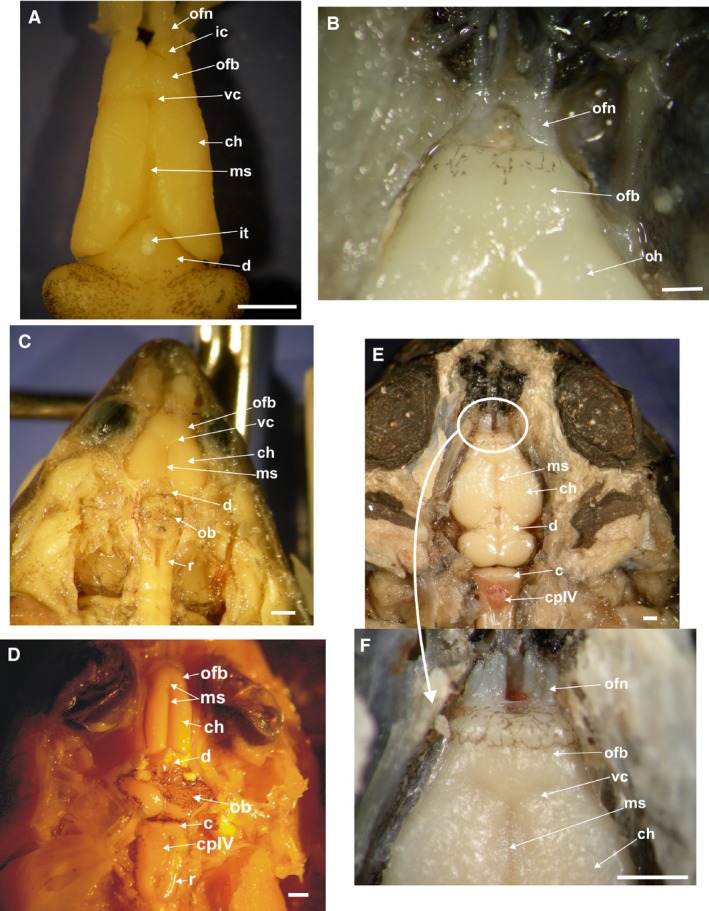
Dorsal view of the brain, telencephalon with details of the olfactory bulbs and nerves. The choroid plexus covering the olfactory bulbs and part of the cerebral hemisphere, was removed: (A) Scinax fuscovarius; (B) Phyllomedusa sauvagii a close view of the branches of the olfactory nerves; (C) Elachistocleis bicolor; (D) Leptodactylus chaquensis; (E) Rhinella fernandezae; (F) Rhinella fernandezae close‐up view of the extremely thick olfactory nerves composed of several fibers. c, cerebellum; ch, cerebral hemisphere; cpIV, choroid plexus of the 4th ventricle; d, diencephalon; ic, implantation cone of the olfactory nerves; it, iridescent tissue; ofn, olfactory nerve; ofb, olfactory bulb; ob, optic bulbs; ms, median sulcus; r, rhombencephalon; vc, V‐shaped superficial constriction. Scale bar: 1 mm.
In the Hylidae and Leptodactylus (Figs 2E,F and 3A,D) each hemisphere is long and cylindrical and longer than wide, with a V‐shaped posterior edge. In all Phyllomedusa, Elachistocleis and Rhinella species (Figs 2A–D and 3C,E), each hemisphere is drop‐shaped and globoid, and wider than long. In all cases both hemispheres are separated by a longitudinal medial sulcus (Fig. 3). A choroid plexus covers the surface of olfactory bulb and the anterior surface of the hemispheres.
In the histological transverse section, an external glomerular layer bordering the bulbs is present in all species, but in some cases it is thicker on the ventral than the dorsal border (Fig. 4D,F,G). In Phyllomedusa species the glomerular layer is thick, surrounding the entire external and rostral border of the bulbs (Fig. 4D,E). In a parasagittal histological section the wide glomerular layer can be observed rostrally after the nerve (Fig. 4E). After the glomerular layer a mass of dispersed cells corresponding to the mitral cell layer, is evident (Fig. 4D, transverse section and 4E longitudinal section). Vomeronasal nerves are present on the ventro‐lateral side of the undivided bulb (Fig. 4D). In the other species, the glomerular layer borders the ventral and ventro‐lateral edge of each bulb (Fig. 4F,G). In Rhinella, the glomerular area is wider and ventrally located, almost reaching the center of each bulb (Fig. 4F). The vomeronasal nerve is located ventrally in each bulb (Fig. 4G).
Figure 4.
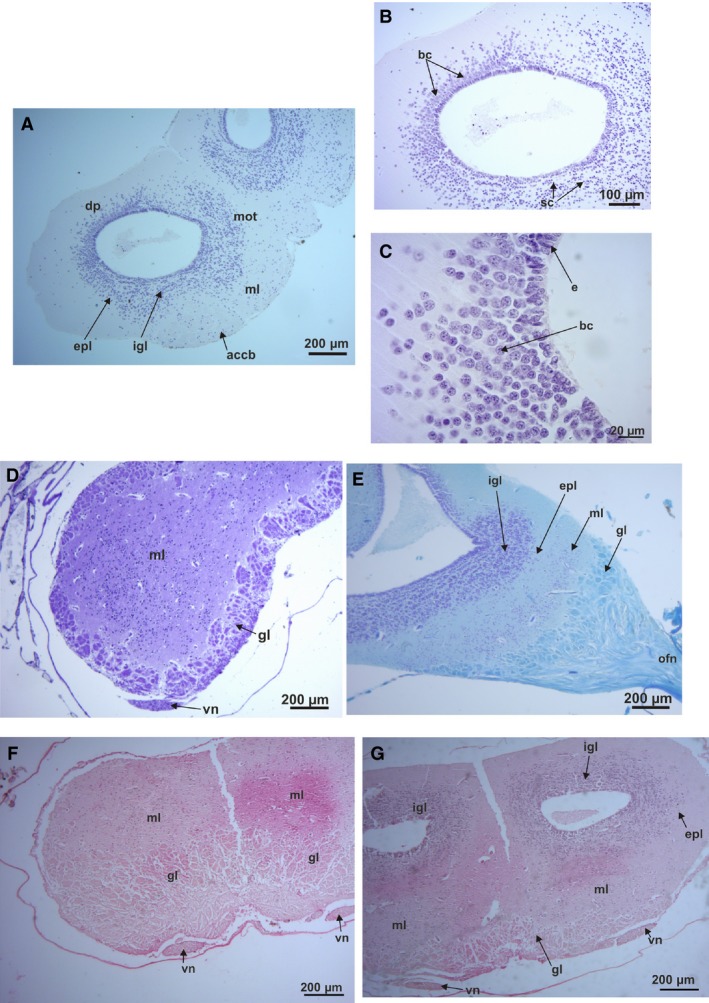
Histological sections of the olfactory bulbs: (A) Hypsiboas pulchellus transverse section, transition between the olfactory bulb and telencephalon of Hypsiboas pulchellus (H‐E stain). (B) Detail of the right olfactory bulbs of H. pulchellus (H‐E stain). (C) Detail of the two types of cells in the latero‐dorsal pallium of the olfactory bulbs of H. pulchellus. (D) Phyllomedusa boliviana transverse section of the rostral area (Nissl stain). (E) Phyllomedusa sauvagii parasagittal section of the rostral area of the olfactory bulbs (cresyl violet and fast blue stain). (F) Rhinella fernandezae transverse section of the rostral area of the olfactory bulbs, showing the big glomerular area and the ventral position of the olfactory nerves. (G) Transverse section of the transition area of the olfactory bulb and the telencephalon of R. fernandezae. accb, accessory bulbs; bc, big cells; dp, dorsal pallium; e, ependymal cells; epl, extragranular plexiform layer; gl, glomerular layer; igl, internal granule layer; ml, molecular layer; mot, medial olfactory tract; on, olfactory nerve; sc, small cells; vn, vomeral nerve.
The ventricles become evident where a separation of the bulbs is present (Fig. 4A,B,G transverse sections and Fig. 4E parasagittal section). At this level, a series of cells are disposed in defined concentric cell layers around the ventricles forming the internal granule layer (Fig. 4A,B,E,G). At the caudal level, two kinds of cells are visible, big cells and smaller ones, concentrated in this internal granular layer of each bulb, very close to the ependymal cells (Fig. 4A–C transverse sections, and Fig. 4E, parasagittal section). Large cells are located at the latero‐dorsal area of the intracellular layer, indicating the beginning of the lateral and dorsal pallium (Fig. 4A–C). Large mitral cells are evident at the ventral and ventro‐lateral areas of the extragranular plexiform layer in almost all studied species. In P. sauvagii, big cells are located along the medial and dorsal surface of the internal granular layer. In H. pulchellus, big cells are evident at the latero‐dorsal area of the pallium (Fig. 4A–C). In a more caudal transverse section of the olfactory bulbs and telencephalon of this species, big cells are located at the medial pallium in a dispersed form, and at the dorsal pallium in a small concentration of cells (Fig. 4A–C).
A pair of correspondent olfactory nerves are present in all studied species, but with differences in the width of the transverse section of the branches (related to the number of fibers that make up each nerve; see Fig. 3B,E,F), and the length of their branches. A cone of implantation of each olfactory nerve in the bulbs is present but varies in size and shape (Fig. 3A). In R. fernandezae, each olfactory nerve is composed of many nerve fibers grouped in a single thick nerve branch that innervates each nasal passage (conducto nasal; Fig. 3E,F). In E. bicolor and H. pulchellus, the nerve is composed of two very thin, long, and separated nerve branches that innervate each nasal passage; the lateral one corresponds to the vomeronasal nerve (Fig. 3C). In Phyllomedusa the olfactory nerve is very thick and long, and also includes the vomeronasal nerve (Fig. 3B). In P. hypochondrialis the nerves are short, wide, and the implantation cone is integrated within the olfactory bulbs. In the case of the aquatic P. minuta the triangular hat‐shaped cones are lateral to each bulb, with short nerves composed of two branches, one of which corresponds to the vomeronasal nerve.
In a histological transverse section of the climber‐walker Phyllomedusa, the ependymal cells covering the lateral edge of the two ventricles of the telencephalon are elongated with short processes (Fig. 5A). The edges of these ventricles are covered by two or more layers of small round cells, except in the above‐mentioned lateral areas. The main concentration of small cells is in the lateral areas of the both ventricles, corresponding to the lateral pallium and reaching part of the dorsal pallium. At the medial pallium, big cells are present also covering part of the dorsal pallium (Figs 4B,C and 5A). The ependymal cells covering both lateral ventricles of the climber‐jumper Hypsiboas are small and round, and are homogeneously dispersed in a layer.
