Abstract
This study examined whether exposure to Hurricane Sandy-related stressors altered children's brain response to emotional information. An average of 8 months (Mage=9.19) before and 9 months after (Mage=10.95) Hurricane Sandy, 77 children experiencing high (n=37) and low (n=40) levels of hurricane-related stress exposure completed a task in which the late positive potential (LPP), a neural index of emotional reactivity, was measured in response to pleasant and unpleasant, compared to neutral, images. From pre- to post-Hurricane Sandy, children with high stress exposure failed to show the same decrease in emotional reactivity to unpleasant vs. neutral stimuli as those with low stress exposure. Results provide compelling evidence that exposure to natural disaster-related stressors alters neural emotional reactivity to negatively valenced information.
Keywords: disaster exposure, children, emotional development, stress, event-related potentials
Recent research has demonstrated the profound influence of environmental stress on neurodevelopment (Lupien, McEwen, Gunnar, & Heim, 2009). Converging evidence from animal and human studies indicates that early life stress has enduring neurobiological consequences across development (Cohen et al., 2013, Ganzel et al., 2013). A history of childhood maltreatment is associated with heightened amygdala reactivity to negative facial cues and reduced grey matter volume in the hippocampus, insula, orbitofrontal cortex, anterior cingulate gyrus, and caudate in adolescents and adults (Dannlowski et al., 2013). Paralleling these findings, maternal deprivation in infancy produces elevated amygdala activity and emotional reactivity in response to stressors in adult laboratory animals (e.g. Cohen et al., 2013). Thus, it has been hypothesized that early adversity may sensitize neural systems underlying negative emotional processing, making individuals more reactive to potential threats in the future (Teicher et al., 2003).
A smaller body of research suggests that stressors may also have long-term effects on mesolimbic dopamine pathways supporting processing of appetitive stimuli that consequently blunt reactivity to pleasurable and rewarding stimuli (for a review see: Pizzagalli, 2014). In humans, individuals with a history of childhood emotional, physical, and/or sexual abuse demonstrate reduced activation in the left basal ganglia—a brain region implicated in reward learning and motivation—compared to controls (Dillon et al., 2009). Additionally, relative to controls, adolescent primates who were deprived of parental care during infancy were found to demonstrate impaired reward learning and reduced reward sensitivity (Pryce, Dettling, Spengler, Schnell & Feldon, 2004). Taken together, these studies suggest that stress may have detrimental but opposing influences on neural systems supporting positive and negative emotional processing.
Research examining the impact of stress on the developing brain has focused largely on childhood maltreatment. However, it is difficult to pinpoint the precise cause of neural changes associated with maltreatment as they are influenced by numerous factors working alone or in tandem (e.g., poverty, substance abuse, low educational attainment, family violence, multiple forms of abuse and neglect). In contrast to maltreatment, environmental stressors associated with exposure to natural disasters provide an opportunity to examine the consequences of fateful negative life events (Dohrenwend, 2006) that are unrelated to the individual's background. The present study examined the impact of Hurricane Sandy—a Category 1 storm system that struck the tri-state area on October 25th, 2012 and became the second costliest hurricane in American history (Neria & Schultz, 2012)—on the neural reactivity to emotional stimuli in children.
Most studies of the effects of stressors on neurodevelopment have assessed neural abnormalities only after the stressor. This makes it difficult to determine whether neurobiological differences are an effect of the stressor or a continuation of pre-existing abnormalities. Furthermore, it is critical to examine emotional reactivity over time, rather than in a single measurement, to understand how the environment impacts neurodevelopment (Casey et al., 2009). The literature suggests that typical development entails a decrease in negative emotional reactivity with age due to maturation of the prefrontal cortex, which provides greater top-down regulatory control of limbic system activation (Gee et al., 2013; Kujawa, Klein & Hajcak, 2012; MacNamara et al., 2015; Swartz et al., 2014; Swartz, Williamson & Hariri, 2015; but see Somerville, Jones & Casey, 2010 for contrary findings). On the other hand, neural reactivity to reward and positively valenced stimuli increases from childhood to adolescence, and this increase has been hypothesized to reflect an imbalance between motivational drive and behavioral control mechanisms (Braams, van Duijvenvoorde, Peper & Crone, 2015; Somerville, Jones & Casey, 2010; Spear, 2011; but see MacNamara et al. 2015 for an exception). Hence, it is important not only to determine whether stress potentiates and blunts neural reactivity to negative and positive emotional stimuli, respectively, but also whether they interfere with normative age-related changes in neural reactivity.
