Abstract
Background
Allergic sensitization affects half of western populations and often precedes the development of allergic disorders including asthma. Despite the critical role of allergens in the pathogenesis of these disorders, little is known about how allergens modulate the immune response. IL-13 receptor α2 (IL-13Rα2) is a decoy receptor for IL-13. Objective: Although the existence of soluble IL-13Rα2 has been documented, the mechanisms underlying its generation are unknown. Many allergens possess protease activity; we investigated whether IL-13Rα2 is solubilized in response to allergen treatment.
Methods
We evaluated the ability of allergens to solubilize IL-13Rα2 in vitro and in vivo and examined the effect on IL-13 signaling and responses.
Results
We determined that treatment of cells with house dust mite (HDM) allergen or purified Dermatophagoides pteronyssinus or Dermatophagoides farinae, but not other allergens, resulted in release of soluble IL-13Rα2 that was biologically active and inhibited IL-13 signaling. Prolonged exposure to HDM or treatment with mold allergens resulted in IL-13Rα2 degradation. This was associated with increased IL-13 signaling. A single treatment of HDM in vivo resulted in release of IL-13Rα2 into the bronchoalveolar lavage (BAL) fluid. BAL fluid from humans also contained IL-13Rα2; BAL fluid from individuals with asthma contained less IL-13Rα2 than that from controls.
Conclusion
Allergen exposure can directly affect the level of soluble IL-13Rα2 in a way that affects IL-13 signaling and responses.
Clinical implications
Soluble IL-13Rα2 may be an important biomarker of environmental allergen exposure and asthma.
Keywords: IL-13Rα2, allergen, house dust mite, asthma, cytokine receptor
Allergic diseases are major public health problems1; over the last 2 decades, high rates of allergen sensitization have been accompanied by an estimated doubling in the incidence of allergic respiratory diseases.2 Allergens encompass diverse protein structures, suggesting that multiple mechanisms are likely responsible for the allergic inflammatory responses that they elicit. Many allergens possess protease activity including fungal allergens and house dust mite (HDM) allergens, Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farinae (Der f). Allergen protease activity may affect immune responses by mediating cleavage of key signaling molecules, altering their distribution across biological compartments, or degrading receptor components.
IL-13 is an immunoregulatory cytokine secreted pre-dominantly by activated TH2 cells3–6 implicated in the pathogenesis of asthma in human and animal studies.7–12 IL-13 has 2 cognate receptors, IL-13 receptor α1 (IL-13Rα1) and IL-13 receptor α2 (IL-13α2).13–18 IL-13Rα1 binds IL-13 with low affinity by itself, but when paired with IL-4 receptor α, it binds IL-13 with high affinity, forming a signaling IL-13 receptor.16
IL-13Rα2 binds IL-13 with high affinity and acts as a decoy receptor, as shown by IL-13Rα2 knockout mice.19,20 IL-13Rα2 may also contribute to IL-13 responses, as suggested by a recent report demonstrating that IL-13–induced TGF-β–mediated fibrosis is dependent on IL-13Rα2.21 Interestingly, IL-13Rα2−/− mice have greatly reduced levels of IL-13 in the serum, but significantly greater tissue levels of IL-13 than wild-type mice. Thus, IL-13Rα2 regulates serum and tissue levels of IL-13. This was supported by a report that treatment of IL-13Rα2−/− mice with soluble IL-13Rα2-Fc increases circulating IL-13,20 demonstrating a complex feedback loop between IL-13 and IL-13Rα2 whereby they each modulate the level of the other, because IL-13 has been shown to induce IL-13Rα2 expression. Thus, regulation of the level of expression of IL-13Rα2 and its relative distribution among the membrane and soluble compartments both likely affect IL-13 responses.
IL-13Rα2 transcripts have been found in the spleen, liver, lung, thymus, and brain.18,22 Soluble IL-13Rα2 fusion proteins have been used in vivo to block IL-13 signaling and prevent allergen-induced airway inflammation,23,24 but the mechanism for the generation of soluble IL-13Rα2 is not known. HDM proteolytic activity has been shown to cleave cellular receptors.25–27 Because IL-13 is a critical mediator of allergy, and allergens often have proteolytic activity, we examined the effect of allergen treatment on the solubilization and degradation of IL-13Rα2 and determined the effect of allergen proteases on IL-13 signaling.
METHODS
Animals
Animals were maintained under Institutional Animal Care and Use Committee-approved procedures and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council). C57BL/6 (Jackson Laboratory, Bar Harbor, Me) mice were anesthetized and administered 0.1 to 100 μg HDM (Greer Laboratories, Lenoir, NC) or PBS alone intratracheally. One day later, blood samples were collected, and the lungs were lavaged twice with 1.0 mL Hanks’ balanced salt solution. Data from mice treated with 10 μg HDM and 100 μg HDM were similar and were combined for statistical analysis.
Cells
Cells overexpressing human IL-13Rα2 were previously described.28
Allergen treatment of cells
Allergen extracts were purchased from Greer Laboratories. Cells were incubated in RPMI-1640 containing allergen extract (10 μg/mL HDM; 1:25 dilution of other allergens) for 60 minutes. In some cases, protease inhibitors (AEBSF, Aprotinin, E-64, and Leupeptin; Sigma-Aldrich, St Louis, Mo) were added per manufacturer recommendations. Conditioned media were collected by centrifugation and soluble IL-13Rα2 quantified by ELISA. For the allergen pretreatment of cells before electrophoretic mobility shift assay (EMSA), cells were incubated with or without HDM (10 μg/mL) for 20 minutes at 37°C or Aspergillus fumigatus 1:25 dilution for 60 minutes, washed 3 times, and stimulated with IL-13 or IL-4 (PeproTech, Rocky Hill, NJ) for 20 minutes.