Figure 5.
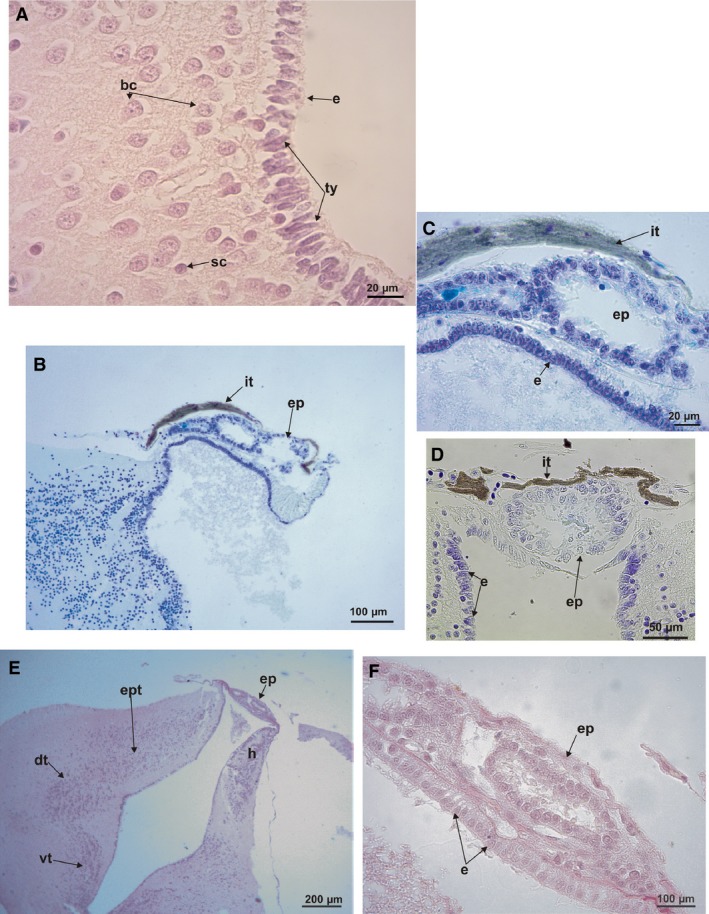
Histological transverse sections. (A) Telencephalon of Phyllomedusa sauvagii showing big cells and small cells at dorsal and medial pallium. Tanycite cells with short processes are signaled at the ependyma (P‐H stain). (B) Parasagittal section of the epiphysis of Phyllomedusa boliviana (cresyl violet and fast blue stains). (C) Detail of the parasagittal section of the epiphysis of Phyllomedusa boliviana (cresyl violet and fast blue stains). (D) Detail of the transverse section of the epiphysis of P. sauvagii (cresyl violet and fast blue stains). (E) Transverse section of the diencephalon at the level of the thalamus and epiphysis of Pseudis minuta (P‐H stain). (F) Detail of the transverse section of the epiphysis of P. minuta (P‐H stain). bc, big cells; sc, small cells; e, ependyma; ep, epiphysis; dt, dorsal thalamus; h, habenula; it, iridescent tissue; ty, tanycytes; vt, ventral thalamus.
Diencephalon
In the climber‐jumper Hypsiboas, the climber‐walker P. boliviana, and the jumper Leptodactylus, the diencephalon is long and wide, partially covered by the posterior edge of the hemispheres. In the rest of the species, it is short (Fig. 2). In the burrower Elachistocleis and hopper‐walker Rhinella the habenular commissure is large (Fig. 2C,D). An iridescent tissue covers the habenular commissure in the climber‐jumper Scinax, and the climber‐walkers P. sauvagii, P. boliviana, and P. hypochondrialis (Figs 2A,B and 3A).
In a histological transverse section, four regions with a vertical distribution of the cell masses can be seen, corresponding to the habenulae, epithalamus, the dorsal and ventral thalamus, and the hypothalamus (Figs 5E and 6). This zone receives visual, auditory, and somato‐sensory information from the midbrain roof (optic tectum and torus semicircularis; Neary, 1990). On the dorsal surface, the epiphysis and the pineal glands are evident and are usually covered by a choroid plexus (Fig. 5). In some cases, part of the paraphysis cerebri can also be observed (Fig. 6C,D,F). The hopper‐walker Rhinella, and burrower Elachistocleis (Fig. 6A,C), and the swimmer‐jumper Pseudis present grouped cells, corresponding to the ventral and dorsal thalamus. These cells are distributed in highly defined layers interspersed by acellular layers (Fig. 5E). The four thalamic areas are well defined in the burrower Elachistocleis, especially at the paraphysis–epiphyseal level; they are grouped in four identifiable areas of parallel lines of cells (Fig. 6C). In all species, at both sides of the epiphysis at the level of the epithalamus, a high concentration of cells can be observed (habenular area; Figs 5E and 6C,E). In the climber‐walker Phyllomedusa and the climber‐jumper Hypsiboas pulchellus the cells layers are not well defined (Figs 5E and 6B,E). The thalamus and hypothalamus areas are represented by tightly grouped, yet unordered cells. In Phyllomedusa, the areas corresponding to the epithalamus, dorsal thalamus, and hypothalamus present a higher concentration of cells compared with the other species (Fig. 6B), except for Elachistocleis.
Figure 6.
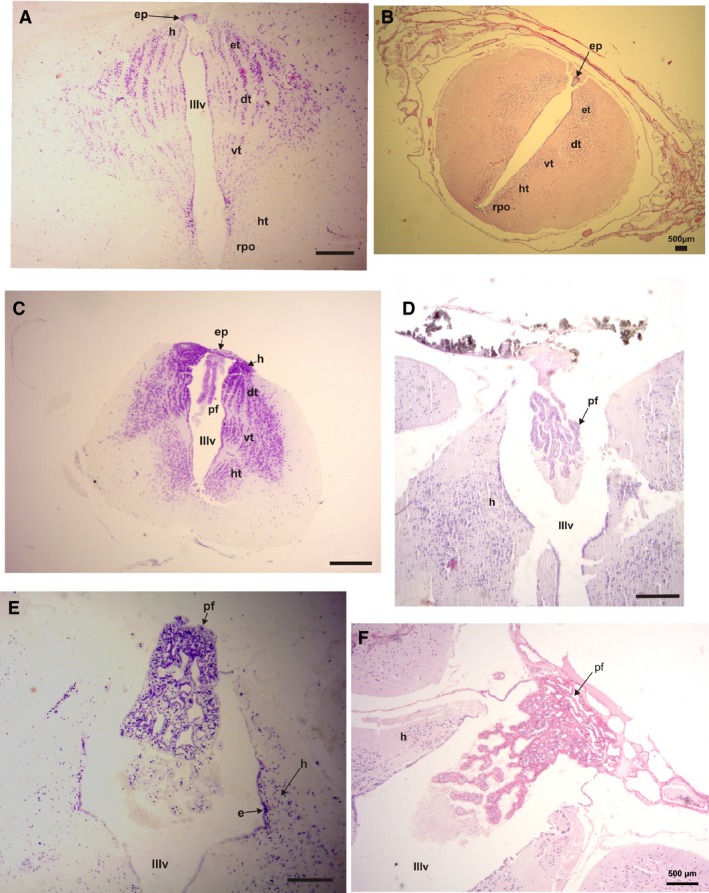
Histological transverse section of the diencephalon. (A) Rhinella fernandezae, showing the thalamic areas composed of highly defined parallel layers of cells interspersed with acellular layers and a remains of the epiphysis (P‐H stain). (B) Phyllomedusa sauvagii, showing the thalamic areas composed of cells layers that are poorly defined (P‐H stain). (C) Elachistocleis bicolor, showing the thalamic areas with a similar distribution of cells as in R. fernandezae and the epiphysis and paraphysis cerebri (P‐H stain). (D) Paraphysis cerebri of Hypsiboas pulchellus (Nissl stain). (E) Paraphysis cerebri of P. sauvagii (H‐E stain). (F) Paraphysis cerebri of Phyllomedusa boliviana (P‐H stain). dt, dorsal thalamus; e, ependymal cells; ep, epithalamus; h, habenula; ht, hypothalamus; pa, paraphysis cerebri; rpo, recessus preopticus; vt, ventral thalamus; IIIv, 3rd ventricle. Scale bars: 200 μm.
Ependymal cells, tanycytes, which cover the floor of the 3rd ventricle in Phyllomedusa, are more elongated than in the other species. The roof of the 3rd ventricle is covered by cells that are also elongated, but most of them have short processes compared with the typical aspect of the tanycytes. At the level of paraphysis there are tanycytes (Fig. 5A), but most of them are ovoid instead of triangular. In the central border of the ventricle, the cells are smaller than in the lateral areas of the roof and floor (Fig. 5D). The ependymal cells covering the 3rd ventricle of the climber‐jumper Hypsiboas are small and rounded, and homogeneously disposed in one layer. Elachistocleis presents tanycytes covering the entire 3rd ventricle. In the swimmer‐jumper Pseudis, tanycytes are distinguishable in the central area of the ependyma of the 3rd ventricle. Only restricted zones of the border of the 3rd ventricle in Rhinella present tanycytes.
In a parasagittal section, a vesicle‐like epiphyseal complex is evident (Fig. 5B,C). A transverse section shows that the epiphysis as a ring‐like structure with a ventricle‐like space in the center. The inner and outer borders of the ring are entirely covered by elongated cells with processes, similar to the ependymal tanycytes, located radially around the lumen. The epiphysis in Phyllomedusa and S. fuscovarius is dorsally and superficially covered by acellular layers that appear mineralized and correspond to the iridescent structure visible at the gross anatomical level (Fig. 5B–D). The ependymal tanycytes of the roof at this level of the 3rd ventricle present processes that are laterally oriented and connected to the epiphysis. Elachistocleis presents a similar organization of the epiphysis, except that there is a wide neuropil between the surface and the ventricle. In Rhinella and Pseudis the epiphyses is similar. The ependymal cells covering the 3rd ventricle are typical cuboid cells. The tissue layer between the external border of the epiphyses and the external border of the diencephalon is thin. No iridescent tissue area is evident (Fig. 5E,F).
Mesencephalon
The mesencephalon consists of the optic bulbs and the tegmentum mesencephali. There are variations in the size of the optic bulbs, being big and oval in the climber‐jumpers Scinax and Hypsiboas (Fig. 7A), the swimmer‐jumper hylid Pseudis (Figs 2E and 7C), and the jumper Leptodactylus (Figs 2F and 7F). In the three climber‐walkers (Phyllomedusa) they are round, large, and have the same width as the telencephalon (Figs 2A,B and 7E). In the hopper‐walker Rhinella fernandezae and the borrower Elachistocleis they are small and round, but narrower than in the telencephalon (Figs 2C,D and 7B,D).