We had the rare opportunity to build on a pre-existing study of child development in a region that was directly affected by Hurricane Sandy enabling us to extend our understanding of the association between environmental stress and the development of neural systems associated with emotion processing. Specifically, we used the late positive potential (LPP), an event-related potential (ERP) indexing emotional reactivity (Schupp, Junghöfer, Weike, & Hamm, 2004), to examine the impact of Hurricane Sandy on 77 children's neural reactivity to neutral, pleasant, and unpleasant images before and after the disaster. The LPP begins around 200 ms after stimulus onset and is potentiated when viewing emotional relative to neutral stimuli; thus it is larger for both pleasant and unpleasant compared to neutral stimuli. The LPP is typically analyzed as the difference between the mean amplitudes to emotional and neutral stimuli to isolate neural reactivity modulated by emotional content. More positive ΔLPPs reflect enhanced attentional engagement with emotional stimuli. Moreover, the LPP can be reliably measured in children and adolescents, and is well-suited to measure emotional reactivity across development (Kujawa et al., 2012; MacNamara et al., 2015). We hypothesized that children with high exposure to Hurricane Sandy-related stressors would show increases (or fail to show the expected decrease) in the ΔLPP to unpleasant emotional stimuli and decreases (or fail to show expected increases) in the ΔLPP to pleasant emotional stimuli.
Method
Participants
The sample included 77 children assessed during middle childhood (Mage = 9.19, SD = 0.35), an average of 8 months before the hurricane, and again an average of 9 months after Hurricane Sandy (Mage = 10.95, SD = 0.77). The sample was derived from a larger longitudinal study of families with young children (N = 609; Olino et al., 2010) who were recruited from the community using commercial mailing lists. Children were eligible to participate in the study if they did not have a significant medical condition or developmental disability and were living with at least one English-speaking biological parent.
Hurricane Sandy struck Long Island shortly before the age 9 wave of assessments were completed. Six weeks after the hurricane, mothers were asked to complete the Hurricane Sandy Stress Exposure Inventory (HSSEI; Kopala-Sibley, Danzig et al., in press; Kopala-Sibley, Kotov et al., in press, Kujawa et al., in press), a web-based questionnaire on the impact of Hurricane Sandy Mothers were asked, “What happened to you as a result of Hurricane Sandy and its aftermath?” The 13 items (Box 1) were drawn from previous measures administered in studies of Hurricane Ike (Norris, Sherrieb & Galea, 2010) and Hurricane Katrina (Galea et al., 2007). Items 1-8 were rated on a 5-point scale (1=not all affected; 5=extremely affected; items 9-10 were rated on duration (1=0 days; 5=2 weeks or more); items 11-13 were rated as present/absent. To create an overall exposure severity score, non-dichotomous items were rescaled such that 0 = absent and 1 = present. We selected cutoffs for each item using a combination of statistical and clinical considerations that distinguished a subgroup of participants with a high and clinically significant level of stress on that item. This involved examining the distribution of responses on each item and considering the nature of the stressor and the response options. For most items, we selected a cutoff of 4 (affected quite a bit; where 3= moderately and 5 = extremely), thinking that for most of these experiences, responses of “quite a bit” and “extremely” would indicate a meaningful degree of stress, whereas the significance of a response of “moderately” was less clear. However, there were two exceptions. “Quite a bit” was the modal response for difficulty finding gasoline. Given the high frequency of this response, we chose to err on the conservative side and required a rating of 5 (“extremely”) for this item. In contrast, responses to the item on financial hardships were extremely skewed, with very few respondents choosing responses of 4 and 5. This may, in part, have been due to the phrasing of the item (“hardships”), which suggested a high threshold for endorsement. Hence, we selected a cutoff of 3 (“moderately”) for this item. Total scores therefore ranged from 0-13. This scale showed adequate internal consistency (Cronbach's alpha = .72).
Box 1. Items Comprising the Hurricane Sandy Stress Exposure Inventory.