ELISA
Human and murine IL-13Rα2 were detected by ELISA as described.28,29
Electrophoretic mobility shift assay
EMSA was performed as previously described.30
Generation of glutathione-S-transferase (GST)–IL-13Rα2 fusion protein and cleavage assay
Mature human IL-13Rα2 cDNA was amplified with primers: 5′-CCCCCGGGAGACACCGAGATAAAAGTTAAC-3′ and 5′-CCC TCGAGTTATTTATCATCATCTTTATAATCTGTATCACAGA AAAATTA-3′ adding a C-terminal FLAG epitope tag, and inserted in the pGEX-KG plasmid (Invitrogen, Carlsbad, Calif), adding a N-terminal GST. This construct was used to transform Escherichia coli, which was treated with 100 mmol/L isopropyl-beta-D-thiogalactopyranoside (IPTG) to induce protein expression. GST–IL-13Rα2 was purified from the supernatant with Glutathione Sepharose 4B (Amersham Biosciences, Piscataway, NJ). The fusion protein was verified by PAGE Coomassie stain and Western blot. This identified a single protein band of appropriate size, immunoreactive to anti-FLAG. Purified fusion protein (300 ng) was treated with allergen extracts at 0.5 μg/mL and resolved by PAGE to assess cleavage.
Flow cytometry
FLAG–IL-13Rα2 expression and IL-13 binding were assessed by flow cytometry as described.28
Trypsin treatment of cells
Cells—1 × 106 (flow cytometry) or 5 × 106 (lysates for ELISA)—were resuspended in 500 μL trypsin-EDTA at 4°C. Cells were then warmed to 37°C for the indicated times. Cells remained >95% viable at all treatment times. Trypsin was inactivated with 1 mL FBS, and the cells were washed once in RPMI and twice in PBS. Cells were stained for surface FLAG–IL-13Rα2 and analyzed by flow cytometry, or lysates were prepared for ELISA as previously described.30
Confocal microscopy
Confocal microscopy was performed as described.31
Subjects
Subjects with asthma recruited from National Jewish Medical and Research Center were diagnosed according to American Thoracic Society guidelines using previously defined criteria.32,33 Normal controls had normal pulmonary function, if able to perform pulmonary function tests, and had no history of any respiratory illness. Normal patients were recruited from National Jewish and from Cincinnati Children’s Hospital Medical Center bronchoalveolar lavage (BAL) specimen bank. Cell free lavage fluid was obtained as previously described32,33 and stored at −70°C until analysis. BAL fluid was analyzed for human IL-13Rα2 by using the DuoSet kit from R&D Systems (Minneapolis, Minn) according to the manufacturer’s instructions. The assay was unaffected by addition of exogenous human IL-13 (50 ng/mL). This study was approved by the respective Institutional Review Boards.
RESULTS
HDM increases soluble IL-13Rα2 release from cells in vitro
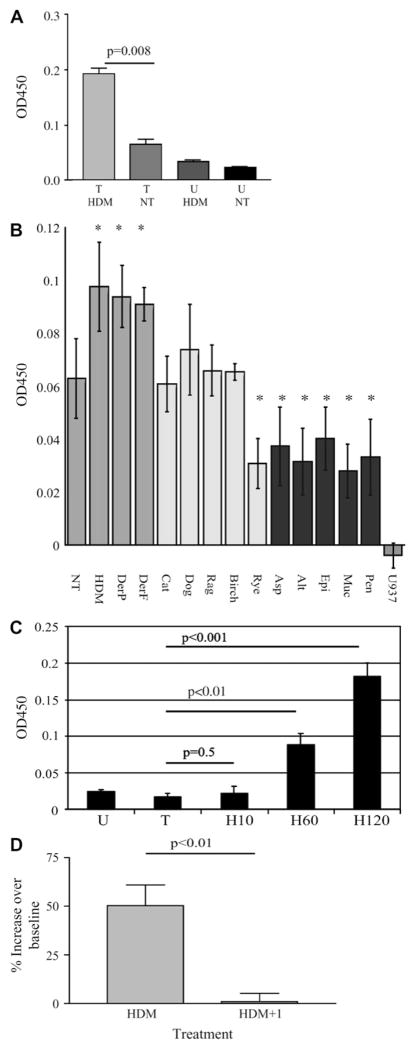
We examined whether soluble IL-13Rα2 could be detected in the conditioned media from U937 cells over-expressing IL-13Rα2.28 We observed a baseline level of soluble IL-13Rα2 released from transfected cells after 60 minutes in culture (Fig 1, A). The amount of receptor released into the media was augmented by the addition of HDM extract.
FIG. 1.