Figure 7.
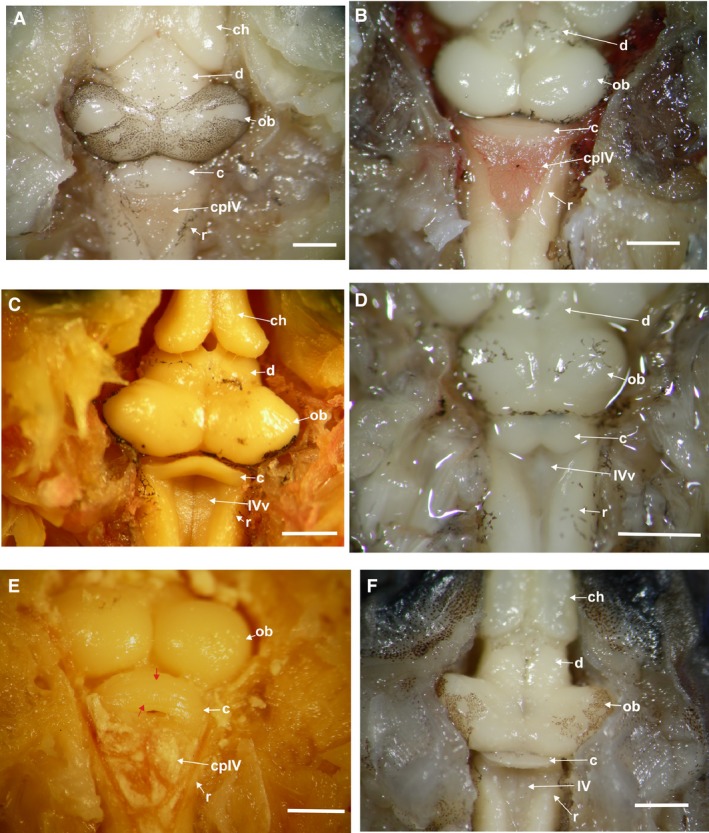
Dorsal view of the optic bulbs and cerebellum. (A) Hypsiboas pulchellus large and oval optic bulbs, wide cerebellum, and the choroid plexus covering the IV ventricle. (B) Rhinella fernandezae with small and round optic bulbs, wide cerebellum, and the choroid plexus covering the IV ventricle. (C) Pseudis minuta with big and oval optic bulbs, thin wedge‐shaped cerebellum. (D) Elachistocleis bicolor with small and round optic bulbs, and the cerebellum with two lateral auricular lobes separated by a medial constriction. (E) Phyllomedusa sauvagii with round and large optic bulbs, a wide cerebellum with sulcal lines running parallel across its surface (red arrows), and the choroid plexus covering the 4th ventricle. (F) Leptodactylus latrans with large and oval optic bulbs, thin wedge‐shaped cerebellum. c, cerebellum; ch, cerebral hemispheres; d, diencephalon; ob, optic bulbs; r, rhombencephalon; IVv, 4th ventricle; cpIV, choroid plexus of the 4th ventricle. Red arrows indicate the cerebellar sulcal lines. Scale bar: 1 mm.
The mesencephalon is a sensory correlation center that includes the tectum mesencephali, the tori semicircularis, and the tegmentum mesencephali (Fig. 8). All the frogs that we sectioned to observe the mesencephalon (the climber‐jumper Hypsiboas, the swimmer‐jumper Pseudis, the climber‐walker Phyllomedusa, the hopper‐walker R. fernandezae, and the jumper Leptodactylus latrans) have eight of the nine layers previously described by Ramon y Cajal (1984) as being present in the tectum mesencephali of frogs (see also Dicke & Roth, 2009) (Fig. 8A–E). At the level of the tegmentum in Phyllomedusa and R. fernandezae there is a higher concentration of cells compared with Hypsiboas pulchellus, which have more dispersed cells (Fig. 8A,E). However, there are differences in the concentration of cells in some layers (layer 5 or 7, for example) and in the width of some other layers (layer 6, for example, and especially layer 8) between Leptodactylus latrans, Rhinella fernandezae (thinner) and Phyllomedusa sp. and Hypsiboas (wide; Fig. 8A–E).
Figure 8.
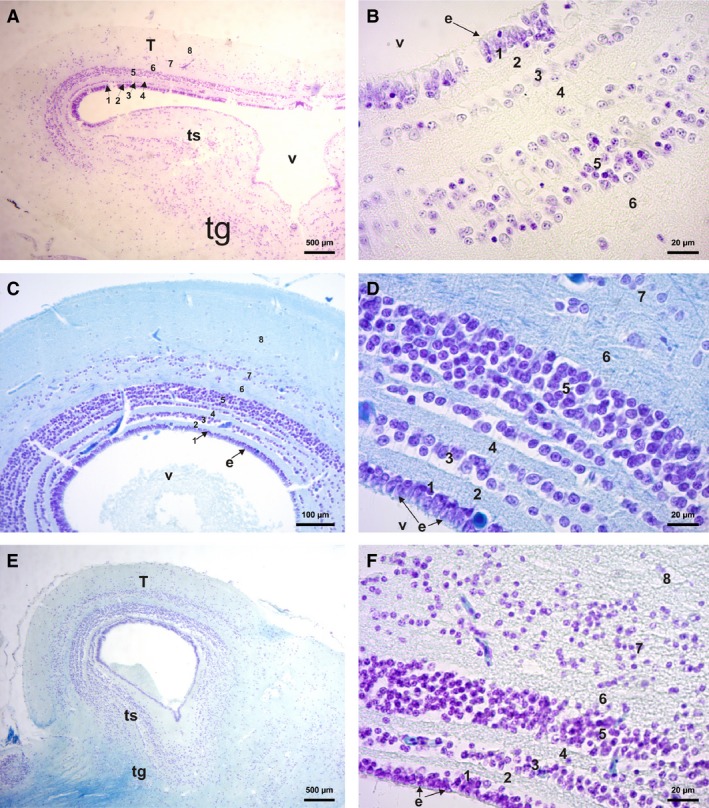
Histological section of the mesencephalon. (A) Transverse section of the Rhinella fernandezae mesencephalon showing the eight lines of the tectum mesencephali, the tegmentum area, and tori semicircularis (cresyl violet stain). (B) Rhinella fernandezae mesencephalon transverse section, detail of six lines (cresyl violet stain). (C) Phyllomedusa sauvagii parasagittal section of optic bulbs showing the eight lines of the tectum mesencephali. (D) Phyllomedusa sauvagii mesencephalon parasagittal section detailing the seven lines (cresyl violet and fast blue stains). (E) Phyllomedusa boliviana parasagittal section of the optic bulbs showing the eight lines of the tectum mesencephali, tori semicircularis and the tegmentum with neuropil arising from cerebellum and brain stem (cresyl violet and fast blue stains). (F) Leptodactylus latrans parasagittal section of the optic bulbs showing the eight lines of the tectum mesencephali (cresyl violet and fast blue stains). e, ependymal cell; T, tectum mesencephali; ts, tori semicircularis; v, 3rd ventricle; tg, tegmentum; IVv, 4th ventricle. Numbers according to Ramon y Cajal's (1984) line classification.
Cerebellum
The cerebellum in Phyllomedusa, Hypsiboas, Scinax, and Rhinella is wedge‐shaped, very wide, and not covered by the choroid plexus (Figs 2A,B,D and 7A,B,E). Both, the jumpers Leptodactylus and the swimmer‐jumper Pseudis, also present a wedge‐shaped cerebellum, but it is very thin and partially covered by the choroid plexus (Figs 2E,F, 3D, and 7C,F). In all climber‐walkers of the genus Phyllomedusa (Figs 2A,B and 7E), the cerebellum has sulcal lines running parallel across its surface, being more conspicuous in P. sauvagii (Figs 7A and 9A,B). In all other observed species the cerebellar surface is smooth (Figs 7A–D,F and 10A–D). The hopper‐walkers Rhinella (Figs 2D and 3E) and the burrower Elachistocleis present a wide cerebellum, which in Elachistocleis has two lateral auricular lobes with a medial constriction fully covered by the choroid plexus (Figs 2C and 7D).
Figure 9.
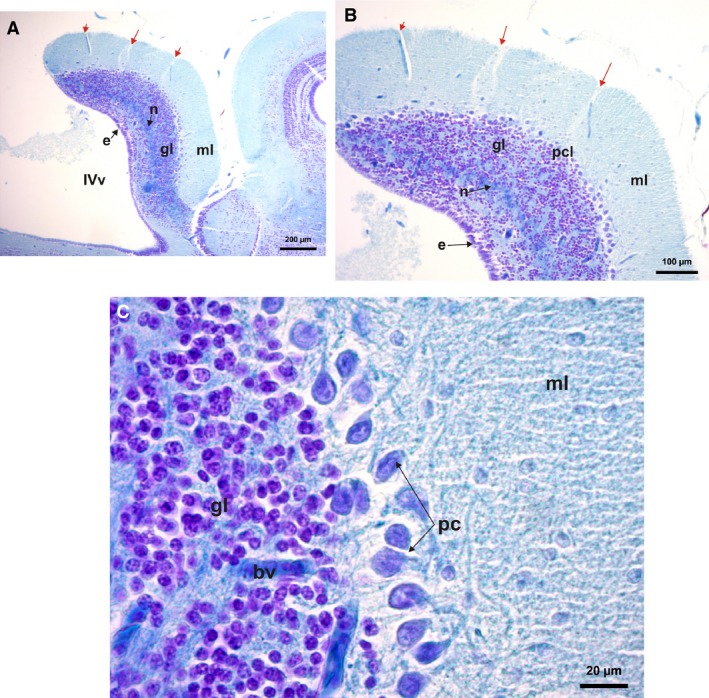
Histological parasagittal sections of cerebellum (cresyl violet and fast blue stains) of Phyllomedusa sauvagii. (A) Cerebellum section showing its layers, molecular layer, granulae layer and Purkinje cell layers and a neuropil layer of nervous projections arising from the rhombencephalon cells. (B) Detail of the sulcal lines in the cerebellum (red arrow), and its layers. (C) Detail of the large Purkinje cells with their projections to the molecular layer, below is the small granulae cell layer with incursions of blood vessels. bv, blood vessel; e, ependymal cell; gl, granulae cells; ml, molecular layer; n, neuropil of cerebellar medulla; IVv, 4th ventricle; pc, Purkinje cells; pcl, Purkinje cell layer.
Figure 10.
