Abstract
Newborn marsupials can be arranged into three grades of developmental complexity based on their external form, as well as based on their organ systems and their cytology. The dasyurids are considered the least developed marsupials at birth, while didelphids and peramelids are intermediate, and macropods are the most developed. Currently there is still little information on caenolestid and microbiotherid development at birth. Developmental stages can be graded as G1, G2 and G3, with G1 being the least developed at birth, and G3 the most developed. Marsupials are also characterized by having an extremely developed craniofacial region at birth compared with placentals. However, the facial region is also observed to vary in development between different marsupial groups at birth. The oral shield is a morphological structure observed in the oral region of the head during late embryological development, which will diminish shortly after birth. Morphological variation of the oral shield is observed and can be arranged by developmental complexity from greatly developed, reduced to vestigial. In its most developed state, the lips are fused, forming together with the rhinarium, a flattened ring around the buccal opening. In this study, we examine the external oral shield morphology in different species of newborn marsupials (dasyurids, peramelids, macropods and didelphids), including the newborn monito del monte young (Dromiciops gliroides – the sole survivor of the order Microbiotheria). The adaptive value of the oral shield structure is reviewed, and we discuss if this structure may be influenced by developmental stage of newborn, pouch cover, species relatedness, or other reproductive features. We observe that the oral shield structure is present in most species of Marsupialia and appears to be exclusively present in this infraclass. It has never been described in Monotremata or Eutherians. It is present in unrelated taxa (e.g. didelphids, dasyurids and microbiotherids). We observe that a well‐developed oral shield may be related to ultra altricial development at birth, large litter size (more than two), and is present in most species that lack a pouch in reproductive adult females or have a less prominent or less developed pouch with some exceptions. We try to explore the evolution of the oral shield structure using existing databases and our own observations to reconstruct likely ancestral character states that can then be used to estimate the evolutionary origin of this structure and if it was present in early mammals. We find that a simple to develop oral shield structure (type 2–3) may have been present in marsupial ancestors as well as in early therians, even though this structure is not present in the extant monotremes. This in turn may suggest that early marsupials may have had a very simple pouch or lacked a pouch as seen in some living marsupials, such as some dasyurids, didelphids and caenolestids. The study's results also suggest that different morphological stages of the oral shield and hindlimb development may be influenced by species size and reproductive strategy, and possibly by yet unknown species‐specific adaptations.
Keywords: Dromiciops gliroides, marsupial, monotreme, newborn, oral shield
Introduction
Marsupials have a unique reproductive system that differs from eutherian mammals. They undergo a short period of gestation with a very short intrauterine development, and give birth to one or a number of highly altricial young that will go through most of their development while attached to their mother's teat. For example, while these newborn appear in most cases to already possess a sense of smell, gravity and touch, the sense of vision and hearing are not yet established (Gemmell et al. 1988; Gemmell & Nelson, 1989; Ashwell et al. 2008; Schneider et al. 2009, 2013; Schneider, 2011; Ashwell & Shulruf, 2014a). However, the level of development at birth in marsupials varies depending on the species, as demonstrated in recent studies (Hughes & Hall, 1988; Nelson, 1988; Schneider, 2011; Ashwell & Shulruf, 2014a,b). Three degrees of development may be distinguished (G1–G3), with G1 being the least developed and G3 the furthest developed (Hughes & Hall, 1988). These grades exhibit differences such as birth size, external and internal morphology, development and locomotion ability (Hughes & Hall, 1988; Ashwell & Shulruf, 2014a,b). They may reflect adaptations related to behavioural requirements of the young to get to the pouch or mammary area, locate and remain attached to the teat.
In order for marsupial neonates to reach the pouch and attach to the teat, they must have somewhat advanced neural connections in their developing brain that allow them to coordinate their movement to the pouch. Most newborn marsupials also have a mature vestibular apparatus at birth with dasyurids such as the Northern quoll (Dasyurus hallucatus) and didelphids such as the Virginia opossum (Didelphis virginiana) having the least developed vestibular system, while macropod diprodontids such as wallabies, kangaroos and pademelons have the most developed (Gemmell & Nelson, 1989; Krause, 1991; Ashwell & Shulruf, 2014a). They have developed sensory mechanisms to allow them to sense the direction of the teat and pouch, and they also possess adaptations such as precociously developed forelimbs to help them reach the teat (Ashwell & Shulruf, 2014a). The forelimbs are developed, the digits separated and there is a deciduous claw at the end of each digit, which allow them to break through the foetal membranes at birth and climb (Lyne, 1964; Cooper & Steppan, 2010; Ashwell, 2013). The neonates predominantly use their forelimbs to move, as at birth all marsupials have very rudimentary developed hindlimbs, lacking claws. As in other mammals, marsupials show forelimb movements even before birth; e.g. the altricial tammar wallaby (Macropus eugenii) embryo shows climbing‐like movements inside the womb before birth with only the forelimbs being prepared for the climb to the pouch (Drews et al. 2013).
Apart from morphology essential for locomotion at birth, newborn marsupials display some morphological adaptations, which appear to be closely related to the secured connection between mother and young via the teat. One is the lateral sealing of the newborn's lips just before birth, leaving only a small triangular mouth‐opening from which the tip of the tongue projects. This opening and the tongue are shaped and adapted to fit the teat securely (Hill & Hill, 1955). This structural feature may also be accompanied by a shield‐like structure formed by the lips and the rhinarium around the buccal opening. This structure was first described by Selenka in 1887 in Virginia opossum embryos and given the German name ‘Schnabelschild’ (literally translated from the German meaning ‘beak shield’). Later it was given the English name oral shield (McCrady, 1938). Overall, marsupials have a very developed cranialfacial system at birth (including early ossification of the membrane bones around the oral cavity, as well as a well‐developed tongue and robust chondocranium), which is functional and an adaptation for suckling (Clark & Smith, 1993; Smith, 1997). In comparison to placental mammals, craniofacial development in marsupials at birth is also advanced (compared with the rest of the body and compared with the development of the CNS), specifically in the development of the oral and facial regions (Smith, 2001). These include a massive robust chondocranium, developed nasal region, differentiated tongue muscles and developed tongue, cartilage present in basicranium, and skeletal bone present in the oral region (maxilla, premaxilla and dentary), which is very accelerated in its development (e.g. Monodelphis; Smith, 2001) as well as the closure of the secondary palate that occurs earlier than in eutherians. These structures that are present in a grey short‐tailed opossum (Monodelphis domestica) embryo at a day before birth are equivalent to a 14–15‐day embryonic mouse (Smith, 2001). The premaxilla, maxilla and palatines are ossified in the grey short‐tailed opossums a day before birth, and in the Tammar wallaby 2 days before birth (Clark & Smith, 1993). In mice (Mus musculus), the ossification of the premaxilla, maxilla and palatines also occurs relatively early. The premaxilla ossifies at E11.5 (embryonic day 11.5) and the maxilla at E12, with the last day of embryonic development being approximately E20 (Kaufman & Bard, 1999) .
While some authors believed that the oral shield is a useless structure (McCrady, 1938), others proposed that it might be of importance in the strong connection between teat and young (Selenka, 1887; Hill & Hill, 1955; Merchant & Sharman, 1966). Another suggestion proposed by Selenka (1887) when studying the Virginia opossum was that the oral shield, which is made up of cornified epidermal cells, was a rudiment of a beak structure (similar to that observed in the adult platypus) and may have served as grasping organ in marsupial ancestors. Furthermore, some authors suggested that the development of the oral shield may be related to the overall morphological development state of the young at birth, whereby larger, more developed marsupial species at birth may be strong enough to hold on to the teat needing no oral shield apparatus (Hill & Hill, 1955).
As there are significant differences in oral morphology and overall development between marsupial species at birth, the aim of this present study is to compare the oral shield morphology in different marsupial groups (dasyurids, microbiotherids, didelphids, peramelids, macropodes) with other specific developmental characters of newborns, and suggest reasons for why this structure is absent or less developed in some species and more developed in others. We will compare these with features in the females such as pouch cover and teat number, which may lead to a greater need of a secure connection between neonate and female. Finally, we will add these findings to information such as birth position, pouch development and other morphological features (skeletal, cranial and soft tissue) in adults to use these in a phylogenetic analysis and reconstruction of the likely ancestral character states.
In this study, we also observe external oral morphology of newborn pouch young from the only living microbiotherian marsupial monito del monte (Dromiciops gliroides). Currently there is very little information on the morphology of microbiotherian pouch young (Muñoz‐Pedreros et al. 2005; Frankham & Temple‐Smith, 2012; Gurovich et al. 2013), this study will also bring together current knowledge and add new information on the only living member of the Microbiotheria (but see D'Elía et al. 2016 and below), a small arboreal marsupial that lives in the Andean valdivian forests of southern Chile and Argentina (Lobos et al. 2005; Amico & Rodríguez‐Cabal, 2009; Celis‐Diez et al. 2012; Gurovich et al. 2015). It has only recently been proposed that two new different species of monito del monte exist, with one of the new species being endemic to Chile, and the other new species occurring in Argentina and Chile (D'Elía et al. 2016). Our study is based on the monito del monte which has the the most southern distribution in Argentina.
The living monito del monte is more phylogenetically related to Australasian marsupials and is part of Australidelphia (including all the Australasian marsupial orders and Microbiotheria). This is a clade supported by morphological evidence predominantly from the ankle region (Szalay, 1982, 1994), and later by skeletal, cranial and dental evidence (Horovitz & Sánchez‐Villagra, 2003), as well as molecular (Amrine‐Madsen et al. 2003; Beck, 2008; Meredith et al. 2008) and total evidence phylogenetic analysis combining molecular and morphological data (Beck et al. 2014). However, phylogenetic relationships between Dromiciops and other Australasian marsupial clades still remain unresolved.
External morphology such as integumental pigmentation and pouch morphology has been described in the past in some marsupial groups (Thomas, 1888; Tate, 1933; Hershkovitz, 1992, 1997, 1999); however, most recent phylogenetic analyses use mainly osteological characters instead of integumental ones (Beck et al. 2014). There are only very few phylogenetic studies that have used soft external morphology characters including the morphology of the pouch, for example, the presence and absence of pouch and mammae arrangement (Horovitz & Sánchez‐Villagra, 2003; Voss & Jansa, 2003, 2009; Horovitz et al. 2009). Many external ‘soft morphology’ characters have been ignored as sources of phylogenetic information (Voss & Jansa, 2003). We are here adding new soft‐bodied morphological characters that can be observed in pouch young and in adult marsupial species, and incorporate these characters into a phylogenetic study to observe the position of Dromiciops in relation to other South American and Australasian marsupials and to estimate the ancestral state of the oral shield.
We therefore will investigate the three following hypotheses: (i) the oral shield may be related to the neonate's overall developmental stage at birth, with developmentally less advanced species at birth showing a more strongly developed oral shield; (ii) the state of the oral shield at birth depends on the possibility to come of the teat once attached, for example, species with less maternal pouch cover would show a more strongly developed oral shield; (iii) oral shield type and pouch type coevolved, and there is a relationship with species that show a strong oral shield. Finally, we will use oral shield morphology and other soft‐body characters in pouch young and in adult females at birth to estimate phylogenetic relationships between extant and extinct marsupials, to investigate the position of Dromiciops within Australidelphia and as well as to investigate character evolution.
Materials and methods
Specimens used for this study
Animals
Monito del monte pouch young used in this study (Table 1) were collected during a field trip to the National Parks in Southern Argentina by R.D. Sage (RDS) and kindly given for study to Y. Gurovich (YG). Two female monito del montes (RDS 18111 and RDS 18110) were trapped with four pouch young each on 7 November 2006, in Parque Nacional Nahuel Huapi, Los Lagos Departamento, Argentina, 4.8 km W, 12.2 km N Villa La Angostura at an elevation of 840 m (39°02′12.48″S, 70°18′25.68″W trapline 9), in mature Coíhue forest with bamboo (Chusqea culeo).
Table 1.
Data of monito del monte (Dromiciops gliroides): date and site of capture, body weights, and meristic of (a) two adult females that were captured with (b) four pouch young (py) each. Animals were captured in Argentina, South America
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID no. | Date of capture | Place | Age | Sex | Weight (g)* | Total length (mm)* | Tail length (mm)* | Hind‐foot length(mm)* | Ear length(mm)* |
| RDS18111 | 7/11/2006 | Neuquén Los Lagos | Adult | F | 25 | 226 | 122 | 20 | 19 |
| RDS18110 | 7/11/2006 | Neuquén Los Lagos | Adult | F | 30 | 213 | 103 | 16 | 18 |
| (b) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ID no. | Date of capture | Place | Age (approx.) | Sex | Weight (g)† | CRL (mm)† | Tail length (mm)† | Head length (mm)† |
| RDS18111A | 7/11/2006 | Neuquén Los Lagos | Newborn | n.a. | 0.12 | 10 | n/a | 5 |
| RDS18111B | 7/11/2006 | Neuquén Los Lagos | Newborn | n.a. | 0.13 | 11 | 6 | 5.5 |
| RDS18110A | 7/11/2006 | Neuquén Los Lagos | Newborn | n.a. | 0.06 | 9 | 3 | 4.5 |
| RDS18110B | 7/11/2006 | Neuquén Los Lagos | Newborn | n.a. | 0.04 | 7 | n/a | 4.5 |
| RDS17491 | 8/10/2005 | Bariloche, Rio Negro | Late stage embryo | n.a. | ? | 5 | ? | n/a |
| RDS17593 | 28/10/2005 | Aluminé, Neuquén | Late stage embryo | n.a. | ? | 9 | ? | n/a |
*Bold laboratory measurements of adult specimens are prefixation taken in 2006.
†All measurements are postfixation taken in 2014. CRL, crown rump length.
Museum special snap traps were placed on the ground (Woodstream Corporation®) baited with oatmeal. These traps were originally destined to trap small terrestrial rodents such as Abrothrix longipilis, but proved useful for trapping the arboreal monito del monte. Traps were set out in the late afternoon and examined in the morning (Herrin & Sage, 2012). The pouch young specimens used in this study were obtained under a permit (Permit 538) from the Administración de Parques Nacionales in Bariloche to RDS and a travel permit (Guia de Transito No. 003669) to YG.
