Abstract
In Caenorhabditis elegans, reduction of insulin/IGF-1 like signaling and loss of germline stem cells both increase lifespan by activating the conserved transcription factor DAF-16 (FOXO). While the mechanisms that regulate DAF-16 nuclear localization in response to insulin/IGF-1 like signaling are well characterized, the molecular pathways that act in parallel to regulate DAF-16 transcriptional activity, and the pathways that couple DAF-16 activity to germline status, are not fully understood at present. Here, we report that inactivation of MBK-1, the C. elegans ortholog of the human FOXO1-kinase DYRK1A substantially shortens the prolonged lifespan of daf-2 and glp-1 mutant animals while decreasing wild-type lifespan to a lesser extent. On the other hand, lifespan-reduction by mutation of the MBK-1-related kinase HPK-1 was not preferential for long-lived mutants. Interestingly, mbk-1 loss still allowed for DAF-16 nuclear accumulation but reduced expression of certain DAF-16 target genes in germline-less, but not in daf-2 mutant animals. These findings indicate that mbk-1 and daf-16 functionally interact in the germline- but not in the daf-2 pathway. Together, our data suggest mbk-1 as a novel regulator of C. elegans longevity upon both, germline ablation and DAF-2 inhibition, and provide evidence for mbk-1 regulating DAF-16 activity in germline-deficient animals.
Keywords: FOXO, DYRK1, aging, phosphorylation, signaling
INTRODUCTION
FOXO transcription factors are evolutionarily conserved regulators of cell proliferation, differentiation, survival and metabolism and play a key role in maintaining cellular homeostasis, particularly under stress conditions [1]. On the organismal level, FOXO orthologs modulate lifespan in a broad variety of species, e.g. in the nematode Caenorhabditis elegans, the fruit fly Drosophila melanogaster and, possibly, in mice, where FOXO family members also have been implicated in age-related diseases such as cancer and type 2 diabetes [1–5]. Interestingly, several studies indicate that polymorphisms in the human FOXO3A-gene are positively associated with longevity in both genders, while one study also found a negative association of FOXO1A-variants with longevity in women [2, 6–8].
In C. elegans, the sole FOXO ortholog, DAF-16, promotes longevity in response to various inputs such as decreased activity of the insulin/IGF1-like receptor DAF-2 or increased signaling through the stress-sensing AMPK-, JNK- and SIR2-pathways [9–12]. In addition, the developmental timing micro-RNA LIN-4 and the ablation of germline stem cells can activate DAF-16 and extend lifespan [13, 14]. On the molecular level, subcellular localization, stability and transcriptional activity of FOXOs are tightly regulated by post-translational modifications (PTMs) such as phosphorylation, acetylation, ubiquitylation and methylation [15]. Most of the currently known FOXO-PTMs have been identified in one of the four mammalian FOXOs, FOXO1, -3, -4 and -6, but the affected residues and the modifying enzymes are frequently conserved across species [15]. Once activated, DAF-16 extends lifespan through inducing or suppressing the expression of many genes encoding, for example, detoxifying enzymes, antimicrobial peptides, chaperones and apolipoproteins [16]. In many contexts, other transcription factors such as HSF-1 and SKN-1/Nrf2 act in concert with DAF-16 to increase lifespan [2, 17].
Germline ablation extends lifespan not only in wild type, but also in daf-2 mutant animals, suggesting that DAF-16 activation and/or function differs between the germline- and the daf-2 longevity pathway [13]. Indeed, reduced activity of the DAF-2/PI3-kinase/AKT pathway promotes nuclear accumulation of DAF-16 in multiple tissues and at all developmental stages [18–20]. In contrast, when germline precursor cells are ablated from L1 larvae, nuclear accumulation of DAF-16 occurs predominantly in the intestine, starts only in early adulthood, and requires the adaptor protein KRI-1 and the nuclear hormone receptor DAF-12 [18, 21, 22]. Of note, nuclear accumulation of DAF-16 is not sufficient to increase C. elegans lifespan, suggesting the existence of additional pathways that directly regulate DAF-16 transcriptional activity [18, 23].
C. elegans MBK-1 (Drosophila melanogaster Minibrain-related kinase) is a member of the evolutionarily conserved DYRK-family of protein kinases and orthologous to human DYRK1A/B [24]. DYRK1A is located in the Down syndrome critical region on chromosome 21 and has been associated with the neurological defects seen in this disease [24, 25]. Through phosphorylation of substrates on serine and threonine residues, DYRK1A/B control various cellular processes, such as cell cycle progression, differentiation and survival [24, 25]. In C. elegans mbk-1 overexpression results in chemotaxis defects while genetic inactivation causes no obvious abnormalities [26]. Yet, there is evidence for MBK-1 being required for resistance to certain pathogens [27]. GFP-reporter studies indicate that mbk-1 is expressed in all somatic tissues throughout development and adulthood and localizes to the nucleus in all cells [26]. In addition to MBK-1, two other DYRK family members have been described in C. elegans, MBK-2 (DYRK2/3) and the more distant relative HPK-1 (HIPK2) [26]. Loss of hpk-1 has been shown previously to shorten lifespan of wild-type and of daf-2(-) worms [28].
Here, we report that in C. elegans, loss of mbk-1 shortens the lifespan of long-lived daf-2 and glp-1 (germline-deficient [29]) mutant animals, while affecting the lifespan of wild-type worms to a lesser extent. Moreover, we provide evidence for mbk-1 contributing to upregulation of some DAF-16 target genes in the glp-1, but not in the daf-2 mutant background. Thus, our findings identify MBK-1 as a novel regulator of lifespan that may function differently in the germline- and in the daf-2 longevity pathways.