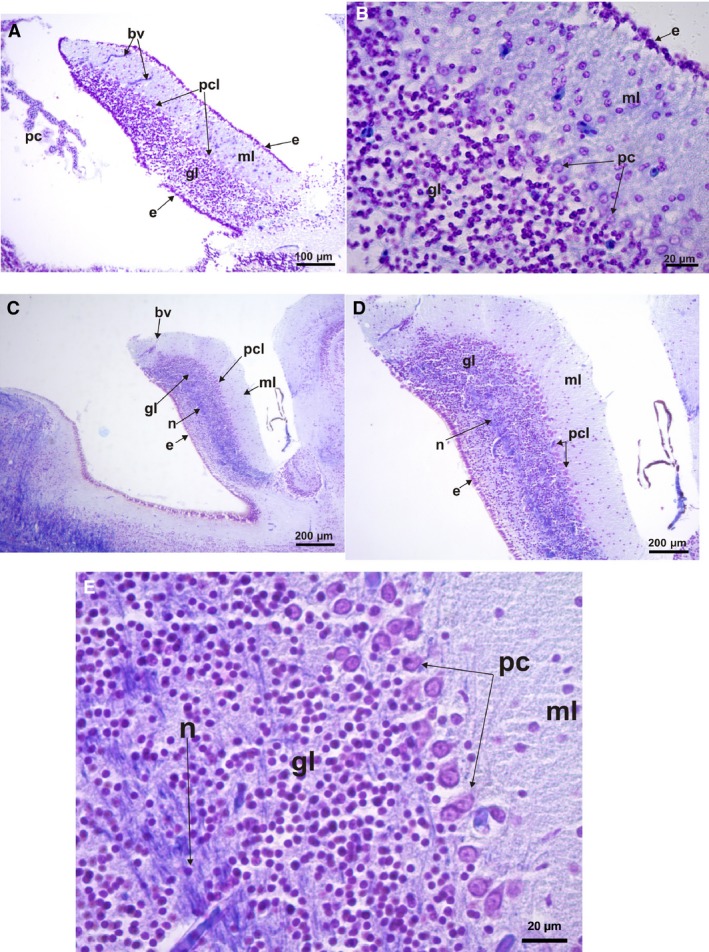
Histological parasagittal sections of the cerebellum (cresyl violet and fast blue stains). (A) Leptodactylus latrans showing its layers, molecular layer, granulae layer, and Purkinje cell layers; ependymal cells covered dorsal and ventral surfaces of the cerebellum. (B) Leptodactylus latrans – a detail of the small ordered Purkinje cells and the ingression of the granular cells to this layer and to the molecular layer. (C) Scinax fuscovarius showing its layers, molecular layer, granula layer, and Purkinje cell layers; ependymal cells covered dorsal surface. (D) Scinax fuscovarius – detail of the large Purkinje cells line‐ordered and the limited ingression of the granular cells to this layer and to the molecular layer. (E) Scinax fuscovarius – detail of the large Purkinje cells with projections to the molecular layer, below is the layer of small granular cells; a detail of the neuropil of nervous projection is shown. bv, blood vessel; e, ependymal cell; gl, granulae cells; ml, molecular layer; n, neuropil of cerebellar medulla; IVv, 4th ventricle; pc, Purkinje cells; pcl, Purkinje cells layer.
The cerebellum includes a superior molecular area, separated from the granular cells layer by a group of big Purkinje cells ordered in a line or two, but sometimes grouped in clusters (Figs 9 and 10). Isolated or clustered cells from the granular layer invade the Purkinje cell (PC) layer and in some cases the molecular layer. Inside the granular cell layer, a neuropil of the cerebellar medulla is evident, formed by projections of Purkinje cell axons (Figs 9A,B and 10C–E). Variations in the size of the Purkinje cells can be seen in the species analyzed by histological sections. Thus Purkinje cells are the largest in Phyllomedusa, intermediate‐sized in Scinax, and the smallest in Leptodactylus (Figs 9 and 10).
From the observation of parasagittal histological sections, variation in size and volume of the cerebellum, and the tendency of folding on top of the 4th ventricle is evident (Figs 9A and 10A,C). Moreover, sulcal lines can be observed in the cerebellum of Phyllomedusa. These sulcal lines seem to be related to the ingression of blood vessels (Fig. 9A,B). This ingression, from the dorsal surface to inside of the cerebellum, is similar in all cases (Figs 9A,C and 10A,B). In Scinax fuscovarius the cerebellum is wider (Fig. 10C) than in Leptodactylus latrans (Fig. 10A) but less than in P. sauvagii and P. boliviana (Fig. 9A). The Purkinje cells (PC) also differ in size and from L. latrans to S. fuscovarius and Phyllomedusa. In L. latrans, PC are smaller than in the other above‐mentioned frogs, and are grouped in clusters that sometimes are mixed with granular cells (Fig. 10B). In the rest, the PC are ordered in a line between granular layer and molecular layer, with less or no ingression of granular cells (Figs 9B,C and 10B,D,E). A visible layer of nervous projections seems to arise from the base of the cerebellum and run to the mesencephalon (Figs 9A,B and 10C–E). The dorsal and ventral surfaces of the cerebellum in L. latrans are covered by small cubical ependymal cells. In the rest of above‐mentioned frogs, ependymal cells only covered the ventral surface of the cerebellum (Figs 9A,B and 10C,D).
Choroid plexus and ependyma
The choroid plexus and ependyma produce the cerebrospinal fluid that surrounds the brain and spinal cord and that penetrates into the brain ventricles. In all species the choroid plexus is composed of a single layer of cuboidal or columnar cells derived from the ependyma and covering the dorsal midline of the central nervous system (Fig. 11A–C). The choroid plexus is formed by a single layer of flattened cells, although some cuboidal cells can be found at the level of the 4th ventricle (Fig. 11B,C). The surface covered by the choroid plexus is variable among species. In all climber‐walkers Phyllomedusa, climber‐jumper Hypsiboas, and the swimmer‐jumper Pseudis the rhombencephalon and 4th ventricle are partially covered by the choroid plexus (Figs 2A,B and 7A,E). In the climber‐jumper Scinax the choroid plexus fully covers the rhombencephalon. In the burrower Elachistocleis and the jumper Leptodactylus, the rhombencephalon is partially covered by the choroid plexus (Fig. 2F). In the terrestrial walker Rhinella, the rhombencephalon is wide and almost completely covered by the choroid plexus (Figs 2D and 7B).
Figure 11.
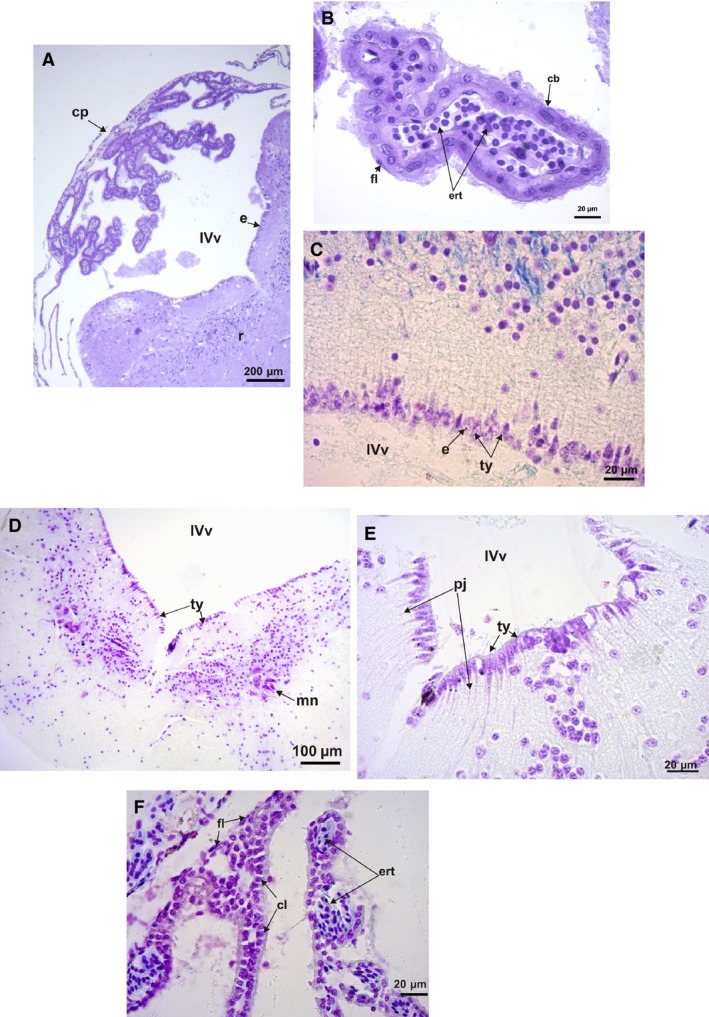
Histological section of the rhombencephalon and IV ventricle. (A) Phyllomedusa boliviana transverse section of the rhombencephalon and 4th ventricle, showing the choroid plexus covering the ventricle (Nissl stain). (B) Transverse section detail of the choroid plexus of P. boliviana showing cubical and flattened ependymal cells, and erythrocytes (Nissl stain). (C) Parasagittal section detail of Phyllomedusa sauvagii ependymal cells of the 4th ventricle, showing tanycytes and their projections and cuboidal ependymal cells (cresyl violet and fast blue stains). (D) Elachistocleis bicolor transverse section of the rhombencephalon and 4th ventricle (H‐E stain). (E) Elachistocleis bicolor transverse section detail of the ependymal cells of the 4th ventricle, showing tanycytes and its projection and cuboidal ependymal cells. (F) Parasagittal section detail of the choroid plexus of Leptodactylus latrans showing columnar and flattened ependymal cells, and erythrocytes (cresyl violet and fast blue stains). cb, cubical cells; cl, columnar cells; cp, choroid plexus; ert, erythrocytes; fl, flattened cells; pj, projections of tanycytes; ty, tanycytes; IVv, 4th ventricle; mn, motor nucleus of the V (trigeminus) nerve.
Tanycytes cover part of the ventricles between the ependymal cells (Fig. 6). At the level of the 3rd ventricle and between the hemispheres, the choroid plexus is intimately related to the paraphysis cerebri forming part of the epiphyseal complex (Fig. 6C–F). The paraphysis cerebri is a glandular organ developed in amphibians and non‐avian reptiles at the level of the 3rd ventricle (Hinton et al. 1990). Variations in the size of the paraphysis cerebri are evident among the climber‐jumper Hypsiboas pulchellus (Fig. 6D) and the climber‐walkers P. sauvagii and P. boliviana, being bigger in the latter two species (Fig. 6E,F), and less developed and cordon‐like in the hopper‐walker R. fernandezae (Fig. 6C).