The other marsupial pouch young specimens (Ameridelphia and Australidelphia; Table 2) used in this study are from the Hubrecht & Hill collection, which is a part of the embryological collection of the Museum für Naturkunde in Berlin, Germany.
Table 2.
List of marsupial and monotreme specimens examined from: (a) the Hubrecht and Hill collection (Museum für Naturkunde Berlin, Germany); and (b) the literature
| (a) | ||||||
|---|---|---|---|---|---|---|
| Species | Common name | Specimen no. | GL (mm) | Head length (mm) | Approx. age | Oral shield type |
| Didelphis virginiana | Virginia opossum | MA79 | 11 | 6 | Newborn | 3 |
| Philander opossum | Grey four‐eyed opossum | MA806F | 8* | 3* | Late stage embryo | 3? |
| Phascolarctos cinereus | Koala | MA489 | 16.5 | – | Newborn | 1 |
| Macropus dorsalis | Black‐striped wallaby | MA664 | 12 | 8 | Late stage embryo | 1 |
| Macropus dorsalis | Black‐striped wallaby | MA700 | 22.5 | – | Shortly after birth? | 1 |
| Macropus robustus | Common wallaroo | MA671a | 17 | – | Shortly after birth? | 1 |
| Petrogale penicillata | Brush‐tailed rock‐wallaby | MA463d† | – | 6* | Newborn | 1 |
| Thylogale thetis | Red‐necked pademelon | MA674 | 15.5 | – | Late stage embryo | 1 |
| Trichosurus vulpecula | Brushtail possum | MA462 | 15 | – | Newborn | 2 |
| Dasyurus spec. | Quoll | MA780 | 5.5 | – | Newborn | 4 |
| Isoodon obesulus | Southern brown bandicoot | MA349 | 14.5 | 6 | Newborn | 2 |
| Myrmecobius fasciatus | Numbat | MA242‡ | 4.4* | 1.7* | Newborn | 4 |
| 7* | 5* | Pouch young | n.a. | |||
| 20* | 13* | pouch young | n.a. | |||
| (b) | ||||||
|---|---|---|---|---|---|---|
| Species | Common name | Reference (specimen no.) | Crl (mm) | Head length (mm) | Approx. age | Oral shield type |
| Tachyglossus aculeatus | Short‐beaked echidna | Griffiths, 1978; p. 253 | 14.7 | Newly hatched | 0 | |
| Ornithorhynchus anatinus | Platypus | Griffiths, 1978; p. 253 | Newly hatched | 0 | ||
| Hughes & Hall, 1998 (WW; Hill collection Utrecht)§ | 16.75 | 6 | Newly hatched | 0 | ||
| Monodelphis domestica | Grey‐short tailed opossum | Schneider, 2011 | Newborn | 3 | ||
| Lasiorhinus latifrons | Southern hairy‐nosed wombat | Taggart et al. 2007 | 5.2 | Newborn | 1 | |
| Macropus eugenii | Tammar wallaby | Hughes & Hall, 1988 | 14.6 | 7.1 | Newborn | 1 |
| Macropus giganteus | Eastern grey kangaroo | Hughes & Hall, 1988 | Newborn | 1 | ||
| Hypsiprymnodon moschatus | Musky rat kangaroo | Keibel, 1906; fig. 48 | Late stage embryo | 1 | ||
| Potorous tridactylus | Long‐nosed potoroo | Hughes, 1962 | 1.8 | 0.6 | Newborn | 1 |
| Antechinus flavipes | Yellow‐footed antechinus | Marlow, 1961 | 4.9 | Newborn | 4 | |
| Sminthopsis macroura | Striped‐faced Dunnart | Frigo & Woolley, 1997 | 4.1 | Newborn | 4 | |
| Dasyurus viverrinus | Eastern quoll | Hughes & Hall, 1988; Schneider, 2011 | 4.7 | 5.5 | Newborn | 4 |
| Sarcophilus harrisii | Tasmanian devil | Hughes & Hall, 1988; Guiler, 1970 | 8 | 4 | Newborn | 4 |
| 12.5 | 6.5 | Intra‐uterine | ||||
| Isoodon macrourus | Northern brown bandicoot | Hughes & Hall, 1988 | 13.83 | Newborn | 2 | |
| Perameles nasuta | Long‐nosed bandicoot | Lyne, 1964 | 12.8 | 6.3 | Newborn | 2 |
| 11.3 | 5.5 | Intra‐uterine | ||||
| Notoryctes typhlops | Marsupial mole | Wood Jones, 1921 | 10 | Newborn | 1? | |
*Postfixation measurement.
†Specimen cut in pieces (head, forelimb, torso and hindlimbs).
‡Two very early aged specimens placed in container with an older pouch young and are not mentioned in the catalogue.
§This specimen appears to be no longer in the collection now situated in Berlin, or may have changed reference number.
crl, crown rump length; gl, greatest length.
Acronyms
RDS – Richard D. Sage Private Collection, Argentina; MA – Hubrecht & Hill collection, which is a part of the embryological collection of the Museum für Naturkunde Berlin, Germany.
Measurements
General morphological measurements of adult female monito del monte (RDS18111 and 18110) were made in the field before fixation (Table 1a). The general morphological measurements of the four pouch young (RDS 18111A, B and RDS 18110A, B) including (weight, crl, head) were taken after fixation (4% formaldehyde; Table 1b).
Measurements of the newborn pouch young from the Hubrecht & Hill collection in Berlin were obtained from the museum's collection catalogue or carried out post‐fixation by NY Schneider (NYS; Table 2a).
Age and sex estimates
Monito del monte pouch young ages were estimated from a comparison of meristic data using a growth curve of the eastern pygmy possum (Cercartetus nanus; Ward, 1990) following Frankham & Temple‐Smith (2012), who also used this growth curve when estimating age for monito del monte pouch young due to similarities in size and other characteristics. There is no published growth curve available for monito del monte or other small South American marsupials. However, the pouch young studied here were much smaller than those presented by Frankham & Temple‐Smith (2012), and so morphological characters such as development of hindlimb, presence of claws, oral shield, presence of hair, etc. were used to determine age. We estimate that based on the very small size these monito del monte were recently born or only a few days old.
The sex of the pouch young could not be determined using criteria by Frankham & Temple‐Smith (2012) and Gurovich et al. (2013) because they are very small in size and newly born. Similarly, Tyndale‐Biscoe & Renfree (1987) observed that it was impossible to determine the sex in the tammar wallaby and Virginia opossum (D. virginiana) at birth externally and only possible histologically.
Comparative morphology
The pouch young presented in Tables 1 and 2 were investigated for their overall appearance, and for their development of: (i) the oral shield; (ii) rhinarium; (iii) fore‐ and hindlimbs (including presence of claws); (iv) cervical swelling (i.e. fine skin and bulge between lower jaw and abdomen); (v) external eye; and (vi) ear.
Observations and measurements of the whole‐mount fixed monito del monte pouch young were made by YG under a dissecting microscope. Drawings were made both free‐hand and with a camera lucida. Digital photographs were taken from a Leica dissecting microscope with camera lucida.
Observations, measurements and drawings of the external morphology of Australiasian and Ameridelphian whole‐mount fixed newborn pouch young from the Hubrecht & Hill collection (Table 2a) were made by NYS under a Leica dissecting microscope with camera lucida. Digital photographs were taken with a Nikon Coolpix.
Drawings of whole‐mount newborns from earlier work by Schneider (2011) were used to complement.
Further observations were obtained from the literature (Table 2b). Search of the literature was conducted using ScienceDirect, PubMed, journals published by CSIRO, google, google scholar and libraries (e.g. Melbourne University, University of New South Wales, Australian Museum, Mammal Library Museum für Naturkunde, Berlin). YG also observed Australian marsupial pouch young at the Australian Museum.
The drawings of the Tasmanian devil (Sarcophilus harrisii), the common opossum (Didelphis marsupialis), the southern marsupial mole (Notoryctes typhlops; Figs 1b,g,o and 2f,l) and the platypus (Ornithorhynchus anatinus; Fig. 3b) were adapted from figures by Hughes & Hall (1988), Osman Hill (1952), Wood Jones (1921) and Hughes & Hall (1998), respectively.
Figure 1.

All marsupial newborns show strong developed forelimbs with claws on the paws, while the hindlimbs are still poorly developed with some not even showing signs of the digits: (a) Eastern quoll; (b) Tasmanian devil; (c) numbat; (d) monito del monte; (e) grey four‐eyed opossum; (f) grey short‐tailed opossum; (g) Virginia opossum; (h) common opossum; (i) southern brown bandicoot; (j) brushtail possum; (k) red‐necked pademelon; (l) black‐striped wallaby; (m) common wallaroo; (n) koala; (o) Southern marsupial mole. [The figure was adapted from Schneider (2011) adding the monito del monte, numbat, grey four‐eyed opossum, red‐necked pademelon, black‐striped wallaby, common wallaroo and the drawings of the Tasmanian devil, common opossum and southern marsupial mole adapted from Hughes & Hall, 1988; Osman Hill, 1952; Wood Jones, 1921, respectively.]
Figure 2.
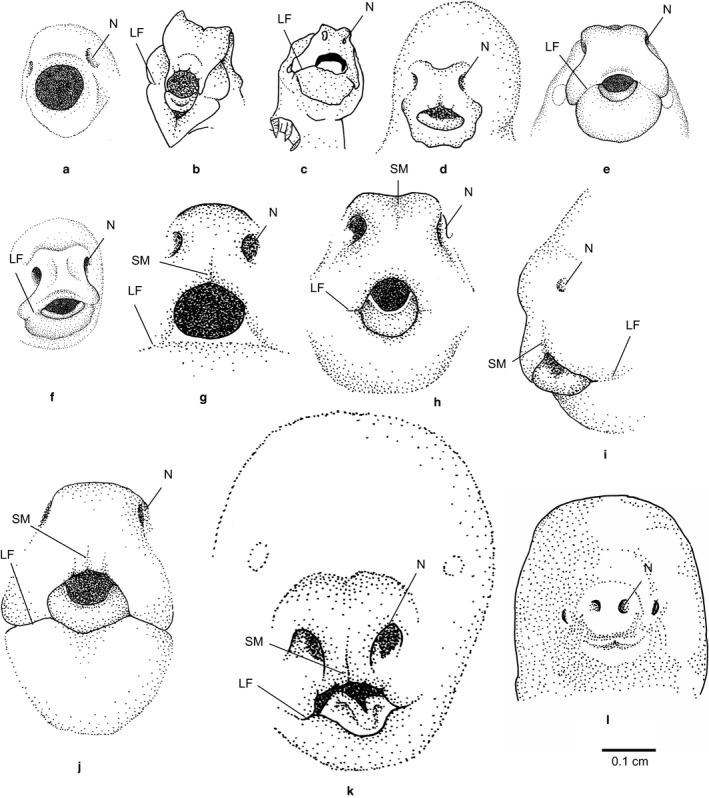
Development of the oral shield. It may be extensively developed like in (a) the Eastern quoll with no difference apparent between the upper and lower mandible. Or extensive to reduced as in the (b) numbat and reduced in (c) the monito del monte, (d) the grey four‐eyed opossum, (e) the grey short‐tailed opossum and (f) the Virginia opossum, which show an oral shield‐like rhinarium structure with slightly visible lib faults. The oral shield is simple in the (g) Southern brown bandicoot and (h) the brushtail possum and vestigial in (i) the red‐necked pademelon, (j) the black‐striped wallaby, (k) koala and (l) Southern marsupial mole (LF, lip fold; N, naris; SM, sulcus medianus) (figure adapted from Schneider, 2011 and marsupial mole redrawn from Wood Jones, 1921).
Figure 3.
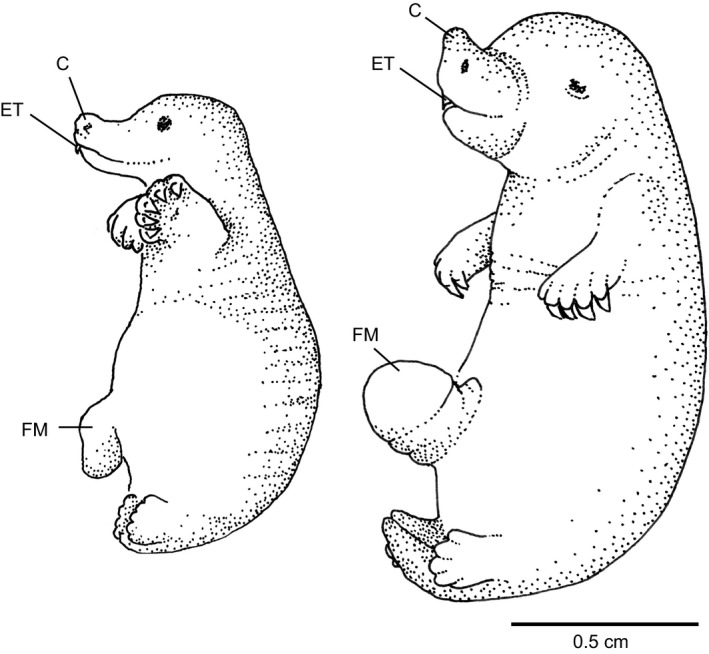
Newly hatched monotremes are at a similar stage of development as marsupial newborns of stage G3: while the hindlimbs are still paddle‐like the forelimbs are well developed and the forepaws exhibit claws. In contrast to marsupial young both echidna (left) and platypus (right) have an egg tooth (ET) and caruncle (C). Their mouth is already a slide‐like opening, which is possibly an adaptation to allow them to sip milk from the milk patch. Parts of the foetal membrane (FM) are still visible. (Echidna drawn after a photo in Griffiths, 1978 p. 242, already presented in Schneider, 2011; platypus adapted from a drawing in Hughes & Hall, 1998.)
