Abstract
Purpose of the study
To determine whether paced vocal fold adduction can check aspiration in patients with various neurological conditions.
Design and method of study and analysis
Five patients with fluoroscopically documented aspiration and repeated pneumonias were enrolled. Two previously reported patients with hemispheric stroke* were compared to three additional subjects with brainstem-basal ganglia and cerebellar stroke (BSBGC), cerebral palsy (CP) and multiple sclerosis (MS). A modified Finetech-Brindey stimulator was implanted subcutaneously and linked to the ipsilateral recurrent laryngeal nerve via perineural electrodes. Vocal fold adduction and glottic closure were effected with pulse trains (42 Hz, 1.2 mA, 188–560 μsec) and recorded with Enhanced Image J ®. Fluoroscopy results with and without stimulation were assessed by two independent blinded reviewers. Pneumonia rates were compared before, after and during the 6–12 months enrollment periods.
Summary of results
There was statistically significant vocal fold adduction (p < .05) for all patients, further verified with bolus arrest (p < .05 for thin, thick liquids and puree depending on the speech-language pathologist). Pneumonia was prevented in four of the five patients during enrollment. The fifth patient with BSBGC was unable to completely seal the glottis and open the cricopharyngeus enough to handle his secretions.
Conclusions
Vocal fold pacing for aspiration pneumonia from a variety of neurological insults appears appropriate as long as the glottis can be sealed. It is not sufficient when the cricopharyngeus must be independently opened.
Keywords: Paced Glottic Closure, Neurological Deficits, Dysphagia, Aspiration Pneumonia, Recurrent Laryngeal Nerve Stimulation
INTRODUCTION
In terrestrial animals, it is normally the role of the vocal folds to seal the larynx, and protect the airway during deglutition. Laryngotracheal separation represents the endpoint of a reflexogenic cascade typical of the second, pharyngeal stage of deglutition, once the first, voluntary oral stage has been completed. This process can be disrupted by a variety of conditions originating in the central nervous system (CNS). Although cerebrovascular accidents (CVA, stroke) constitute the bulk of these insults, other neurologic diseases are usually included in a common approach to rehabilitation (1). Traditionally, therapy has been implemented by speech-language pathologists (SPL) and usually comprises special risk reduction diets of variable consistencies and compensatory strategies such as chin tuck, head turn, supersupraglottic swallow, double supraglottic swallow, the Mendelsohn maneuver, etc. (2) and exercises such as the Masako or Shaker (3).
This study compares the results of laryngeal stimulation and its effect on pneumonia rates in 5 patients with aspiration resulting from diverse neurological impairments. We include additional data from two cases previously presented in a preliminary feasibility report (4). Our approach has followed information from the Agency for Health Care, Policy and Research (AHCPR) linking aspiration to the occurrence of pulmonary infections (1).
It is attractive to use the recurrent laryngeal nerve (RLN) to stimulate the vocal fold adductors since they comprise the bulk of the intrinsic laryngeal muscles (ILMs). Sphincteric vocal fold closure is the most important safety mechanism in preventing aspiration (5) and controls the “final” expression of any disrupted swallow at the glottic endpoint, regardless of the level and degree of failure involved along the swallowing cascade.
MATERIAL AND METHODS
Five patients with varying neurologic conditions and chronic aspiration pneumonia despite traditional therapies were entered into the study. Their ages varied between 54 and 81 years, (mean 68.8 yrs+/− SD 10.8). The indications for enrollment in the study are listed in Table 1. All were unable to ingest any food, and they complained of decreased quality of life.
Table 1.
Inclusion/Exclusion criteria for the study.
| CLINICAL CRITERIA FOR PATIENTS INCLUSION |
| 1. Documented aspiration after a neurological insult |
| 2. No improvement after 6 months of swallowing therapy |
| 3. Tracheotomy either planned or already in place |
| 4. Can understand the research and manipulate a switch |
| CLINICAL CRITERIA FOR PATIENTS EXCLUSION |
| 1. Heart condition (cardiac pacemaker) |
| 2. Less than 18 years of age |
| 3. Women already or planning to become pregnant |
Clinical histories were retrieved from primary care physicians, patient and family interviews at enrollment time, and verified on medical records as authorized by HIPAA. Chest X rays (CXRs) read by an attending radiologist were further rated in terms of the absence (0) or presence specifying magnitude, of infiltrates either as absent [0], mild [1], moderate [2] or severe [3]. As one of the conditions for enrollment, all candidates failed fluoroscopic examination (modified barium swallow, MBS) in terms of exhibiting significant physiological indicators of dysphagia coupled with frequent aspiration of boluses of various consistencies. Fluoroscopy has been traditionally, if arguably, considered as the gold standard for objectively documenting aspiration (1,6–7).