HDM treatment results in solubilization of IL-13Rα2. A, U937 (U) and FLAG–IL-13Rα2 transfected U937 cells (T) were treated with HDM. Soluble IL-13Rα2 was quantified. B, FLAG–IL-13Rα2 transfected U937 cells were treated with the indicated allergen extracts. Soluble IL-13Rα2 was quantified. C, Media from FLAG–IL-13Rα2 transfected cells treated with HDM for minutes as indicated. D, FLAG–IL-13Rα2 transfectants were treated with HDM, alone or with protease inhibitors (+I). Soluble IL-13Rα2 was quantified by ELISA. Significant differences noted. NT, No treatment. *Significantly different from untreated, P < .05.
Induced release of soluble IL-13Rα2 is allergen-specific
We next examined the ability of purified dust mite allergens, Der p and Der f, to result in IL-13Rα2 solubilization and compared this with other allergens (Fig 1, B). Treatment of cells with either HDM extract or purified Der p or Der f extracts resulted in significantly increased soluble IL-13Rα2 released into the media. This effect was dose-dependent (data not shown). Other allergens, including pollens (ryegrass, birch, ragweed), molds (Penicillium, Mucor, Epicoccus, Alternaria, Aspergillus), and pet dander (cat, dog) failed to induce solubilization of IL-13Rα2 above baseline levels. Interestingly, treatment with the mold allergens decreased soluble IL-13Rα2 in the media (P < .05), likely because of degradation of IL-13Rα2 by the highly proteolytic molds.
We examined the kinetics of release of IL-13Rα2 after HDM treatment by ELISA (Fig 1, C). IL-13Rα2 was below the threshold of detection in the media 10 minutes after HDM treatment but became significant after 60 and 120 minutes.
Protease inhibitor reduces HDM-induced release of soluble IL-13Rα2
To examine the role of protease activity in the HDM-induced release of soluble IL-13Rα2, we treated FLAG–IL-13Rα2 transfectants with HDM extract in the presence or absence of protease inhibitors (Fig 1, D). After 60 minutes, soluble IL-13Rα2 in the conditioned media was increased 50% over baseline. Treatment with protease inhibitors completely blocked the HDM-induced increase in soluble IL-13Rα2. Protease inhibitor treatment had no effect on the release of soluble IL-13Rα2 in the absence of HDM.
Direct cleavage of IL-13Rα2 by HDM
To examine whether HDM could cleave IL-13Rα2 directly, we generated a GST-human IL-13Rα2 fusion protein. In an acellular assay, this protein was incubated with allergens for 60 minutes (Fig 2, A). HDM was able to cleave IL-13Rα2 directly. Birch pollen had some effect, but dog, ragweed, and rye allergens had little or no effect. Treatment with mold allergens resulted in loss of IL-13Rα2. However, mold treatment decreased soluble IL-13Rα2 (Fig 1, B). We suspected this was a result of degradation of IL-13Rα2 by mold allergens. We therefore directly compared the ability of HDM and Alternaria to degrade IL-13Rα2. As shown in Fig 2, B, HDM resulted in cleavage but not degradation of IL-13Rα2. In contrast, no IL-13Rα2 was detected after treatment with Alternaria. We then investigated the effect of prolonged exposure to HDM antigen to determine whether the proteolytic activity of HDM would eventually degrade IL-13Rα2. Fig 2, C, demonstrates decreased IL-13Rα2 during HDM treatment, with essentially complete loss of IL-13Rα2 within 24 hours.
FIG. 2.
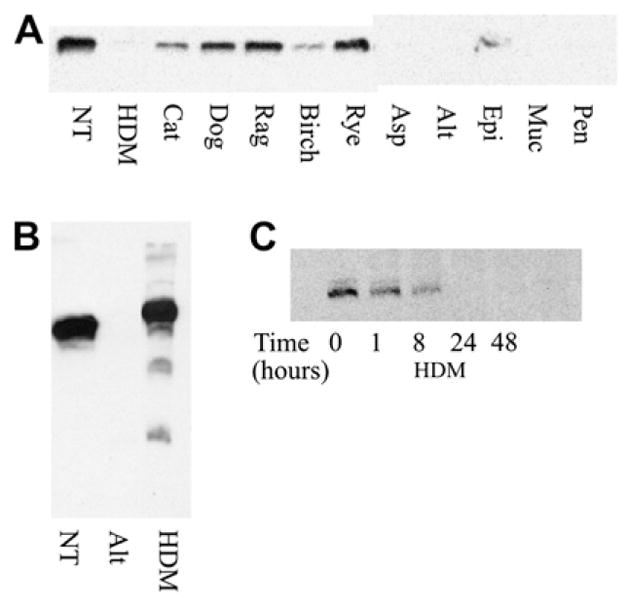
Direct cleavage of FLAG–IL-13Rα;2 by allergens. GST–FLAG–IL-13Rα2 was incubated with the indicated allergens or media for 1 hour, analyzed by PAGE, and detected by Western blot using anti-FLAG (A and C) or anti–IL-13Rα2 antibodies (B). Western blot is representative of 3 experiments. NT, No treatment.