Character evolution analysis
Phylogenetic affinities of extant and extinct marsupials
A phylogenetic analysis was performed to establish marsupial higher‐level relationships based on new soft tissue morphological characters, including the oral morphology in very young marsupial pouch young. This phylogenetic analysis was performed using the morphological information from previous studies (Voss & Jansa, 2003, 2009; Horovitz et al. 2009; Beck, 2012; Beck et al. 2014). The matrix consists of combined postcranial, cranial and soft‐body tissue anatomical characters (268 characters), including 38 taxa representing all the major marsupial radiations. The two extant monotremes Tachyglossus and Ornithorynchus were used as the outgroups following Beck et al. (2014). Many of the 38 taxa (including the extinct taxa) are scored on cranial and dental material only, and not on soft‐body morphology. This results in a significant amount of missing data, which corresponds to a poor phylogenetic resolution.
All characters are unordered, and multistate characters are treated as polymorphic (matrix available upon request to the authors).
Most parsimonious trees were sought using a heuristic procedure. Initial trees were built using 1000 random addition‐sequence replicates. TBR searches were conducted on the initial trees. Decay or Bremer support values were estimated using the same heuristic procedure described above using PAUP*. The parsimony analyses consisted of 1000 heuristic replicates, saving 10 trees per replicate, and the multiple most parsimonious trees were summarized using strict consensus, with bootstrap values.
Morphological matrix
The matrix used in this analysis (part of which is reproduced in Table 3) is based on the morphological matrix of Beck et al. (2014), which is one of the most comprehensive matrices currently available for investigating higher‐level metatherian relationships. This matrix uses morphological, osteological and dental characters as well as soft‐bodied characters that unfortunately cannot be coded in fossil taxa. As noted by Beck et al. (2014), taxon sampling is still somewhat limited, including morphological soft‐bodied characters. In this study, new soft‐bodied morphological characters were added (see below).
Table 3.
Matrix with soft‐body characters used for establishment of a phylogenetic tree modified from Voss & Jansa (2009 and Beck et al. (2014): monotreme and marsupial species with oral shield morphology at birth, and number of teats, litter size and pouch morphology of mother. Some specific taxa are added to this matrix but not used in the phylogenetic study
| Species | Position of cloaca | Oral shield group | Pouch (present/absent) | Pouch type | Mammae/areola arrangement | Unpaired median teat | Mammary count | Mammary anlagen male pouch young and/or adult | Urogenital and rectal opening position | Urogenital and rectal openings separation | Gular glands | Birth position | Pouch coverage | Number of teats/litter size | Number of teats (mammary) | Litter size | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ornithorhynchus | 0 | 0 | 0 | 3 | 0 | 0 | 0 | ? | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 1–2 | 1 |
| Tachyglossus | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 1 |
| Didelphis | 1 | 3 | 1 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 3 | 3 | 1.30 | 17 | 13 | 2, 3, 4, 9, 11 |
| Monodelphis | 1 | 3 | 0 | 3 | 0 | 1 | 4 | 0 | 0 | 0 | 1 | 1 | 1 | 1.57 | 22 | 14 | 2, 9, 14, 20 |
| Caluromys | 1 | ? | 1 | 0/1 | 0 | 1 | 2 OR 3 | ? | 0 | 0 | 0 | ? | 2 | 1.33 | 4 | 3 | 26, 27 |
| Glironia | 1 | ? | 0 | 0 | 0 | 0 | 2 | ? | 0 | 0 | ? | ? | 0 | ? | 5 | ? | 26, 27 |
| Caenolestes | 1 | ? | 0 | 0 | 0 | 0 | 2 | ? | 0 | ? | 0 | ? | 0 | ? | 4 | ? | 26, 28 |
| Dasyuroides | 1 | ? | 1 | 0 | 0 | 0 | 3 | ? | 0 | ? | 1 | 2 | 2 | 1 | 6 | 5–6 | 19, 9 |
| Dasyurus | 1 | 4 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | ? | 1 | 2 | 1 | 1.33 | 8 | 6 | 2, 5, 17, 18 |
| Phascogale | 1 | ? | 1 | 0 | 0 | 0 | 3 | 1 | 0 | ? | ? | ? | 1.33 | 8 | 1–6 | 29 | |
| Notoryctes | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | ? | 0 | ? | 3 | 0.33 | 2 | 1–6 | 7, 9 |
| Echymipera | 1 | ? | 1 | 2 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 1 | 2 | 3 | 1.5 | 25, 26, 30, 31 | |
| Perameles | 1 | 1–2? | 1 | 2 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 1 | 3 | 1.6 | 8 | 1–5 | 2, 5, 6, 9, 21 |
| Dromiciops | 0 | 3 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | ? | 3 | 1 | 4 | 4 | 10 |
| Trichosurus | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ? | 1 | 3 | 3 | 2 | 2 | 1 | 2, 8, 9, 12 |
| Phalanger | 1 | ? | 1 | 1 | 0 | 0 | 2 | 1 | 0 | ? | ? | ? | 3 | 0.66 | 2 | 1–3 | 9 |
| Petaurus | 1 | ? | 1 | 1 | 0 | 0 | 2 | 1 | 0 | ? | 1 | 3 | 3 | 2.5 | 2–4 | 1.6 | 9, 27, 39 video, 43, 44 |
| Pseudochirops | 1 | ? | 1 | 1 | 0 | 0 | 2 | 1 | 0 | ? | ? | ? | 3 | 2 | 4 | 2 | 32 |
| Cercartetus | 1 | ? | 1 | 1 | 0 | 0 | 2 | 1 | 0 | ? | ? | ? | 3 | 1 | 4 | 4 | 33 |
| Macropus | 1 | 1 | 1 | 1 | 0 | 0 | 2 | ? | 0 | ? | ? | 3 | 3 | 4 | 4 | 1 | 5, 9, 10, 13, 14, 24 |
| Dendrolagus | 1 | ? | 1 | 1 | 0 | 0 | 2 | ? | 0 | ? | ? | ? | 3 | 4 | 4 | 1 | 35 |
| Dorcopsis | 1 | ? | 1 | 1 | 0 | 0 | 2 | ? | 0 | ? | ? | ? | 3 | 4 | 4 | 1 | 34, 35 |
| Thylogale | 1 | ? | 1 | 1 | 0 | 0 | 1 | ? | 0 | ? | 0 | 3 | 3 | 2 | 4 | 1–2 | 35 |
| Vombatus | 1 | ? | 1 | 2 | 0 | 0 | 1 | ? | 0 | ? | 1 | 3 | 3 | 2 | 2 | 1 | 22, 23 |
| Phascolarctos | 1 | 1 | 1 | 2 | 0 | 0 | 2 | ? | 0 | ? | 1 | 3 | 3 | 2 | 2 | 1 | 2, 25, 42 |
| Potorous tridactylus | 1 | 1 | 1 | 5 | 0 | 0 | 2 | ? | 0 | ? | ? | 3 | 3 | 4 | 4 | 1 | 35 |
| Hypsiprymnodon moschatus | 1 | 1 | 1 | 4 | 0 | 0 | 2 | ? | 0 | ? | ? | ? | 2 | 2 | 4 | 2 | 9 |
| Isoodon macrourus | 1 | 2 | 1 | 6 | 0 | 0 | 3 | ? | 0 | ? | ? | ? | 3 | 2 | 8 | 4 | 9, 40, 41 |
| Isoodon obesulus | 1 | 2 | 1 | 6 | 0 | 0 | 3 | ? | 0 | ? | ? | ? | 3 | 2.66 | 8 | 3 | 9 |
| Macrotis lagotis | 1 | ? | 1 | 6 | 0 | 0 | 3 | ? | 0 | ? | ? | ? | ? | 2.66 | 8 | 1–3 | 9 |
| Sarcophilus harrisii | 1 | 4 | 1 | 2 | 0 | 0 | 2 | ? | 0 | ? | ? | ? | 1 | 1.33 | 4 | 3 | 36 |
| Sminthopsis macroura | 1 | 4 | 1 | 5 | 0 | 0 | 3 | ? | 0 | ? | 1 | ? | 3 | 1 | 8 | 1–8 | 9, 36 |
| Antechinus flavipes | 1 | ? | 1 | 0 | 0 | 0 | 3 | ? | 0 | ? | 1 | ? | 1 | 0.66 | 8 | 3–12 | 9, 16, 38 |
| Myrmecobius fasciatus | 1 | 3–4 | 0 | 0 | 0 | 0 | 2 | ? | 0 | ? | 1 | ? | 0 | 1 | 4 | 4 | 10, 37 |
Position of cloaca: 0 – basicaudal; 1 – precaudal or inguinal.
Oral shield groups: 0 – no oral shield; lips are separated and mouth can be opened wide; 1 – oral shield vestigial but lips still closed but clearly separated from the rhinarium; 2 – oral shield simple; lips not swollen and rhinarium forming separate structure; 3 – structure reduced to group 4 and the lips were becoming visible as folds of the skin. Area of the lips is slightly swollen around the buccal opening and the rhinarium is well developed; 4 – upper and lower lips form together with the rhinarium a flattened ring structure that surrounds the buccal opening.
Pouch present/absent during reproductive season: 0 – pouch absent; 1 – pouch present.
Pouch opening/type [as described in Tyndale–Biscoe & Renfree, 1987 (reproductive physiology of marsupials) redrawn from Woolley, 1974 (1–4) and Russell, 1982]: 0 – type 1: the mammary area has no covering fold of skin and the teats are exposed. Marginal, usually lateral, ridges of skin develop during the breeding season. Pouch may become deeper and evaginated in the breeding season; 1 – type 5: the mammary area is completely covered by a fold of skin. The deep pouch so formed opens at its anterior margin; 2 – type 6: the mammary area is completely covered by a fold of skin. The deep pouch so formed opens at its posterior margin; 3 – no marsupium or skin folds develop during the reproductive period; 4 – thin marsupium‐like structure develops during reproductive period; 5 – the mammary area is covered by a circular fold of skin with a central opening and all the teats are enclosed (did not use this character state in the phylogeny as specific to certain species of Dasyurids – see Woolley, 1974).
Mammae/areola arrangement: 0 – mammae/areola all abdominal/inguinal, more or less confined to pouch region; 1 – mammae/areola extending anteriorly beyond pouch region to thoracic region.
Unpaired median teat: 0 – absent; 1 – present.
Mammary count: 0 – 0 teats; 1 – 2 teats; 2 – 4 teats; 3 – 5 to 8 teats; 4 – 9 teats or more.
Mammary anlagen male pouch young and or adult male: 0 – present; 1 – absent.
Urogenital and rectal opening position: 0 – inguinal; 1 – basicaudal.
Urogenital and rectal openings separation: 0 – urogenital and rectal openings closely juxtaposed, and sharing a common mucosa; 1 – urogenital and rectal openings widely separated by furred skin.
Gular glands: 0 – absent; 1 – present.
Birth position: 0 – mother delivers egg curls up and lays the egg into the incubatorium formed between tail and abdomen (Burrell, 1927; Schneider et al. 2013) 1 – mother lies on side to deliver neonate; 2 – mother stands on all fours to deliver neonate; 3 – mother sits to deliver neonate.
Pouch coverage: 0 – no pouch; 1 – not covering; 2 – partly covering; 3 – fully covering.
References: 1Griffiths, 1978; 2Schneider, 2011; 3McCrady, 1938; 4Osman Hill, 1952; 5Hughes & Hall, 1988; 6Lyne, 1964; 7Wood Jones, 1921; 8Hughes & Hall, 1984; 9Tyndale–Biscoe & Renfree, 1987; 10this article; 11Reynolds, 1952; 12Veitch et al. 2000; 13Renfree et al. 1989; 14Sharman & Calaby, 1964; 15personal observations by NYS; 16Selwood, 1980; 17Gemmell et al. 2002; 18Nelson & Gemmell, 2003; 19Hutson, 1976; 20Rose & Fadem, 2000; 21Smith et al. 2001; 22Hogan et al. 2013; 23West, 2002; 24Poole, 1975; 25Ashwell, 2010; 26Voss & Jansa, 2009; 27Gardner, 2008; 28Patterson, 2015; 29Soderquist, 1993; 30Cuthbert & Denny, 2014; 31Aplin et al. 2010; 32Wood Jones, 1922; 33Harris, 2008; 34Collins, 1973; 35Nowak, 1999; 36Cooper et al. 2005; 37Cooper, 2011; 38Marlow, 1961; 39Smith, 1973; 40Gemmell, 1982; 41Hall, 1990.
Taxa
All (38) taxa used in the matrix here were maintained from the original matrix by Beck et al. (2014). These taxa include extant and extinct species of marsupials (Australasian – diprotodontids, peramelids, dasyurids, notoryctids; and American – caenolestids, didelphids and the extant microbiotherid, monito del monte) with two extant monotremes, Ornithorynchus and Tachyglossus, as outgroups. The 33 metatherian ingroup taxa include 23 extant species and 10 fossil species; two fossil eutherian species (Asioryctes and Ukhaatherium) and one stem‐therian Vincelestes.
Morphological characters
A total of 268 characters were used in this matrix, with most characters being related to the skeletal, dental and cranial morphology in adult marsupials. In this analysis, 15 of the 268 characters were soft tissue characters and most are related to the reproductive system (sperm pairing in epididymis, pouch presence, pouch type, mammary arrangement, mammary count, urogenital and rectal opening position, urogenital separation, cloaca position), and were obtained from Beck et al. (2014), Horovitz & Sánchez‐Villagra (2003) and Voss & Jansa (2003, 2009).
However, characters 258 (oral shield in pouch young), 264 (mammary anlagen in male pouch young or adult male) and 268 (birth position) defined below are new, and character 260 (pouch morphology) is modified.
Character 258: oral shield in pouch young
(0): No oral shield; lips are separated and mouth can be open wide; (1): oral shield vestigial but lips still closed but clearly separated from the rhinarium; (2): oral shield simple; lips not swollen and rhinarium forming separate structure; (3): structure reduced compared with state 4 the lips becoming visible as folds of the skin, the lips are slightly swollen around the buccal opening and the rhinarium is well developed; (4): upper and lower lips form together with the rhinarium a flattened ring structure that surrounds the buccal opening.
Character 260: pouch type in mammary area
(0): In non‐breeding adults the mammary area has no covering fold of skin and the teats are exposed. Marginal, usually lateral, ridges of skin develop during the breeding season and pouch may become deeper and evaginated. These lateral ridges of skin can be connected posteriorly. (1): Pouch consists of lateral folds of skin connected posteriorly; pouch opening anteriorly and covers the pouch young and teats.
