Abstract
Background
Early-onset ventilator-associated pneumonia (EO-VAP) is the leading cause of morbidity and mortality in comatose patients. However, VAP prevention bundles focus mainly on late-onset VAP and may be less effective in preventing EO-VAP in comatose patients. Systemic antibiotic administration at the time of intubation may have a role in preventing EO-VAP. Therefore, we evaluated the effectiveness of systemic antibiotic administration in VAP prevention in comatose patients through a systematic review and meta-analysis.
Methods
We searched for studies published through December 2015 that evaluated systemic antibiotic prophylaxis in comatose patients. Two authors independently selected and evaluated full-length reports of randomized clinical trials or prospective cohorts in patients aged >16 years that evaluated the impact of systemic antibiotics at the time of intubation on EO-VAP compared to placebo or no prophylaxis. The outcome variables were the incidence of EO-VAP, the duration of mechanical ventilation, ICU length of stay, and ICU mortality.
Results
We identified 10,988 citations, yielding 26 articles for further analysis; three studies with 267 patients were finally analyzed. Most patients (n = 135) were comatose due to head trauma. Systemic antibiotic administration was associated with decreased incidence of EO-VAP (RR 0.32; 95% CI 0.19–0.54) and shorter ICU LOS (standardized mean difference −0.32; 95% CI −0.56 to −0.08), but had no effect on mortality (RR 1.03; 95% CI 0.7–1.53) or duration of mechanical ventilation (standardized mean difference −0.16; 95% CI −0.41 to 0.08).
Conclusions
Antibiotic prophylaxis in comatose patients reduced the incidence of EO-VAP and decreased the ICU stay slightly. Future trials are needed to confirm these results.
Keywords: Systematic review, Meta-analysis, Ventilator-associated pneumonia, Coma
Background
Ventilator-associated pneumonia (VAP) is a frequent cause of morbidity and mortality in comatose patients. In this population, pneumonia usually occurs within the first four days of mechanical ventilation and is termed early-onset pneumonia (EO-VAP) [1]. The incidence of EO-VAP ranges from 21 to 60% [2, 3] in critically ill patients with traumatic brain injury (TBI) and is about 48% in those with subarachnoid hemorrhage. In a mixed population of patients in coma due to various causes, EO-VAP accounted for 70% of all cases of pneumonia [4]. In neurosurgical patients, the incidence of VAP peaks in the first three days after admission [5]. Pneumonia is associated with higher mortality in acute neurological patients [6]; a recent meta-analysis by the Cochrane Group found that pneumonia in stroke patients is associated with mortality (OR 3.62) [7]. Jovanovic et al. [8] found that VAP was associated with higher and earlier mortality in comatose patients with TBI.
The predominance of EO-VAP in comatose patients is in striking contrast to general critical care patients, in whom accounts for 62–73% of all cases of VAP are late onset [9, 10]. Many risk factors are related to the increased incidence of EO-VAP in brain-injured patients. Massive or microbronchoaspiration, leakage of colonized subglottic secretions around the cuff of the endotracheal tube, and brain injury-induced immunosuppression may all play significant roles [11]. Moreover, it is not always feasible to implement VAP prevention bundles in brain-injured patients [12], and preventive measures that are effective for late-onset VAP might not be effective for EO-VAP [13]. Thus, alternative prophylactic measures should be explored in comatose patients.
Among other measures, antibiotic administration at the time of intubation seems a reasonable alternative. Systemic antibiotics have been reported to protect against EO-VAP [14]. However, the role of antibiotic prophylaxis in comatose patients remains unclear. Therefore, we performed a systematic review and meta-analysis of prospective studies to answer the following question: Is the administration of systemic antibiotics at the time of intubation superior to placebo or no prophylaxis in preventing VAP and decreasing all-cause mortality reduction in comatose patients?