Allergen-induced release of soluble IL-13Rα2 does not affect surface IL-13Rα2 levels, but decreases total cellular IL-13Rα2
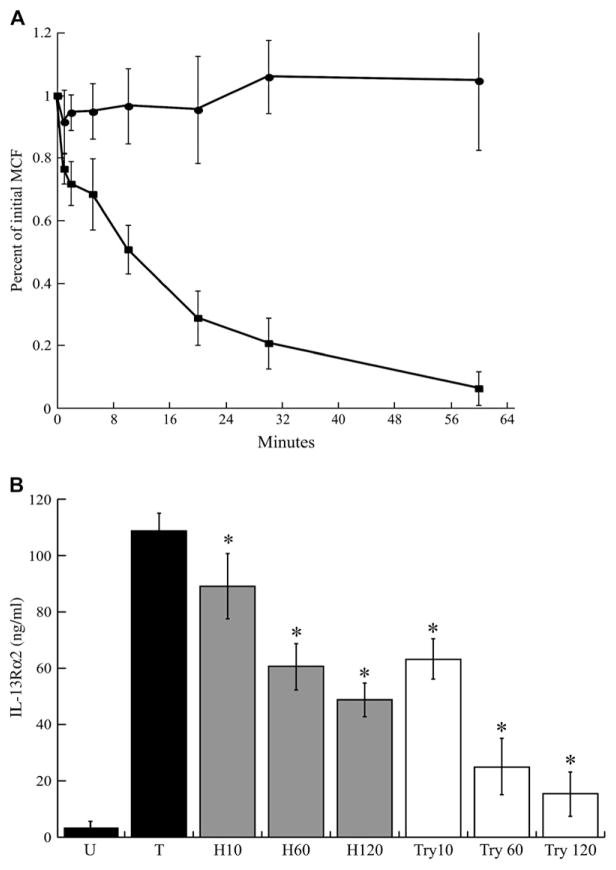
We examined the effect of HDM on level of surface IL-13Rα2 by using flow cytometry. As shown in Fig 3, A, HDM treatment did not result in a detectable decrease in the surface expression of IL-13Rα2. In contrast, trypsin treatment of the cells resulted in rapid and complete loss of surface IL-13Rα2.
FIG. 3.
Effect of HDM treatment on surface IL-13Rα2. A, Surface IL-13Rα2 expression was determined after exposure to HDM (circles) or trypsin (squares) for the indicated times. B, Total IL-13Rα2 in cellular lysates was determined after HDM (gray bars) or trypsin (white bars) treatment (*P < .05 compared with transfected untreated lysate). Means ± SDs of 4 experiments shown. MCF, Mean channel fluorescence.
We determined whether HDM-induced release of receptor affected the total cellular IL-13Rα2 despite unchanging surface IL-13Rα2. HDM-treated cells demonstrated a decrease in the total cellular IL-13Rα2 after 60 and 120 minutes. Interestingly, although trypsin resulted in near complete removal of surface IL-13Rα2 after 60 minutes (Fig 3, A), total cellular IL-13Rα2 from cells treated with trypsin for 60 minutes decreased by only 77% (Fig 3, B). Also, total cellular IL-13Rα2 continued to decrease at 120 minutes of trypsin treatment, by 86%. Thus, additional IL-13Rα2 receptors are becoming accessible to trypsin at the cell surface, presumably from intracellular pools replenishing the cleaved membrane receptors. HDM resulted in a decrease in total cellular IL-13Rα2 at 60 minutes of 45%, or 58% of the amount removed by trypsin treatment at the same time.
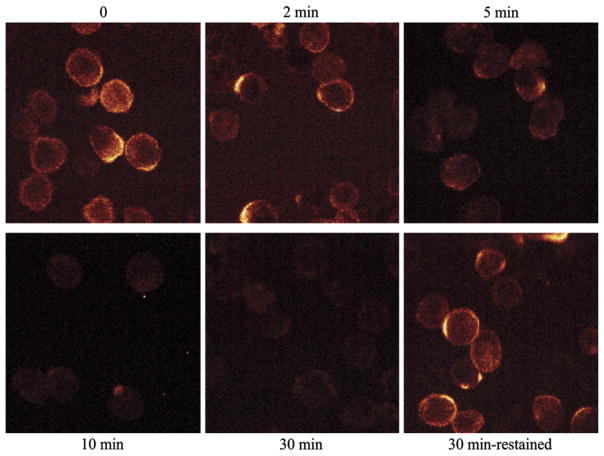
Rapid turnover of surface IL-13Rα2
Our data demonstrate significant IL-13Rα2 is located intracellularly and that surface IL-13Rα2 is replenished after solubilization. We examined the fate of surface IL-13Rα2 by confocal microscopy, investigating the kinetics of the loss of surface receptor. Cell surface IL-13Rα2 was labeled with anti-FLAG antibodies. Then the cells were washed and incubated at 37°C, and residual surface FLAG antibody was detected by flow cytometry. The loss of surface IL-13Rα2 was rapid (Fig 4). Interestingly, restaining the cells after this loss of labeled surface receptor demonstrates that the steady state of FLAG–IL-13Rα2 is unchanged. This most likely represents replenishing of surface IL-13Rα2 from intracellular pools.
FIG. 4.
Confocal microscopy reveals maintenance of steady-state surface level of IL-13Rα2. FLAG–IL-13Rα2 transfectants were labeled with anti-FLAG antibodies at 4°C, washed, and incubated at 37°C for the indicated times. Remaining surface-bound FLAG antibodies were detected by confocal microscopy. After the 30-minute incubation, some cells were restained for surface FLAG–IL-13Rα2. Representative images from 3 independent experiments shown.