Damage occurred to home and/or possessions
Self or family's safety threatened
Financial hardships
Children fear for safety
Life disruption
Difficulty finding gasoline
Difficulty getting enough water, food, and heat
Increased quarrelling/complaining by children
Duration of power loss
Duration of school closing
Injury/robbery of family members
FEMA/Red Cross application
Evacuation
Of the 407 mothers whose child completed the age 9 ERP assessment prior to Hurricane Sandy, 323 (79.3%) families were in the area at the time of Hurricane Sandy and completed the post-hurricane questionnaire. Questionnaires were completed an average of 8.4 (SD = 1.5) weeks after the hurricane. Although all families included in the current study lived in FEMA-declared disaster areas, there was considerable variation in the degree to which participants were affected by the hurricane. The most commonly endorsed exposures were extended periods of time without school (50.2%) and power (35.0%), followed by a serious disruption of life due to Hurricane Sandy (23.2%) and children fearing for their safety during and/or after the hurricane (23.8%). A number of families also experienced difficulty finding food or warmth (19.2%), difficulty finding gasoline (16.4%), children complaining more than usual (16.1%), family's safety threatened (13.3%), financial hardship (12.4%) and damage to home or possessions (11.8%). The least commonly endorsed items were home evacuation (4.3%), requesting FEMA aid (4.3%), and family members and/or pets being injured, victimized, robbed, or lost (3.4%). The mean number of stressors reported were 2.33 (SD= 2.26)
During the summer after Hurricane Sandy (8-10 months post-Sandy (June 2012-August 2012), 48 children who were below and 45 children who were above the sample mean of hurricane-related stress exposure were invited to the lab to repeat the identical ERP assessment of emotional reactivity. Among children above the mean, we oversampled those with greater stress. Of those 93 children, 9 participants were excluded due to poor EEG data quality at the age 9 assessment and 7 participants were excluded from analyses due to task refusal or data loss or poor quality EEG data at the post-Hurricane Sandy assessment, resulting in a final sample of 77 participants (40 with low and 37 with high exposure). This reports final sample was 90.9% Caucasian, 5.2% Black or African American, 2.6% Asian and 1.3% Native American. Ethnically, 11.7% of the final sample was of Hispanic or Latino origin. The research protocol was approved by the Stony Brook University Institutional Review Board. At each assessment, parents provided written informed consent and children provided verbal assent. Families were financially compensated for their time.
Procedure
Emotion Reactivity Task
The LPP was measured using the emotion interrupt task (Kujawa et al., 2012), which is a computerized paradigm that requires participants to press either the left or right mouse button in response to a target (a left or right arrow) presented in-between the presentation of developmentally appropriate neutral, pleasant, or unpleasant images from the International Affective Picture System (IAPS; Lang, Bradley & Cuthbert, 2008). The emotional interrupt task provides advantages over a passive picture-viewing task because it can confirm that the participants are paying attention by only examining trials in which their response to the target was correct. A total of 60 images were presented: 20 neutral (e.g., outdoor scenes, household objects), 20 pleasant (e.g., children playing, cute animals, babies), and 20 unpleasant (e.g., sad or angry people, weapons, scary animals). The task included 120 total trials and each image was randomly presented once in each of two blocks. Each trial began with an 800 ms fixation (+), and then an image was presented for 1000 ms followed by a target (< or >) presented for 150 ms and the same picture presented for an additional 400 ms. The inter-trial interval varied randomly between 1500 and 2000 ms. Participants were instructed to respond as quickly as possible to the target (left or right arrow) by clicking the corresponding left or right mouse button (see Kujawa et al., 2012 for further details).
EEG Recording and Analysis
Continuous electroencephalography (EEG) was recorded using a 34-channel Biosemi system based on the 10/20 system (32-channel cap with the addition of Iz and FCz). Two electrodes were placed on the left and right mastoids, and the electrooculogram (EOG) generated from eye blinks and movements was recorded from two pairs of facial electrodes. One pair was approximately 1 cm above and below the participant's left eye to detect vertical eye movement; the other electrode pair was placed approximately 1 cm to the left of the left eye and 1 cm to the right of the right eye to detect horizontal eye movements. The Common Mode Sense active electrode and the Driven Right Leg passive electrode formed the ground electrode during acquisition. The data were digitized using ActiView software at 24-bit resolution with a LSB value of 31.25 nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. Off-line analyses were performed using Brain Vision Analyzer software (Brain Products). All data were converted to a mastoid reference and band-pass filtered with cutoffs of 0.1 and 30 Hz. The EEG was segmented for each trial, beginning 200 ms before each picture onset and continuing for 1000 ms after the initial image presentation. The EEG was corrected for eye blinks (Gratton, Coles & Donchin, 1983), and semi-automated artifact rejection was used to remove artifacts with a voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, or a maximum voltage difference of less than 0.5 μV within 100 ms intervals. Visual inspection was then used to reject trials in which additional artifacts were observed.