Methods
Data sources and study selection
Following the methodological recommendations of the Cochrane Collaboration and the PRISMA statement [15], two authors (CR and IML) independently searched PubMed and the Cochrane Library (2015) for the terms aspiration pneumonia, pneumonia, ventilator-associated pneumonia (VAP), coma, altered level of consciousness, and depressed level of consciousness, cross-referenced to the terms antibiotic prophylaxis, and preemptive antibiotic therapy. The search strategy performed was the following:
First Search: #1
Search ((((((((((aspiration pneumonia[MeSH Terms]) OR “pneumonia”[MeSH Terms]) OR “pneumonia, ventilator associated”[MeSHTerms]) OR respiratory infections[MeSH Terms]) AND coma[MeSH Terms])OR altered level of consciousness[MeSH Terms]) OR depressed level of consciousness[MeSH Terms]) OR consciousness disorder[MeSH Terms]) OR consciousness, loss of[MeSH Terms]) AND antibiotic prophylaxis[MeSHTerms]) OR antibiotic premedication[MeSH Terms].
Second Search: #2
Search (((((((((“aspiration pneumonia”[Title/Abstract]) OR”pneumonia”[Title/Abstract]) OR “ventilator associated pneumonia”[Title/Abstract]) OR “respiratory infection”[Title/Abstract])AND “coma”[Title/Abstract]) OR “depressed level of consciousness”[Title/Abstract]) AND “antibiotic prophylaxis”[Title/Abstract]) OR “antibiotic premedication”[Title/Abstract]) OR “preemptive antibiotic treatment”[Title/Abstract]) OR “preemptive antibiotic therapy”[Title/Abstract].
Third Search:
#1 OR #2
Then, we manually searched personal files for full-length articles published in peer-reviewed journals by May 12 (2017). We selected the inclusion criteria for articles using the PICO approach. The inclusion criteria were: (1) clinical trials or prospective cohorts; (2) population analyzed—adult (>18 years) comatose patients; (3) intervention—systemic antibiotic prophylaxis at the time, or just before orotracheal intubation; (4) control group—patients who did not receive antibiotics for intubation; and (5) outcome—studies that evaluated VAP incidence, ICU and hospital mortality, as well as length of hospital stay and length of mechanical ventilation. We excluded studies that did not report enough data to estimate the odds ratio (OR) or relative risk (RR) and their variance.
Two authors (CR and IML) screened citations and articles identified by the initial search, selecting potentially relevant titles, reviewing their abstracts, and determining whether the articles met the inclusion criteria. We also searched the reference lists in the selected articles to look for any study that was not identified in the original search. The protocol was published in the International Prospective Register of Systematic Reviews (PROSPERO identifier: CRD42016033698).
Data extraction and study quality assessment
Two authors (CR and IML) independently abstracted data from the selected articles, recording the following information, when available:
Study characteristics (study location, period of enrollment, criteria for patient enrollment, number of patients enrolled, duration of follow-up);
Study design;
Patients’ characteristics (age, sex, mechanical ventilation, disease severity, cause of coma, and Glasgow Outcome Scale);
VAP definition (early and late onset);
Antibiotic therapy and controls;
Outcomes (incidence of VAP (early and late); ICU and hospital mortality; duration of mechanical ventilation; ICU and hospital length of stay).
Any discrepancies were resolved by discussion among authors (CRS, IML, FAB). If data were not reported, we planned to contact first or senior authors by email.
To assess the methodological quality of the studies included, we used the Cochrane Risk of Bias Tool (for RCT) [16] and the Newcastle-Ottawa Score, for observational studies.
Outcomes
The main outcomes of interest were incidence of VAP (early and late) and ICU and hospital mortality. The secondary outcomes were ICU and hospital length of stay as well as duration of mechanical ventilation.
Patient involvement
This review and meta-analysis did not involve patients directly.
Statistical analysis
We compared patients’ characteristics and outcomes between the group of patients who received antibiotic prophylaxis and those who did not (control group). Primary outcome variables were the incidence of EO-VAP and ICU mortality; secondary outcome variables were ICU length of stay and duration of mechanical ventilation.
Primary outcome variables are reported as relative risks (RR) with their corresponding 95% confidence intervals (CI), analyzed with the Mantel–Haenszel fixed-effects method. Secondary outcome variables are reported as standardized mean differences (SMD) with their respective 95% CI. To assess the impact of heterogeneity across studies on the meta-analysis, we used the I 2 statistic, which reflects the amount of heterogeneity between studies over and above the sampling variation and is robust to the number of studies and choice of effect measure. We used the R statistical package for all analyses.