(2): The mammary area is completely covered by a fold of skin. The so‐formed deep pouch opens at its anterior margin. (3): The mammary area is completely covered by a fold of skin. The so‐formed deep pouch opens at its posterior margin and covers the pouch young and teats.
(4): No pouch develops. No skin folds during breeding/reproductive season. The mammary area, as well as the pouch young that are attached to the teats are not covered by any skin folds during the first and second phase of lactation (Tyndale‐Biscoe, 2005). (5): Thin pouch‐like structure develops during reproductive period. Small evagination in the mammary area.
Character 264: mammary anlagen male pouch young and or adult male
(0): Present; (1): absent.
Character 268: birth position
(0): Mother delivers egg, curls up and lays the egg into the incubatorium formed between tail and abdomen or thin pouch‐like structure (Burrell, 1927).
(1): Mother lies on side to deliver neonate; (2): mother stands on all fours to deliver neonate; (3): mother sits to deliver neonate (Gemmell et al. 2002).
Ancestral state reconstruction
We coded the taxa based on our own collected data and used the literature available (Voss & Jansa, 2003, 2009; Horovitz et al. 2009; Beck, 2012; Beck et al. 2014).
Oral shield character states were coded as mentioned above (character 258). If no data were available, we used the (‘?’) symbol for an uncertain state. Parsimony reconstruction and ancestral state reconstruction were performed using MESQUITE software (Maddison & Maddison, 2015). An ancestral state reconstruction was performed using the most parsimonious tree and the step ‘traces all characters’ to summarize reconstructed ancestral states for a series of characters at each of many node changes in the trait evolutionary history.
Statistics
Multiple correspondence analyses (MCA) were performed using SPSS for species for which the categories: oral shield, pouch type, pouch cover and number of young, where known. The MCA included data from 22 species, including four American species [grey short‐tailed opossum (M. domestica), Virginia opossum, grey four‐eyed opossum (Philander opossum), monito del monte (Dromiciops gliroides) and 18 Australian species [tammar wallaby, eastern grey kangaroo (Macropus giganteus), black‐striped wallaby (Macropus dorsalis), common wallaroo (Macropus robustus), red‐necked pademeleon (Thylogale thetis), brush‐tailed rock wallaby (Petrogale penicillata), long‐nosed potoroo (Potorous tridactylus), musky rat kangaroo (Hypsiprymnodon moschatus), koala (Phascolarctos cinereus), Southern hairy wombat (Lasiorhinus latifrons), brushtail possum (Trichosurus vulpecula), Tasmanian devil, yellow‐footed antechinus (Antechinus flavipes), numbat (Myrmecobius fasciatus), Southern brown bandicoot (Isoodon obesulus), Northern brown bandicoot (Isoodon macrourus), Eastern quoll (Dasyurus viverrinus), striped‐faced dunnart (Sminthopsis macroura)]. All categories were ranked by grade of development. This representation was used to detect and represent the underlying structures in the data set.
Results
Morphological description of newborn marsupial pouch young examined
All marsupial newborn species examined appear similar in development but varying in size (Fig. 1a–o). In all marsupial specimens examined (Tables 1b and 2), the eyes are not yet open or developed and only visible in some species as dark retinal pigmentation showing through the transparent skin (e.g. D. gliroides; Fig. 1d). In all specimens examined, the orifice of the ear is not visible and in most of the larger specimens the area is covered by the pinna and the epitrichium (also observed in newborn bandicoot species by Lyne, 1964). The nostrils are well developed and open in all specimens (Sharman, 1973). An oral shield in differing states is observed in all examined species (Tables 2, 3, 4). Its development will be discussed in a subsequent paragraph. In all specimens the forelimbs are pronated, probably allowing digitopalmar prehension, and are more developed than the hindlimbs (Manger et al. 1998; Figs 1a–o and 3a,b), whereas the hindlimbs have their plantar surfaces closely opposed in the sagittal plane.
Table 4.
Stages of gestation (G1–G3) of marsupials at birth by development of external morphology (adapted from Hughes & Hall, 1988; Pask & Renfree, 2010) compared with monotremes at hatching
| Gestation stages | G1 | G2 | G3 | Monotreme hatchling |
|---|---|---|---|---|
| Species |
Eastern quoll
d,k
Tasmanian devil d Striped‐faced Dunnart a Yellow‐footed antechinus i Numbat l |
Virginia opossum
j,k,l
Philander opossum l Common opossum l Grey short‐tailed opossum l,k Monito del monte l Brushtail possum c,l,k Northern brown bandicoot d Southern brown bandicoot g,k |
Tammar wallaby
d
Eastern grey kangaroo d Long‐nosed potoroo e Red‐necked pademelon l Black‐striped wallaby l Common wallaroo l Brush‐tailed rock‐wallaby l Koala l,k Musky rat kangaroo f |
Echidna
b
Platypus b , h |
| Feature | ||||
| Eye primordia | Barely visible | Visible | Prominent | Prominent |
| Eye lids | Not present | Slight/not present | Visible | Not present |
| Retinal pigmentation | Absent/visible | Absent/visible | Prominent ring | Visible |
| Ear primordia | Barely visible | Visible/barely visible | Prominent elevation | Visible |
| Oral shield | Extensive complex | Reduced/simple | Vestigial | Vestigial, mouth open (slit in coronal plane) |
| Definition of mandible | Slight | Moderate | Pronounced | Pronounced |
| Prominence of nasal swelling | Extreme | Extreme/moderate | Slight | Slight |
| Cervical swelling | Present/absent | Absent | Absent | Absent |
| Toes of the hindfoot | Not visible | Visible as folds/formed | Formed | Visible as folds/formed |
At closer inspection it becomes obvious that hindlimb development in particular is not quite the same in all species. In most species the digits of the forelimbs and hindlimbs are already well separated, this can be noted in the koala, various macropodidae (Eastern grey kangaroo, common wallaroo, tammar wallaby, black‐striped wallaby, brush‐tailed rock wallaby), potoroidae (long‐nosed potoroo, musky rat kangaroo), brushtail possum, northern and southern brown bandicoot, Didelphidae (Virginia opossum, grey short‐tailed opossum; McCrady, 1938; Hughes, 1962; Hughes & Hall, 1984, 1988; Schneider, 2011; Chew et al. 2014; this study). Furthermore, specific morphological features of the hindfeet found in the adult are already visible in the newborn, for example, in the macropod newborn digit I is missing, digits II and III fused, and digit IV elongated, and in the koala newborn's digits II and III are fused.
Other species, such as the monito del monte (Gurovich, in prep.) and possibly the Philander opossum, only show folds or slight crevices in the hindfeet indicating the developing digits. However, as the Philander opossum specimen (MA806F; Table 2a) is a late stage embryo, it cannot be ruled out that the digits of the hindlimbs are more developed at birth. Finally, in some species the digits of the hindlimb are not visible or differentiated at all. This is observed in the Tasmanian devil, the Eastern quoll and the yellow‐footed antechinus (Marlow, 1961; Hughes & Hall, 1988; Schneider, 2011), and can be described as very much ‘paddle like’. We were also very fortunate to find three pouch young of the endangered numbat in the Hill collection (Figs 1c and 4b–d), of which two were unknown to exist. We assume that all these three pouch young are numbats as the structure of the snout in the young of this species is very peculiar and this morphology is also observed in these younger specimens (Wood Jones, 1923). As a white substance, likely to be milk, was noticed under the skin of the two smaller young (Fig. 4b,c), it could be concluded that all of them are newborn or slightly older pouch young. We consider the smallest of these young, measuring less than 1 cm in greatest length, as a recently born specimen (Fig. 4b). Here, we describe for the first time a neonate of this species. The newborn numbat appeared slightly dried and dehydrated, and its very small size did not allow one to ascertain the presence of claws on the forelimbs and the hindlimb structure. Nevertheless, the hindlimbs are likely to be paddle‐like as the overall development is similar to that of the three above‐mentioned species besides the peculiar oral shield described later on. Furthermore, the hindlimbs of the elusive Southern marsupial mole (N. typhlops) pouch young (GL about 10 mm) appear to be paddle like (Fig. 1o; Table 2b). The Southern marsupial mole was first described and illustrated by Wood Jones (1921), but the age of this specimen is unknown. However, due to its overall developmental stage, well‐developed forelimbs but still paddle‐like hindlimbs, it may be suggested that this illustrated specimen represents an only recently born pouch young. Interestingly, this specimen only has two prominent digits (III and VI) armoured with claws on each digit of the front pedes (Fig. 1o).
Figure 4.
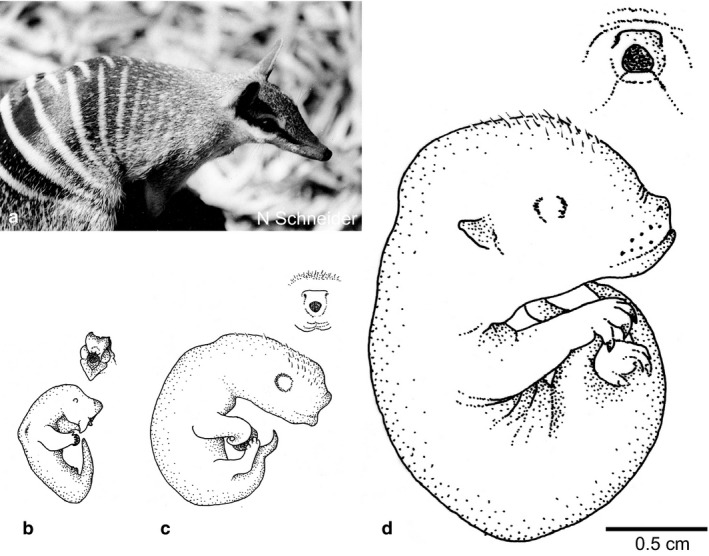
While the adult numbat (a) has a thin and pointy muzzle, this is not the case in pouch young shown here (b–d) (all MA242 from Hill Collection). Even though the oral shield structure disappears the muzzle remains short, which is likely to be an adaptation to allow the young to stay securely attached to the teat. This may be especially important as female numbats do not possess a pouch.
Furthermore, four dasyurids (numbat, striped‐faced dunnart, Tasmanian devil and the Eastern quoll) studied here show a pronounced cervical swelling between the forelimbs (i.e. fine skin and bulge between lower jaw and abdomen) and below the head (Hughes & Hall, 1988; Frigo & Woolley, 1997; Schneider, 2011; this study). The pronounced cervical swelling is not observed in any other species.
No external genitals were observed in the newborn pouch young examined.
We found that newborn marsupials present external developmental differences at birth. These differences were especially observed in hindlimb development, oral morphology, and in the presence or absence of a cervical swelling. This observation is also supported by other authors (Hughes & Hall, 1988; Smith, 2001; Ashwell, 2010) who note that diprotodont marsupials at birth have more prominent external ear and eye primordia, retinal pigmentation, hindlimbs with digits and pronounced mandibles in comparison to dasyurids, which are born highly altricial with no external ears, no visible eye primordia and a very pronounced oral morphology. These differences may reflect the differences in the mode of transport of the newborn from the urogenital sinus to the pouch and teat (Ashwell, 2010).
State of the oral shield
The oral shield is a very enigmatic structure so far only observed in newborns of some marsupial species. Below we will discuss the different states of the oral shield observed in different taxa. We observe oral shield type 0 in monotremes, and four types of oral shield state are distinguished in marsupials.
Oral shield type 0
Newborn monotremes hatchlings do not have an oral shield, even though there are similarities in the overall external morphology and body shape between newborn marsupials and monotreme hatchlings (Griffiths, 1978; Hughes & Hall, 1998).
Four types of oral shield states could be distinguished in marsupials.
Oral shield type 1
In some marsupials the oral shield is vestigial and consists of fused lips, which are clearly separated from the rhinarium. Oral shield type 1 is observed in the neonates of the koala (Fig. 2k), various macropodidae [Eastern grey kangaroo, common wallaroo, tammar wallaby, black‐striped wallaby (Fig. 2j), red necked pademelon (Fig. 2i) and brush‐tailed rock wallaby] and potoroidae (long‐nosed potoroo, musky rat kangaroo; Hughes, 1962; Hughes & Hall, 1988; Schneider, 2011; this study). Furthermore, the southern marsupial mole may also have a vestigial oral shield (Wood Jones, 1921). Interestingly, the mouth of the southern marsupial mole pouch young (Fig. 2l) appears slit‐like, and the rhinarium is already more morphologically reminiscent of the nasal morphology seen in adults. Very little is known about this specimen, including when it was captured and its neonatal age.
Oral shield type 2
A slightly more developed oral shield with a less prominent separation from the rhinarium is observed in the northern and southern brown bandicoot (Fig. 2g) and the brushtail possum (Fig. 2h; Hughes & Hall, 1988, 1984; Schneider, 2011).
Oral shield type 3
An even more prominent oral shield with lips still visible as folds but slight swelling of the lips around the buccal opening is seen in a number of Didelphidae [grey four‐eyed opossum (Fig. 2d), grey short‐tailed opossum (Fig. 2e) and Virginia opossum (Fig. 2f)], as well as in the microbiotherid monito del monte (Fig. 2c; McCrady, 1938; Schneider, 2011; this study). The grey four‐eyed opossum in this study is a late stage embryo (about stage 34 described for the Virginia opossum by McCrady, 1938) as no neonate specimen was available. It may therefore be that the structure of the neonate is slightly different. In this embryo we observed six pointy ends on the lower lip part of the collar‐like oral shield structure, similar to the structures observed by Selenka (1887) and McCrady (1938) in the Virginia opossum late stage embryo, which are no longer visible in the neonate. While the oral shield is also more prominent in the preterm embryo of the grey short‐tailed opossum (personal observations by NYS), it does not show any pointy ends as observed in the Virginia and grey four‐eyed opossum.
The newborn numbat's oral shield is well‐developed and does not resemble any of the so‐far described structures in other marsupials (Figs 2b and 4b). The lower part of the swollen lips joins to a medial ventral point. A slight depression runs from the round mouth opening towards the point of the lower lip. The upper lip is separated from the lower by a slight groove. The rhinarium is set apart from the upper lip and shows dorsally two bulges on either side, which are formed around the opening of the naris (Fig. 4b). We consider this a type 3–4 oral shield as lips are visible as folds as in type 3 but the shield is prominent (type 4) as described in other dasyurids such as the Eastern quoll (Fig. 1a) and Tasmanian devil (Fig. 1b).