ERPs were constructed by separately averaging the responses to neutral, pleasant, and unpleasant images. Only correct trials were included in averages. ERPs were baseline corrected to the 200 ms interval prior to stimulus onset. The LPP was scored as the mean activity 400–1000 ms after the onset of the pre-target image averaged at occipital (Oz, O1, and O2) and parietal (Pz, P3, and P4) sites, which is consistent with previous research (Kujawa et al., 2012) and is where the difference between emotional and neutral images was maximal.
Results
Table 1 presents descriptive statistics for children with low and high Hurricane Sandy exposure. There were no differences between the groups in the LPP to neutral images at either the pre- (p = .51) or post- (p = .90) disaster assessment. Therefore, we calculated difference scores (i.e., pleasant-neutral, unpleasant-neutral) to examine ΔLPP potentiation. To examine the influence of Hurricane Sandy exposure on the ΔLPP, we conducted a Time (pre- vs. post-disaster) × Valence (pleasant-neutral vs. unpleasant-neutral) × Hurricane Sandy Exposure (low vs. high) mixed-measures analysis of variance (ANOVA). There were significant main effects of valence, F(1, 75) = 12.52, p < .001, and time F(1, 77) = 4.47, p = .038, that were qualified by a Time × Valence interaction F(1, 75) = 4.08, p = .047 and a Time × Valence × Hurricane Sandy Exposure interaction, F(1, 75) = 4.90, p = .03, ηp2 = .06. There was no significant main effect of Hurricane Sandy Exposure, F(1, 75) = 0.39, p = .54, or significant Valence × Exposure, F(1,75) = 0.23, p = .63 or Time × Exposure, F(1,75) = 0.90, p = .35, interactions. The significant Time × Valence × Hurricane Sandy Exposure interaction was followed-up by conducting separate Time × Hurricane Sandy Exposure mixed-measures ANOVAs for unpleasant and pleasant stimuli. For ΔLPPs to unpleasant stimuli, there was a main effect of time, F(1, 75) = 9.46, p < .01, that was qualified by a Time × Hurricane Sandy Exposure interaction, F(1, 75) = 4.79, p = .03, ηp2 = .06. As shown in Figure 1, participants with low Hurricane Sandy exposure exhibited the expected decrease in the ΔLPP to unpleasant stimuli from the pre- to post-Hurricane Sandy assessment, F(1, 39) = 8.08, p = .007, Cohen's d = .61. In contrast, for high exposure participants, the ΔLPP to unpleasant stimuli did not differ between the assessments, F(1, 36) = 0.01, p = .93. There were no significant main or interaction effects in the ANOVA examining changes in the ΔLPP to pleasant images for either group. (The analysis of independent developmental data documenting LPP reduction with age is included as supplementary material.)
Table 1. Descriptive Statistics for Participants with Low and High Hurricane Sandy Exposure.
| Low Exposure (n = 40) | High Exposure (n = 37) | χ2 or t | |
|---|---|---|---|
| Demographics | |||
| Age pre-Hurricane assessment (yrs) | 9.23 (0.42) | 9.14 (0.27) | t = 1.00 |
| Age post-Hurricane assessment (yrs) | 11.02 (0.78) | 10.87 (0.78) | t = 0.63 |
| Sex (% females) | 52.5% | 56.8% | χ2 = 0.14 |
| Race (% Caucasians) | 87.5% | 78.4% | χ2 = 1.14 |
| Parental education (% with at least one parent graduated from college) | 70.0% | 78.4% | χ2 = 0.70 |
| Socioeconomic Statusa | 42.47 (9.36) | 46.24 (11.31) | t = 2.55 |
| Hurricane Sandy exposures | 0.98 (0.80) | 5.65 (2. 21) | t = 156.34** |
| Pre-Hurricane Sandy | |||
| LPP | |||
| Neutral | 4.47 (10.17) | 5.93 (9.03) | t = .44 |
| Pleasant | 7.16 (10.21) | 9.44 (8.85) | t = 1.09 |
| Unpleasant | 10.99 (10.13) | 11.48 (7.35) | t = 0.06 |
| ΔLPP | |||
| Pleasant | 2.37 (6.80) | 3.51(8.02) | t = 0..23 |
| Unpleasant | 6.52 (6.50) | 5.55(6.23) | t = 0.45 |
| Post-Hurricane Sandy | |||
| LPP | |||
| Neutral | 4.39 (6.25) | 4.57 (6.23) | t = 0.15 |
| Pleasant | 6.43 (5.94) | 6.51 (6.69) | t < .01 |
| Unpleasant | 7.40 (7.36) | 10.21 (6.42) | t = 3.18† |
| ΔLPP | |||
| Pleasant | 2.03 (4.75) | 1.94 (4.76) | t < .01 |
| Unpleasant | 3.00 (4.92) | 5.64 (6.12) | t = 4.38* |
Figure 1.