Effect of HDM and A fumigatus on IL-13–dependent signal transducer and activator of transcription 6 activation
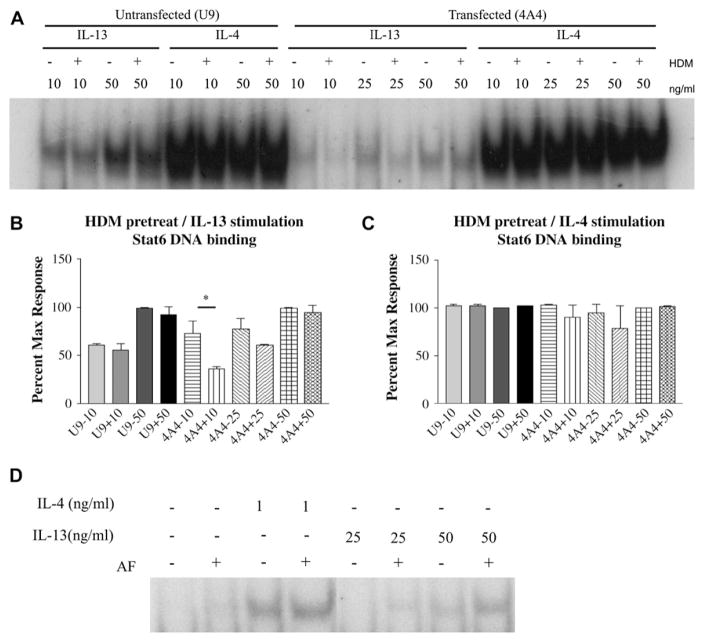
Because HDM-induced receptor release occurs in the absence of changes in the levels of surface expression of IL-13Rα2, we examined the effects of increased solubilization of IL-13Rα2 on IL-13 signaling. Cells pretreated with HDM to induce soluble IL-13Rα2 release were stimulated with either IL-13 or IL-4 for 20 minutes. Signal transducer and activator of transcription 6 (Stat6) activation was assessed (Fig 5, A). As shown in Fig 5, B, the Stat6 activity was significantly reduced at 10 ng/mL IL-13 stimulation in HDM pretreated transfectants compared with non-HDM treated transfectants. No change in Stat6 activity was observed in nontransfected cells. The inhibition was specific to IL-13, because no change in Stat6 activation was seen after IL-4 stimulation with or without HDM pretreatment (Fig 5, C). Thus, IL-13Rα2 released after HDM treatment inhibits IL-13 responses.
FIG. 5.
HDM treatment and A fumigatis treatment have opposite effects on IL-13–dependent Stat6 activation. A, Cells were incubated with or without HDM and stimulated with IL-13 or IL-4 for 20 minutes. Stat6 activation by EMSA. B and C, Means ± SEMs of densitometry of 3 experiments. *P = .02. D, FLAG–IL-13Rα2 transfected cells were incubated with or without A fumigatus and stimulated with cytokine. Stat6 activation by EMSA. AF, Aspergillus fumigatus.
In contrast with HDM treatment, exposure of cells to A fumigatus resulted in degradation of IL-13Rα2 and decreased soluble IL-13Rα2. Thus, we next investigated the effect of A fumigatus pretreatment of cells expressing IL-13Rα2 on IL-13–dependent and IL-4–dependent Stat6 activation. As shown in Fig 5, D pretreatment with A fumigatus has no effect on Stat6 activation in response to IL-4 but augmented Stat6 activation in response to IL-13.
HDM treatment increases soluble IL-13Rα2 in vivo
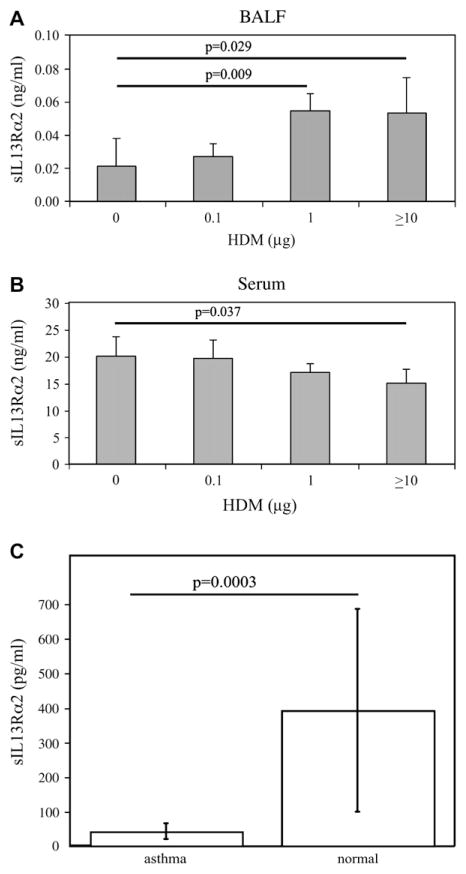
Because we have demonstrated that HDM cleaves IL-13Rα2 with in vitro and acellular models, we next investigated the ability of HDM to solubilize IL-13Rα2 in vivo. We treated naive C57BL/6 mice with a single dose of HDM (0.1–100 μg) intratracheally. HDM treatment resulted in increased IL-13Rα2 in the BAL fluid compared with controls in a dose-dependent manner (Fig 6, A). Serum IL-13Rα2 was decreased in response to HDM exposure (Fig 6, B).
FIG. 6.