Oral shield type 4
The most prominent oral shield state consists of the upper and lower lips joined together with the rhinarium to form a flattened ring structure that surrounds the buccal opening. This type of oral shield is observed only in dasyurid species, the Tasmanian devil, the Eastern quoll, striped faced dunnart and yellow‐footed antechinus, as well as the numbat (Marlow, 1961; Hughes & Hall, 1988; Frigo & Woolley, 1997; Schneider, 2011; this study).
We determine here that the oral shield is an important feature of the newborn marsupial, and its morphology is likely related to the overall development of the newborn.
Character evolution analysis
Phylogenetic tree construction
Maximum parsimony heuristic analyses were carried out using both extant and extinct taxa, and 22 most parsimonious trees were recovered with tree length of the best tree at 1028.
Figure S1 shows the strict consensus of 22 most parsimonious trees. All taxa are included (extinct and extant), the relationship of Dromiciops to other Australian marsupials is poorly resolved, and the clade that includes all extant and extinct Metatheria has a low support value (bootstrap = 52). The clade that includes all theria has a very high support value (bootstrap = 100). Most extant Australian clades are well supported with Peramelemorphia (bootstrap = 100), but including Notoryctes this clade does not have a good support (bootstrap = 56), Dasyurimorphia has a very high support value (bootstrap = 99). Vombatiformes (bootstrap = 98) and Diprotodontia are unresolved with moderate support values (bootstrap = 71).
Reconstruction of ancestral oral shield morphology
Parsimony ancestral state reconstruction for the character of oral shield supports the inference that an oral shield (type 2–3) may have been present in early marsupials as seen in Fig. 5b, and may have been lost secondarily in more recent clades. It is a character that is found in many different groups of extant marsupials and thus seems plausible that newborn marsupial ancestor had an oral shield type structure as is present in newborn didelphids, dasyurids, micrbiotherians, etc. It is absent in extant monotremes and perhaps was absent in monotreme ancestors.
Figure 5.
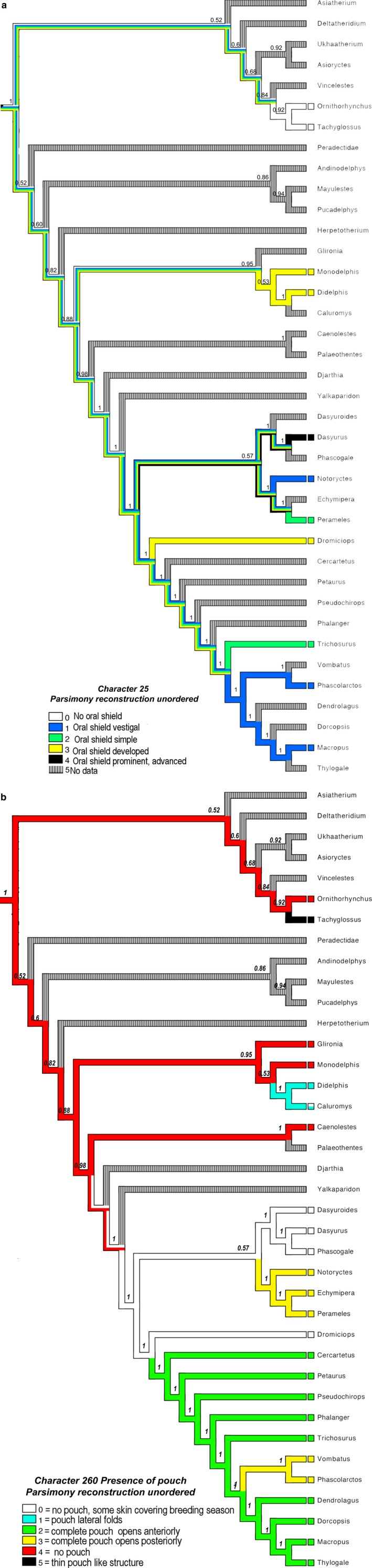
Phylogenetic relationships of Dromiciops and extant marsupials with extinct species based on maximum parsimony analysis of the morphology‐only matrix. (a) Parsimony ancestral state reconstruction using MESQUITE software (Maddison & Maddison, 2015) demonstrating the prevalence of character 258 (oral shield) based on majority‐rule consensus of 100 trees. Consistency index = 0.36194416, tree length = 967, retention index = 0.634262. Character states are: (0): no oral shield; lips are separated and mouth can be open wide; (1): oral shield vestigial but lips still closed but clearly separated from the rhinarium; (2): oral shield simple; lips not swollen and rhinarium forming separate structure; (3): structure reduced compared with state 4, the lips becoming visible as folds of the skin, the lips are slightly swollen around the buccal opening and the rhinarium is well developed; (4): upper and lower lips form together with the rhinarium a flattened ring structure that surrounds the buccal opening. (b) Parsimony ancestral state reconstruction using MESQUITE software (Maddison & Maddison, 2015) demonstrating the prevalence of character 260 (Pouch type in mammary area) based on majority‐rule consensus of 100 trees. Consistency index = 0.36194416, tree length = 967, retention index = 0.634262. Character states are (0): in non‐breeding adults the mammary area has no covering fold of skin and the teats are exposed. Marginal, usually lateral, ridges of skin develop during the breeding season and the pouch may become deeper and evaginated. These lateral ridges of skin can be connected posteriorly. (1): Pouch consists of lateral folds of skin connected posteriorly; pouch opening anteriorly and covers the pouch young and teats. (2): The mammary area is completely covered by a fold of skin. The so‐formed deep pouch opens at its anterior margin. (3): The mammary area is completely covered by a fold of skin. The so‐formed deep pouch opens at its posterior margin and covers the pouch young and teats. (4): No pouch develops. No skin folds during breeding/reproductive season. The mammary area, as well as the pouch young that are attached to the teats are not covered by any skin folds during the first and second phase of lactation (Tyndale‐Biscoe, 2005). (5): Thin pouch‐like structure develops during reproductive period. Small invagination in the mammary area.
Parsimony ancestral state reconstruction for the character of pouch (Fig. 5b) supports the inference that early mammals did not have a pouch, or may have had a very simple pouch as seen in some extant didelphids. More complex pouches are found in extant Australian clades that have a more recent diversification and younger fossil record.
Pouch type vs. oral shield type
It seems that most species studied here that have a fully covered pouch (e.g. kangaroos, possums and bandicoots), possess a type 1 or type 2 oral shield (vestigial or non‐prominent) in newborn young (Figs 5a,b and 6a). Hughes & Hall (1988) first proposed to classify the development of marsupials by three grades, G1, G2 and G3, with G1 being the ‘least developmentally advanced’ at birth and G3 ‘the most developmentally advanced’ at birth. This staging system was supported by Pask & Renfree (2010) who propose that it coincides with the length of intrauterine organogenesis and birth weight, with less developed G1 having the shortest organogenesis and the lowest birth weight. The different states of the oral shield can also be associated to the different grades of development (Hughes & Hall, 1988; this study; Table 4), whereby a strongly developed oral shield is considered as G1, a reduced oral shield is considered as G2, and a simple or vestigial oral shield is considered as G3. The MCA (Fig. 6a; dimension 1: 0.962 Cronbachs alpha, variance: eigenvalue 4.335, inertia 0.867, percentage of variance 86.69; dimension 2: 0.803 Cronbachs alpha, variance: eigenvalue 2.794, inertia 0.559, percentage of variance 55.88) shows which traits are more likely to occur in combination with the different oral shield types. Giving birth to a great number of young (more than two), high number of teats and partial coverage of the mammary area appears to coincide with a strongly developed oral shield. On the contrary, the vestigial to simple oral shield is found in species that have a low number of young (one–two), and with pouch types that have fully covered mammary area and a low number of teats (two–four). Interestingly, it is relatively difficult to separate the traits situated around the reduced oral shield and strongly developed oral shield.
Figure 6.
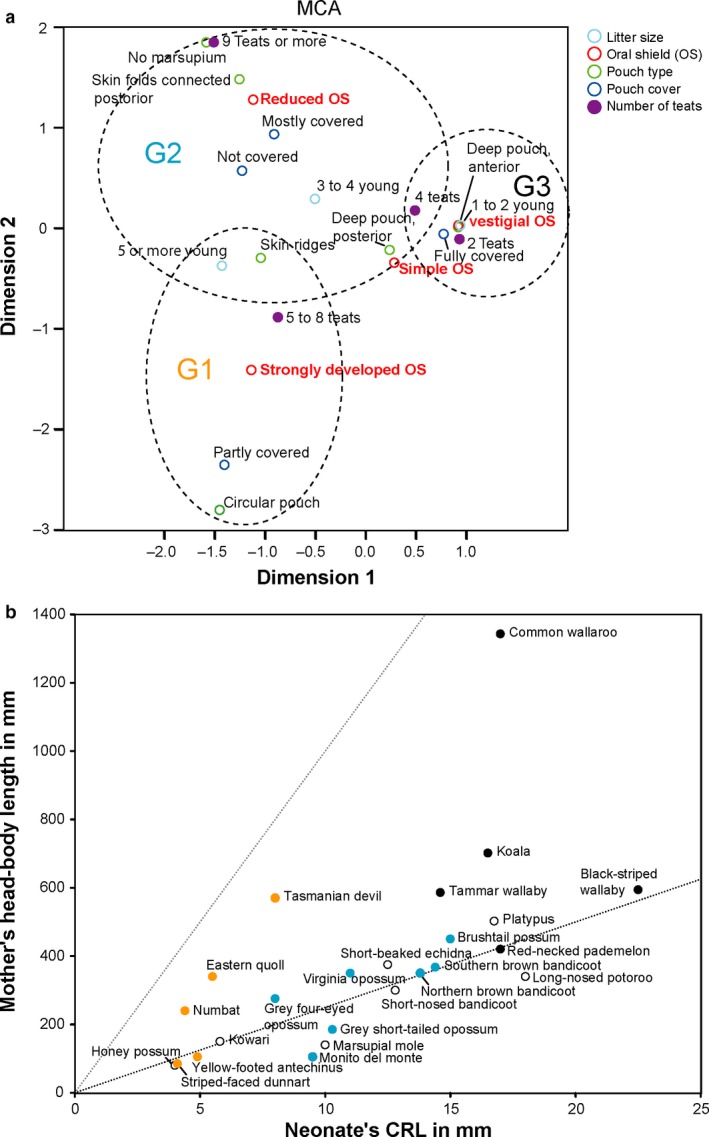
(a) Results of multiple correspondence analyses (MCA) using SPSS for species for which the categories: oral shield, pouch type, teat coverage, and number of young were known. The MCA included data from 22 species, including four American species (grey short‐tailed opossum, Virginia opossum, grey four‐eyed opossum, monito del monte) and 18 Australian species (tammar wallaby, eastern grey kangaroo, black‐striped wallaby, common wallaroo, red‐necked pademeleon, brush‐tailed rock wallaby, long‐nosed potoroo, musky rat kangaroo, koala, Southern hairy wombat, brushtail possum, Tasmanian devil, yellow‐footed antechinus, numbat, Southern brown bandicoot, Northern brown bandicoot, Eastern quoll, striped‐faced dunnart). The results can be grouped into the three stages G1, G2 and G3 of pouch young development. (b) Marsupial adult female head and body length (mm) vs. crl (crown rump length) of neonate young (mm). Orange circles represent G1 species, blue circles G2 and black G3. White filled circles with black outline are species in which the G group is unknown or n.a. (e.g. monotremes). The dark dotted line represents the average % of birth size of the adult size (4%). The light grey dotted line marks the 1%. Australian adult female length was taken from Van Dyck & Strahan (2008), Fadem et al. (1982) for grey short tailed opossum; Castro‐Arellano et al. (2000) for grey four‐eyed opossum and Marshall (1978) for monito del monte. Measurement of newborn honey possum taken from Oates et al. (2007).
The two consensus phylogenetic trees for character 258 (oral shield; Fig. 5a) and 260 (pouch type; Fig. 5b) show a similar result. While Dasyurus has a type 4 oral shield, which is very prominent and distinct in the newborn, and the adult female has a partially covered pouch with exception of the Tasmanian devil, Dromiciops and Didelphis have a type 3 oral shield and a partially covered pouch or mostly covered pouch. Some didelphids such as Glironia and Monodelphis have no pouch cover and have a type 3 oral shield (Fig. 5a).
Based on this limited information (Figs 5a,b and 6a), it seems that a vestigial oral shield such as observed in Macropus (type 1) coincides with a well‐developed pouch and a small number of young (one or two), while a strongly developed oral shield (type 3 developed and type 4 prominent) corresponds to a partially covered pouch or no pouch and a greater number of young (more than two).
Here we observe that the state of the oral shield is similar in more closely related taxa (Fig. 5a,b), i.e. a more developed oral shield is found in different species of dasyurids, while a vestigial oral shield is found in the larger Australasian macropod marsupials. The vestigial oral shield is considered here the more derived condition (i.e. it is not found in the common ancestor to all species, and thus evolved more recently in certain taxa). None of the extant South American marsupials observed had a vestigial oral shield; all observed species had a developed oral shield. We also observe that taxa that have the less developed or vestigial oral shield morphology also have a more developed pouch in breeding females (Fig. 5b). However, further data and research on other species are needed to verify these two points, to see that there is a correlation between the state of development of the oral shield in newborns and the state of the pouch in adults, and to observe this morphology in a large distribution of extant marsupials including newborn pouch young and breeding females. If our two hypotheses are right, we would expect that early marsupials may have had a very simple pouch or even lacked a pouch during the breeding season, they would have given birth to a large number of pouch young and pouch young that were born very small and highly altricial, and we hypothesize that early marsupials may have been born with a somewhat developed oral shield. Previous studies also hypothesis that early marsupials were pouchless and that pouches may have evolved independently in several lineages from pouchless ancestors (Sharman, 1976; Kirsch, 1977). If indeed early marsupials were pouchless and they gave birth to highly altrical young, how would these young be able to stay attached to the teat and survive close to the mother's body? They would need to be attached very firmly indeed.