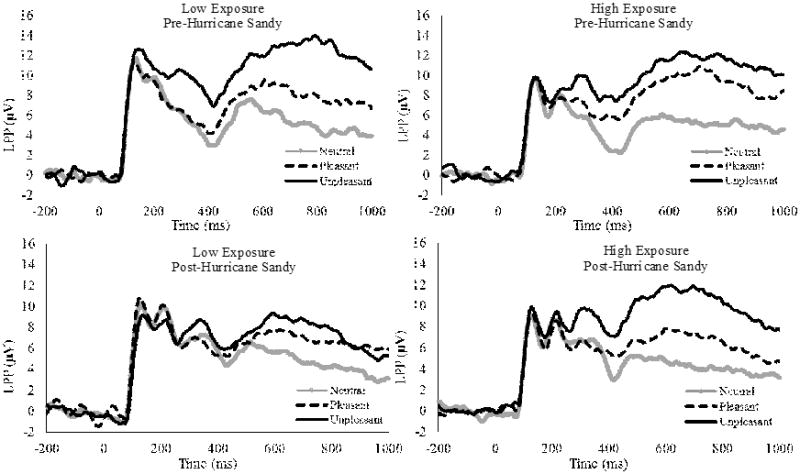
Waveforms displaying the LPP to neutral, pleasant, and unpleasant stimuli. The LPP waveforms were pooled across occipital (Oz, O1, and O2) parietal (Pz, P3, and P4) electrodes.
Discussion
The present study examined whether Hurricane Sandy-related stress exposure impacted the neural processing of positive and negative, compared to neutral, emotional stimuli. Consistent with our hypothesis, we found that high Hurricane Sandy-related stress exposure altered the trajectory of emotional reactivity to unpleasant vs. neutral stimuli, such that children with high hurricane-related stress exposure did not show the same reduction in the ΔLPP to unpleasant stimuli exhibited by children with low exposure. This is consistent with previous research indicating that stressful life events (SLE) are associated with greater negative emotional reactivity (e.g., Cohen et al., 2013; Ganzel et al., 2013). However, very few studies (e.g., Swartz, Williamson & Hariri, 2015), and to our knowledge none in children, have taken into account prior neural functioning to examine the effect of stress on changes in the neural processing of emotional stimuli. This is also the first study to do this while also examining stress associated with a fateful (i.e. independent of an individual's behavior) stressor like a natural disaster. Thus, we were able to both minimize the contribution of preexisting participant characteristics to the occurrence of the stressor and rule out the possibility that the observed effects were merely reflective of prior neural abnormalities. These are particularly informative findings given the general paucity of research examining the effects of natural disasters on children's neural functioning (see Weems, 2015).
Taking into account pre-disaster baseline data also enables us to examine how disaster-related stress exposure influences emotional reactivity in youth disaster victims. Whereas the majority of studies suggest that SLE facilitate defensive responses by augmenting vigilance to threatening information (Ganzel et al., 2014), our findings suggest that fateful negative life events (Dohrenwend, 2006), like Hurricane Sandy, do not in fact appear to enhance emotional reactivity to negative emotional information in preadolescent children. Consistent with prior research (MacNamara et al., 2015; see supplementary data), findings from the present study suggest that there are age-related reductions in the ΔLPP to unpleasant compared to neural stimuli. Thus, it appears that SLE may disrupt typical neurodevelopment in a manner that maintains relatively heightened levels of vigilance to threatening and more emotionally arousing information in the environment in a manner that is characteristic of younger children (Teicher et al., 2003). This raises the possibility that stressors associated with fateful events like natural disasters interact with the ongoing dynamic development of the brain to impact emotional reactivity at the neural level differently for children, adolescents, and adults. An alternative explanation is that children with high levels of disaster-related stress exposure failed to habituate to a repeated administration of unpleasant stimuli after Hurricane Sandy. However, there is little evidence to suggest that the ΔLPP to negative vs. neutral stimuli exhibits habituation over extended periods of time (in this case, a mean of 1.8 years) between assessments. Regardless, the fact that this pattern of reactivity was evident nine months after Hurricane Sandy suggests that disaster-related stress exposure may have a persisting impact on brain functioning.