RESULTS
Evidence for DAF-16 Ser326 phosphorylation in vivo
In order to investigate how DAF-16 activity in the intestine is regulated by phosphorylation in different longevity pathways, we used mass spectrometry to analyze immunoprecipitates of intestinally expressed GFP::DAF-16 (encoded by transgene muIs194, daf-16 isoform c, also known as isoform a1) from lysates of three different strains: (1) daf-16(mu86), muIs194 (referred to as wild-type in the context of mass spectrometry experiments), (2) daf-16(mu86); daf-2(e1370); muIs194 (referred to as daf-2 mutant), and (3) daf-16(mu86); glp-1(e2144ts); muIs199 (referred to as glp-1 mutant). We identified a phosphopeptide spanning Ser326 in a sample from wild-type worms. ClustalΩ alignments mapped this phosphopeptide to a region downstream of the DNA-binding (forkhead) domain (Figure 1A) and revealed that Ser326 corresponds to Ser329 in human FOXO1 and to Ser326 in murine FOXO1, previously described phosphorylation sites for the mammalian kinases DYRK1A and NLK, respectively [30, 31]. Additional sequence analysis indicated that the residues surrounding Ser326/Ser329 are well conserved between DAF-16 and FOXO1/3/4 and match the DYRK target motif RX1-2S/TP [32, 33] (Figure 1B). On the other hand, NLK-regulation of murine FOXO1 apparently involves concurrent phosphorylation of Ser326 and up to seven additional S/TP-sites [31], all of which are not conserved in DAF-16 (Supplementary Figure S1). Together, our observation of in vivo phosphorylation of DAF-16 at Ser326, conservation of phosphorylated motifs between DAF-16 and FOXO, and phosphorylation data on human FOXO1 [30] raised the possibility that a DYRK1A ortholog modulates DAF-16 activity in C. elegans.
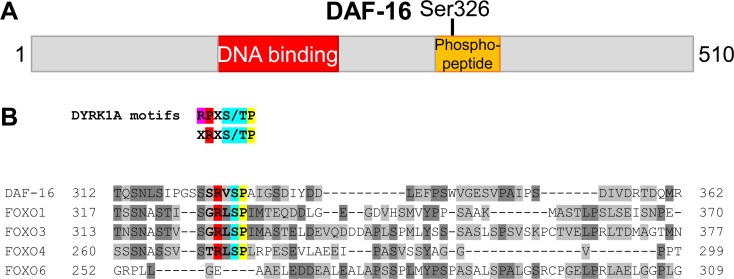
Figure 1. Evidence for phosphorylation of Ser326 in C. elegans DAF-16.
(A) Schematic drawing (to scale) of the DAF-16 protein (isoform c/a1). The location of a phosphopeptide derived from immunoprecipitated GFP::DAF-16 by tryptic digest, is shown in orange. The phosphorylation site was mapped to Ser326. (B) ClustalΩ alignment of the full length sequences of human FOXO family members and C. elegans DAF-16. Only the part spanning the Ser326-containing phosphopeptide is shown. The phosphorylated Serine in DAF-16 (Ser326), and its corresponding sites in FOXO1 (Ser329), FOXO3 (Ser325), FOXO4 (Ser273) and FOXO6 (not present) are highlighted in blue. Additional residues specifying the DYRK1A consensus motifs [32, 33, 65] are highlighted in red and yellow.
Loss of mbk-1 shortens lifespan of long-lived C. elegans mutants
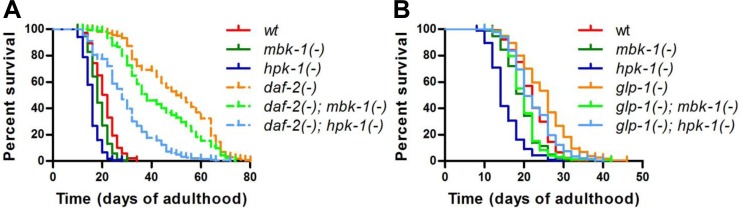
To address the question whether the DYRK1A ortholog MBK-1 plays a role in C. elegans lifespan regulation, we introduced a predicted null mutation, mbk-1(pk1389) (representing 1.8-kb deletion that spans the first intron to the sixth exon and disrupts majority of the kinase domain) [26] into the long-lived daf-2(e1370) and glp-1(e2144ts) backgrounds (hereafter referred to as mbk-1(-), daf-2(-) and glp-1(-), respectively) and compared the lifespans of mbk-1(-) worms to that of the corresponding mbk-1(+) animals (Figure 2). The lifespan effects of a predicted null mutation in another DYRK-family member, hpk-1(pk1393) (1.5-kb deletion that disrupts the respective kinase domain) [26] were examined in parallel. Mbk-1(-) animals were smaller and shorter-lived than their mbk-1(+) counterparts in all genetic backgrounds tested, although to different extents. While mbk-1 mutation decreased glp-1(-)-lifespan almost back to wild-type level, the reduction of lifespan in daf-2(-) and especially in wild-type animals was more modest (Figure 2, Table 1). On the other hand, hpk-1(-) animals appeared less healthy and were, as reported previously, substantially shorter-lived than wild-type worms [34]. Also in agreement with an earlier study [28], hpk-1 loss strongly reduced lifespan of daf-2(-) worms, as well as their speed of development and viability of progeny. Interestingly, in the glp-1(-) background, hpk-1(-) mutation appeared to cause a more moderate decrease in longevity than in the other backgrounds (Figure 2, Table 1). Taken together, our lifespan analyses suggested MBK-1 as a novel factor required for full longevity of daf-2- and glp-1-deficient C. elegans and confirm the previously described role of hpk-1 in maintaining normal lifespan [34].