The causative neurological deficits are reported in Table 2. While the first three subjects shared a diagnosis of stroke, their CNS insults were anatomically distinct on computerized axial tomography (CT) scans as involving the right hemisphere (# 1, CVA R HEM), the left hemisphere (#2, CVA L HEM), and the brain stem, basal ganglia and cerebellum (# 3, CVA BSBGC). The other two patients had more generalized disease. Patient # 4 was born with cerebral palsy (CP) and presented with a three year history of progressive dysphagia, while patient # 5 had a long term history of multiple sclerosis (MS) and had had diminished swallowing capability for two years. Four of the five patients had percutaneous endoscopic gastrostomies (PEGs) at the time of enrollment. Patient #4 was advised to have a PEG, but refused.
Table 2.
Patients’ distribution. Times prior to enrollment relate to duration of documented aspiration for the diagnosed conditions. CVA: cerebrovascular accident. R and LHEM: right and left cerebral hemisphere. BSBGC: brain stem basal ganglia, cerebellar. CP: cerebral palsy. MS: multiple sclerosis.
| PATIENT/GENDER | AGE | ETIOLOGY | ASP TIME PRIOR TO ENROLLMENT |
|---|---|---|---|
| 1/F | 54 | CVARHEM | 8 years |
| 2/M | 81 | CVALHEM | 16 months |
| 3/M | 70 | CVABSBGC | 12 years |
| 4/F | 76 | CP | 3 years + |
| 5/M | 62 | MS | 2 years + |
All candidates received an FDA-approved (IDE # G980179) modified Vocare ® stimulator (Finetech Medical Ltd, Welwyn Garden City, UK) originally used for voiding in paraplegics. The stimulator was implanted subcutaneously in the anterior chest wall and linked to a Huntington Medical Research Institute (HMRI, Pasadena, CA) bipolar electrode similar to those used for vagal stimulation in epilepsy. Surgery was carried out as previously described (4).
After implantation, the patients were subjected repeated examinations at weeks 1,2 and 4, followed by examinations at 2, 3, 6 and 12 months. These sessions included videotaped flexible laryngoscopy (Machida ENT 3-L flexible laryngoscope, Machida Endoscope C., Ltd., Tokyo, Japan), without and with stimulation, CXR and MBS examinations. Vocal fold positions were evaluated frame by frame on Image J ® software from the videotapes. Occasionally, there were scheduling difficulties due to patient illness or an inability to find transportation (7). Therefore, some CXRs were independently implemented by the treating physician either at his office or the nursing facilities. Infiltrates on chest X ray (CXR) radiology reports read by an attending radiologist were rated as absent (0), mild [present, 1], moderate [significant, 2] or severe [when massive or bilateral [3].
For fluoroscopy (MBS), the patients were given boluses of 3–15 ml and a variety of consistencies (thin, thick liquid, puree, pudding and peanut butter and jelly, when applicable). Patient were instructed to hold the bolus orally just before stimulation onset (the circuit is set to fire with a 2 sec delay). MBS studies were first read by the administering SPL in conjunction with the attending radiologist. They were subsequently blindly evaluated by two independent SPLs extraneous to the parent institution (University Hospitals of Cleveland, Cleveland, Ohio). Data collected following original speech-language pathologist (ORIG SLP) review of patients #s 1 and 2 in the preliminary study (4) were also considered. The number of sessions was 7, 8, 14, 8 and 10 sessions for patients 1–5 respectively. Speech Language Pathologist I (SLP I) recorded 59, 64, 43, 106 and 88 evaluable trials for patients 1–5(total N=360), and Speech Language Pathologist II (SLP II) recorded 29, 62, 64, 122, and 142 (total N=419) trials for patients 1–5 I. Findings were reported as either present (+) or absent (−) for an array of symptoms (e.g. premature pharyngeal entry, delayed swallowing response etc., see further). For each category, the total number of trials, the total number of evaluable trials, the presence or absence of stimulation, the consistency of the bolus and the results (positive, negative or unevaluable) were transcribed on Excel™ spread sheets. The findings for each category were then expressed as the number of positive results for each patient divided by the number of evaluable trials for each patient.
We then examined the effect of stimulation on aspiration. Since stimulation started just at time of oral bolus introduction, aspiration was categorized into three groups (prior, during and after swallowing) by the SPLs. These results were added to give one cumulative tally per individual swallowing trial. The cumulative number of aspirations divided by the total number of swallows in each subject were compared for stimulation ON and stimulation OFF and submitted to statistical analysis (X2). We did not have enough numbers and too many variables to specifically analyze the effect of bolus consistency in individual patients. In addition, there is a lack of consensus as to what viscosity liquids should be used (8) and viscosity was quite variable in our study. Thus, evaluation of individual patients was replaced by a global assessment. The findings were further compared to as of yet unpublished data calculated from ORIG SLP (4).
Finally, changes in quality of life related to the study were directly verified from the patients, close family and allied personnel, letters of testimony and objective observation by the investigators recorded at the completion of each visit. When conditions were considered safe enough for home usage, some patients (#s 2, 4 and 5) were allowed to use the device during meals before each swallow for a period limited to six months. Patient # 4 originally did well with the device, but unfortunately was not considered sufficiently compliant for home use because of a craving for peanut butter and jelly sandwiches. The two who did not qualify for home use were non-verbal patient # 1 who lacked sufficient family and local SLP support to sustain regular use, and # 3 where control of aspiration, aggravated by copious gastropharyngeal reflux had not been found fully satisfactory in terms of glottic seal and fluoroscopy in the radiological suite.