Thus, the state and morphology of the oral shield in newborn marsupials is similar in phylogenetically more closely related taxa (Table 2a,b). For example, we observed four species of dasyurids to have the same oral shield type 4, three species of didelphids to have oral shield type 3, one species of microbiotherian to have oral shield type 3, two bandicoot species to have oral shield type 2, and four species of macropods to have oral shield type 1. Finally, the two species of monotremes have oral shield type 0. From this preliminary analysis it seems that didelphids, microbiotherians and dasyurids have a more developed oral shield type 4 to 3, while other Australasian marsupials (macropods, bandicoots, possums) have a less developed oral shield (type 2–1). We have no data on paucituberculata marsupials newborns; however, all extant caenolestids lack a pouch (Patterson, 2015). As many extant South American marsupials do lack a pouch (e.g. order Didelphimorphia, order Paucituberculata), we may expect them to have a developed oral shield if there is a correlation between lacking or having a less developed pouch and having newborn born that are small, highly altricial and with a developed oral shield. Finally, this would lead us to believe that in the past early metatherians may have had a very simple or absent pouch, they would have given birth to a large number of pouch young and pouch young that were born highly altricial, and with a prominent oral shield to help them attach to a teat and stay attached, similar to what is seen today in pouchless extant didelphids and caenolestids.
Pouch type vs. relatedness
Our study shows that pouch morphology may be related to oral shield morphology. We also observe that having a well‐developed pouch is not necessarily a characteristic present in all marsupials and is not a specific trait that aids in rearing of the altricial young as previously stated by Edwards & Deakin (2012). What seems to be more important in survival of highly altricial newborn marsupials is the presence of an oral structure that allows a secure attachment to the teat, regardless whether there is a pouch or not. Pouches vary markedly between marsupial species, as they can be deep or shallow and contain different numbers of teats (Tyndale‐Biscoe, 2005).
In this study, we observe a pattern in which the majority of basal Australasian marsupials (possums, kangaroos, bandicoots) all share the presence of a very prominent fully covered deep pouch (Fig. 5b). On the other hand, other Australasian marsupials such as some carnivorous dasyurids and most Ameridelphia marsupials have a different pouch morphology that consists of an uncovered mammary area with no folds at all, or a mammary area that is covered by folds that form only during the breeding season. Dromiciops is nested within these Australidelphia and has a pouch morphology that is more similar to some carnivorous Australasian marsupials (dasyurids) and Ameridelphia marsupials. The Dromiciops pouch is shallow, undeveloped and the mammary area is not covered (i.e. the teats are visible) while the female is not breeding. However, during the breeding season and when the female is lactating and small newly born pouch young are present, the pouch is very strongly covered (YG, personal communication). Pouch morphology is a character that needs to be studied in more detail, for example, in Dromiciops the pouch changes morphology, colour and size throughout the reproductive cycle, and in the Australian antechinus (A. flavipes) the pouch is normally not present, it is only formed 16 days after parturition and then atrophies after weaning (Marlow, 1961).
As mentioned previously in other phylogenetic analyses that use both soft‐body morphology and fossil taxa (Beck et al. 2014), a major weakness presented here is the lack of fossil taxa sampling for Australidelphia, and the lack of coding of soft morphological characters for all extant taxa.
Discussion
This study compares oral shield morphology of newborns of different marsupial species with other newborn external characteristics and with reproductive adaptations found in the females. The general newborn marsupial body plan was similar in all species studied here. Marsupials are born: (i) furless and with underdeveloped vibrissae; (ii) undeveloped eyes; (iii) undeveloped ears; (iv) a deeply innervated snout with touch receptors (Merkel cells; Hughes & Hall, 1988); (v) a prominent craniofacial development specifically in the oral‐nasal region; (vi) a c‐curved body; (vii) strongly developed cartilaginous forelimbs with differentiated digits that bear curved epitrichial claws (Gemmell et al. 1988; Martin & Mackay, 2003); (viii) paddle‐like cartilaginous hindlimbs whose digits may not be differentiated (Barthélemy & Cabana, 2005; Weisbecker et al. 2008); and (ix) in most cases no external genitalia (Bolton, 1983; Renfree et al. 1990, 1996; Ullmann, 1993). In the following we will discuss the relationship between the newborn's oral shield morphology and typical morphological features of the young. We will look into how the state of the oral shield may depend on the female's reproductive features, such as pouch morphology, number of young and female size. Finally, we will look at the evolution of the oral shield, especially focussing on the monito del monte and its placement in the here established phylogeny and its oral shield type at birth compared with closely related species.
The state of the oral shield and its relationship to other traits of the newborn at birth
The oral shield appears to be a marsupial‐specific structure. A similar structure has to our knowledge not been described in eutherians or monotremes. To understand whether the state of the oral shield is related to the overall state of development of the young at birth, we will discuss in the following the concurrently occurring states of other typical features in newborn marsupials.
First of all it may be noted that some newborn features appear to be at similar state in all the here‐studied marsupials. So while the development of the structure formed by the lips and the rhinarium (oral shield) varies between marsupial species, all of the species studied here presented fused lips at birth, which is likely an adaptation to the long teat attachment period after birth.
Another example is the ‘forelimb development’. Almost all here‐studied species showed well‐developed forelimbs bearing five digits equipped with claws. Exceptions to the rule are the bandicoots and the fossorial subterranean marsupial mole. The bandicoots such as the newborn I. macrourus (Lyne, 1974) and Perameles nasuta (Lyne, 1964 only have three deciduous claws (that fall off after birth) on the three middle digits (digits II, III and IV) of the manus, and I. macrourus has a rudimentary digit I and small apparently non‐functioning digit V with no claws (Driessen & Rose, 2015). Thylacomyids also lack digit I. The marsupial mole has forelimbs at birth that only show two prominent digits (III and IV) with deciduous claws, the other three being stub‐like (Wood Jones, 1921; Fig. 1), resembling the morphology observed in adults. Throughout its life adult marsupial moles spend most of their time underground in underground burrows (Warburton, 2003), and the specialized forelimb development at birth may be an early adaption that will later help juvenile young move through the sand. It is likely that the newborn may come into contact with sand particles (if parturition occurs in underground burrows) during its journey to the rear‐opening pouch, but the distance the newborn must travel from the urogenital sinus to enter the rear opening pouch is perhaps minimal and the young may be aided by the mother (Johnson, 1995). Newborn marsupial moles must have the same forelimb morphology as the adult, as the forelimb morphology is already well established in marsupial newborns before birth.
No difference in the overall external forelimb (palmar, dorsal) morphology was apparent in the other marsupial species observed in this study. Therefore, it is likely that muscular and skeletal development differs at birth, depending on the overall development of the young at birth (highly altricial as in dasyurids, less altricial as in macropods) and perhaps the distance the young has to travel after birth in order to get to the teat. Some differences in muscle and skeletal development have been observed by other authors (Hughes & Hall, 1988). In summary, the overall lack of obvious differences of forelimb development may be due to the fact that this structure is essential for all young to be able to reach the teat and does not stand in any relationship to the overall state of development or that of the oral shield.
While there were very little differences in forelimb morphology, differences in ‘hindlimb development’ could be established. The least developed hindlimbs were observed in the dasyurids (G1) with no digits apparent, while the most developed were observed in macropods (G3) in which digits were already visible and showed adult‐specific features (Hughes & Hall, 1988; Chew et al. 2012; this study). It has been suggested that slower hindlimb development may allow for resources and energy to be used for development of anterior structures that are essential for survival (energy trade off hypothesis; Chew et al. 2014). The difference in the grade of development is probably related to gestation length, with species with a shorter gestation showing greater differences between anterior and posterior development. Less hindlimb development coincides with a more prominent oral shield that may point to the fact that the state of the oral shield may be an adaptation of less developed young or directly related to the developmental stage of the young.
The ‘cervical swelling’ appears to be an adaptation of certain marsupial species as it has so far only been observed in some newborn dasyurids (Hill & Hill, 1955; Marlow, 1961; Hughes & Hall, 1988; Frigo & Woolley, 1997; this study) and it is not part of the embryological development of any other marsupial species as far known (McCrady, 1938; tammar wallaby personal observations NYS). It is suggested that this structure supports the head, so that it is at the right angle to the body, facilitating connection with the teat (Hill & Hill, 1955). It may be related to the relatively poor state of development of these species’ newborns compared with other species’ newborn. The presence of a cervical swelling coincides with a strongly developed oral shield. Its presence is though not specific to all G1 marsupials, and is only observed in one family, and could therefore be a family‐specific adaptation.
So while the overall development of the newborn appeared similar, three different grades of development, G1 to G3, could be observed as described in earlier studies (Hughes & Hall, 1988; Pask & Renfree, 2010). Newborn of developmental grade G1 being the least developed with the most prominent oral shield, while those of grade G3 being the most developed with vestigial oral shield, resembling in their overall development those of newly hatched monotreme (Table 4). Furthermore, it appears that species size (leading to smaller young in smaller species that are less developed at birth) may correlate to neonate developmental state (Ashwell & Shulruf, 2014a,b; Fig. 6b). It may therefore be another important trait to look at to further understand the developmental grade of the neonate at birth including the state of the oral shield.
In summary, it appears that the oral shield state of newborn marsupials is related to the overall developmental state of the newborn and probably the species size.
Correlations between female reproductive adaptations and oral shield morphology
The next big question would be why less developed newborn marsupials need a stronger developed oral shield compared with more developed newborns. We hypothesis that this may be important in order to establish a more secure connection between mother and young. This again may be related to the females pouch structure. Newborn of marsupials with less pouch cover or no cover would show more strongly developed oral shields as the newborns are at greater risk to get detached and would be unable to reattach.
Using external newborn morphology and reproductive information from the literature, we observed at least two extreme forms of reproductive adaptations in marsupials (Figs 5a,b and 6a; Table 4). The first one consists of the female producing many offspring (more than two; often insufficient teats for all newborns). These newborns are born with significantly less developed hindlimbs, a strongly developed oral shield and, in most cases a cervical swelling. This form of pouch young development coincides with females having a large number of teats and a pouch area that is less covered to not covered. These newborns are born highly altricial and the lactation period is usually shorter than in the other form. The second form of development includes marsupials that have a litter size that ranges between one and two newborns. The newborns show well‐developed hindlimbs with visible differentiated digits, in some instances there is early digit separation and differentiation (e.g. macropods; Hughes & Hall, 1988), no cervical swelling and a simple to vestigial oral shield at birth. Females of these species have a deep well‐developed pouch with enough teats for all newborn. The newborn offspring stays attached to the teat for a long period, and lactation is extended as in macropods.
An explanation for the development of the oral shield may therefore be that it serves to provide stronger attachment to the teat, and thus increase survival rate. This is important in species that are not protected by a deep developed pouch, and are at a greater risk to become unattached from the teat and to be unable to reattach. Numbat newborns have a very developed oral shield, and even older pouch young have a shortened and flattened snout, while adult numbats have a very elongated snout (Wood Jones, 1923; this study; Figs 2b and 4a–d). Similarly, adult bandicoots (e.g. Echymipera and Isoodon) also have a distinctly elongated snout while their newborns show a shortened snout with a simple oral shield type (tables 2 and 4; Lyne, 1951). The young of these two species do not have the same developmental grade at birth (numbat: G1; bandicoot: G2), but these findings appear to underline the crucial importance of an adapted oral‐nasal morphology at birth, especially in numbat females that do not have a pouch (Cooper, 2011). This is further supported by information that pouch young, from taxa that have a secure pouch, seem to be able to detach and reattach to the teat early on (e.g. tammar wallaby; Collie, 1830; personal communication by Brandon Menzies). But on the other hand there are species such as the Tasmanian devil that has a well‐developed rear opening pouch but newborns of G1 development as other dasyurids with little to no pouch cover. Therefore, it appears that other factors than adaptation to the pouch type may also play a role, such as species relatedness (Fig. 5a).
Finally, our results suggest that the oral shield is important for secure teat attachment and therefore more strongly developed in species in which females have less pouch cover.
The evolution of the oral shield and the monito del monte's placement with the Australidelphia
In order to better understand the evolutional development of an oral shield and its relation to pouch development in marsupials, we constructed a phylogenetic tree including oral shield and pouch developmental data.
Based on morphological external features, we here place the monito del monte (D. gliroides) newborn in stage G2 (Table 4), along with opossums, bandicoots and the brushtail possum. Newborn marsupial young in stage G2 have well‐developed forelimbs while the hindlimbs are less developed with visible digits. The oral shield structure is present at birth but reduced (type 3) in the monito del monte. Interestingly, even though the overall development of the newborn is very similar in development to the Australasian phalangeroid (possums), the oral shield of the monito del monte resembles more that of the Ameridelphid (opossums).
Our maximum parsimony analysis using postcranial, cranial and soft‐body morphology in adults and newborn pouch young does not place Dromiciops within the Australidelphia, and this clade is unresolved (Fig. S1). This could be due to the lack of morphological data available for this present analysis. However, other authors have placed Dromiciops nested within Australidelphia (Horovitz & Sánchez‐Villagra, 2003; Beck et al. 2014). These authors use very little soft‐body morphology in their analyses. In our analysis (Fig. S1), Australidelphia (Beck et al. 2014) as well as the very limited soft‐body morphological characters were used for extant taxa. More studies are needed on soft‐body anatomy of all extant South American and Australasian marsupials, and these characters can then be used to test and support more robust phylogenetic relationships.
Finally, our results using character state reconstruction suggest that ancestral marsupials may have had no pouch but may have had an oral shield type of 3 in newborn pouch young. This result suggests that the oral shield in newborn marsupials may not have been developed as an adaptation for the lack of a pouch in adult females, but may be related to other characteristics. In the specimens observed it seems that the development of the oral shield occurs just before birth, and this suggests that this feature is essential for survival of the pouch young at birth and for remaining attached to the teat. It is also present in species regardless of whether the mother has a pouch or not, but more so in species in which the neonate is very altrical at birth (e.g. dasyurids, didelphids) and not as highly developed. Another explanation for this result could be due to the limited number of species in which this structure could be investigated as our observations are based solely on museum specimens and what is available in the literature.