Soluble IL-13Rα2 in vivo. C57BL/6 mice were treated once with intratracheal HDM or PBS, and levels of soluble IL-13Rα2 (sIL-13Rα2) were determined in (A) BAL fluid and (B) serum by ELISA. Means ± SDs shown (N = 5 mice in each group). C, sIL-13Rα2 in BAL fluid obtained from subjects with asthma (N = 11) or control subjects (N = 23) was quantified. Means ± SDs shown.
Decreased IL-13Rα2 in BAL fluid of subjects with asthma
Because IL-13 is a critical mediator of asthma and allergic inflammation, we speculated that soluble IL-13Rα2 may be deficient in subjects with asthma. We analyzed BAL fluid of individuals with asthma or normal controls for IL-13Rα2 (Fig 6, C). Strikingly, subjects with asthma had lower levels of IL-13Rα2 in their BAL fluid compared with controls without asthma.
DISCUSSION
Allergic diseases are a major public health problem. Increases in the rates of allergen sensitization have been accompanied by rises in the incidence of allergic respiratory diseases.2 Understanding environmental risks related to these spiraling rates for allergic disease is important because these factors can be more easily manipulated than other risk factors, such as genetics. We demonstrate that environmental allergens can act directly on IL-13Rα2 and affect IL-13 signaling, a novel mechanism by which environmental exposure may to contribute to the development of allergic disorders. Our findings are consistent with environmental HDM and mold allergens potentially having multiple roles in the allergic response. First, they are antigens. Second, HDM protease activity facilitates trans-epithelial allergen delivery.34 Third, we have shown that HDM and mold allergens are able to cleave/degrade IL-13Rα2 receptors, influencing the local milieu of cytokine-cytokine receptor interactions. The resultant modifications of cytokine responses could affect immune inflammatory responses to HDM and mold, as well as to other coexisting antigens. Finally, cleaved IL-13Rα2 receptors are released into biological fluids where they may traffic to distant sites and affect cytokine signaling remotely. Other antigens with proteolytic activity may also be able to modify the cytokine receptors in a similar manner, presenting a novel mechanism for antigens to influence immune responses.
The importance of both HDM and IL-13 in the development of asthma has been well studied. In large population studies, HDM exposure was found to affect directly the development of asthma in a dose-dependent fashion.35 IL-13 has been shown to be a critical mediator of allergic inflammation in human and animal studies.3,23,24 The ability of IL-13Rα2 to modulate IL-13 signaling in local and remote ways will depend on its distribution in cytoplasmic, membrane, and soluble compartments. Mechanisms that contribute to the generation of soluble IL-13Rα2 have not yet been elucidated. Our data establish that brief HDM exposure results in solubilization of IL-13Rα2, whereas prolonged exposure (or exposure to molds) results in IL-13Rα2 degradation. Thus, these environmental exposures will affect the relative distribution of IL-13Rα2 and the microenvironment in which IL-13 responses are determined.
In mouse models of allergic inflammation, proteolytically active Der p significantly enhanced IgE production compared with inactive Der p,36 supporting a role for protease activity in allergic sensitization. Soluble IL-13Rα2 released by HDM proteases was biologically active and inhibited IL-13 responses, likely by binding IL-13 and blocking its binding to IL-13Rα1. The inhibitory effect of IL-13Rα2 was a result of the increase in soluble receptor and not a loss of membrane receptor because the level of membrane receptor remained constant after HDM treatment. The ability of HDM to solubilize IL-13Rα2 was observed in vivo, whereby a single treatment with HDM resulted in release of soluble IL-13Rα2 in a dose-dependent manner. Interestingly, we discovered that humans with asthma have decreased IL-13Rα2 in BAL compared with normal controls. It is possible that the differences between the human and mouse findings are a result of the temporal nature of the exposure to allergen, the murine model representing single exposure to relatively high concentrations of allergen whereas the human findings represent chronic environmental allergen exposure. Possibly, differences in cumulative environmental allergen exposure or differences in the intrinsic regulation of the production of soluble IL-13Rα2 could lead to decreased IL-13Rα2 in subjects with asthma and increased susceptibility to IL-13. IL-13Rα2 may represent an important bio-marker for asthma and allergic diseases. Population studies may address these possibilities.
The effect of HDM treatment on IL-13Rα2 may involve a direct cleavage of IL-13Rα2 by HDM. Release of IL-13Rα2 into the media required HDM protease activity. An acellular assay using a GST–IL-13Rα2 fusion protein demonstrated that HDM allergen can directly cleave IL-13Rα2 and that prolonged exposure of IL-13Rα2 to HDM results in the degradation of IL-13Rα2. It is possible that indirect mechanisms may also be playing a role in the effect of HDM on IL-13 signaling. Fungal allergens caused digestion of IL-13Rα2 in the acellular assay, but did not increase IL-13Rα2 in cellular supernatants. This is likely a result of the fact that molds possess considerable proteolytic activity and result in degradation of IL-13Rα2. Furthermore, pretreatment of cells with A fumigatus resulted in enhanced IL-13 signaling, presumably because of degradation of IL-13Rα2 and loss of this decoy activity. In contrast, HDM pretreatment resulted in inhibition of IL-13 responses, likely because of release of functional IL-13Rα2. HDM allergen exposure may initially be protective for the development of allergic inflammation, but chronic HDM or mold exposure may promote allergic disease pathogenesis.