RESULTS
Pneumonias and chest radiographs
All candidates presented with a substantial number of symptomatic pneumonias (as one of the conditions for enrollment) prior to treatment. The cumulative numbers of both clinical pneumonias and infiltrates were clearly decreased during the arbitrarily designated pacing period of 6–12 months when compared to the 6–12 months prior to the stimulation period. In addition, in four of the five patients there appeared to be a persistent beneficial effect, albeit to a lesser degree, for 6 to 12 months after the end of stimulation (p= .001 on the X2 test). The only exception was patient # 3 who kept presenting with ongoing pneumonia (Fig. 1, table 3).
Fig. 1.
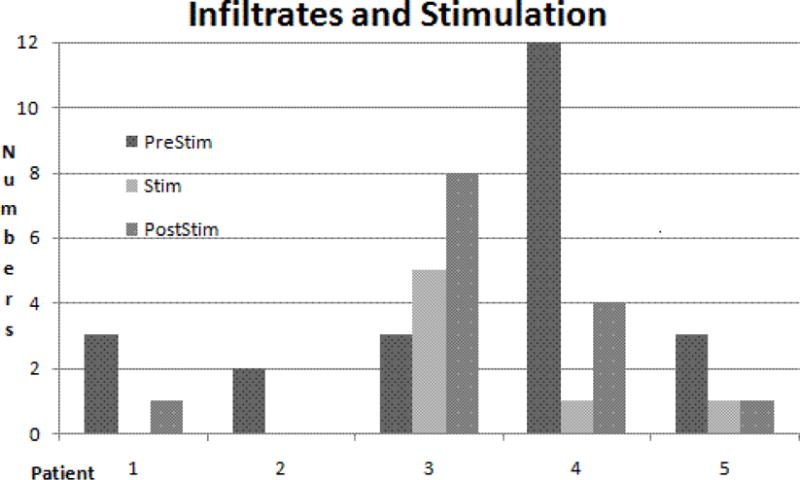
Distribution of numbers of cumulated pulmonary infiltrates over time. See Table 3 for numbers. Average was 4.6 before, 1.4 during and 2.8 after stimulation for all patients. There was no infiltrate during stimulation in patients #s 1 and 2, and marked reduction in the others except for patient # 3 where infiltrates increased in numbers with time. Note high prestim number in patient # 4 followed by sharp decline. X2 test indicates statistically significant differences between pre vs. stim and post stim (p = .001) when calculated for roughly equal observation times.
Table 3.
Decreases in pneumonias and infiltrates during and after as compared to prior stimulation times Left to right: 4 sets of columns: patient identification number, and numbers of clinical pneumonias, chest radiographs and months followup, before, during and after stimulation. CP: clinical pneumonias (before, B, during, D, and after, A, stimulation); INF:number of cumulative infiltrates; CXR: number of chest radiographs during observation time; F/U: follow up (months); rec: recurring; est: estimated (based on pneumonia counts); Patients #s 2,4 and 5 had home use of the stimulator. Clinical information: referring physican, patient, family and medical records.
| P | CPB | CXR | INF | F/U | CPD | CXR | INF | F/U | CPA | CXR | INF | F/U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | > 3 | 3 | 3 est | 6 | 0 | 4 | 0 | 6 | 0 | 6 | 1 | 18 |
| 2 | > 2 | 2 | 2 est | 11 | 0 | 4 | 0 | 12 | 1 | 4 | 0 | 11 |
| 3 | rec | 5 | 3 | 9 | rec | 9 | 9 | 8 | rec | 1 | 1 | 12 |
| 4 | > 5 | 7 | 12 | 9 | 0 | 5 | 2 | 6 | 0 | 11 | 3 | 23 |
| 5 | > 5 | 19 | 3 | 23 | 0 | 8 | 1 | 10 | 8 | 1 | 10 |
Paced vocal fold adduction
Charges necessary to implement glottic seal varied from patient to patient and encounter to encounter. However, the smallest necessary amount was used, usually producing laryngeal tingling or mild coughing. While closure angles (degrees) were stimulus dependent (1–4 normalized amplitude, 88–560 μsec pulse duration) (p < .05 on the paired t-test), we remained unable to achieve tight, workable glottic closure in patient # 3 in spite of substantial adduction from a paramedian position (Fig. 2).
Fig. 2.