Concluding remarks
Our morphological and phylogenetic study provides for the first time an extensive assessment of the marsupial newborn‐specific oral shield states and hypotheses of its possible functions.
Firstly, the oral shield appears to depend on the overall development of the young at birth (with less developed young – prominent oral shield) and its morphology may be species dependent (most prominent oral shield only found in dasyurids). Developmental differences between newborns are also seen in other external characters such as hindlimb development and cervical swelling, but not in forelimb development, which is at least externally very similar between all investigated species. Forelimb development is therefore not species related with two exceptions: the bandicoots (e.g. golden bandicoot Isoodon auratus with only three clawed toes on forelimb) and the marsupial mole, and this consistency in overall forelimb morphology throughout Marsupialia reflects the need of the young for functional forelimbs to reach the teat. The functional requirements of this climb to the pouch have imposed a developmental constraint on marsupial forelimb evolution (Cooper & Steppan, 2010).
We also observed that less developed newborns are born in smaller species. We therefore formulate as a new hypothesis that species size influences reproductive strategy (e.g. seasonal or non‐seasonal, one or more litters per year, litter size, nest or no nest), which impact on body size and the level of development of the young at birth (hindlimb, oral shield and cervical swelling), where this is not influenced by other species‐specific adaptations. However, this needs to be further supported by anatomical and life history studies in marsupial neonates as there are still limited data available.
Secondly, the oral shield seems to be important for a secure attachment to the teat. The state of the oral shield appears to correlate with the pouch cover present in the females (e.g. deep pouch and vestigial oral shield; no pouch and prominent oral shield).
Thirdly, we describe for the first time the state of the oral shield in some species including the monito del monte. These soft tissue data allowed us to create phylogenetic trees based on both soft‐body and postcranial and cranial data. The character evolution analysis suggests that the oral shield in newborn marsupials may not have been developed as an adaptation for the lack of a pouch in adult females, but may be related to other characteristics. Another explanation may be the still relatively low number of investigated species. Finally, while our phylogenetic results place the enigmatic monito del monte nested in the Australidelphia, its oral shield resembles more those of the Ameridelphid. This underlines the significance of external soft‐body morphology of extant marsupial adult and pouch young as an important source of phylogenetic information (Voss & Jansa, 2003), which should be included in future phylogenetic studies to help determine more robust marsupial relationships.
Author contributions
Both authors contributed equally to experimental design, data collection (specimens and literature) and the writing of the manuscript. YG collected data of the monito del monte specimens from the National Parks in Southern Argentina kindly lent for examination to her by R.D. Sage. She also prepared drawings of the monito del monte and re‐drawings of the Tasmanin devil, the common opossum and the marsupial mole. NYS collected the data from the Hubrecht and Hill collection (Museum für Naturkunde Berlin, Germany), and made drawings of those specimens and re‐drawings of the echidna and platypus.
Supporting information
Fig. S1. Phylogenetic relationships of Dromiciops and extant marsupials with extinct species based on maximum parsimony analysis of the morphology‐only matrix.
Acknowledgment
The authors would like to thank Dr Richard D. Sage for lending us the monito del monte specimens for observation, and Dr Peter Giere for allowing us to use the Hubrecht and Hill collection (Museum für Naturkunde Berlin, Germany). The authors thank Dr Sandy Ingleby (Mammalogy Collection) and Dr Anja Divljan for providing access to the Mammalogy Collection, Australian Museum, Sydney, and also Dr Jacqueline Nguyen for help with PAUP.
YG would like to thank Dr Francisco J. Goin for his support and discussion on marsupial evolution and development.
Furthermore, the authors would like to thank CONICET for funding provided to YG for this research project.
References
- Amico GC, Rodríguez‐Cabal MA and Aizen MA (2009) The potential key seed‐dispersing role of the arboreal marsupial Dromiciops gliroides . Acta Oecol 35, 8–13. [Google Scholar]
- Amrine‐Madsen H, Scally M, Westerman M, et al. (2003) Nuclear gene sequences provide evidence for the monophyly of australidelphian marsupials. Mol Phylogenet Evol 28, 186–196. [DOI] [PubMed] [Google Scholar]
- Aplin KP, Helgen KM, Lunde DP (2010) A review of Peroryctes broadbenti, the giant bandicoot of Papua New Guinea. Am Mus Novit 3696, 1–41. [Google Scholar]
- Ashwell KWS ed. (2010) The Neurobiology of Australian marsupials: Brain Evolution in the Other Mammalian Radiation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Ashwell KWS (2013) Neurobiology of Monotremes: Brain Evolution in Our Distant Mammalian Cousins. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- Ashwell KWS, Shulruf B (2014a) Vestibular development in marsupials and monotremes. J Anat 224, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell KWS, Shulruf B (2014b) Spinal cord development in marsupials in relation to birthing strategies and in comparison with monotremes and the laboratory rat. Somatosens Mot Res 31, 152–165. [DOI] [PubMed] [Google Scholar]
- Ashwell KWS, Marotte LR, Cheng G (2008) Development of the olfactory system in a wallaby (Macropus eugenii). Brain Behav Evol 71, 216–230. [DOI] [PubMed] [Google Scholar]
- Barthélemy D, Cabana T (2005) Postnatal development of limb motor innervation in the opossum Monodelphis domestica: immunohistochemical localization of acetylcholine. Dev Brain Res 155, 87–98. [DOI] [PubMed] [Google Scholar]
- Beck RMD (2008) A dated phylogeny of marsupials using a molecular supermatrix and multiple fossil constraints. J Mammal 89, 175–189. [Google Scholar]
- Beck RMD (2012) An ‘ameridelphian’ marsupial from the early Eocene of Australia supports a complex model of Southern Hemisphere marsupial biogeography. Naturwissenschaften 99, 715–729. [DOI] [PubMed] [Google Scholar]
- Beck RMD, Travouillon KJ, Aplin K, et al. (2014) The osteology and systematics of the enigmatic Australian Oligo‐Miocene metatherian Yalkaparidon (Yalkaparidontidae; Yalkaparidontia; Australidelphia; Marsupialia). J Mammal Evol 21, 127–172. [Google Scholar]
- Bolton PM (1983) Gonadal development in Antechinus stuartii (Marsupialia: Dasyuridae). MSc thesis. Sydney, Australia: Macquarie University. [Google Scholar]
- Burrell H (1927) The Platypus. Sydney: Angus and Robertson. [Google Scholar]
- Castro‐Arellano I, Zarza H, Medellín RA (2000) Philander opossum. Mammal Species 638, 1–8. [Google Scholar]
- Celis‐Diez JL, Hetz J, Marín PA, et al. (2012) Population abundance, natural history, and habitat use by the arboreal marsupial Dromiciops gliroides in rural Chiloé Island, Chile. J Mammal 93, 134–148. [Google Scholar]
- Chew KY, Yu H, Pask AJ, et al. (2012) HOXA13 and HOXD13 expression during development of the syndactylous digits in the marsupial Macropus eugenii . BMC Dev Biol 12, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew KY, Shaw G, Yu H, et al. (2014) Heterochrony in the regulation of the developing marsupial limb. Dev Dyn 243, 324–338. [DOI] [PubMed] [Google Scholar]
- Clark CT, Smith KK (1993) Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae). J Morphol 215, 119–149. [DOI] [PubMed] [Google Scholar]
- Collie A (1830) On some particulars connected with the natural history of the kangaroo. Zool J 5, 238–241. [Google Scholar]
- Collins LR (1973) Monotremes and Marsupials: a Reference for Zoological Institutions. Washington: Smithsonian Institute Press. [Google Scholar]
- Cooper EC (2011) Myrmecobius fasciatus (Dasyuromorphia: Myrmecobiidae). Mammal Species 43, 129–140. [Google Scholar]
- Cooper WJ, Steppan SJ (2010) Developmental constraint on the evolution of marsupial forelimb morphology. Aust J Zool 58, 1–15. [Google Scholar]
- Cooper CE, McAllan BM, Geiser F (2005) Effect of torpor on the water economy of an arid‐zone marsupial, the stripe‐faced dunnart (Sminthopsis macroura). J Comp Physiol B 175, 323–328. [DOI] [PubMed] [Google Scholar]
- Cuthbert RJ, Denny MJ (2014) Aspects of the ecology of the kalubu bandicoot (Echymipera kalubu) and observations on Raffray's bandicoot (Peroryctes raffrayanus), Eastern Highlands Province, Papua New Guinea. Austr Mammal 36, 21–28. [Google Scholar]
- D'Elía G, Hurtado N, D'Anatro A (2016) Alpha taxonomy of Dromiciops (Microbiotheriidae) with the description of 2 new species of monito del monte. J Mammal 97, 1136–1152. [Google Scholar]
- Drews B, Roellig K, Menzies BR, et al. (2013) Ultrasonography of wallaby prenatal development shows that the climb to the pouch begins in utero . Sci Rep 3, 1458. doi:10.1038/srep01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen MM, Rose RK (2015) Isoodon obesulus (Peramelemorphia: Peramelidae). Mammal Species 47, 112–123. [Google Scholar]
- Edwards MJ, Deakin JE (2012) The marsupial pouch: implications for reproductive success and mammalian evolution. Aust J Zool 61, 41–47. [Google Scholar]
- Fadem BH, Trupin GL, Maliniak E, et al. (1982) Care and breeding of the gray short‐tailed opossum (Monodelphis domestica). Lab Anim Sci 23, 405–409. [PubMed] [Google Scholar]
- Frankham GJ, Temple‐Smith PD (2012) Absence of mammary development in male Dromiciops gliroides: another link to the Australian marsupial fauna. J Mammal 93, 572–578. [Google Scholar]
- Frigo L, Woolley PA (1997) Growth and development of pouch young of the striped‐faced dunnart, Sminthopsis macroura (Marsupialia: Dasyuridae), in captivity. Aust J Zool 45, 157–170. [Google Scholar]
- Gardner AL (2008) Mammals of South America, Volume 1: Marsupials, Xenarthrans, Shrews, and Bats. Chicago: University of Chicago Press. [Google Scholar]
- Gemmell RT (1982) Breeding bandicoots in Brisbane (Isoodon macrourus; Marsupialia, Peramelidae). Aust Mammal 5, 187–193. [Google Scholar]
- Gemmell RT, Nelson J (1989) Vestibular system of the newborn marsupial cat, Dasyurus hallucatus . Anat Rec 225, 203–208. [DOI] [PubMed] [Google Scholar]
- Gemmell RT, Peters B, Nelson J (1988) Ultrastructural identification of Merkel cells around the mouth of the newborn marsupial. Anat Embry 177, 403–408. [DOI] [PubMed] [Google Scholar]
- Gemmell RT, Veitch C, Nelson J (2002) Birth in marsupials. Comp Biochem Physiol B Biochem Mol Biol 131, 621–630. [DOI] [PubMed] [Google Scholar]
- Griffiths M (1978) The Biology of the Monotremes. New York: Academic Press. [Google Scholar]
- Guiler ER (1970) Observations on the tasmanian devil, Sacophilus harrisii (Marsupialia: Dasyuridae) II. Reproduction, breeding, and growth of pouch young. Aust J Zool 18, 63–70. [Google Scholar]
- Gurovich Y, Sage R, Goin F (2013) Morphological description and age estimate in pouch young in Dromiciops gliroides (Marsupialia: Microbiotheria) from Patagonia, Argentina. Australian Mammal Society Conference 8–10 July 2013, p. 68.