To understand how IL-13Rα2 modulates IL-13 responses, it is necessary to elucidate the connections among surface, intracellular, and soluble forms of IL-13Rα2, and their collective and distinct roles. Despite the release of IL-13Rα2 in response to HDM, surface expression of surface IL-13Rα2 remained constant whereas total IL-13Rα2 in lysates declined. Trypsin treatment rapidly removed all surface IL-13Rα2 and decreased total IL-13Rα2 in lysates. Significant amounts of IL-13Rα2 are located intra-cellularly and were not accessible at the cell surface. The reduction in total cellular IL-13Rα2 after trypsin treatment continued beyond 1 hour, when all surface IL-13Rα2 had been removed by trypsin. This shows that additional IL-13Rα2 receptors are being shuttled to the cell surface where they are accessible to trypsin cleavage, presumably from cytoplasmic pools, where the majority of IL-13Rα2 is located,31 demonstrating communication between intra-cellular pools and the cell surface. This was confirmed when we investigated the kinetics of the receptor turnover by confocal microscopy. A proposed model for HDM and mold allergens and their effect on IL-13 signaling is shown in Fig 7. After brief HDM exposure, soluble IL-13Rα2 is initially increased by the proteolytic activity of HDM, inhibiting IL-13 dependent Stat6 activation. Prolonged exposure to HDM degrades IL-13Rα2, potentially increasing IL-13 responses. With mold exposure, IL-13Rα2 is degraded, resulting in decreased soluble IL-13Rα2, enhancing IL-13 signaling. The effect of allergens on IL-13Rα2 receptor level and distribution depends on the proteolytic activity of the antigen and the duration of exposure.
FIG. 7.
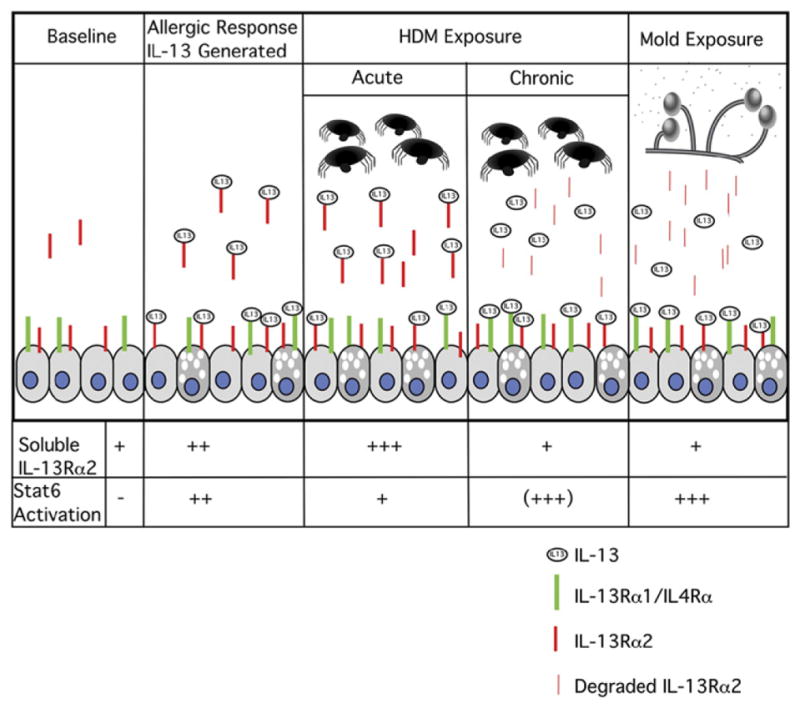
Schematic representation of effects of HDM and mold allergen exposure on IL-13 receptors and signaling.
Exposure to mold or HDM allergens resulted in degradation of IL-13Rα2. The effect of this on the development of allergic inflammation is not clear. It is intriguing to speculate that HDM and/or mold-dependent degradation of IL-13Rα2 and resultant decreased levels of IL-13Rα2 contribute to the pathogenesis of allergic disorders in individuals with allergy because of the loss of IL-13Rα2 inhibition of IL-13 responses. We observed lower levels of IL-13Rα2 in BAL fluid from subjects with asthma versus controls. This could be a result of chronic exposure to proteolytic allergens in subjects with asthma leading to degradation of IL-13Rα2, or subjects with asthma having impaired generation of soluble IL-13Rα2. Larger studies are warranted to investigate further the utility of soluble IL-13Rα2 as a biomarker of allergic disease.
Acknowledgments
Supported by NIH R01 AI058157 (G. K. K. H.) and NIH K08 AI053150-01 (M. O. D.).
We thank Dr Marc E. Rothenberg for critical review of this manuscript.