Rhombencephalon
The rhombencephalon is funnel‐shaped and has a posterior indentation projecting to the medulla in all species analyzed (Figs 2 and 7). In all climber‐walkers of the genus Phyllomedusa (Fig. 2A,B), in the climber‐jumpers Scinax and Hypsiboas (Fig. 7A), and in the swimmer–jumper Pseudis (Figs 2E and 7C), the rhombencephalon is wider than in the other species analyzed.
In parasagittal histological sections, a clear disposition of the motor cell nuclei in a line along the rhombencephalon below the 4th ventricle between the cellular area and the tract can be observed (Fig. 12A,C). Interestingly the length of the rhombencephalon at the level of the 4th ventricle is variable, being longer in Phyllomedusa species (Fig. 12D).
Figure 12.
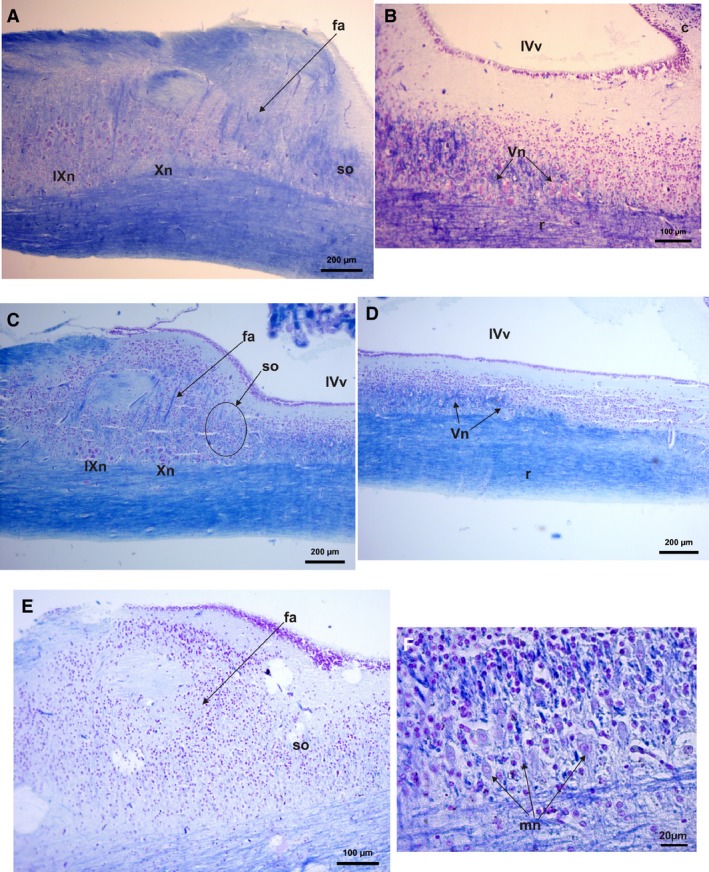
Histological parasagittal section of the rhombencephalon and 4th ventricle (cresyl violet and fast blue stains). (A) Phyllomedusa sauvagii caudal area of the rhombencephalon showing nucleus of nerves IX and X cells and the superior olive neuropil and cell area. Fibrae arquatiae fibers are evident. (B) Phyllomedusa sauvagii rhombencephalon showing nucleus and fibers of nerve V. (C) Scinax fuscovarius caudal area of the rhombencephalon showing nucleus of nerves IX and X cells and the superior olive neuropil and cell area. Fibrae arquatiae fibers are evident. (D) Scinax fuscovarius rhombencephalon showing nucleus and fibers of nerve V. (E) Leptodactylus latrans caudal area of the rhombencephalon showing nucleus of nerves IX and X cells and the superior olive neuropil and cells area. Fibrae arquatiae fibers are evident. (F) A close view of the nerve V cells at the rhomboenceplon. Fa, fibrae arquatiae fibers; IVv, 4° ventricle; IXn, spinal nerve IX nucleus of cells; mn, motor neurons of the spinal nerve V; Vn, spinal nerve V; r, rhombencephalon; SO, superior olive fibers and cells; X, spinal nerve X nucleus of cells.
In L. latrans the nuclei of the nerves IX and X are hard to distinguish compared with the rest of the analyzed species (Fig. 12E). In most them, two nuclei of medium to large motor cells of nerves IX and X are located caudal to the superior olive (a crucial part of the auditory olivary complex system, that integrate signals from the two ears and localize sound sources. Templin & Simmons, 2005), which also are big cells (Fig. 12A,C).
Plexus brachialis
In all species the relationship of the cranial nerve II (hypoglossus), spinal nerves III (brachial), and IV (thoracicus‐abdominal) can present variations after the spinal nerve III passes its spinal ganglion. The nerves II and IV can be connected along the spinal nerve III, such as in the climber‐walker Phyllomedusa, swimmer‐jumper P. minuta, and climber‐jumper Scinax fuscovarius. The nerve II can also be unconnected from the spinal nerve III, as in the burrower Elachistocleis bicolor, the hopper‐walker Rhinella fernandezae, the jumper Leptodactylus, and the climber‐jumper Hypsiboas pulchellus (Fig. 13A–F). In Elachistocleis there is also a branch of the brachialis nerve III that runs forward to the otic region of the head (Fig. 13E).
Figure 13.
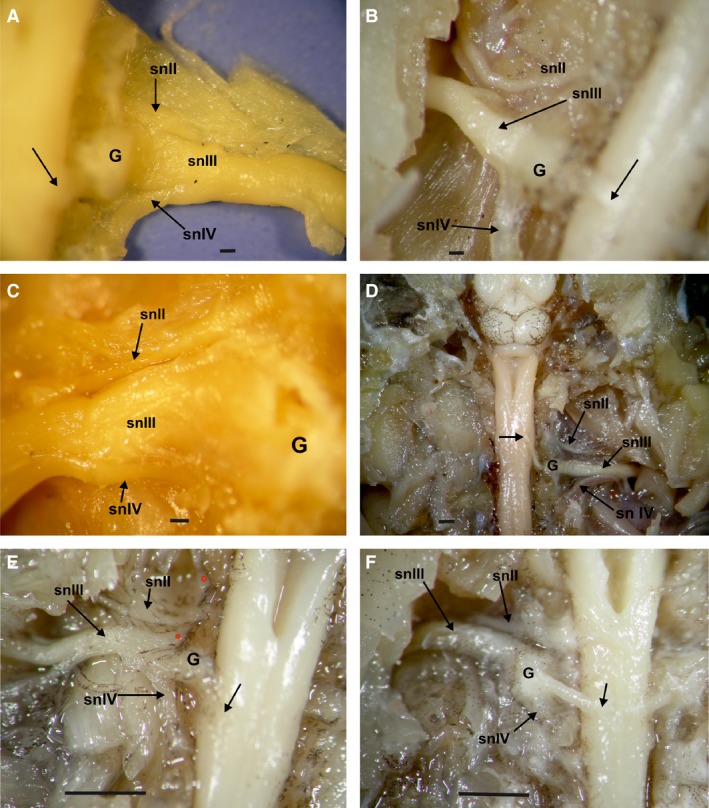
Dorsal view of spinal cord at the level of spinal nerve III and the plexus brachialis complex; the vertebra was removed exposing the dorsal ganglia. (A) Scinax fuscovarius dorsal view of the dorsal root of the spinal nerve III and its relation to the ganglia and the spinal nerve II and IV. (B) Hypsiboas pulchellus dorsal view of the dorsal root of the spinal nerve III and its relation to the ganglia and the spinal nerve II and IV. (C) Phyllomedusa sauvagii dorsal view of the dorsal root of the spinal nerve III and its relation to the ganglia and the spinal nerve II and IV. (D) Rhinella fernandezae dorsal view of the dorsal root of the spinal nerve III and its relation to the ganglia and the spinal nerve II and IV. (E) Elachistocleis bicolor dorsal view of the dorsal root of the spinal nerve III and its relation to the ganglia and the spinal nerve II and IV. A branch of the spinal nerve II is directed to the rostral area (red dots). (F) Leptodactylus latrans dorsal view of the dorsal root of the spinal nerve III and its relation to the ganglia and the spinal nerve II and IV. G, ganglion; H, Hypoglossus nerve (spinal nerve II); snII, spinal nerve II (Hypoglossus); sn III, spinal nerve III (brachial); snIV, spinal nerve IV. Arrows indicate the afferent branch of spinal nerve III, after the dorsal ganglia. Scale bar: 1 mm.
Quantitative analysis
A mancova with head width as covariate indicated significant effects of locomotor mode on brain compartment volume (Wilks’ λ = 0.004; F 28,33.87 = 4.37; P < 0.001). Similarly, a mancova with snout‐vent length (SVL) as covariate also showed significant effects of locomotor mode on brain volume (Wilks’ λ = 0.002; F 28,33.87 = 5.74; P < 0.001). However, whereas the univariate anovas suggested differences for diencephalon volume, the volume of the optic bulbs, the cerebellum, and the rhombencephalon when using SVL as a covariate, the rhombencephalon volume was not different between locomotor modes when using head width as a covariate (P = 0.07).
A multivariate analysis of variance (manova) similarly detected a significant effect of locomotor mode on brain dimensions (Wilks’ λ = 0.005; F 28,37.48 = 4.39; P < 0.001). Subsequent univariate analyses of variance (anovas) showed that locomotor modes differed in the volume of the olfactory (F 4,16 = 3.03; P = 0.049) and optic bulbs (F 4,16 = 4.90; P = 0.009), the volume of the telencephalon (F 4,16 = 3.17; P = 0.042), the diencephalon (F 4,16 = 5.65; P = 0.005), the cerebellum (F 4,16 = 10.41; P < 0.001), as well in overall brain size (F 4,16 = 3.77; P = 0.024). Post‐hoc tests showed that slow terrestrial species (burrowers and hoppers) differ from arboreal species (both arboreal walkers and jumpers) in diencephalon volume, with slow terrestrial species having the smallest diencephalon volume. The optic bulbs differed between slow terrestrial species and arboreal jumpers, with the latter having the largest optic bulbs. The cerebellum volume was different between the arboreal species (walkers and jumpers) and all terrestrial species (hoppers, jumpers, and burrowers), with the arboreal species having the largest cerebellar volume. No differences in telencephalon volume between groups were detected in our post‐hoc tests.