In comparison to the observed changes in the ΔLPP to unpleasant images and the impact of high exposure to Hurricane Sandy-related stressors on its development, we did not find differences between children with low and high Hurricane-related stress exposure on changes in the ΔLPP to pleasant stimuli. While early adversity has been shown to be associated with diminished positive valence system functioning (e.g., Dillon et al., 2009), this association may be more closely associated with chronic stress rather than a relatively acute stressful life event, like Hurricane Sandy. Indeed, one recent study found that among healthy controls, acute stress did not impact reward functioning, and even increased reward sensitivity among depressed individuals (Kumar et al., 2015). Additionally, our findings conflict with other studies that find sensitivity to reward and pleasurable stimuli increase from childhood to adolescence (Braams, van Duijvenvoorde, Peper & Crone, 2015; Somerville, Jones & Casey, 2010; Spear, 2011). Differences in sample ages, stimuli and measures used may account for this discrepancy. As the majority of children in the current sample had not yet reached adolescence, it is possible that such increases are only apparent later in development. Moreover, appetitive images in the present study (e.g., cute animals and babies) were relatively low in salience compared to studies using monetary reward or other stimuli that would be developmentally inappropriate for our sample (e.g., erotica). Mounting evidence also suggests that premorbid vulnerabilities influence developmental trajectories of reward functioning (e.g., Urosevic, Collins, Schissel, Lim & Lucian, 2015), thus acute stress may impact reward system development or functioning only among a subset of vulnerable individuals. It is important to note, however, that we did not assess whether Hurricane Sandy-related stressors (e.g. damage to the home and financial burden) were still impacting children at the time of their second lab visit. Thus, it is possible, that at least for some children, Hurricane Sandy-related stressors persisted.
The present study's focus on 9-11 year-old children may inform our understanding of the effects of adversity on brain development as a function of timing. Late childhood is characterized by a global shift in cognition, motivation, and social behavior. It is theorized that this shift is accompanied by heightened brain plasticity in which the brain is collecting input from the environment in order to promote development that serves to increase the biological fitness within that milieu (Del Guidice, 2014). Thus, late childhood may mark a developmental phase during which the brain is more vulnerable to stressors like Hurricane Sandy (Casey et. al., 2010; Lupien, McEwen, Gunnar & Helm, 2009). This vulnerability to acute stressors may be greater for negative or threatening than positive stimuli, as having the capacity to detect and respond to environmental dangers and threats effectively may be more pertinent to improving an individual's ability to successfully navigate future environmental threats. However, future studies are needed to examine whether children of this age are particularly vulnerable to the effects of stress compared to younger children and older adolescents.
Although the occurrence of stressors like Hurricane Sandy are unrelated to an individual's background, prior research suggests that an individual's susceptibility to the influence of high levels of disaster-related stress exposure may depend on preexisting individual differences, as well as environmental factors like parenting and socioeconomic status (Weems, 2015). Future research would benefit from investigating which preexisting features make an individual's emotional reactivity to negative information more or less susceptible to the influence of disaster-related stress exposure.
While the observed decrease in the ΔLPP to unpleasant images among children with low Hurricane Sandy-related stress exposure was consistent previous studies also finding that negative emotional processing measured via the LPP declines with age (Kujawa, Klein & Hajcak, 2012. 2013; MacNamara et al., 2015; see supplementary data), few studies have examined these changes in the restricted age range (9-11) of children included in the present study. Thus, more longitudinal research is necessary to elucidate patterns of normative developmental changes of the ΔLPP.