- Gurovich Y, Stannard HJ, Old JM (2015) The presence of the marsupial Dromiciops gliroides in Parque Nacional Los Alerces, Chubut, Southern Argentina, after the synchronous maturation and flowering of native bamboo and subsequent rodent irruption. Revista chilena de historia natural 88, 1–12. [Google Scholar]
- Hall LS (1990) Growth and a description of the development of external features of pouch young of captive Isoodon macrourus In: Bandicoots and Bilbies. (eds Seebeck JH, Brown PR, Wallis RL, Kemper CM.), pp. 123–133. Australia, Sydney: Surrey Beatty and Sons. [Google Scholar]
- Harris JM (2008) Cercartetus nanus (Diprotodontia: Burramyidae). Mamm Species 815, 1–10. doi:10.1644/815.1. [Google Scholar]
- Herrin CS, Sage RD (2012) Description of a new species of Haemogamasus (Mesostigmata, Laelapidae, Haemogamasinae) from Chubut, Río Negro and Neuquén Provinces, Argentina. ZooKeys 173, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz P (1992) The South American gracile mouse opossums, genus Gracilinanus Gardner and Creighton, 1989 (Marmosidae, Marsupialia): a taxonomic review with notes on general morphology and relationships. Fieldiana: Zool 70, 1–56. [Google Scholar]
- Hershkovitz P (1997) Composition of the family Didelphidae Gray, 1821 (Didelphoidea: Marsupialia) with a review of the morphology and behavior of the included four‐eyed opossums of the genus Philander Tiedemann, 1808. Fieldiana Zool 86, 1–103. [Google Scholar]
- Hershkovitz P (1999) Dromiciops gliroides Thomas, 1894, Last of the Microbiotheria (Marsupialia), with a review of the family Microbiotheriidae. Fieldiana. Zoology 93, 1–60. [Google Scholar]
- Hill JP, Hill WCO (1955) The growth stages of the native cat (Dasyurus viverrinus) together with observations on the anatomy of the newborn young. Trans Zool Soc Lond 28, 349–452. [Google Scholar]
- Hogan LA, Janssen T, Johnston SD (2013) Wombat reproduction (Marsupialia; Vombatidae): an update and future directions for the development of artificial breeding technology. Reproduction 145, R157–R173. [DOI] [PubMed] [Google Scholar]
- Horovitz I, Sánchez‐Villagra MR (2003) A morphological analysis of marsupial mammal higher‐level phylogenetic relationships. Cladistics 19, 181–212. [Google Scholar]
- Horovitz I, Martin T, Bloch J, et al. (2009) Cranial anatomy of the earliest marsupials and the origin of opossums. PLoS ONE 4, e8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RL (1962) Reproduction in the macropod marsupial Potorous tridactylus (Kerr). Aust J Zool 10, 193–224. [Google Scholar]
- Hughes RL, Hall LS (1984) Embryonic development in the common brushtail possum Trichosurus vulpecula In: Possums and Gliders. (eds Smith AP, Hume ID.), pp. 197–212. Sydney: Australian Mammal Society. [Google Scholar]
- Hughes RL, Hall LS (1988) Structural adaptations of the newborn marsupial In: The Developing Marsupial. (eds Tyndale‐Biscoe CH, Janssens PA.), pp. 8–27. Berlin: Springer. [Google Scholar]
- Hughes RL, Hall LS (1998) Early development and embryology of the platypus. Philos Trans R Soc Lond B Biol Sci 353, 1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson GD (1976) Grooming behavior and birth in the Dasyurid marsupial Dasyuroides byrnei . Aust J Zool 24, 277–282. [Google Scholar]
- Johnson KA (1995) Marsupial mole Notoryctes typhlops In: The Mammals of Australia. (ed. Strahan R.), pp. 409–411. Chatswood: Reed Books. [Google Scholar]
- Kaufman MH, Bard JBL (1999) The Anatomical Basis of Mouse Development. San Diego: Academic Press. [Google Scholar]
- Keibel F (1906) Chapter 6. Die Entwicklung der äusseren Körperform der Wirbeltierembryonen, insbesondere der menschlichen Embryonen aus den ersten 2 Monaten In: Handbuch der vergleichenden und experimentellen Entwicklungslehre der Wirbeltiere, Vol. 1 (ed. Hertwig O.), 1–176. Jena: Verlag von Gustav Fischer. [Google Scholar]
- Kirsch JA (1977) The six‐percent solution: second thoughts on the adaptedness of the Marsupialia: features of their physiology and diversity suggest that marsupials represent an alternative but not inferior kind of mammal, valuable in understanding the course of mammalian evolution. Am Sci 65, 276–288. [PubMed] [Google Scholar]
- Krause WJ (1991) The vestibular apparatus of the opossum (Didelphis virginiana) prior and immediately after birth. Acta Anat 142, 57–59. [DOI] [PubMed] [Google Scholar]
- Lobos G, Charrier A, Carrasco G, et al. (2005) Presence of Dromiciops gliroides (Microbiotheria: Microbiotheriidae) in the deciduous forests of central Chile. Mamm Biol 70, 376–380. [Google Scholar]
- Lyne AG (1951) Notes on external characters of the barred bandicoot (Perameles gunnii Gray), with special reference to the pouch young. Proc Zool Soc Lond 121, 587–598. [Google Scholar]
- Lyne AG (1964) Observations on the breeding and growth of the marsupial Perameles nasuta Geoffroy, with notes on other bandicoots. Aust J Zool 12, 322–339. [Google Scholar]
- Lyne AG (1974) Gestation period and birth in the marsupial Isoodon macrourus . Aust J Zool 22, 303–309. [Google Scholar]
- Maddison WP, Maddison DR (2015) Mesquite: a modular system for evolutionary analysis. Version 2.75 http://mesquitepro- ject.org.
- Manger PR, Hall LS, Pettigrew JD (1998) The development of the external features of the platypus (Ornithorhynchus anatinus). Phil Trans Roy Soc Lon B Biol Sci 353, 1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow BJ (1961) Reproductive behaviour of the marsupial mouse, Antichinus flavipes (Waterhouse) (Marsupialia) and the development of the pouch young. Aust J Zool 9, 203–218. [Google Scholar]
- Marshall LG (1978) Dromiciops australis. Mammalian Species 99, 1–5. [Google Scholar]
- Martin KE, Mackay S (2003) Postnatal development of the fore‐ and hindlimbs in the grey short‐tailed opossum, Monodelphis domestica. J Anat 202, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrady E (1938) The Embryology of the Opossum. Philadelphia: Wister Institute Press. [Google Scholar]
- Merchant JC, Sharman GB (1966) Observations on the attachment of marsupial pouch young to the teats and on the rearing of pouch young by foster‐mothers of the same or different species. Aust J Zool 14, 593–609. [Google Scholar]
- Meredith RW, Westerman M, Case JA, et al. (2008) A phylogeny and timescale for marsupial evolution based on sequences for five nuclear genes. J Mamm Evol 15, 1–36. [Google Scholar]
- Muñoz‐Pedreros A, Lang BK, Bretos M, et al. (2005) Reproduction and development of Dromiciops gliroides (Marsupialia: Microbiotheriidae) in temperate rainforests of southern Chile. Gayana 69, 225–233. [Google Scholar]
- Nelson JE (1988) Growth of the brain In: The Developing Marsupial. (eds Tyndale‐Biscoe CH, Janssens PA.), pp. 86–100. Berlin: Springer. [Google Scholar]
- Nelson JE, Gemmell RT (2003) Birth in the northern quoll, Dasyurus hallucatus (Marsupialia: Dasyuridae). Aust J Zool 51, 187–198. [Google Scholar]
- Nowak RM (1999) Walker's Mammals of the World, Vol. 1 Baltimore, USA: JHU Press. [Google Scholar]
- Oates JE, Bradshaw FJ, Bradshaw SD, et al. (2007) Reproduction and embryonic diapause in a marsupial: insights from captive female Honey possums, Tarsipes rostratus (Tarsipedidae). Gen Comp Endocrinol 150, 445–461. [DOI] [PubMed] [Google Scholar]
- Osman Hill WC (1952) V. Observations on marsupials in the Royal Scottish Museum, with special reference to the fœtal material. Trans Roy Soc Edinb 62, 145–167. [Google Scholar]
- Pask AJ, Renfree MB (2010) Molecular regulation of marsupial reproduction and development In: Marsupial Genetics and Genomics. (ed. Waters PD, Deakin JE, Marshall Graves JA.), pp. 285–316. The Netherlands: Springer. [Google Scholar]
- Patterson BD (2015) Family Caenolestidae (Shrew Opossums) In: Handbook of the Mammals of the World. Volume 5. Monotreme and Marsupials. (ed. Wilson DE, Mittermeier RA.), pp. 188–197.Lynx Editions, ISBN: 978‐84‐96553‐99‐6. [Google Scholar]
- Poole WE (1975) Reproduction in the two species of grey kangaroos, Macropus giganteus Shaw, and M. fuliginosus (Desmarest). II. Gestation, parturition and pouch life. Aust J Zool 23, 333–353. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Fletcher TP, Blanden DR, et al. (1989) Physiological and behavioural events around the time of birth in macropodid marsupials In Kangaroos, Wallabies and Rat–Kangaroos. (eds Grigg G, Jarman P, Hume I.). pp 323–337. New South Wales, Australia: Surrey Beatty, Sons Pty. [Google Scholar]
- Renfree MB, Robinson ES, Short RV, et al. (1990) Mammary glands in male marsupials: I. Primordia in neonatal opossums Didelphis virginiana and Monodelphis domestica . Development 110, 385–390. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Short RV, Shaw G (1996) Sexual differentiation of the urogenital system of the fetal and neonatal tammar wallaby, Macropus eugenii . Anat Embryol 194, 111–134. [DOI] [PubMed] [Google Scholar]
- Reynolds HC (1952) Studies on Reproduction in the Opossum (Didelphis virginiana virginiana). University of California Publ Zool; 52, 223–284. [Google Scholar]
- Rose R, Fadem BH (2000) The hormonal control of birth behavior in the gray short‐tailed opossum (Monodelphis domestica). Horm Behav 37, 163–167. [DOI] [PubMed] [Google Scholar]
- Russell EM (1982) Patterns of parental care and parental investment in marsupials. Biol Rev 57, 423–486. [DOI] [PubMed] [Google Scholar]
- Schneider NY (2011) The development of the olfactory organs in newly hatched monotremes and neonate marsupials. J Anat 219, 229–242. doi:10.1111/j.1469‐7580.2011.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider NY, Fletcher TP, Shaw G, et al. (2009) The olfactory system of the tammar wallaby is developed at birth and directs the neonate to its mother's pouch odours. Reproduction 138, 849–857. [DOI] [PubMed] [Google Scholar]
- Schneider NY, Shaw G, Renfree MB (2013) The role of olfaction at birth in Marsupial and Monotreme mammals In: Chemical Signals in Vertebrates XII. (eds East ML, Dehnhard M.), pp.87–96. New York: Springer. [Google Scholar]
- Selenka E (1887) Das Opossum (Didelphys virginiana) In: Studien über die Entwicklungsgeschichte der Thiere, Vol. 4, pp. 101–172. Wiesbaden: C.W. Kreidels. [Google Scholar]
- Selwood L (1980) A timetable of embryonic development of the dasyurid marsupial Antechinus stuartii (Macleay). Aust J Zool 28, 649–668. [Google Scholar]
- Sharman GB (1973) Adaptations of marsupial pouch young for extra‐uterine existence In: The Mammalian Fetus in vitro, pp. 67–90. Dordrecht, Neverlands: Springer. [Google Scholar]
- Sharman GB (1976) Evolution of viviparity in mammals In: Reproduction in Mammals, Vol. 6 (ed. Austin CR.), pp.32–70. London: Chapman & Hall. [Google Scholar]
- Sharman GB, Calaby JH (1964) Reproductive behaviour in the red kangaroo, Megaleia rufa, in captivity. CSIRO Wildl Res 9, 58–85. [Google Scholar]
- Smith MJ (1973) Petaurus breviceps. Mamm Species 30, 1–5. [Google Scholar]
- Smith KK (1997) Comparative patterns of craniofacial development in eutherian and metatherian mammals. Evolution 51, 1663–1678. [DOI] [PubMed] [Google Scholar]
- Smith KK (2001) Early development of the neural plate, neural crest and facial region of marsupials. J Anat 199, 121–131. doi:10.1017/S0021878201008202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, MacFadyen A, Rose R (2001) Hormonal control of birth behavior in the bandicoot (Perameles gunnii: Marsupialia) and other marsupials. Physiol Behav 72, 527–532. [DOI] [PubMed] [Google Scholar]
- Soderquist TR (1993) Maternal strategies of Phascogale tapoatafa (Marsupialia, Dasyuridae). 1. Breeding seasonality and maternal investment. Aust J Zool 41, 549–566. [Google Scholar]
- Szalay FS (1982) A new appraisal of marsupial phylogeny and classification In: Carnivorous marsupials. (ed. Archer M.), pp. 621–640. Mosman: Royal Zoological Society of New South Wales. [Google Scholar]
- Szalay FS (1994) Evolutionary History of the Marsupials and an Analysis of Osteological Characters. Cambridge: Cambridge University Press. [Google Scholar]
- Taggart DA, Finlayson GR, Shimmin G, et al. (2007) Growth and development of the southern hairy‐nosed wombat, Lasiorhinus latifrons (Vombatidae). Aust J Zool 55, 309–316. [Google Scholar]
- Tate GHH (1933) A Systematic Revision of the Marsupial Genus Marmosa: with a Discussion of the Adaptive Radiation of the Murine Opossums (Marmosa). Cambridge, USA: Bulletin of the AMNH. v. 66, article 1. [Google Scholar]
- Thomas O (1888) Catalogue of the Marsupialia and Monotremata in the collection of the British Museum (Natural History). London: Trustees of the British Museum (Natural History). [Google Scholar]
- Tyndale‐Biscoe H (2005) Life of Marsupials. Collingwood: CSIRO Publishing. [Google Scholar]
- Tyndale‐Biscoe H, Renfree M (1987) Reproductive Physiology of Marsupials. Cambridge: Cambridge University Press. [Google Scholar]
- Ullmann SL (1993) Differentiation of the gonads and initiation of mammary gland and scrotum development in the brushtail possum Trichosurus vulpecula (Marsupialia). Anat Embryol 187, 475–484. [DOI] [PubMed] [Google Scholar]
- Van Dyck S, Strahan R (eds 2008) The Mammals of Australia. Sydney, Australia: New Holland Pub Pty Limited. [Google Scholar]
- Veitch CE, Nelson J, Gemmell RT (2000) Birth in the brushtail possum, Trichosurus vulpecula (Marsupialia: Phalangeridae). Aust J Zool 48, 691–700. [Google Scholar]
- Voss RS, Jansa SA (2003) Phylogenetic studies on didelphid marsupials II. Nonmolecular data and new IRBP sequences: separate and combined analyses of didelphine relationships with denser taxon sampling. Bull Am Mus Nat Hist 276, 1–82. [Google Scholar]
- Voss RS, Jansa SA (2009) Phylogenetic relationships and classification of Didelphid marsupials, an extant radiation of New World Metatherian mammals. Am Mus Nat Hist 322, 1–177. [Google Scholar]
- Warburton NM (2003) Functional morphology and evolution of marsupial moles (Marsupialia; Notoryctemorphia). Sort 50, 500. [Google Scholar]
- Ward SJ (1990) Life‐history of the Eastern Pygmy‐Possum, Cercartetus nanus (Burramyidae, Marsupialia), in South‐Eastern Australia. Aust J Zool 38, 287–304. [Google Scholar]
- Weisbecker V, Goswami A, Wroe S, et al. (2008) Ossification heterochrony in the therian postcranial skeleton and the marsupial‐placental dichotomy. Evolution 62, 2027–2041. [DOI] [PubMed] [Google Scholar]
- West MMR (2002) The oestrous cycle and manipulation of reproduction in the common wombat (Vombatus ursinus), Doctoral dissertation. Clayton, VIC, Australia: Monash University, Institute of Reproduction and Development. [Google Scholar]
- Wood Jones FW (1921) The external characters of pouch embryos of marsupials. No. 2 Notoryctes typhlops . Trans R Soc S Aust 55, 36–39. [Google Scholar]
- Wood Jones F (1922) The external characters of pouch embryos of Marsupials. No. 4. Pseudochirops dahli . Trans R Soc S Aust 46, 119–130. [Google Scholar]
- Wood Jones F (1923) The external characters of pouch embryos of marsupials. No. 7 Myrmecobius fasciatus . Trans R Soc S Aust 47, 195–200. [Google Scholar]
- Woolley P (1974) 3.‐The pouch of Planigale subtilissima and other dasyurid marsupials. J Roy Soc West Aust 57, 11–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Phylogenetic relationships of Dromiciops and extant marsupials with extinct species based on maximum parsimony analysis of the morphology‐only matrix.