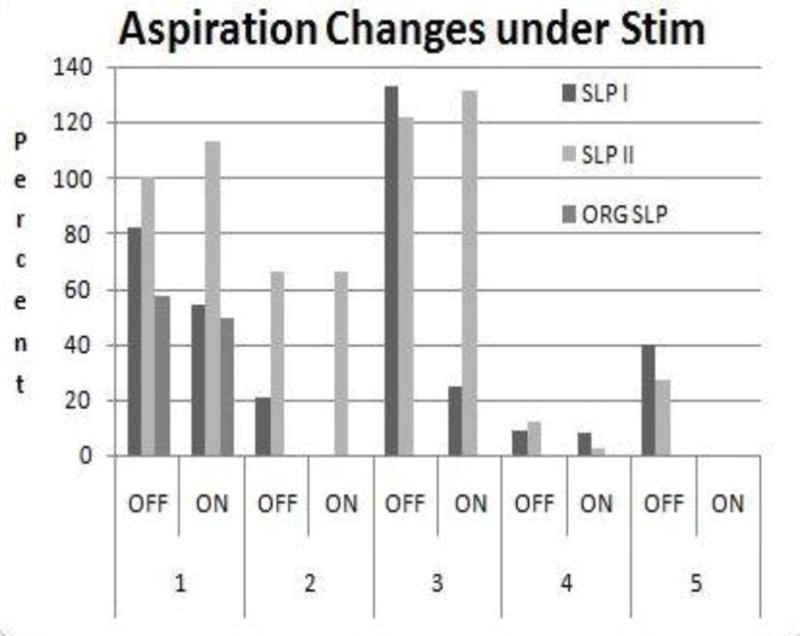
Stimulation outcomes on aspiration (%) in patients #s 1–5 based on SLP (I, II, ORIG) information. Aspiration decreased with stimulation ON in all cases except for minor differences in patients # 1 (SLP II), # 2 (no differences for SLP II) and # 3 (SLP II). ORIG SLP data also indicate decrease (See table 5 for numbers).
Speech-Language Pathologist MBS Reviews
The total number of reviewed trials amounted to 394 and 460 for SLP I and SLP II respectively. However, information was unevaluable in 34 (SLP I) and 41 (SLP II) cases, due to skipped images, poor quality video, or limited full screen view. Thus, 360 evaluable trials were found by SLP I and 419 evaluable trials were recorded by SLP II for all 5 patients. These numbers roughly compare to the totals (N=128) evaluated by ORIG SLP distributed as Ns = 65 and 63 for our first 2 patients.
Interreliability between SLPs was high for some frequent symptoms, but was not as good for less frequently encountered symptoms. Premature pharyngeal entry (PPE) was present in all subjects, although in various proportions. Decreased swallowing response (DSR), inadequate airway closure (IAC), penetration in the laryngeal vestibule (PLV), vallecular residue (VLR) and pyriform sinus residue (PSR) were also frequent. Some of these data are in agreement with ORIG SLP (table 4).
Table 4.
Physiological signs (symptoms) (N/trials, %) observed on fluoroscopy by two independent blinded speech-language pathologists (SLPs I and II). PPE: premature pharyngeal entry, ASR: absent swallow response, DSR: delayed swallow response, APS: aspiration prior to swallow, RPC: reduced pharyngeal contraction, RLE: reduced laryngeal excursion, REI: reduced epiglottic inversion, IAC: inadequate airway closure, PLV: penetration laryngeal vestibule, PSR: pyriform sinus residue, VLR: vallecular residue, ADS: aspiration during swallow, RPC: reduced pharyngeal contraction, APS: aspiration prior to swallow, REI: reduced epiglottic inversion, ASR: absent swallow response, PWR: pharyngeal wall residue, CPD: cricopharyngeal dysfunction, AWS: aspiration with swallow.
| Patient | 1 | 1 | 2 | 2 | 3 | 3 | 4 | 4 | 5 | 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | SLP I | SLP II | SLP I | SLP II | SLP I | SLP II | SLP I | SLP II | SLP I | SLP II |
| PPE | 100 | 100 | 100 | 100 | 95 | 93 | 56 | 56 | 68 | 64 |
| ASR | 5 | 20 | 2 | 3 | 83 | 3 | 0 | 0 | 0 | 0 |
| DSR | 85 | 100 | 83 | 100 | 12 | 93 | 0 | 12 | 62 | 45 |
| APS | 11 | 15 | 2 | 3 | 67 | 79 | 0 | 1 | 0 | 2 |
| RPC | 12 | 100 | 8 | 57 | 33 | 99 | 0 | 5 | 8 | 30 |
| RLE | 48 | 88 | 28 | 75 | 18 | 97 | 0 | 53 | 9 | 41 |
| REI | 9 | 96 | 3 | 27 | 33 | 95 | 0 | 25 | 2 | 7 |
| IAC | 73 | 76 | 33 | 43 | 70 | 94 | 0 | 3 | 8 | 18 |
| PLV | 75 | 75 | 43 | 53 | 81 | 96 | 10 | 18 | 9 | 26 |
| ADS | 22 | 46 | 3 | 35 | 13 | 44 | 3 | 2 | 8 | 9 |
| VLR | 24 | 63 | 71 | 77 | 66 | 90 | 22 | 32 | 28 | 10 |
| PSR | 37 | 96 | 27 | 23 | 89 | 93 | 9 | 0 | 26 | 14 |
| PWR | 2 | 3 | 5 | 2 | 25 | 21 | 2 | 0 | 23 | 7 |
| CPD | 4 | 35 | 81 | 0 | ||||||
| AWS | 27 | 3 | 34 | 70 | 79 | 0 | 63 | 13 | 7 |
Note: Considering also salient symptoms from ORIG SPL (not represented here), PPE amounted to 95 and 92 % for patients #s 1 and 2, but varied as 93.8 and 40 % for VLR and 93 and 40 % for PLV respectively. In patient # 1, RPC was 63 % and IAC 49 % for patient # 2. Interreliability correlations between SLPs I and II varied (%) as 99 for PPE, 59 for DSR, 98 for PLV, 76 for VLR and 77 for PSR.