Figure 2. Loss of mbk-1 decreases lifespan of long-lived daf-2 and glp-1 mutant C. elegans.
The effect of loss of function mutations in mbk-1 and hpk-1, mbk-1(pk1389) and hpk-1(pk1393), respectively, on lifespan relative to mbk-1(+) and hpk-1(+) animals was examined in different genetic backgrounds. (A) daf-2(-) [daf-2(e1370)] and corresponding daf-2(+) animals were grown continuously at 20°C. (B) glp-1(-) [glp-1(e2144ts)] and corresponding glp-1(+) animals were grown at 25°C for the first 24 h of postembryonic development to eliminate germ cells in glp-1(-) strains, and subsequently, were cultured at 20°C for the remainder of the experiment. See Table 1 for statistical analysis.
Table 1. Lifespan data Related to Figure 2.
| Experiment | Strain | Mean survival | SEM | Deaths | Total worm number | Relative to control | Relative to wt | ||
|---|---|---|---|---|---|---|---|---|---|
| % Lifespan change | p-Value | % Lifespan change | p-Value | ||||||
| # 1 | wt | 20.56 | 0.40 | 151 | 180 | N/A | N/A | N/A | N/A |
| daf-2 set, graphed in Fig 2A | mbk-1(-) | 18.40 | 0.27 | 180 | 200 | −10.51 | <0.0001 | −10.51 | <0.0001 |
| hpk-1(-) | 15.55 | 0.25 | 174 | 200 | −24.37 | <0.0001 | −24.37 | <0.0001 | |
| daf-2(-) | 49.40 | 1.20 | 173 | 200 | N/A | N/A | 140.27 | <0.0001 | |
| daf-2(-); mbk-1(-) | 42.47 | 1.06 | 207 | 210 | −14.03 | <0.0001 | 106.57 | <0.0001 | |
| daf-2(-); hpk-1(-) | 29.09 | 0.83 | 196 | 200 | −41.11 | <0.0001 | 41.49 | <0.0001 | |
| # 1 | wt | 21.50 | 0.41 | 123 | 200 | N/A | N/A | N/A | N/A |
| glp-1 set | mbk-1(-) | 19.21 | 0.28 | 200 | 200 | −10.65 | <0.0001 | −10.65 | <0.0001 |
| hpk-1(-) | 15.37 | 0.28 | 196 | 200 | −28.51 | <0.0001 | −28.51 | <0.0001 | |
| glp-1(-) | 26.39 | 0.54 | 154 | 200 | N/A | N/A | 22.74 | <0.0001 | |
| glp-1(-); mbk-1(-) | 20.37 | 0.36 | 166 | 200 | −22.81 | <0.0001 | −5.26 | 0.0008 | |
| glp-1(-); hpk-1(-) | 22.88 | 0.36 | 190 | 200 | −13.30 | <0.0001 | 6.42 | 0.3459 | |
| # 2 | wt | 16.94 | 0.22 | 136 | 200 | N/A | N/A | N/A | N/A |
| mbk-1(-) | 17.80 | 0.20 | 220 | 220 | 5.08 | 0.0888 | 5.08 | 0.0888 | |
| hpk-1(-) | 12.72 | 0.14 | 176 | 200 | −24.91 | <0.0001 | −24.91 | <0.0001 | |
| glp-1(-) | 24.14 | 0.62 | 187 | 240 | N/A | N/A | 42.50 | <0.0001 | |
| glp-1(-); mbk-1(-) | 20.81 | 0.18 | 360 | 400 | −13.79 | <0.0001 | 22.85 | <0.0001 | |
| glp-1(-); hpk-1(-) | 20.32 | 0.43 | 167 | 180 | −15.82 | <0.0001 | 19.95 | <0.0001 | |
| # 3 | wt | 20.76 | 0.35 | 199 | 220 | N/A | N/A | N/A | N/A |
| mbk-1(-) | 19.63 | 0.31 | 239 | 250 | −5.44 | 0.0028 | −5.44 | 0.0028 | |
| hpk-1(-) | 13.68 | 0.23 | 165 | 200 | −34.10 | <0.0001 | −34.10 | <0.0001 | |
| glp-1(-) | 22.23 | 0.46 | 150 | 200 | N/A | N/A | 7.08 | 0.0025 | |
| glp-1(-); mbk-1(-) | 18.39 | 0.15 | 234 | 300 | −17.27 | <0.0001 | −11.42 | 0.0028 | |