Results
The literature search produced 11,340 citation titles, yielding 26 articles for detailed analysis; three studies including a total of 267 patients met the inclusion criteria and were included in the systematic review (Fig. 1).
Fig. 1.
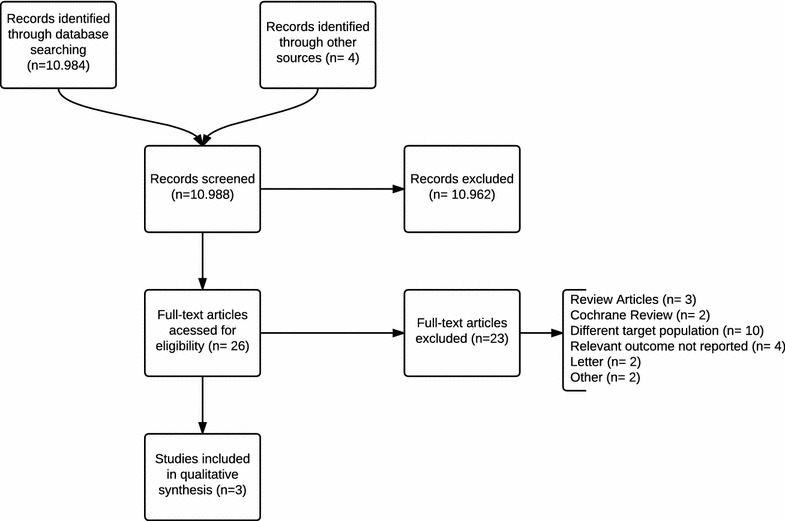
Selection of studies on antibiotic use for VAP prevention in comatose patients
Definitions
All studies defined EO-VAP as pneumonia developed within the first four days of mechanical ventilation. Clinical criteria for VAP definition varied among the studies; however, all studies required a microbiological confirmation of pneumonia—either by bronchoalveolar lavage (BAL) or by protected brush sampling or by tracheal aspirate. The definition of coma also varied—Vallés et al. [17] and Acquarolo et al. [18] defined as a Glasgow Coma Scale (GCS) ≤ 8 and Sirvent et al. [4] defined it as a GCS ≤ 12. The definitions are summarized in Table 1.
Table 1.
Studies’ characteristics
| Sirvent JM et al. | Acquarolo A et al. | Vallés J et al. | ||||
|---|---|---|---|---|---|---|
| Year published | 1997 | 2007 | 2013 | |||
| Country | Spain | Italy | Spain | |||
| Study design | RCT | RCT | Prospective study with historical control or non-randomized control | |||
| Inclusion criteria | Head injury or coma due to stroke or surgery for space occupying lesions with Glasgow ≤ 12 | Adults, comatose patients (GCS ≤ 8) in mechanical ventilation | Adults, comatose patients (GCS ≤ 8) in mechanical ventilation | |||
| Tested antibiotic | Cefuroxime | Ampicillin–sulbactam | Ceftriaxone or ertapenem or levofloxacin | |||
| Antibiotics used in control group?b | Yes | Yes | No | |||
| Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | |
| Number of subjects | 50 | 50 | 19 | 19 | 71 | 58 |
| Age (mean ± SD), years | 42 ± 20 | 37 ± 21 | 54.8 (18.0)a | 54.6 (17.7)a | 56 ± 19 | 59 ± 16 |
| Male gender, n (%) | 34 (68%) | 40 (80%) | 13 (68.4%) | 12 (63.2%) | 48 (67.6%) | 43 (74.1%) |
| Glasgow Coma Scale (mean ± SD) | 7.5 ± 2.4 | 8.0 ± 1.8 | 5 (3–7)a | 5 (4–7)a | 5 ± 2 | 5 ± 2 |
| APACHE II (mean ± SD) | 14 ± 5 | 13 ± 5 | 20 (17–24)a | 22 (18–23)a | 17 ± 7 | 18 ± 7 |
| Early VAP, n (%) | 8 (16%) | 18 (36%) | 4 (21%) | 11 (57.8%) | 2 (2.8%) | 13 (22.4%) |
| Late VAP, n (%) | 4 (8%) | 7 (14%) | 10 (episodes) | 9 (episodes) | 6.5 (incidence/1000 days MV) | 5.3 (incidence/1000 days MV) |
| Total VAP, n (%) | 12 (24%) | 25 (50%) | 14 (episodes) | 20 (episodes) | 10.8 (incidence/1000 days MV) | 28.4 (incidence/1000 days MV) |
| Duration of mechanical ventilation (mean ± SD), days | 4.6 ± 1.5 | 4.4 ± 2.1 | 9.9 (6.9)a | 10.6 (9.4)a | 6.4 ± 6.5 | 9.7 ± 9.6 |