From the available fluoroscopy information, the CVA patients (#s 1, 2 and 3) presented with a greater frequency and a wider range of physiological signs of dysphagia (up to 100 %) when compared to those with less focused neurological deficits (i.e. # 4 with CP and # 5 who had MS). However, relatively high rates of PPE stand out for those two patients (56–68 %), as DSR for # 5 (68 %), when counts are compared to their other own symptoms (Table 4).
Aspiration arrest
Focusing then on aspiration proper, while arrest ratios varied, those were statistically verified in each paced candidate based on at least one SLP data, # 3 being an exception. In patient # 1, there was an overall decrease in aspiration with the stimulation ON (N=18/22 vs. 20/37, p < .05 per X2) for SLP I, while the contrary was true (more aspiration) for SLP II (N=11/11 vs. 25/22, p < .01). However, our preliminary study per ORIG SLP (4), indicated bolus arrest (N=16/28 vs. 19/39, X2 p < .05;. Patient # 2 also experienced arrest based on SPL I (N= 5/17 vs. 0/19, p < .02) but no significant change based on SLP II data (N= 19/29 vs. 21/32, NS [non-significant]). Here again, however, original SLP information indicated arrest (N=0/18 vs. 0/19, p < .05), although this patents does not seem to have aspirated in the first place for ORIG SLP. Patient # 3 aspirated less when stimulated (N= 8/6 vs.1/4, NS) for SLP I, while the contrary was verified based on SLP II data (N= 33/27 vs. 46/35, NS). For patient #4, stimulation produced arrest considering both SLPs I (N=3/32 vs. 3/36, NS), and II (N=5/42 vs. 1/59, p < .05) data. Finally, patient # 5 also showed arrest based on both SLPs (N= 2/5 vs. 0/5, NS for I) and (N= 19/70 vs. 0/71, p < 0.01 for II). See Fig. 3 and table 5 for percents of arrests when present.
Fig. 3.
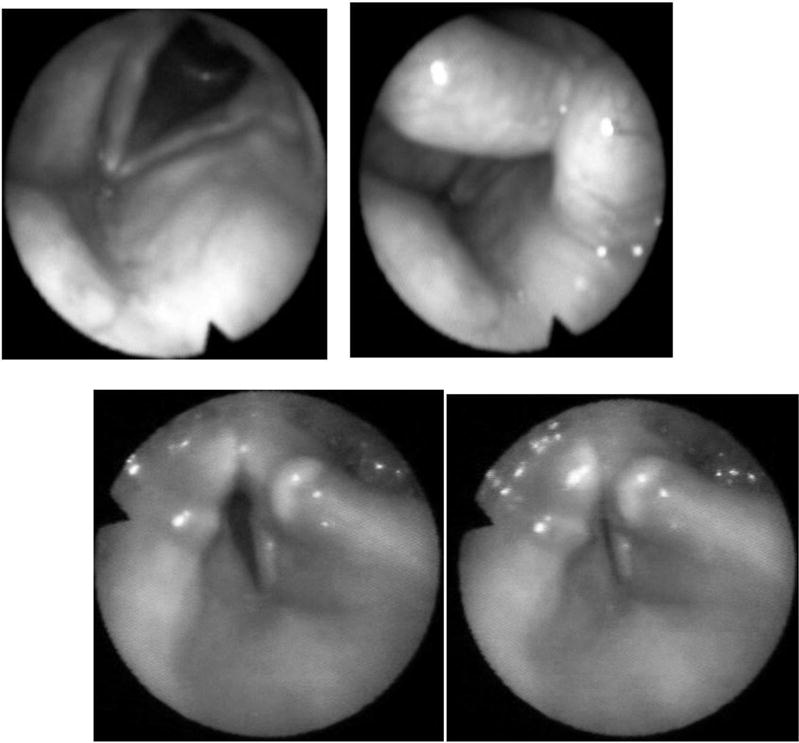
Up: Stimulation of right vocal fold resulting in tight glottic seal (right) in patient # 5. Down: Stimulation of left vocal fold resulting in incomplete adduction insufficient for tight glottic closure (right). Note poor abduction in patients #3 (CVABGBSC) in resting position (left).