Youth who live through natural disasters experience higher rates of psychiatric problems that can continue into adulthood (McFarlane & Van Hoof, 2009). Additionally, early life stress enhances vulnerability to stressors in adulthood (Dich et al., 2014). Altered neural reactivity to negative emotional information may be one mechanism through which disasters lead to these outcomes. Enhanced reactivity to negative emotional information is concurrently associated with internalizing disorders in both children and adults (Kujawa et al., 2015; Weinberg & Hajcak, 2011) and predicts increases in stress-related symptoms in response to stressful and traumatic life events (Admon et al., 2009, Kujawa et al., in press; McLaughlin et al., 2014; Swartz, Knodt, Radke & Hariri, 2015). It remains to be seen whether the present study's observed disruption in the development of emotional reactivity to unpleasant stimuli persists, and whether it relates to subsequent psychopathology and functioning. To address these questions, we will continue to study these children in order to examine the long-term impact of the disaster and the role of altered development of emotional reactivity to negative stimuli and risk for psychopathology.
In sum, the present study provides novel evidence that exposure to natural disaster-related stressors alters the trajectory of emotional reactivity to negative stimuli. The failure to exhibit the expected declines in emotional reactivity to negative relative to neutral stimuli in childhood was evident nine months after the disaster and may be a marker of increased vulnerability for subsequent maladaptive outcomes later in life.
Supplementary Material
Figure 2.
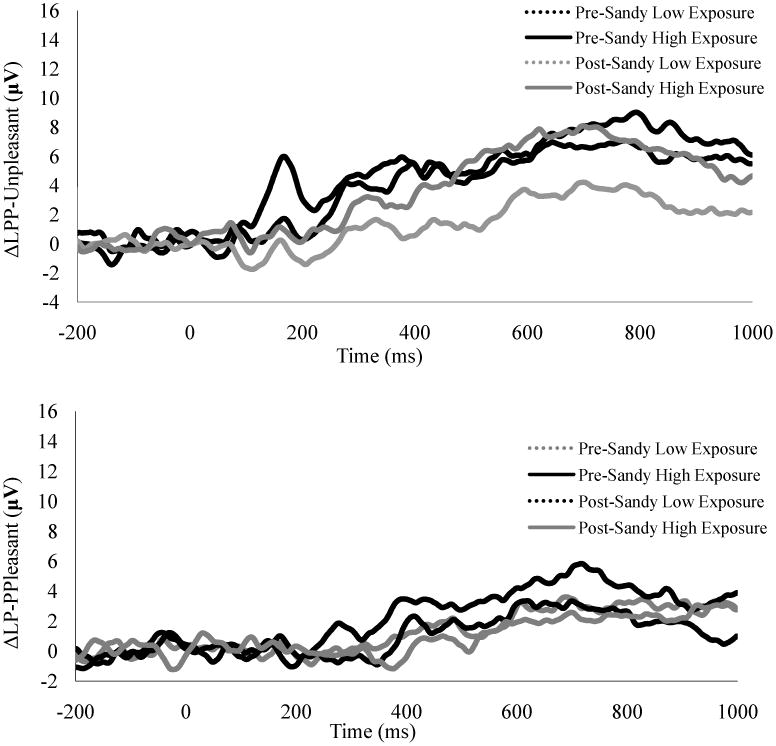
Waveforms displaying the ΔLPP to unpleasant (top) and pleasant (bottom) stimuli pre- and post-Hurricane Sandy in children with high and low Hurricane Sandy-related stress exspore. The LPP waveforms were pooled across occipital (Oz, O1, and O2) parietal (Pz, P3, and P4) electrodes.
Acknowledgments
This work was supported by National Institute of Mental Health grant R01 MH069942 (DNK).