| ICU LOS (mean ± SD), days | 13 ± 8 | 16 ± 11 | 12.8 (8.7)a | 12.6 (9.7)a | 9.7 ± 9.8 | 14.9 ± 13.9 |
| Hospital LOS (mean ± SD), days | 27 ± 16 | 28 ± 13 | Not informed | Not informed | 17.5 ± 17.7 | 23.5 ± 24.3 |
| ICU mortality, n (%) | 10 (20%) | 7 (14%) | 7 (36.8%) | 8 (42.1%) | 21 (29.6%) | 18 (31%) |
RCT randomized controlled trial, VAP ventilator-associated pneumonia, LOS length of stay, APACHE II Acute Physiology and Chronic Health Evaluation II, MV mechanical ventilation
aMedian (interquartile range)
bAntibiotics used mainly as surgical prophylaxis
Characteristics of the studies included
The meta-analysis included two randomized clinical trials and one prospective observational cohort with a non-randomized historical control group. Table 2 provides detailed information about the three studies. Study populations ranged from 38 to 129 patients. The most common cause of coma was head trauma (n = 135), followed by stroke (n = 49) and cardiac arrest (n = 37). All studies evaluated EO-VAP, defined as VAP acquired within four days after intubation for mechanical ventilation. All studies reported short-term outcomes (ICU mortality, duration of mechanical ventilation, and ICU LOS). Two studies also evaluated hospital mortality. Table 3 provides details about the Jadad Scale evaluation of the methodological quality of the studies. There was no heterogeneity among the studies in the main outcomes.
Table 2.
Summary of the quality evaluation by Jadad Scale of clinical trials of antibiotic prophylaxis in comatose patients
| Randomization | Blinding | Description of withdrawals and dropout | Score | |
|---|---|---|---|---|
| Sirvent JM | 2 | 0 | 1 | 3 |
| Acquarolo A | 2 | 2 | 1 | 5 |
| Vallés J | 0 | 0 | 1 | 1 |
Table 3.
VAP microbiology
| Early VAP | Late VAP | |
|---|---|---|
| Sirvent et al. | S. aureus—14 | Enterobacter—1 |
| H. influenzae—11 | Serratia—2 | |
| Strep pneumoniae—1 | Proteus—1 | |
| P. aeruginosa—4 | ||
| Acinetobacter sp—3 | ||
| Vallés et al. | S. aureus—3 | E cloacae—3 |
| Anaerobes—1 | S. aureus—1 | |
| Mixed flora—1 | P. aeruginosa—3 | |
| Streptococcus sp—1 | ||
| Total | S. aureus—17 | P. aeruginosa—7 |
| H. influenza—11 | E cloacae/Acinetobacter sp—3 each | |
| S. pneumoniae/Anaerobes/mixed flora—1 each | Serratia—2 | |
| Enterobacter/Proteus/S. aureus/Streptococcus sp—1 each |
Main outcomes
Figure 2 shows the association between systemic antibiotic administration and the outcomes of interest. The RR of EO-VAP was 0.32 (95% CI 0.19–0.54, p < 0.01), favoring the intervention group, which suggests a protective effect, and the RR of ICU mortality was 1.03 (95% CI 0.7–1.53, p = 0.88), showing no protective effect.
Fig. 2.