| glp-1(-); hpk-1(-) | 20.27 | 0.44 | 184 | 200 | −8.82 | 0.0013 | −2.36 | <0.0001 | |
| # 4 | wt | 19.12 | 0.42 | 170 | 200 | N/A | N/A | N/A | N/A |
| glp-1(-) | 24.72 | 0.70 | 181 | 200 | N/A | N/A | 29.29 | <0.0001 | |
| glp-1(-); mbk-1(-) | 17.83 | 0.46 | 129 | 150 | −27.87 | <0.0001 | −6.75 | 0.0192 | |
| glp-1(-); hpk-1(-) | 19.45 | 0.64 | 99 | 150 | −21.32 | <0.0001 | 1.73 | 0.6228 | |
| composite | wt | 19.83 | 0.22 | 458 | 620 | N/A | N/A | N/A | N/A |
| combined glp-1 sets from # 1/2/3, graphed in Fig 2B | mbk-1(-) | 18.89 | 0.16 | 659 | 670 | −4.74 | 0.0004 | −4.74 | <0.0001 |
| hpk-1(-) | 13.98 | 0.14 | 537 | 600 | −29.50 | <0.0001 | −29.50 | <0.0001 | |
| glp-1(-) | 24.26 | 0.33 | 491 | 640 | N/A | N/A | 22.34 | <0.0001 | |
| glp-1(-); mbk-1(-) | 19.97 | 0.13 | 760 | 900 | −17.68 | <0.0001 | 0.71 | 0.0181 | |
| glp-1(-); hpk-1(-) | 21.21 | 0.24 | 541 | 580 | −12.57 | <0.0001 | 6.96 | 0.0049 | |
The effect of the mbk-1(pk1389) and hpk-1(pk1393) loss of function mutations on lifespan relative to mbk-1(+) and hpk-1(+) animals was examined in different genetic backgrounds. % change in lifespan and p-Values from Mantel-Cox-tests were calculated relative to mbk-1(+) and hpk-1(+) control animals of the same genetic background (wt, daf-2(-) or glp-1(-)), and relative to the wild-type strain N2E. Experiment #1, daf-2 set was plotted in Figure 2A, combined data for the glp-1 sets. Experiments 1, 2 and 3 were plotted in Figure 2B. Lifespan increases observed for glp-1 relative to wild-type are consistent with experiments in the literature [51, 63, 64].
Loss of mbk-1 reduces DAF-16 target gene expression
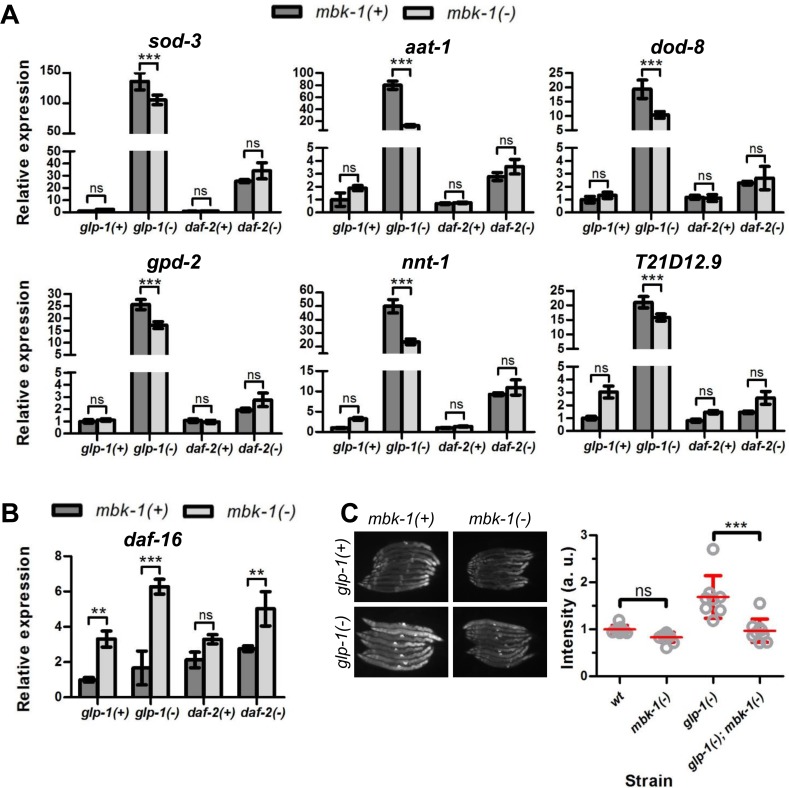
To investigate whether the reduction of glp-1(-) and daf-2(-) longevity upon mbk-1 inactivation is due to DAF-16-inhibition, we used qPCR to measure the mRNA levels of eight genes that previously have been reported to be upregulated by DAF-16 upon germline ablation and/or daf-2 mutation [16, 35, 36], in wild-type, glp-1(-) and daf-2(-) worms. Transcripts of six genes, sod-3, aat-1, dod-8, gpd-2, nnt-1 and T21D12.9, were strongly induced in germline-deficient mbk-1(+) worms but consistently lowered when mbk-1 was inactivated in these animals (Figure 3A). In contrast, F52H3.5 and K07B1.4 expression levels were not significantly affected by mbk-1 loss (data not shown). In daf-2(-) animals, expression of all genes analyzed was also elevated relative to wild-type worms, but not significantly reduced in the absence of mbk-1 (Figure 3A). Similarly, in wild-type background, mbk-1 loss also did not suppress DAF-16 target genes (Figure 3A). Of note, mRNA levels of daf-16 itself were not decreased, but rather, increased in mbk-1(-) animals in the three genetic backgrounds examined (Figure 3B).
Figure 3. Effect of C. elegans mbk-1 on DAF-16 target gene expression.