The principal component analysis performed on the log10‐transformed brain measurements extracted three axes jointly explaining 97.52% of the overall variation in the dataset (Table 3, Fig. 14). All variables loaded moderately negatively on the first axis, suggesting that this axis is an overall indicator of brain volume, with species such as E. bicolor having smaller brains and species such as P. sauvagii and P. boliviana having the largest overall brain size (Fig. 14). The second axis is determined by olfactory bulb volume, with arboreal species such as P. sauvagii and the burrower E. bicolor having the largest olfactory bulb volume, and the arboreal jumper H. pulchellus and the terrestrial jumper L. latrans having the smallest volume. The third axis is determined by the volume of the cerebellum, with species such as the aquatic P. minuta and the terrestrial jumper, L. latrans having a small cerebellum, in contrast to arboreal species such as H. pulchellus and P. hypochondrialis, which have a large cerebellum. When plotting the phylogeny in the morphospace it becomes clear that some phylogenetic structuring is present, with closely related species occupying similar areas of the morphospace. However, despite this phylogenetic clustering, the locomotor ecology of the species clearly also impacts the distribution of taxa as illustrated, for example by the different position of the aquatic hylid P. minuta compared with the closely related arboreal hylids on the third axis of the principal component analysis (PCA).
Figure 14.
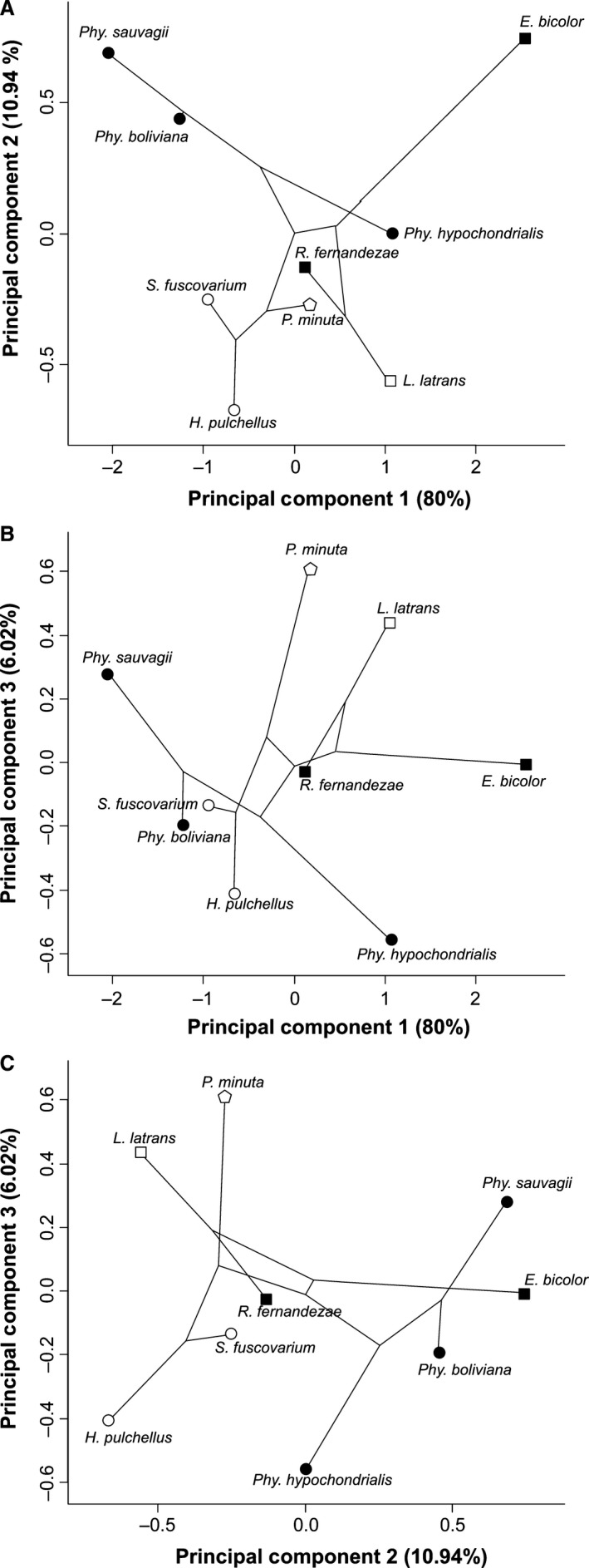
Scatterplot representing the results of a principal component analysis performed on the brain measurements. The phylogenetic tree is plotted with the morphospace to explore how phylogeny may structure differences among taxa. Top: scatterplot of principal components one and two. Middle: scatterplot of principal components one and three. Bottom: scatterplot of principal components two and three.
Discussion
Although a great deal of research has been devoted to our understanding of the nervous system, and especially the brain, it is remarkable that the majority of the studies are concentrated on a small number of amphibian species (Butler & Hodos, 1996; but see Liao et al. 2015). It is commonly considered that amphibians have a simplified brain organization, compatible with their supposedly limited behavioral complexity and reduced motor skills (Butler & Hodos, 1996). In fact, the brain in amphibians is often considered to represent the ancestral state in vertebrates (Leghissa, 1962; Dicke & Roth, 2009). The organization of the central nervous system in amphibians resembles that of other vertebrates (Dicke & Roth, 2009). However, our results show variations in brain morphology among species (but not within species) that are related to locomotor behavior, as suggested by our preliminary quantitative analyses. Arboreal animals appear to be different from others in showing larger cerebella, larger optic bulbs, and larger diencephala.We also detected an impact of locomotor mode on telencephalon volume, but the effect was weak and post‐hoc tests did not discriminate between groups. In previous studies, telencephalon volume was found to differ between frogs that live in different habitats, with arboreal species having larger telencephala (Liao et al. 2015). A qualitative inspection of our data suggest that our results show a similar trend for a very different set of taxa. Thus, overall our results suggest that an arboreal lifestyle may be driving differences in brain volume, with arboreal species being characterized by larger brains. Quite often, there was a contrast between terrestrial species and arboreal species, suggesting that the complex three‐dimensional nature of the arboreal habitat impacts the evolution of the brain. The volume of the diencephalon was, however, specifically different between slow terrestrial species such as hoppers and burrowers and arboreal species, suggesting that locomotor speed may also impact the evolution of brain morphology; however, this remains to be investigated further.
In all examined species the olfactory bulbs are prominent and perfectly identifiable structures located anterior to both cerebral hemispheres, being considered an evagination of them (Northcutt & Kicliter, 1980; Jungblut et al. 2011, 2012, 2013). Interestingly, there are variations in the relation to the vomeronasal nerve and the accessory bulb, and also in the timing of their development (Jungblut et al. 2011). The main olfactory nerve is variable in size and length, and in the form of its implantation cone. A thick olfactory nerve is present in Rhinella. A large glomerular area is particularly evident in hopper‐walkers such as Rhinella and burrowers such as Elachystocleis. The location of this structure, commonly related to the pheromone reception, is variable with respect to the main olfactory bulbs. The vomeronasal system appears to participate as a receptor of stimuli from the water in semi‐aquatic species (Jungblut et al. 2013). This organ is also functional in pre‐metamorphic anuran larvae as well as after the metamorphic climax. Cells of the olfactory bulbs are, in contrast, totally replaced by new neurons (Jungblut et al. 2011, 2013). The possible absence of the vomeronasal system in Pseudis, a secondarily aquatic species, is of interest but should be corroborated by additional samples. Olfactory nerves are especially wide in phyllomedusines and R. fernandezae. In this last species of frog, the localization of their burrows after long‐distance migration for reproduction is crucial (Sanchez & Busch, 2008). A wider olfactory nerve could be related to a better receptive olfactory system, helping them find their burrows. Although both groups share a walking mode of locomotion, they live in different habitats. Indeed, whereas phyllomedusines are arboreal frogs, Rhinella are strictly terrestrial, suggesting that other features such as predation risk may be driving the evolution of the olfactory bulb and nerve (Liao et al. 2015).
Two morphs of telencephalon are evident. Most of the studied species have a globoid shape. An elongated telencephalon is present in those species that not share a close common ancestor but do share a common locomotion such as jumping. In the case of a globoid telencephalon, they share mostly a walking mode of locomotion, otherwise they are climber‐jumpers, belonging to the Arborana group of frogs (Duellman et al. 2016). The diencephalon is a bipartite structure that is dorsally covered by the choroid plexus of the 3rd ventricle, forming the paraphysis cerebri. Interestingly, we found differences in the size of the diencephalon between jumpers and hopper‐walkers and burrowers, suggesting that jumpers develop a larger diencephalon. However, climber‐walkers also had a long diencephalon and aquatic swimmer‐jumpers a short diencephalon, suggesting that relationships between locomotor mode and the size of the diencephalon are not straightforward.
The epiphyseal complex in amphibians (on the dorsal side of the diencephalon) includes the paraphysis cerebri located anterior to the epiphysis (Norris & Carr, 2013). In general, the paraphysis cerebri is underestimated in the epiphyseal complex studies and its function is believed to be related to calcium metabolism, essential for the regulation of melatonin (Falcón, 1999) and almost all neuronal activities. Although considered part of the epiphyseal complex, the paraphysis cerebri develops from the telencephalon as a ‘sac‐like diverticulum’ (Norris & Carr, 2013).The anatomical differences could be related to differences in physiology; however, the small size of the samples prevent us from discarding the idea that the studied specimens were in a different metabolic stage. Variations have also been reported, for example, at the level of the dorsal thalamus, with asymmetries commonly present in amphibians and other vertebrates (Kemali & Braitenberg, 1969; Harris et al. 1996). These asymmetries are sexually dimorphic and are mediated by gonadal hormones.
We observed an iridescent tissue covering the epiphyseal complex in some hylid species (S. fuscovarius and Phyllomedusa). The function of this tissue is unclear but may be related to the frontal organ in frogs. Indeed, it has been stressed that the pineal complex is externally covered by a photosensitive tissue underlying the skin in amphibians (Bentley, 1998). Thus, the iridescent tissue could have a photosensory function. Nevertheless, a direct cell association with this tissue could not be observed. The evidence presented here suggests that structural asymmetry in the brain may be linked to the evolution of highly specialized systems. For example, the sulcal asymmetry in lizards and frogs appears to be linked to the entrainment of biological and behavioral rhythms to light cycles by extra‐retinal photoreceptive organs (Kemali & Braitenberg, 1969; Harris et al. 1996). Epiphyseal cells are derived from the ependymal ones during development in frogs and could differentiate as a sensory organ or endocrine gland, depending on the proliferation of the cell elements (Oksche, 1965). The optic bulbs and tectum play an important sensory role, mediated via the reticulo‐spinal pathways (Fagerstedt et al. 2001; Saitoh et al. 2007).