Abbreviations used
- BAL
Bronchoalveolar lavage
- Der f
Dermatophagoides farinae
- Der p
Dermatophagoides pteronyssinus
- HDM
House dust mite
- IL-13Rα1
IL-13 receptor α1
- IL-13Rα2
IL-13 receptor α2
- Stat6
Signal transducer and activator of transcription 6
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56:835–8. doi: 10.1136/thorax.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberg N, Hesselmar B, Aberg B, Eriksson B. Increase of asthma, allergic rhinitis and eczema in Swedish schoolchildren between 1979 and 1991. Clin Exp Allergy. 1995;25:815–9. doi: 10.1111/j.1365-2222.1995.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 3.Minty A, Chalon P, Derocq J-M, Dumont X, Guillemot J-C, Kaghad M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie AN, Culpepper JA, de Waal Malefyt R, Briere F, Punnonen J, Aversa G, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci USA. 1993;90:3735–9. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokota T, Otsuka T, Mosmann T, Banchereau J, DeFrance T, Blanchard D, et al. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell-and T-cell-stimulating activities. Proc Natl Acad Sci USA. 1986;83:5894–8. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noma Y, Sideras P, Naito T, Bergstedt-Lindquist S, Azuma C, Severinson E, et al. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986;319:640–6. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- 7.Rankin JA, Picarella DE, Geba GP, Temann UA, Prasad B, DiCosmo B, et al. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci USA. 1996;93:7821–5. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura Y, Azuma M, Okano Y, Sano T, Takahashi T, Ohmoto Y, et al. Upregulatory effects of interleukin-4 and interleukin-13 but not interleukin-10 on granulocyte/macrophage colony-stimulating factor production by human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;15:680–7. doi: 10.1165/ajrcmb.15.5.8918375. [DOI] [PubMed] [Google Scholar]
- 10.Tang ML, Coleman J, Kemp AS. Interleukin-4 and interferon-gamma production in atopic and non-atopic children with asthma. Clin Exp Allergy. 1995;25:515–21. doi: 10.1111/j.1365-2222.1995.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–94. [PubMed] [Google Scholar]
- 12.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts: implication in asthma. J Clin Invest. 1998;101:2129–39. doi: 10.1172/JCI741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aman M, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ. CDNA cloning and characterization of the human interleukin 13 receptor alpha chain. J Biol Chem. 1996;271:29265–70. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 14.Caput D, Laurent P, Kaghad M, Lelias JM, Lefort S, Vita N, et al. Cloning and characterization of a specific interleukin (IL)-13 binding protein structurally related to the IL-5 receptor alpha chain. J Biol Chem. 1996;271:16921–6. doi: 10.1074/jbc.271.28.16921. [DOI] [PubMed] [Google Scholar]
- 15.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miloux B, Laurent P, Bonnin O, Lupker J, Caput D, Vita N, et al. Cloning of the human IL-13R alpha1 chain and reconstitution with the IL4R alpha of a functional IL-4/IL-13 receptor complex. FEBS Lett. 1997;401:163–6. doi: 10.1016/s0014-5793(96)01462-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JG, Hilton DJ, Willson TA, McFarlane C, Roberts BA, Moritz RL, et al. Identification, purification, and characterization of a soluble interleukin (IL)-13-binding protein: evidence that it is distinct from the cloned Il-13 receptor and Il-4 receptor alpha-chains. J Biol Chem. 1997;272:9474–80. doi: 10.1074/jbc.272.14.9474. [DOI] [PubMed] [Google Scholar]
- 18.Guo FH, Uetani K, Haque SJ, Williams BR, Dweik RA, Thunnissen FB, et al. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100:829–38. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood N, Whitters MJ, Jacobson BA, Witek J, Sypek JP, Kasaian M, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197:703–9. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, et al. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha(2) receptor is involved in induction of TGF-beta(1) production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, et al. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161:2317–24. [PubMed] [Google Scholar]
- 23.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 25.Schulz O, Sewell HF, Shakib F. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med. 1998;187:271–5. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz O, Laing P, Sewell HF, Shakib F. Der p I, a major allergen of the house dust mite, proteolytically cleaves the low-affinity receptor for human IgE (CD23) Eur J Immunol. 1995;25:3191–4. doi: 10.1002/eji.1830251131. [DOI] [PubMed] [Google Scholar]
- 27.Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173:5343–8. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- 28.Daines MO, Tabata Y, Walker BA, Chen W, Warrier MR, Basu S, et al. Level of expression of IL-13R alpha 2 impacts receptor distribution and IL-13 signaling. J Immunol. 2006;176:7495–501. doi: 10.4049/jimmunol.176.12.7495. [DOI] [PubMed] [Google Scholar]
- 29.Tabata Y, Chen W, Warrier M, Gibson A, Daines M, Khurana Hershey G. Allergy-driven alternative splicing of interleukin-13 receptor alpha 2 yields distinct membrane and soluble forms. J Immunology. 2006 doi: 10.4049/jimmunol.177.11.7905. In press. [DOI] [PubMed] [Google Scholar]
- 30.Andrews RP, Ericksen MB, Cunningham CM, Daines MO, Hershey GK. Analysis of the life cycle of stat6: continuous cycling of STAT6 is required for IL-4 signaling. J Biol Chem. 2002;277:36563–9. doi: 10.1074/jbc.M200986200. [DOI] [PubMed] [Google Scholar]
- 31.Daines MO, Hershey GK. A novel mechanism by which interferon-gamma can regulate interleukin (IL)-13 responses: evidence for intracellular stores of IL-13 receptor alpha-2 and their rapid mobilization by interferon-gamma. J Biol Chem. 2002;277:10387–93. doi: 10.1074/jbc.M108109200. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–8. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 33.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma: persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156:737–43. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 34.Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–33. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, et al. House dust mite allergens: a major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996;153:141–6. doi: 10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- 36.Gough L, Schulz O, Sewell HF, Shakib F. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J Exp Med. 1999;190:1897–902. doi: 10.1084/jem.190.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]