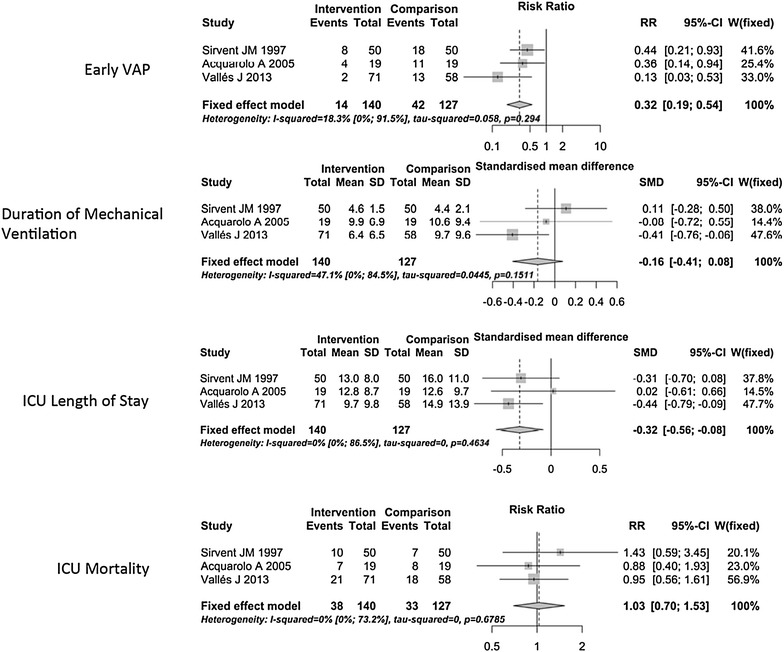
Impact of antibiotic prophylaxis on early VAP, ICU mortality, duration of mechanical ventilation, and ICU length of stay
Antibiotic administration did not affect the duration of mechanical ventilation (SMD: −0.16; 95% CI −0.41–0.08, p = 0.18), but decreased ICU LOS slightly (SMD: −0.32; 95% CI −0.56 to −0.08, p < 0.01), indicating that antibiotic prophylaxis reduced the ICU stay by 9 h on average.
Discussion
Antibiotic prophylaxis seems effective in preventing EO-VAP in a mixed cohort of comatose patients and may also decrease the ICU LOS slightly; however, it had no effect on the length of mechanical ventilation or ICU mortality.
One explanation for the decrease in the incidence of EO-VAP in comatose patients receiving antibiotic prophylaxis is the reduction of bacterial inoculum in the lungs. After brain injury, many concurrent phenomena act to increase the bacterial burden within the alveolar space: micro- or macroaspiration, brain-induced immunosuppression, or even increased capillary leakage from sympathetic overstimulation [19]. Antibiotic administration may prevent the propagation of bacteria into the lung, thereby preventing EO-VAP, whereas many traditional prophylactic measures included in the VAP bundle may be less effective in brain-injured patients.
EO-VAP is frequent in critically ill acute neurological patients. Although we found no impact of antibiotic prophylaxis on ICU mortality, preventing EO-VAP may reduce overall antibiotic use and costs, improve functional prognosis, and indirectly decrease long-term mortality. Finlayson et al. [20] showed that, in patients with ischemic stroke, pneumonia is associated with higher 30-day and 1-year mortality, as well as with a poorer functional outcome. While mortality from infection is estimated to account for up to 30% of stroke deaths and infection is an independent predictor of neurological deterioration, patients that have a decrease in EO-VAP did not show significantly different mortality [21]; this kind of cases are specially complex and might present some complications especially common in this subset of patients. In addition, while the mortality represents a very robust outcome, we consider that mortality could be analyzed cautiously just because of the impact of this intervention. Moreover, infection is the primary cause of readmission after stroke [22]. Hospital-acquired pneumonia is independently associated with poor functional outcome up to 5 years after TBI [23].
Our meta-analysis also found that antibiotic prophylaxis decreased ICU LOS slightly, possibly due to the decrease in EO-VAP. Just as treating tracheobronchitis can lead to a reduction in VAP and consequent reduction in ICU LOS [24, 25], prophylaxis against EO-VAP may have an impact in reducing ICU LOS and may also affect functional outcomes. However, none of the studies included in the meta-analysis was designed to evaluate functional outcome, so we could not assess the effect of antibiotic prophylaxis on long-term prognosis.