(A) Loss of mbk-1 decreases expression of a panel of DAF-16 target genes in glp-1(-) [glp-1(e2144ts)], but not in wild-type or daf-2(-) [daf-2(e1370)] animals as determined by qPCR (representative experiment shown, n=2). Error bars indicate standard deviations of three technical replicates. Statistical significance of expression level differences was determined by two-way ANOVA with Bonferroni post tests. (B) Loss of mbk-1 does not decrease daf-16 mRNA levels as determined by qPCR (representative experiment shown, n=2; error bars and statistical analysis as in panel A). (C) Loss of mbk-1 decreases Psod-3::gfp-expression in glp-1(-), and –to a lesser extent- in wild-type background (representative experiment shown, n=3). Error bars indicate standard deviations. Statistical significance of fluorescence intensity differences was determined by two-way ANOVA with Bonferroni post tests. All experiments in (A)-(C) were performed on day-2 adult worms. Images in (C) were taken at 100x magnification.
The role of mbk-1 in modulating expression of the well-characterized daf-16 regulated gene sod-3 [37, 38] was also analyzed using a Psod-3::gfp reporter-construct [36]. In agreement with the qPCR results, mbk-1(pk1389) consistently lowered Psod-3::gfp fluorescence in the glp-1(-) background and also in wild-type, although to a lesser extent and not statistically significantly (Figure 3C, Supplementary Table 3). For the lifespan-shortening hpk-1(-) allele, there seemed to be trend towards decreased Psod-3::gfp expression in glp-1(-); hpk-1(-) worms, while the opposite was observed in hpk-1(-) single mutant worms (Supplementary Figure S2A and Supplementary Table 4). When mbk-2, the third DYRK-family member in C. elegans, was depleted by RNAi (null mutations in mbk-2 cause embryonic lethality [26]), we consistently observed elevated Psod-3::gfp levels in glp-1(-) animals relative to control RNAi-treated animals, and similar trends were seen in wild-type worms (Supplementary Figure S2B, Supplementary Table 5). Of note, RNAi-depletion of mbk-2 in wild-type worms also caused the prominent mbk-2 phenotype of almost 100% dead eggs [26, 39]. Taken together, our qPCR and reporter gene analyses indicate that mbk-1 loss prevents full induction of a subset of DAF-16 target genes in glp-1(-)-animals but does not attenuate expression of the same DAF-16 targets in the wild-type or daf-2(-) background.
Loss of mbk-1 does not block DAF-16 nuclear accumulation in germline-deficient C. elegans
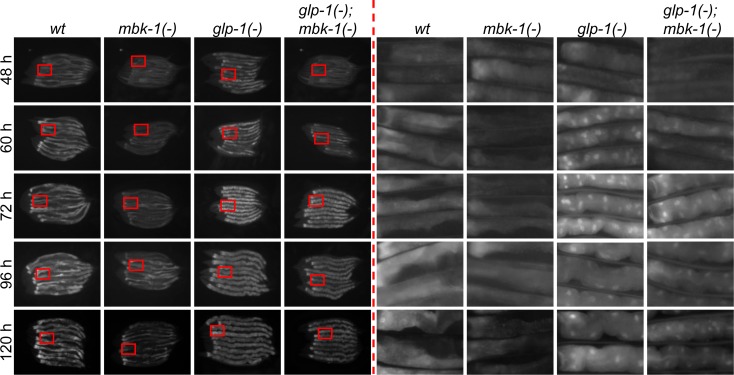
To examine whether MBK-1 affects DAF-16 target gene expression in glp-1(-) worms by altering DAF-16 subcellular localization, we analyzed nuclear accumulation of GFP::DAF-16 expressed specifically in the intestine in the presence and absence of the mbk-1(-) mutation in wild-type and glp-1(-) worms by fluorescence microscopy. In all glp-1(+) animals, the intestine-specific (ges-1 promoter-driven) GFP::DAF-16 protein was predominantly cytoplasmic at all time points analyzed (48 h - 120 h post plating of L1 larvae, i.e. from the L4 stage until day 3 of adulthood, Figure 4). In agreement with a previous report [18], nuclear accumulation of GFP::DAF-16 in glp-1(-) single-mutant animals began in early adulthood and was essentially complete 60 h after plating of L1 larvae. On the other hand, in glp-1(-); mbk-1(-) double-mutant animals, nuclear accumulation of GFP::DAF-16 was slightly delayed and completed only 72 h after plating. However, this delay in GFP::DAF-16 nuclear accumulation appeared to parallel the general slight delay in postembryonic development that is conferred by mbk-1 loss (data not shown). Since blocking phosphorylation of the DAF-16 ortholog FOXO1 at the site regulated by the MBK-1 ortholog DYRK1A in human cells has been reported to further increase FOXO1 nuclear accumulation under conditions of low IGF-1 signaling [30], we also examined GFP::DAF-16 localization in daf-2(-) animals and found that it was also not altered by the mbk-1 mutation (Supplementary Figure S3). Therefore, we conclude that in the conditions tested, MBK-1 does not regulate DAF-16 subcellular localization and instead, may control its transcriptional activity through other mechanisms.
Figure 4. Loss of mbk-1 does not affect DAF-16 subcellular localization in germline-deficient C. elegans.
The effect of the mbk-1 loss of function mutation mbk-1(pk1389) on subcellular localization of an intestine-specific GFP::DAF-16 protein (encoded by transgene muIs145[Pges-1::gfp::daf-16]) was determined at the times indicated in wild-type and germline-deficient glp-1(-) [glp-1(e2144ts)] animals. Images on the left were taken at 128x (48 h), 100x (60-96h) or 80x (120 h) magnification, images on the right are 6.5x magnifications of the areas boxed in red.