Our histological data show that the tectum mesencephali is conservative and is organized in a series of eight alternating layers of cells and fibers, as previously described by Ramon y Cajal (1984), Gaupp (1896), Potter (1965), and Lazar (1973). In general, anurans are characterized by an elaborated tectum (Butler & Hodos, 1996) and the layers are named from the periphery to the ventricle according to a functional pattern (Huber & Crosby, 1933). For example, cells forming lines 8 and 9 are related to the reception of retinal afferent fibers (Lazar, 1989). The degree of layer differentiation has been related to the differences in visual capacity (Ten Donkelaar, 1998). If it is considered that the tectum in reptiles is more laminated than in lobe‐finned fishes and salamanders, and that it does not appear to be more laminated than in anurans (Northcutt, 2002), it could be inferred that anuran visual capacities are more highly developed than those of salamanders (Roth et al. 1992). The even smaller number of tectal laminae of mammals were related to the possible nocturnal origins of mammals characterized by a decreased importance of visual stimuli and an increased reliance on audition and olfaction (Northcutt, 2002). Vision also plays a dominant role in prey‐capture behavior in frogs and is so specialized that frogs can see the object in movement from far away, in contrast to salamanders (An der Heiden & Roth, 1989).
Arboreal walker frogs exhibit skilled forelimb movements that may have originated early in tetrapod evolution, possibly as early as the divergence between amphibians and amniotes (Iwaniuk & Whishaw, 2000). The frogs in our sample usually inhabit semi‐arid or seasonally arid regions and have low rates of evaporative water loss, which seem to be favored by the lipids distributed on the skin by wiping (Blaylock et al. 1976). The most complex limb movements during wiping have been described in a group of frogs with opposable digits that include Polypedates maculatus and Phyllomedusa species (Lillywhite et al. 1998; Blaylock et al. 1976). Their opposable digit is also strongly associated to grasping in the context of locomotion (Manzano et al. 2008; Herrel et al. 2013). The primates, which have always been considered the most skilled of all vertebrates, the hand is coordinated by specific areas of the cortex of the telencephalon (Georgopoulos et al. 1982). Interestingly, the comparable dexterity of the hand in the arboreal walker frogs must have another coordination center, since frogs lack a true cortex (Northcutt, 1981) and corticospinal tract (Iwaniuk & Whishaw, 2000). It has been suggested that all the four descendent pathways probably act synergistically to mediate skilled forelimb movements in mammals (Iwaniuk & Whishaw, 2000). Yet, frogs are perfectly capable of executing skilled forelimb movements without one of these pathways. Rubrospinal, tectospinal, and reticulospinal tracts are present in frogs and are important in coordinating skilled forelimb movements, but not necessarily executing them (Iwaniuk & Whishaw, 2000). Arboreal walkers exploit a complex 3D narrow branch environment and need fine postural control of the forelimb during locomotion (Herrel et al. 2008, 2013; Manzano et al. 2008), which may at least partly explain the strongly developed cerebellum in these species. However, not only climber‐walkers (see also Taylor et al. 1995), but also hopper‐walkers and burrowing frogs have a large cerebellum.
Our data also show that the frog cerebellum is not a simple lamellar structure as previously described by Llinás & Precht (1976). Cerebellar circuits are also involved with the timing and coordination of movements and with the learning of motor skills (Ten Donkelaar, 1998). The cerebellum is particularly important in arboreal frogs with variation in the size, volume, and form being present in the species analyzed. The cerebellum is largest in Phyllomedusa frogs and the arboreal jumpers and, in addition, shows parallel folds or sulci. The cerebellum is folded in different angles: almost straight in Leptodactylus, a jumping frog; concave in Scinax, a climber‐jumper frog; and totally concave with sulci in Phyllomedusa. Some authors (Taylor et al. 1995; Ten Donkelaar, 1998) also related the size of the cerebellum to an arboreal mode of life. In fact, it has been stressed that damage in the cerebellum produced marked changes in posture and locomotion in tree frogs, whereas the consequences in terrestrial frogs seem minor (Steiner, 1885; Lutteroti, 1934). The layers made up by Purkinje cells in arboreal and terrestrial walkers such as Phyllomedusa, Elachistocleis, and Rhinella, are greater in number than in terrestrial jumpers and aquatic frogs. Moreover, Phyllomedusa shows the biggest group of large Purkinje cells distributed in several layers. The number of Purkinje cells in the cerebellum of arboreal frogs is double that in other frogs (Larsell, 1925, 1967; Lutteroti, 1934; Ten Donkelaar, 1998). The folds present in the cerebellum of Phyllomedusa could represent an increase in surface area that may translate into the number of the neurons and their interconnections, thus expanding the possibilities for processing information (Northcutt, 2002). An increasing complex network of Purkinje cells could be related to the need to generate complex or subtle movements.
In conclusion, variation is present in the central nervous system of frogs. We were able to identify variation at the histological level for some structures such as the cerebellum. Our data suggest that these variations may be related to locomotor mode, but this remains to be tested further. Moreover, the relationship of the hypoglossus nerve and spinal nerve III at the brachial plexus at the level of the pectoral girdle varies. A classification of nerve IV and its relation to the other two nerves that compose the plexus was given by Gaupp (1896). The nerve branches responsible for the innervations of the pectoral and fore limb muscles also show considerable variation, perhaps reflecting different muscle activities in specialized arboreal walkers (Herrel et al. 2008). Our data suggest that frogs are an excellent model to study variation in the central nervous system. Despite the significant differences in brain morphology detected, our results also suggest that brain morphology is at least partly structured by phylogeny. As such, the inclusion of a broader sample of frogs and other measures such as cell number would be of interest to test the idea that an arboreal locomotor mode drives brain evolution in frogs.
Author contributions
All authors contributed in a same way to the acquisition of the specimens and data; data analysis; elaborating interpretation, drafting of the manuscript, its critical revision and approval of the article.
Supporting information
Fig. S1. Dorsal view of the brain and telencephalon with details of the olfactory bulbs and nerves.
Fig. S2. Dorsal view of the brain of Hypsiboas pulchellus: (a) Lines through the landmarks used to brain measures, (b) same view of the brain but with a scale.
Acknowledgements
We would like to thank two anonymous reviewers for helpful and constructive comments on an earlier version of our manuscript. A.‐C. Fabre thanks the Marie‐Skłodowska Curie Fellowship (EU Project 655694 ‐ GETAGRIP) for funding. We also thank CONICET and SECYT: PIP CONICET 11220110100284; PIP 11220150100389 for funding. We thank Silvia Etcheverry for her assistance with the specimens and Pablo Aceñolaza for his assistance in statistics.
Appendix 1.
All specimens belong to the Herpetological collection of CICyTTP‐CONICET corresponding to the acronym DIAM.
Elachistocleis bicolor DIAM 395 (1 specimen) DIAM389 (1 specimen) DIAM 386 (1 specimen) DIAM 413 (1 specimen).
Hypsiboas pulchellus DIAM lot 434 (3 specimens) DIAM 478 (1 specimen) DIAM 039 (1 specimen), DIAM081 (1 specimen).
Leptodactylus chaquensis DIAM 041(1 specimen), DIAM 040 (1 specimen).
Leptodactylus latrans DIAM 126 (5 specimens).
Phyllomedusa boliviana, DIAM lote 452 (3 specimens).
Phyllomedusa hypocondrialis DIAM lote 336 (3 specimens).
Pseudis minutus DIAM 338 (3 specimens).
Phyllomedusa sauvagii DIAM lote 337 (2 specimens) DIAM lote 494 (2 specimens) DIAM lote 490 (3 specimens).
Rhinella fernandezae DIAM 146 (2 specimens), DIAM 451 (1 specimen) DIAM 434 (2 specimens).
Scinax fuscovarious DIAM 060 (5 specimens).
References
- An der Heiden U, Roth G (1989) Retina and optic tectum in amphibians: a mathematical model and stimulation studies In: Visuomotor Coordination: Amphibians, Comparisons, Models, and Robots. (eds Ewert JP, Arbib MA.), pp. 243–265. New York: Plenum Press. [Google Scholar]
- Bancroft JD, Gamble G (2001) Theory and Practice of Histological Techniques. London: Churchill Livingstone. [Google Scholar]
- Bentley PJ (1998) Comparative Vertebrate Endocrinology, 3rd edn, 527 pp. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Blaylock L, Ruibal R, Platt‐Aloia K (1976) Skin structure and wiping behavior of Phyllomedusinae frogs. Copeia 2, 283–295. [Google Scholar]
- Borchers HW, Burghagen H, Ewert J‐P (1978) Key stimuli of prey for toads (Bufo bufo L.): configuration and movement patterns. J Comp Physiol 128, 189. [Google Scholar]
- Bruschi DP, Busin CS, Toledo LF, et al. (2013) Evaluation of the taxonomic status of populations assigned to Phyllomedusa hypochondrialis (Anura, Hylidae, Phyllomedusinae) based on molecular, chromosomal, and morphological approach. BMC Genet 14, 70 http://www.biomedcentral.com/1471-2156/14/70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AB, Hodos W (1996) Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. Chichester: John Wiley. [Google Scholar]
- Deban SM, O'Reilly JC, Nishikawa KC (2001) The evolution of the motor control of feeding in amphibians. Am Zool 41, 1280–1298. [Google Scholar]
- Dicke U, Roth G (2009) Evolution of the amphibian nervous system In: Evolutionary Neuroscience. (ed. Kaas JH.), pp. 169–233, USA: Elsevier and American Press. [Google Scholar]
- Duellman WE, Marion AB, Blair Hedges S (2016) Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa 4104, 001–109. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Kelley DB (2001) Auditory and lateral line inputs to the midbrain of an aquatic anuran; neuroanatomic studies in Xenopus laevis . J Comp Neurol 438, 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert J‐P (1976) The visual system of the toad: behavioral and physiological studies on a pattern recognition system In: The Amphibian Visual System. (ed. Fite KV.). New York: Academic Press. [Google Scholar]
- Ewert J‐P (1987) Neuroethology of releasing mechanisms: prey catching in toads. Behav Brain Sci 10, 337–405. [Google Scholar]
- Fagerstedt P, Orlovsky GN, Deliagina TG, Grillner S, Ullen F (2001) Lateral turns in the lamprey. II. Activity of reticulospinal neurons during the generation of fictive turns. J Neurophysiol 86, 2257–2265. [DOI] [PubMed] [Google Scholar]
- Falcón J (1999) Cellular circadian clocks in the pineal. Prog Neurobiol 58, 121–162. [DOI] [PubMed] [Google Scholar]
- Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125, 1–15. [Google Scholar]
- Frost DR, Grant T, Faivovich J, et al. (2006) The amphibian tree of life. Bull Am Mus Nat Hist 297, 1–370. [Google Scholar]
- García del Moral R (1993) Manual de laboratorio de Anatomía Patológica. McGraw‐Hill/Interamericana Madrid, España.