Table 5A.
Tally of aspiration arrests (all consistencies) over number of trials in individual (#s 1–5) patients. Top: SLP I, middle: SLP II, bottom: ORIG SLP (4). While most patients experienced aspiration rate decreases under stimulation, #s 1 and 3 had small increases according to SLP II only. Statistical significance (bold) was verified between ON and OFF except in patient # 2 and for SLP II and # 3, or when the sample was too small as in # 5, SLP I).
| SLP I/Pt | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| ASP OFF | 18 | 5 | 8 | 3 | 2 |
| # Trials | 22 | 17 | 6 | 32 | 5 |
| ASP ON | 20 | 0 | 1 | 3 | 0 |
| # Trials | 37 | 19 | 4 | 36 | 5 |
| X2 | p< .05 | p< .02 | NS | NS | NS |
| SLP II/Pt | 1 | 2 | 3 | 4 | 5 |
| ASP OFF | 11 | 19 | 33 | 5 | 19 |
| # Trials | 11 | 29 | 27 | 42 | 70 |
| ASP ON | 25 | 21 | 46 | 1 | 0 |
| # Trials | 22 | 32 | 35 | 59 | 71 |
| X2 | p< .01 | NS | NS | P< .05 | p< .01 |
| ORIG SLP/Pt | 1 | 2 | |||
| ASP OFF | 16 | 0 | |||
| # Trials | 28 | 18 | |||
| ASP ON | 19 | 0 | |||
| # Trials | 39 | 18 | |||
| X2 | p< .05 | p< .05 | |||
Concentrating on evaluation of thin, thick liquids and puree which were the more often used consistencies overall, cumulative aspiration arrests (i.e. between stimulation prior, during and after swallowing and overall numbers of trials) indicate a statistically significant difference for thin (N= 11/44 vs. 2/52) and thick (N= 4/21 vs. 1/27) liquids (p < .05 on the X2 test) for SLP I, and for thick liquid (N= 9/61 vs. 17/70) and puree (N= 14/35 vs. 21/40 for SLP II) (p < .05), further verified (p < .05) for thin (N=13/20 vs. 8/24) and thick liquids (13/24 vs. 8/52) based on ORIG SLP evaluation on the 2 first patients (table 6).
Table 6.
Tally of aspirations overall for boluses of various consistencies based on each the three SLP data. Only significant results are reported.
| CONS | STIM OFF | STIM ON | X2 | |||
|---|---|---|---|---|---|---|
| SLP I | Thin | 11 | 44 | 2 | 52 | p < .05 |
| Thick | 4 | 21 | 11 | 27 | p < .05 | |
| SLP II | Thick | 9 | 61 | 17 | 70 | p < .05 |
| Puree | 14 | 35 | 21 | 40 | p < .05 | |
| OR SLP | Thin | 13 | 20 | 8 | 24 | p = .05 |
| Thick | 13 | 24 | 8 | 52 | p < .05 |
Finally, 4 out of the 5 patients (# 3 being the exception) expressed satisfaction in terms of improved quality of life for their ability to eat.
DISCUSSION
This limited series of patients with aspiration pneumonias from a variety of neurological conditions is admittedly not fully representative of any of its constituting parts. A clean definition of laryngeal pacing outcomes in individual subjects would require larger samples, a task currently difficult to fulfill (1). Since there are currently no national guidelines for the control of aspiration pneumonia (1,6), we wondered whether shared symptoms observed between diverse populations such as this series could be alleviated by a common approach. Whatever their differences in etiology and clinical presentation, our patients already constituted a pre-selected group with each individual representing his/her own control. Considering the promising results of our pilot study in terms of arresting aspiration (4), we hypothesized that the benefits of evoked dynamic laryngotracheal separation could be expanded to subjects sharing more similarities than could be expected from apparently unrelated causative factors.
Based on clinical and radiological criteria and core fluoroscopic information, we have shown that laryngeal pacing was able to check aspiration in 4 out of our 5 cases associated with a tight glottic seal. Particularly encouraging are results in those patients who were allowed to carry the device for home use, although most seemed to have been long term beneficiaries beyond the stimulation period. The one exception was patient # 3, who was unable to handle secretions from lack of glottic seal even under higher stimulation levels (Fig. 2) associated with documented cricopharyngeal dysfunction (table 4). In our opinion, this failure illustrates an inability to check ongoing pneumonia (or possibly non-bacterial chemical pneumonitis from associated gastropharyngeal reflux) further illustrated by associated radiological infiltrates (table 3).