DISCUSSION
In this study, we report the first evidence for the DYRK1A ortholog MBK-1 contributing to lifespan extension in response to germline ablation and decreased insulin-like signaling in C. elegans. Moreover, our data indicate that MBK-1 exerts at least parts of its lifespan-modulatory function in germline-less/glp-1(-) worms by maximizing the activity of the FOXO-transcription factor DAF-16. On the other hand, in insulin receptor/daf-2(-) animals, mbk-1 inactivation did not reduce the expression of a subset of DAF-16 target genes. Thus, an MBK-1/DAF-16 signaling axis may act specifically in the context of germline deficiency to promote longevity, while contribution of MBK-1 to daf-2(-) longevity may be mediated by other factors.
Our study was initiated by the observation that DAF-16 Ser326 is phosphorylated in wild-type worms. Intriguingly, the DAF-16 ortholog FOXO1 has been reported to be inhibited by phosphorylation at the corresponding site, Ser329 in unstimulated and IGF-1 stimulated cultured cells [30]. Moreover, FOXO1-Ser329 has been identified as a major in vitro phosphorylation site of the mammalian kinase DYRK1A [30]. Thus, its C. elegans ortholog MBK-1 appeared to be a good candidate negative regulator of daf-16 dependent longevity pathways. However, our results in wild-type and daf-2(-) worms, which parallel IGF-1 treated and untreated cells examined previously [30], indicated that MBK-1 does not influence DAF-16 transcriptional activity and subcellular localization under these conditions. We note that our analysis focused on DAF-16 target genes reported previously to be induced in response to lifespan-extending genetic mutations [16, 35, 36]. Moreover, the daf-16 locus, through the use of different promoters and transcriptional start sites and through alternative splicing, gives rise to several isoforms with partially different expression patterns and target gene profiles [40, 41]. Longevity of daf-2(-) and also of glp-1(-) worms (cf. below) appears to be predominantly mediated by isoform a (referred to as isoform c in this study), while contributions from DAF-16f are controversial [40, 41]. Thus, our data cannot rule out the possibility that MBK-1 in wild-type and daf-2(-) worms regulates DAF-16 isoforms and target genes that were not examined by us. Yet, our C. elegans results in combination with currently available mammalian cell data, are also consistent with the notion that DYRK1A-regulation of FOXO transcription factors is not conserved across species and/or may even be specific to FOXO1. Indeed, potential DYRK1A-phosphorylation of FOXO3 and FOXO4, which share the DYRK1A-site but not all of their organismal functions with FOXO1 [1], has not been investigated yet.
On the other hand, our observation that mbk-1 loss reduces longevity and DAF-16 target gene expression in glp-1 deficient C. elegans is consistent with the model that MBK-1 is a positive regulator of DAF-16 activity and lifespan extension. Moreover, conservation of the DYRK1A-site between FOXO1 and DAF-16 supports the hypothesis that DAF-16 is a substrate of MBK-1. However, such a model in C. elegans substantially differs from the model suggested by previous work in mammalian cells [30], which implies that DYRK1A is an inhibitor of FOXO1. This discrepancy raises the possibility that a potential MBK-1/DAF-16 signaling axis in C. elegans does not parallel the apparent DYRK1A/FOXO1 kinase-substrate relationship in mammalian cells in all details. Interestingly, recent reports already suggested that regulatory pathways can differ between C. elegans and mammals although they engage orthologous factors. For example, the deubiquitylase MATH-33 recently has been reported to stabilize/activate DAF-16 by antagonizing polyubiquitylation, while its mammalian counterpart USP7/HAUSP inhibits FOXO1 and FOXO4 by decreasing their nuclear localization and transcriptional activity, respectively, by removing monoubiquitin moieties [42–44]. Moreover, MBK-1 itself may function differently from its human orthologs DYRK1A and DYRK1B, at least in certain contexts. Specifically, MBK-1 promotes transcriptional activity of HIF-1, C. elegans’ only hypoxia-inducible factor α subunit [45], independently of the HIF-1 destabilizing E3-ligase VHL-1, thereby contributing to Pseudomonas aeruginosa resistance [27]. In contrast, in glioma stem cells, one of the human HIF−1 homologs, HIF-2α/EPAS1, is inhibited by DYRK1A/B in a VHL-dependent manner [46]. It will be interesting to examine the role of DAF-16 Ser326 phosphorylation and of other Ser326 candidate kinases, such as the MBK-1 relative MBK-2 [26] and the NLK-ortholog LIT-1 [47], on C. elegans lifespan and on global DAF-16 target gene expression. Such studies will, together with biochemical studies on DAF-16 and putative Ser326 kinases, further clarify the mechanistic links between longevity, DAF-16 Ser326-phosphorylation, and MBK-1 in C.elegans.
Although mbk-1 loss in our study only partially suppressed DAF-16 target gene expression in glp-1(-) worms, it completely prevented lifespan extension in these animals. Accordingly, MBK-1 may regulate other germline-longevity promoting factors in addition to DAF-16, for example SKN-1, PHA-4, DAF-12 or NHR-80 [17]. In contrast, in daf-2(-) animals, mbk-1 loss shortened lifespan without significantly attenuating the induction of the DAF-16 target genes analyzed. Therefore, as discussed above, mbk-1 may contribute to daf-2(-) longevity by engaging factors other than DAF-16, for example SKN-1 or HSF-1 [2]. Interestingly, similar to MBK-1 in this study, the transcription elongation factor TCER-1 and the adaptor protein KRI-1, have been reported previously to modulate DAF-16 activity only in glp-1(-), but not in daf-2(-) animals [21, 36]. The concept that daf-2 and glp-1 mutations influence DAF-16 activity through different signaling mediators is further supported by a recent study that provided evidence for both, daf-2(-) and glp-1(-) longevity being primarily dependent on the same DAF-16 isoform, DAF-16a [40].