References
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proceedings of the National Academy of Science. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde AC, Peper JS, Crone EA. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. The Journal of Neuroscience. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, et al. Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guidice M. Middle childhood: an evolutionary-developmental synthesis. Child Development Perspectives. 2014;8(4):193–200. doi: 10.1111/cdep.12084. [DOI] [Google Scholar]
- Dich N, Hansen ÅM, Avlund K, Lund R, Mortensen EL, Bruunsgaard H, Rod NH. Early life adversity potentiates the effects of later life stress on cumulative physiological dysregulation. Anxiety, Stress, & Coping. 2015;28(4):372–390. doi: 10.1080/10615806.2014.969720. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin. 2006;132(3):477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Brewin CR, Gruber M, Jones RT, King DW, King LA, et al. Kessler RC. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Archives of General Psychiatry. 2007;64(12):1427–1434. doi: 10.1001/archpsyc.64.12.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel BL, Kim P, Gilmore H, Tottenham N, Temple E. Stress and the healthy adolescent brain: evidence for the neural embedding of life events. Development and Psychopathology. 2013;25:879–889. doi: 10.1017/S0954579413000242. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. Tottenham N. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. The Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55(83):468–484. 90135–9. doi: 10.1016/0013-4694. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Unpublished manuscript. Yale University; 1975. Four Factor Index of Social Status. [Google Scholar]
- Kopala-Sibley DC, Kotov R, Bromet EJ, Carlson GA, Danzig AP, Black SR, Klein DN. Personality diatheses and Hurricane Sandy: effects on post-disaster depression. Psychological Medicine. :1–11. doi: 10.1017/S0033291715002378. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Danzig AP, Kotov R, Bromet EJ, Carlson GA, Olino TM, Bhatia V, Black SR, Klein DN. Negative emotionality and its facets moderate the effects of exposure to hurricane sandy on children's post-disaster depression and anxiety symptoms. Journal of Abnormal Psychology. doi: 10.1037/abn0000152. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Hajcak G. Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience. 2012;2:458–467. doi: 10.1016/j.dcn.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald K, Monk C, Phan KL. Enhanced neural reactivity to threatening faces in anxious youth: evidence from event-related potentials. Journal of Abnormal Child Psychology. 2015 doi: 10.1007/s10802-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Danzig AP, Black SR, Bromet EJ, Carlson GA, Kotov R, Klein DN. Neural reactivity to emotional stimuli prospectively predicts the impact of a natural disaster on psychiatric symptoms in children. Biological Psychiatry. doi: 10.1016/j.biopsych.2015.09.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Slavich GM, Berghorst LH, Treadway MT, Brooks NH, Dutra SJ, et al. Pizzagalli DA. Perceived life stress exposure modulates reward-related medial prefrontal cortex responses to acute stress in depression. Journal of Affective Disorders. 2015;180:104–111. doi: 10.1016/j.jad.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instructional manual. Unpublished manuscript. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Vergés A, Kujawa A, Fitzgerald KD, Monk CS, Phan KL. Age-related changes in emotional face processing across childhood and into young adulthood: Evidence from event-related potentials. Developmental Psychobiology. 2015 doi: 10.1002/dev.21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane AC, Van Hooff M. Impact of childhood exposure to a natural disaster on adult mental health: 20-year longitudinal follow-up study. The British Journal of Psychiatry. 2009;195:142–148. doi: 10.1192/bjp.bp.108.054270. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and Anxiety. 2014;31:834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neria Y, Shultz JM. Mental health effects of Hurricane Sandy: characteristics, potential aftermath, and response. Journal of the American Medical Association. 2012;308:2571–2572. doi: 10.1001/jama.2012.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris FH, Sherrieb K, Galea S. Prevalence and consequences of disaster-related illness and injury from hurricane Ike. Rehabilitation Psychology. 2010;55(3):221. doi: 10.1037/a0020195. [DOI] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. Journal of Abnormal Psychology. 2010;119(3):468. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biological Psychiatry. 2004;56(2):72–79. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS. Age-related changes in the structure and function of prefrontal cortex–amygdala circuitry in children and adolescents: A multi-modal imaging approach. NeuroImage. 2014;86:212–220. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85:505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdale reactivity during adolescence: effects of family history of depression and stressful life events. The American Journal of Psychiatry. 2015;172(3):276–283. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews. 2003;27(03):33–44. 00007–1. doi: 10.1016/S0149-7634. [DOI] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Schissel A, Lim KO, Luciana M. Effects of reward sensitivity and regional brain volumes on substance use initiation in adolescence. Social Cognitive and Affective Neuroscience. 2015;10(1):106–113. doi: 10.1093/scan/nsu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF. Biological correlates of child and adolescent responses to disaster exposure: a bio-ecological model. Current Psychiatry Reports. 2015;17(7):1–7. doi: 10.1007/s11920-015-0588-7. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Electrocortical evidence for vigilance-avoidance in Generalized Anxiety Disorder. Psychophysiology. 2011;48(6):842–851. doi: 10.1111/j.1469-8986.2010.01149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