While there has been a relative scarcity of information on how dysphagia can be explained on the base of specific CNS insults (1), our limited series points to a common core of physiological signs (symptoms) in patients presenting with pneumonia. Ding and Logeman reviewed 378 stroke (CVA) patients who did and did not experience pneumonia (9). In the latter group, (of interest here) aspiration was shared by all patients although with variable frequencies. Multiple CVAs (as in patient # 3) aspirated in 72.3 % of the cases followed (in this order) by left cortical (i.e. hemispheric) (# 2), cerebellar (# 3), right cortical (# 1), multiple (# 3), unspecified, and brainstem (# 3) locations (50 %). Our data also support the observations of Johnson et al (10) who reported that those who aspirate often exhibit increased pharyngeal transit times (DRS, as in all our patients except #4), reduced pharyngeal elevation (RLE, all), vestibule closure (PLV, mostly CVAs), cricopharyngeal opening (CPD, typically in patient # 3), and weakness and increased incidence of delayed pharyngeal swallow (DSR, all).
Other causes of non-CVA dysphagia (as in patients #s 4 and 5) may manifest themselves with more diffuse or unpredictable profiles. Rapp reports that the little available data on CP in adults (in spite of their increasing numbers) must be extrapolated from children, with whom they share poor muscle coordination and rapid fatigue (11). In De Pauw’s review of 309 MS patients, as many as 24 % of those mildly impaired had dysphagia, while 59 % already had pneumonia (12). For Calgagno et al, out of 143 consecutive subjects, 34.3 % had dysphagia, (13), a roughly similar rate (43 %) as found by Abraham who studied 525 subjects (14).
The fact that our patients presented overall with fewer pneumonias during, but also to some significant degree after stimulation as compared to enrollment times (Table 3) raises the question as to how evoked dynamic laryngotracheal separation could possibly control the problem on a long term basis. Admittedly, it is difficult to draw conclusions from this small sample or patients (1).
The first issue, of a quantitative nature, pertains to the predictive value of aspiration arrests on pneumonia. Unfortunately, there may not be a direct relation between both factors when solely based on fluoroscopy (even assuming observed aspiration arrest), particularly due to false positives and negatives, lack of consensus about bolus viscosities (see table 6) and original SLP evaluation (1) as may all have possibly occurred in our (or for that matter any) series. Also, patients always swallow more often than they ingest food, leaving residual to be silently aspirated (15), as variously noted in our swallow trials (table 4). However, while our CP and MS patients stood out as having had the highest numbers of pneumonias, and also “failed” fluoroscopies before enrollment, they aspirated less during and after stimulation compared to some of their CVA counterparts (table 4). Pacing in fact reduced pneumonia rates to quasi nil in spite of the multifactorial natures of responsible CNS deficits. This progress, which may well reflect spared brain plasticity (16) possibly denied to some more pathologically focused CVA cases could offer promise in expanded SLP therapies.
There is another, qualitative aspect to this issue. According to Campbell-Taylor (17), one of the most common misrepresentations about swallowing is that aspiration should be recognized and treated to prevent pneumonia. A corollary of this assertion is that there has been too much emphasis on the importance of aspiration which has come to obscure all other causes of pneumonia. For that matter, it has been recognized that pneumonia is more probably due to malnutrition, dehydration, and reduced immune system defense as a result of impaired swallowing (1, 6–8). It is, therefore, probably not surprising that those of our patients who were able to use the stimulator during their meals at home did better than during fluoroscopy since the radiological setting does not mimic normal eating (5, 17).
There are obviously multiple variables involved with pneumonia associated with CNS deficits. Clearly, small case series such as ours should be broadened to large randomized control trials whew aspiration should not be considered as a definitive marker for the patients outcomes of pneumonia (1).
CONCLUSION
From our data, and particularly in the absence of better avenues, we submit that paced laryngotracheal separation offers promise for the control of pneumonia based on 1) a unified strategy for a common core of symptoms from diverse etiologies, 2) an objective outcome in terms of producing a glottic seal on demand, 3) documented clinical and radiological progress and 4) enhanced quality of life. However, larger series must be studied to determine the optimal approach to the problem.
Table 5B.
Percentiles of aspiration counts for stimulation OFF and ON. In spite of reduction overall, there were increases with patient #s 1 (statistical) and patient # 3 (non statistical) based on SLP II data, and non statistical changes in patient # 3 for both evaluators. For ORIG SLP, patient #2 did not aspirate. When present, increases were due to residuals (table 4) irrespectively of stimulation status.
| Pt # | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| St/% | OFF | ON | OFF | ON | OFF | ON | OFF | ON | OFF | ON |
| SLP I | 82 | 54 | 21 | 0 | 133 | 25 | 9 | 8 | 40 | 0 |
| SLP II | 100 | 113 | 66 | 66 | 122 | 131 | 12 | 2 | 27 | 0 |
| ORIG SLP | 57 | 49 | 0 | 0 |
Acknowledgments
Supported by the NeuroControl Corporation NIH SBIR grant # 1 R43 NS38776-01 and NIH grant R 21 DC0066703-2.
Footnotes
Presented at the Annual Meeting of the American Broncho-Esophagological Association, Phoenix, AZ, May 28, 2009
Broniatowski M, Grundfest-Broniatowski S, Tyler DJ, et al. Dynamic Laryngotracheal Closure for Aspiration: A Preliminary Report. Laryngoscope 111: 2032-40, 2001.