Mbk-1 has been implicated in several longevity-relevant processes, including pathogen resistance, H2S resistance and HIF-1 activation [27]. For daf-16 a role in antibacterial immunity has also been described, which involves protection against strains that kill C. elegans slowly by gut colonization [48, 49]. Mbk-1, on the other hand, counteracts fast-killing of worms by the HCN-producing Pseudomonas aeruginosa strain PAO1 [27]. Whether daf-16 contributes to the mbk-1 mediated defense mechanism or vice versa, has not been examined. Since mbk-1 mediated resistance against the PAO1 strain likely reflects a function of MBK-1 in increasing HCN-tolerance, MBK-1 may also protect C. elegans from other toxic compounds with similar modes of action to HCN, such as H2S [27, 50]. Interestingly, elevated levels of endogenous H2S have been observed in germline-deficient worms and have been reported to be required for their longevity [51, 52]. Thus, it is tempting to speculate that MBK-1 enables germline-deficient worms to tolerate higher endogenous H2S levels. However, the described mechanism for MBK-1 mediated resistance against HCN, and by extension H2S, further involves the transcription factor HIF-1 [27, 53], which is not required for longevity of both, glp-1 and daf-2 mutant C. elegans [54, 55]. It will be interesting to examine the role of MBK-1 in protection from H2S in the future.
In summary, the data reported here establish an unanticipated positive role for the conserved protein kinase MBK-1 in the longevity of daf-2 and germline-deficient C. elegans and point to regulatory connections between MBK-1 and DAF-16 that are different form the DYRK1A-FOXO1 axis in mammalian cells.
MATERIALS AND METHODS
C. elegans strains and culture
Strains used in this study are listed in Supplementary Table 1. Worms were cultured on NG agar plates seeded with E. coli OP50 according to standard protocols. To eliminate germ cells in worms carrying the glp-1(e2144ts) allele, these animals and corresponding glp-1(+) control animals were incubated at 25°C for the first 24 h of postembryonic development at then shifted to 20°C. daf-2(e1370) worms and corresponding daf-2(+) control worms were continuously cultured at 20°C.
Bioinformatics analysis
Protein sequence alignments of human FOXO1/3/4/6 (UniProt accession numbers Q12778, O43524, P98177, A8MYZ6, last retrieval on 05/01/2016) and DAF-16 isoform c/a1 (O16850-3) were performed using the ClustalΩ program at www.uniprot.org. All daf-16 transgenes used in this study and numbering in DAF-16 sequences correspond to isoform c/a1.
GFP::DAF-16 immunoprecipitation
For mass spectrometry experiments, worms expressing GFP or GFP-tagged DAF-16 in the intestine (zcIs18[Pges-1::gfp(cyt) or muIs194[Pges-1::ha::gfp::daf-16 + Podr-1::rfp]) were synchronized by hypochlorite treatment and grown at a density of 4,000 worms/10 cm plate until day 1 of adulthood. Approx. 200,000 worms were grown in three batches, harvested, flash frozen and combined upon lysis by bead-beating (BioSpec Products, Bartlesville, OK, USA) with 0.7 mm Zirconia beads in 2 pellet volumes of lysis buffer (modified from [66]: 50 mM HEPES pH 7.4, 100 mM NaCl, 1 mM EGTA, 10% glycerol) containing 2x protease and phosphatase inhibitors (2 mM PMSF, complete and PhosSTOPTM tablets, Roche Diagnostics, Rotkreuz, Switzerland). Then, detergents were added to final concentrations of 1% Triton X-100, 1% Sodium Deoxycholate and 0.1% SDS and lysates were incubated under rotation at 4°C for 15 min. Lysates were cleared by 4 rounds of centrifugation at 14,000 rpm, 4°C, 15 min and incubation with unconjugated agarose beads. GFP/GFP::DAF-16 was immunoprecipitated from 30 mg of total protein lysate (20 mg/ml) using an anti-GFP nanobody coupled to agarose beads (GFPtrap, ChromoTek, Planegg-Martinsried, Germany). Beads were washed four times with lysis buffer with detergents and 1x inhibitors, once with high salt buffer (10 mM Tris, pH 7.4, 500 mM NaCl) and once with low salt buffer (10 mM Tris, pH 7.4, 100 mM NaCl). For mass spectrometry analyses, beads were eluted with 2% SDS, 50 mM Tris, pH 6.8, 5% v/v beta-Mercaptoethanol.
Protein digestion
Eluates were diluted to 8 M urea - 100 mM Tris(hydroxyethylamine) pH 8.4 for denaturation and reduction of proteins with 5 mM Tris(2-carboxyethyl) phosphine for 30 min. Cysteine residues were acetylated with 10 mM iodoacetamide for 15 min in the dark. The sample was diluted to 2 M urea with 100 mM Tris(hydroxyethylamine) pH 8.5. Trypsin (0.5 µg) and CaCl2 (1 mM) were added for a 4 hour digestion at 37°C. The peptide sample was acidified to 5% formic acid and spun at 18,000 x g and loaded directly onto a MudPIT column.