- Gaupp E (1896) Anatomie des Frosches. Braunschweig: Friedrich Vieweg und Sohn. [Google Scholar]
- Georgopoulos A, Kalaska JF, Caminiti R, et al. (1982) On the relations between the direction of two‐dimensional arm movements and cell discharge in primate motor cortex. J Neuroscience 2, 527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafen A (1989) The phylogenetic regression. Philos Trans R Soc Lond B Biol Sci 326, 119–157. [DOI] [PubMed] [Google Scholar]
- Gray LA, Nishikawa KC (1995) Feeding kinematics of phyllomedusine tree frogs. J Exp Biol 198, 457–463. [DOI] [PubMed] [Google Scholar]
- Gray L, O'Reilly JC, Nishikawa KC (1997) Evolution of forelimb movement patterns for prey manipulation in anurans. J Exp Zool 277, 417–424. [DOI] [PubMed] [Google Scholar]
- Grillner S, Kozlov A, Dario P, et al. (2007) Modeling a vertebrate motor system: pattern generation, steering and control of body orientation. Prog Brain Res 165, 221–234. [DOI] [PubMed] [Google Scholar]
- Grobstein P, Comer C, Kostyk SK (1983) Frog prey capture behavior: between sensory maps and directed motor output In: Advances in Vertebrate Neuroethology. (eds Ewert J‐P, Capranica R, Ingle D.), pp. 331–347. New York: Plenum Press. [Google Scholar]
- Herrel A, Schaerlaeken V, Ross CF, et al. (2008) Electromyography and the evolution of motor control: limitations and insights. Integr Comp Biol 48, 261–271. [DOI] [PubMed] [Google Scholar]
- Herrel A, Perrenoud M, Decamps T, et al. (2013) The effect of substrate diameter and incline on locomotion in an arboreal frog. J Exp Biol 216, 3599–3605. [DOI] [PubMed] [Google Scholar]
- Harris JA, Guglielmotti V, Bentivoglio M (1996) Diencephalic asymmetries. Neurosci Biobehav Rev 20, 637–643. [DOI] [PubMed] [Google Scholar]
- Hinton DA, Nelson SR, Gatone VH (1990) Vasculature of the paraphysis cerebri of the frog. J Submicrosc Cytol Pathol 22, 345–351. [PubMed] [Google Scholar]
- Huber GC, Crosby EC (1933) A phylogenetic consideration of the optic tectum. Proc Natl Acad Sci U S A 19, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk AN, Whishaw IQ (2000) On the origins of skilled forelimb movements. Trends Neurosci 23, 372–376. [DOI] [PubMed] [Google Scholar]
- Jungblut L, Pozzi A, Paz D (2011) Larval development and metamorphosis of the olfactory and vomeronasal organs in the toad Rhinella arenarum (Hansel 1867). Acta Zool 92, 305–315. [Google Scholar]
- Jungblut LD, Pozzi AG, Paz DA (2012) A putative functional vomeronasal system in anuran tadpoles. J Anat 221, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungblut LD, Pozzi AG, Paz DA (2013) El sistema vomeronasal y su posible funcionalidad en larvas de anuros. Cuad Herpetol 27, 47–56. [Google Scholar]
- Junqueira LC, Carneiro J, Kelley RO (1995) Basic Histology, 8th edn Connecticut, USA: Appleton & Lange Press. [Google Scholar]
- Kemali M, Braitenberg V (1969) Atlas of the Frog's Brain. Berlin: Springer‐Verlag. [Google Scholar]
- Larsell O (1925) The development of the cerebellum in the frog (Hyla regilla) in relation to the vestibular and lateral‐line systems. J Comp Neurol 39, 249–289. [Google Scholar]
- Larsell O (1967) The Comparative Anatomy and Histology of the Cerebellum Form Myxinoids Through Birds. Minneapolis: University of Minnesota Press. [Google Scholar]
- Lazar G (1973) The development of the optic tectum in Xenopus laevis: a Golgi study. J Anat 116, 347–355. [PMC free article] [PubMed] [Google Scholar]
- Lazar G (1989) Cellular architecture and connectivity of the frog's optic tectum and pretectum In: Visuomotor Coordination: Amphibians, Comparisons, Models, and Robots. (eds Ewert JP, Arbib MA.), pp. 175–195. New York: Plenum Press. [Google Scholar]
- Leghissa S (1962) L'evolutine del tetto ottico nei bassi vertebrati (1). Arch Ital Anat Embriol 67, 343–413. [Google Scholar]
- Lettvin JY, Maturana HR, McCulloch WS, et al. (1968) What the frog's eye tells the frog's brain In: The Mind: Biological Approaches to its Functions. (eds Corning WC, Balaban M.), pp. 233–258. New York: Interscience Publishers. [Google Scholar]
- Liao WB, Lou LS, Zeng Y, et al. (2015) Evolution of anuran brains: disentangling ecological and phylogenetic sources of variation. J Evol Biol 28, 1986–1996. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB, Mittal AK, Garg TK, et al. (1998) Basking behavior, sweating and thermal ecology of the Indian tree frog, Polypedates maculatus . J Herpetol 32, 169–175. [Google Scholar]
- Llinás R, Precht W (1976) Frog Neurobiology: A Handbook. New York: Springer‐Verlag. [Google Scholar]
- Lutteroti M (1934) Kleinhirnextirpation bei Hyla arborea . Z Wiss Zool 145, 66–78. [Google Scholar]
- Manzano AS, Barg M (2005) The iliosacral articulation in Pseudinae (Anura: Hylidae). Herpetologica 61, 259–267. [Google Scholar]
- Manzano A, Abdala V, Herrel A (2008) Morphology and function of the forelimb in arboreal frogs: specializations for grasping ability? J Anat 213, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauwelaerts S, Aerts P (2006) Take‐off and landing forces in jumping frogs. J Exp Biol 209, 66–77. [DOI] [PubMed] [Google Scholar]
- Neary TJ (1990) The pallium of anuran amphibians In: Cerebral Cortex: Comparative Structure and Evolution of Cerebral Cortex. (eds Jones EG, Peters A.), pp. 107–138. New York: Plenum Press. [Google Scholar]
- Nishikawa KC, Anderson CW, Deban SM, et al. (1992) The evolution of neural circuits controlling feeding behavior in frogs. Brain Behav Evol 40, 125–140. [DOI] [PubMed] [Google Scholar]
- Norris D, Carr J (2013) Vertebrate Endocrinology, 5th edn Boulder, Co, USA: Academic Press. [Google Scholar]
- Northcutt RG (1981) Evolution of the telencephalon in non‐mammals. Annu Rev Neurosci 4, 301–350. [DOI] [PubMed] [Google Scholar]
- Northcutt RG (2002) Understanding vertebrate brain evolution. Integr Comp Biol 42, 743–756. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Kicliter E (1980) Organization of the amphibian telencephalon In: Comparative Neurology of the Telencephalon. (ed. Ebbesson SOE.), pp. 203–255. New York: Plenum Publishing Corporation. [Google Scholar]
- Northcutt RG, Royce JG (1975) Olfactory bulb projections in the bullfrog Rana catesbeiana . J Morphol 145, 251–267. [DOI] [PubMed] [Google Scholar]
- Oksche A (1965) Survey of the development and comparative morphology of the pineal organ. Prog Brain Res 10, 3–29. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K (2004) ape: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. [DOI] [PubMed] [Google Scholar]
- Potter DH (1965) Mesencephalic auditory region of the bullfrog. J Neurophysiol 28, 1132–1154. [DOI] [PubMed] [Google Scholar]
- R Core Team (2016) R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- Ramon y Cajal S (1984) Neuroglia In: The Neuron and the Glial Cell. (eds De La Torre J, Gibson WC.), pp. 263–290. Springfield: Charles C Thomas. [Google Scholar]
- Revell LJ (2012) Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3, 217–223. [Google Scholar]
- Roelants K, Gower DJ, Wilkinson M, et al. (2007) Global patterns of diversification in the history of modern amphibians. Proc Natl Acad Sci U S A 104, 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G, Dicke U, Nishikawa K (1992) How do ontogeny, morphology and physiology of sensory systems constrain and direct the evolution of amphibians? Am Nat 139, S105–S124. [Google Scholar]
- Saitoh K, Menard A, Grillner S (2007) Tectal control of locomotion, steering, and eye movements in lamprey. J Neurophysiol 97, 3093–3108. [DOI] [PubMed] [Google Scholar]
- Sanchez LC, Busch M (2008) Population traits of the burrowing toad Rhinella fernandezae (Gallardo, 1957) (Anura, Bufonidae). Braz J Biol 68, 137–140. [DOI] [PubMed] [Google Scholar]
- Steiner J (1885) Die Funktion des Centralnervensystems und Ihre Phylogenese. Braunschweig: Friedrich Vieweg und Sohn. [Google Scholar]
- Sustaita D, Pouydebat E, Manzano A, et al. (2013) Getting a grip on tetrapod grasping: form, function, and evolution. Biol Rev 88, 380–405. [DOI] [PubMed] [Google Scholar]
- Taylor GM, Nol E, Boire D (1995) Brain regions and encephalization in anurans: adaptation or stability? Brain Behav Evol 45, 96–109. [DOI] [PubMed] [Google Scholar]
- Templin T, Simmons AM (2005) Cellular and spatial changes in the anuran superior olive across metamorphosis. Hear Res 207, 87–98. [DOI] [PubMed] [Google Scholar]
- Ten Donkelaar HJ (1998) Anurans In: The Central Nervous System of Vertebrates. (eds Nieuwenhuys R, Ten Donkelaar HJ, Nicholson C.), pp. 1151–1314. London: Springer. [Google Scholar]
- Ullmann J, Cowin G, Collin SP (2010) Quantitative assessment of brain volumes in fish: comparison of methodologies. Brain Behav Evol 76, 261–270. [DOI] [PubMed] [Google Scholar]
- Wicht H, Himstedt W (1988) Topologic and connectional analysis of the dorsal thalamus of Triturus alpestris (Amphibia, Urodela, Salamandridae). J Comp Neurol 267, 545–561. [DOI] [PubMed] [Google Scholar]
- Yopak K, Lisney TJ (2012) Allometric scaling of the optic tectum in cartilaginous fishes. Brain Behav Evol 80, 108–126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Dorsal view of the brain and telencephalon with details of the olfactory bulbs and nerves.
Fig. S2. Dorsal view of the brain of Hypsiboas pulchellus: (a) Lines through the landmarks used to brain measures, (b) same view of the brain but with a scale.