Contributor Information
Michael Broniatowski, Department of Otolaryngology-Head and Neck Surgery, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Nina S Moore, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio.
Sharon Grundfest-Broniatowski, Department of General Surgery, Associate Professor of Surgery, Cleveland Clinic Lerner College of Medicine at CWRU, Cleveland, Ohio.
Harvey M Tucker, Department of Otolaryngology-Head and Neck Surgery, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Ellen Lancaster, St Vincent Charity Hospital, University Hospitals Health System, Cleveland, Ohio.
Kate Krival, School of Speech Pathology and Audiology, Kent State University, Kent, Ohio.
Aaron J Hadley, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio.
Dustin J Tyler, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio.
References
- 1.Diagnosis and Treatment of Swallowing Disorders (Dysphagia) in Acute-Care Stroke Patients. Agency for Health Care Policy and Research (AHCPR). US Department of Health and Human Services. Evidence Report/Technology Assessment: Number 8. 1999 [PMC free article] [PubMed] [Google Scholar]
- 2.Neuman S, Bucholz D, Prosiegel M. Swallowing therapy of neurological patients: correlation of outcome with pretreatment variables and therapeutic methods. Dysphagia. 1995;10:1–5. doi: 10.1007/BF00261272. [DOI] [PubMed] [Google Scholar]
- 3.Huckabee ML, Pelletier CA. Management of Adult Neurogenic Dysphagia: Delmar Learning 1999 [Google Scholar]
- 4.Broniatowski M, Grundfest-Broniatowski S, Tyler DJ, Scolieri P, Abass F, Tucker HM, Brodsky S. Dynamic laryngortrachal Closure for Aspiration.: A preliminary Report. Laryngoscope 2001. 2001;111:2032–40. doi: 10.1097/00005537-200111000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Kidder TM. Esophago/pharyngo/laryngeal rehabilitation. Airway protection mechanisms. Dysphagia. 1995;10:228–31. doi: 10.1007/BF00431414. [DOI] [PubMed] [Google Scholar]
- 6.Carnaby-Mann G, Lenius K, Crary MA. Update on Assessment and management of Dysphagia Post Stroke. North East Florida Medicine. 2007;58:31–34. [Google Scholar]
- 7.Hageman C. Feasibility of Videofluoroscopy and Acccelerometry for Dysphagia Patients. University of Iowa; Unpublished data. www.elixirresearch.com. [Google Scholar]
- 8.Cook IJ. Treatment of oropharyngeal dysphagia. Curr Treat Options Gastroenterol. 2003;6:273–81. doi: 10.1007/s11938-003-0019-4. [DOI] [PubMed] [Google Scholar]
- 9.Ding L, Logeman JA. Pneumonia in Stroke Patients: A Retrospective Study. Dysphagia. 2000;15:51–57. doi: 10.1007/s004550010001. [DOI] [PubMed] [Google Scholar]
- 10.Johnson ER, McKenzie SW, Sievers A. Aspiration pneumonia in stroke. Arch Phys Med Rehab. 1993;74:773–76. [PubMed] [Google Scholar]
- 11.Rapp CE, Jr, Torres MM. The Adult with Cerebral Palsy. Arch Fam Med. 2000;9:466–72. doi: 10.1001/archfami.9.5.466. [DOI] [PubMed] [Google Scholar]
- 12.De Pauw A, Dejaeger E, D’hooghe B, Carton H. Dysphagia in multiple sclerosis. Clinical Neurology and Neurosurgery. 2002;104:345–51. doi: 10.1016/s0303-8467(02)00053-7. [DOI] [PubMed] [Google Scholar]
- 13.Calgagno P, Ruopollo G, Grasso MG, De Vincentiis M, Paolucci S. Dysphagia in multiple sclerosis-prevalence and prognostic factors. Acta Neurol Scandinavica. 2002;105:40–43. doi: 10.1034/j.1600-0404.2002.10062.x. [DOI] [PubMed] [Google Scholar]
- 14.Abraham S. Neurological Impairment and Disability Status in Outpatients with Multiple Sclerosis Reporting Dysphagia Symptomatology. Neurorehabilitation and Neural Repair. 1997;11:1–7. [Google Scholar]
- 15.Smith CH, Logeman JA, Colangelo LA, Rademaker AW, Roa Pauloski B. Incidence and Patient Caracteritics Associated with Silent Aspiration in the Acute Care Setting. Dysphagia. 1999;14:1–7. doi: 10.1007/PL00009579. [DOI] [PubMed] [Google Scholar]
- 16.Johaussen BB. Brain plasticity- stroke rehabilitation. Stroke. 2000;31:23–30. [Google Scholar]
- 17.Campbell-Taylor I. Oropharyngeal Dysphagia in Long-Term Care: Misperceptions of Treatment efficacy. J Am Med Dir Assoc. 2008;9:523–31. doi: 10.1016/j.jamda.2008.06.001. [DOI] [PubMed] [Google Scholar]