Whether antibiotic prophylaxis induces bacterial resistance is a well-founded concern. An association between broad-spectrum systemic antibiotics and the development of antibiotic resistance was pointed out a decade ago [26]. Depuydt et al. [27] showed VAP involving multidrug-resistant pathogens was associated with higher mortality and that the risk of developing VAP involving multiresistant bacteria was associated with previous antibiotic use. These findings have led to the development of antibiotic stewardship programs that minimize antibiotic exposure to diminish resistance to antibiotics and improve outcomes [28].
However, the use of one- or two-dose prophylactic antibiotic regimens may not be as deleterious to the patient and ICU ecology as prolonged, inadequate antibiotic administration. In a landmark randomized controlled trial, Chastre et al. [29] showed that multidrug-resistant pathogens emerged less frequently in recurrent infections developing in patients assigned to an 8-day course of antibiotic than in those developing in patients assigned to a 15-day course (42.1 vs. 62.3%). In neutropenic patients, quinolone prophylaxis was not associated with increased antimicrobial resistance [30], and in cardiac surgery patients, antibiotic resistance was associated only with antibiotic prophylaxis for more than 48 h [31]. Although our meta-analysis was not planned to analyze this issue, none of the studies included reported any increase in multidrug-resistant pathogens. This finding suggests that very short antibiotic regimens may not lead to greater resistance, but this hypothesis must be confirmed in future trials.
Antibiotic prophylaxis is a simple and cheap measure that can be easily reproduced all around the world. Other prophylactic measures have not proven effective. Corticosteroid administration was not effective in preventing nosocomial pneumonia in TBI patients, although the overall incidence of pneumonia in this study was lower than expected [32]. The effectiveness of beta-blockers [33, 34], or statins [35, 36], in preventing pneumonia in stroke patients is controversial and must be tested in future trials.
This meta-analysis has some limitations. First and most importantly, numbers of studies are low, which has consequences for the interpretation of the data and may amplify a hypothetically minor impact on pneumonia prevention. We would, however, highlight our surprise in such an easy intervention and the low number of studies conducted when compared to the literature of more complex interventions [37, 38]. Based on the low number of patients in each subgroup and the lack of individual information, we considered that more exploratory analyses such as: (1) dose of antibiotic; (2) time of initiation; and (3) type of antibiotic would increase the heterogeneity and would not allow robust conclusions. Moreover, most patients were admitted to intensive care for TBI, and this patient mix might limit the generalizability of our results. Finally, the broad-spectrum antibiotic regimens tested varied among the different studies. Despite the common goal of preventing EO-VAP, these differences in antibiotic use, dosage, and timing of administration may preclude the analysis of their impact as a single group. However, the results of this meta-analysis support the hypothesis that antibiotic prophylaxis reduces EO-VAP without increasing antimicrobial resistance.
Conclusion
This meta-analysis found that antibiotic prophylaxis in comatose patients reduced the incidence of EO-VAP and decreased ICU LOS slightly. However, a larger randomized trial focusing on measuring both the decrease in the incidence of EO-VAP and possible improvements in long-term functional outcomes is needed to confirm these findings. The 2005 ATS guidelines on hospital-acquired pneumonia concluded that although administering antibiotics at the time of intubation may prevent EO-VAP, its routine use could not be recommended until more evidence was available [39]. The current IDSA/ATS guidelines do not make any recommendation regarding antibiotic prophylaxis against EO-VAP [40]. We were surprised that a non-complex intervention has been not widely studied. We think that the results of this meta-analysis strongly support undertaking new clinical trials in the incidence of EO-VAP in other subsets of critically ill patients and possible improvements in long-term functional.
Authors’ contributions
IML had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CR and IML independently searched and assisted in collection of the data. PA assisted in analysis and interpretation of the results. JV, IML, CR, PA, FB contributed substantially to the study design, interpretation of the data, and writing and critical revision of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
Clinical Research Collaboration (CRC) by European Respiratory Society.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cássia Righy, Email: cassiarighy@gmail.com.
Pedro Emmanuel Americano do Brasil, Email: pedro.brasil@ini.fiocruz.br.
Jordi Vallés, Email: jvalles@tauli.cat.
Fernando A. Bozza, Email: bozza.fernando@gmail.com
Ignacio Martin-Loeches, Email: drmartinloeches@gmail.com.
References
- 1.Zilahi G, Artigas A, Martin-Loeches I. What’s new in multidrug-resistant pathogens in the ICU? Ann Intensive Care. 2016;6:96. doi: 10.1186/s13613-016-0199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronchard R, Albaladejo P, Brezac G, et al. Early onset pneumonia: risk factors and consequences in head trauma patients. Anesthesiology. 2004;100:234–239. doi: 10.1097/00000542-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Lepelletier D, Roquilly A, Demeure dit latte D, et al. Retrospective analysis of the risk factors and pathogens associated with early-onset ventilator-associated pneumonia in surgical-ICU head-trauma patients. J Neurosurg Anesthesiol. 2010;22:32–37. doi: 10.1097/ANA.0b013e3181bdf52f. [DOI] [PubMed] [Google Scholar]
- 4.Sirvent JM, Torres A, El-Ebiary M, et al. Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med. 1997;155:1729–1734. doi: 10.1164/ajrccm.155.5.9154884. [DOI] [PubMed] [Google Scholar]
- 5.Berrouane Y, Daudenthun I, Riegel B, et al. Early onset pneumonia in neurosurgical intensive care unit patients. J Hosp Infect. 1998;40:275–280. doi: 10.1016/S0195-6701(98)90303-6. [DOI] [PubMed] [Google Scholar]
- 6.Ruhnke AM, Paiva J, Meersseman W, et al. Online Supplementary Appendix Article title: Anidulafungin for the treatment of candidaemia/invasive candidiasis in selected critically ill patients.
- 7.Westendorp WF, Vermeij J-D, Vermeij F, et al. Antibiotic therapy for preventing infections in patients with acute stroke. Cochrane Database Syst Rev. 2012;1:CD008530. doi: 10.1002/14651858.CD008530.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Jovanovic B, Milan Z, Djuric O, et al. Twenty-eight-day mortality of blunt traumatic brain injury and co-injuries requiring mechanical ventilation. Med Princ Pract Int J Kuwait Univ Health Sci Cent. 2016;25:435–441. doi: 10.1159/000447566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giard M, Lepape A, Allaouchiche B, et al. Early- and late-onset ventilator-associated pneumonia acquired in the intensive care unit: comparison of risk factors. J Crit Care. 2008;23:27–33. doi: 10.1016/j.jcrc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Vallés J, Pobo A, García-Esquirol O, et al. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intensive Care Med. 2007;33:1363–1368. doi: 10.1007/s00134-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 11.Winklewski PJ, Radkowski M, Demkow U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J Neuroinflamm. 2014;11:213. doi: 10.1186/s12974-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croce MA, Brasel KJ, Coimbra R, et al. National Trauma Institute prospective evaluation of the ventilator bundle in trauma patients: Does it really work? J Trauma Acute Care Surg. 2013;74:354–360. doi: 10.1097/TA.0b013e31827a0c65. [DOI] [PubMed] [Google Scholar]
- 13.Seguin P, Laviolle B, Dahyot-Fizelier C, et al. Effect of oropharyngeal povidone-iodine preventive oral care on ventilator-associated pneumonia in severely brain-injured or cerebral hemorrhage patients: a multicenter, randomized controlled trial. Crit Care Med. 2014;42:1–8. doi: 10.1097/CCM.0b013e3182a2770f. [DOI] [PubMed] [Google Scholar]
- 14.Bornstain C, Azoulay E, De Lassence A, et al. Sedation, sucralfate, and antibiotic use are potential means for protection against early-onset ventilator-associated pneumonia. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;38:1401–1408. doi: 10.1086/386321. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallés J, Peredo R, Burgueño MJ, et al. Efficacy of single-dose antibiotic against early-onset pneumonia in comatose patients who are ventilated. Chest. 2013;143:1219–1225. doi: 10.1378/chest.12-1361. [DOI] [PubMed] [Google Scholar]
- 18.Acquarolo A, Urli T, Perone G, et al. Antibiotic prophylaxis of early onset pneumonia in critically ill comatose patients. A randomized study. Intensive Care Med. 2005;31:510–516. doi: 10.1007/s00134-005-2585-5. [DOI] [PubMed] [Google Scholar]
- 19.Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care. 2009;11:417–426. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 20.Finlayson O, Kapral M, Hall R, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 21.Boehme AK, Kumar AD, Dorsey AM, et al. Infections present on admission compared with hospital-acquired infections in acute ischemic stroke patients. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2013;22:e582–e589. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SV, Corado C, Bergman D, et al. Impact of poststroke medical complications on 30-day readmission rate. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2015;24:1969–1977. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 23.Kesinger MR, Kumar RG, Wagner AK, et al. Hospital-acquired pneumonia is an independent predictor of poor global outcome in severe traumatic brain injury up to 5 years after discharge. J Trauma Acute Care Surg. 2015;78:396–402. doi: 10.1097/TA.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Loeches I, Povoa P, Rodríguez A, et al. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med. 2015;3:859–868. doi: 10.1016/S2213-2600(15)00326-4. [DOI] [PubMed] [Google Scholar]
- 25.Nseir S, Martin-Loeches I, Makris D, et al. Impact of appropriate antimicrobial treatment on transition from ventilator-associated tracheobronchitis to ventilator-associated pneumonia. Crit Care Lond Engl. 2014;18:R129. doi: 10.1186/cc13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould CV, Rothenberg R, Steinberg JP. Antibiotic resistance in long-term acute care hospitals: the perfect storm. Infect Control Hosp Epidemiol Off J Soc Hosp Epidemiol Am. 2006;27:920–925. doi: 10.1086/507280. [DOI] [PubMed] [Google Scholar]
- 27.Depuydt PO, Vandijck DM, Bekaert MA, et al. Determinants and impact of multidrug antibiotic resistance in pathogens causing ventilator-associated-pneumonia. Crit Care Lond Engl. 2008;12:R142. doi: 10.1186/cc7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuts EC, Hulscher MEJL, Mouton JW, et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:847–856. doi: 10.1016/S1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 29.Chastre J, Wolff M, Fagon J-Y, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 30.Imran H, Tleyjeh IM, Arndt CAS, et al. Fluoroquinolone prophylaxis in patients with neutropenia: a meta-analysis of randomized placebo-controlled trials. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2008;27:53–63. doi: 10.1007/s10096-007-0397-y. [DOI] [PubMed] [Google Scholar]
- 31.Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916–2921. doi: 10.1161/01.CIR.101.25.2916. [DOI] [PubMed] [Google Scholar]
- 32.Asehnoune K, Seguin P, Allary J, et al. Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): a double-blind, multicentre phase 3, randomised placebo-controlled trial. Lancet Respir Med. 2014;2:706–716. doi: 10.1016/S2213-2600(14)70144-4. [DOI] [PubMed] [Google Scholar]
- 33.Maier IL, Karch A, Mikolajczyk R, et al. Effect of beta-blocker therapy on the risk of infections and death after acute stroke—a historical cohort study. PLoS ONE. 2015;10:e0116836. doi: 10.1371/journal.pone.0116836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sykora M, Siarnik P, Diedler J, Acute Collaborators VISTA. β-Blockers, pneumonia, and outcome after ischemic stroke: evidence from virtual international stroke trials archive. Stroke J Cereb Circ. 2015;46:1269–1274. doi: 10.1161/STROKEAHA.114.008260. [DOI] [PubMed] [Google Scholar]
- 35.Scheitz JF, Endres M, Heuschmann PU, et al. Reduced risk of poststroke pneumonia in thrombolyzed stroke patients with continued statin treatment. Int J Stroke Off J Int Stroke Soc. 2015;10:61–66. doi: 10.1111/j.1747-4949.2012.00864.x. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez de Antonio LA, Martínez-Sánchez P, Martínez-Martínez MM, et al. Previous statins treatment and risk of post-stroke infections. Neurol Barc Spain. 2011;26:150–156. doi: 10.1016/j.nrl.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 37.Pileggi C, Bianco A, Flotta D, et al. Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Crit Care Lond Engl. 2011;15:R155. doi: 10.1186/cc10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bo L, Li J, Tao T, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD009066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 40.Kalil AC, Metersky ML, Klompas M, et al. Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63:575–582. doi: 10.1093/cid/ciw504. [DOI] [PMC free article] [PubMed] [Google Scholar]