MudPIT analysis
Capillary columns were prepared in-house for LC-MS/MS analysis from particle slurries in methanol. An analytical RPLC column was generated by pulling a 100 µm ID/360 µm OD capillary (Polymicro Technologies, Inc, Phoenix, AZ) to a 5 µm ID tip. Reverse phase particles (Jupiter C18, 4 µm dia., 90 Å pores, Phenomenex, Torrance, CA) were packed directly into the pulled column at 800 psi until 15 cm long. The column was further packed, washed, and equilibrated at 100 bar with buffer B followed by buffer A. MudPIT and analytical columns were assembled using a zero-dead volume union (Upchurch Scientific, Oak Harbor, WA). LC-MS/MS analysis was performed using an Agilent 1200 HPLC pump and Thermo LTQ-Orbitrap XL using an in-house built electrospray stage. Electrospray was performed directly from the analytical column by applying the ESI voltage at a tee (150 µm ID, Upchurch Scientific) directly downstream of a 1:1000 split flow used to reduce the flow rate to 250 nL/min through the columns. 3-step MudPIT [56] was performed where each step corresponds to 0, 25, and 100% buffer C being run for 5 min at the beginning of a 2 hr gradient. The repetitive 2 hr gradients were from 100% buffer A to 60% buffer B over 70 min, up to 100% B over 20 min, held at 100% B for 10 min, then back to 100% A for a 10 min column re-equilibration. Buffer A was 5% acetonitrile 0.1% formic acid, B was 80% acetonitrile 0.1% formic acid, and C was 500 mM ammonium acetate. Electrospray directly from the LC column was done at 2.5 kV with an inlet capillary temperature of 250°C. Precursor scanning in the Orbitrap XL was performed from 400 - 2000 m/z with the following settings: 5 × 105 target ions, 50 ms maximum ion injection time, and 1 microscan. Data-dependent acquisition of MS/MS spectra with the LTQ on the Orbitrap XL were performed with the following settings: MS/MS on the 8 most intense ions per precursor scan, 30K automatic gain control target ions, 100 ms maximum injection time, and 1 microscan. Dynamic exclusion settings used were as follows: repeat count: 1; repeat duration: 30 sec; exclusion list size: 500; and exclusion duration: 60 sec. Protein and phosphopeptide identification and phosphorylation analysis were performed using Integrated Proteomics Pipeline (IP2, www.integratedproteomics.com/). Tandem mass spectra were extracted to MS2 files from raw files using RawExtract 1.9.9 [57] and searched against a non-redundant UniProt human database with reversed sequences using ProLuCID [58]. The search space included all fully- and half-tryptic peptide candidates. Carbamidomethylation (+57.02146) of cysteine was considered as a static modification; phosphorylation (+79.9663) on serine, threonine, and tyrosine were considered as variable modifications. Peptide candidates were filtered to 0.1% FDR using DTASelect [59].
Lifespan analysis
To obtain synchronized populations, gravid adults were treated with hypochlorite and eggs were allowed to hatch in M9 buffer overnight. L1 larvae were plated on NG agar plates seeded with E. coli strain OP50. At the late L4 stage, and every 10 days thereafter, worms were transferred to fresh OP50-seeded NG agar plates containing 20 µM 5-fluoro-2′-deoxyuridine (FUDR) to prevent development of progeny and desiccation, respectively. Animals were maintained at a density of 40 worms/6 cm plate and scored for survival every other day starting on day 8 of adulthood. Worms were considered dead if they did not respond to gentle touching with a worm pick. Animals that showed a protruding vulva, or had ruptured, died from internal progeny hatching (bagging) or escaped from the plate, were censored. Kaplan-Meier survival analysis was performed using, GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
RNAi experiments
All RNAi clones were from the Ahringer library (Source BioScience, Nottingham, UK) and verified by sequencing. The empty vector L4440 served as control. Experiments were performed as described previously [51]. RNAi treatment was initiated in the L1 stage unless otherwise noted.
Fluorescence imaging
Worms expressing muIs84[Psod-3::gfp] [38] or muIs145[Pges-1::gfp::daf-16+Podr-1::rfp] (integrated version of muEx268 [38]) were synchronized by timed egg laying for 2 h and analyzed on day 2 of adulthood (unless otherwise noted) using a fluorescence microscope equipped with a standard GFP bandpass filter (MF16, Leica Microsystems, Wetzlar, Germany). GFP signal intensity in muIs84-expressing animals was quantified with Cellprofiler (http://cellprofiler.org) [60].
qPCR
RNA was extracted from 200 synchronized day 2 adults using TRIzol Reagent (Life Technologies/Thermo Fisher Scientific, Waltham, MA, USA), and 0.5-2 µg total RNA were reverse-transcribed using the Protoscript First Strand Synthesis kit (New England Biolabs, Ipswich, MA, USA). qPCR was performed on an AbiPrism 7300 instrument (Applied Biosystems®/Thermo Fisher Scientific, Waltham, MA, USA) with SYBR® Green (Power SYBR® Green Master Mix, Applied Biosystems®/Thermo Fisher Scientific, Waltham, MA, USA). Data were analyzed by the ΔΔCt method and target gene expression levels were normalized to the geometric mean of cdc-42, tba-1 and Y45F10D.4 [61, 62]. Primers for qPCR analysis of DAF-16 target genes have been published previously [36].
SUPPLEMENTARY MATERIAL
Acknowledgments
We thank Cynthia Kenyon, in whose former lab at the University of California, San Francisco, this work was initiated, for advice and support, and all members of the Kenyon lab, especially Richard Parenteau, Vikram Narayan and Yuehua Wei, for helpful discussion. We also thank Rigo Roman-Albarran and Werner Kapferer for excellent technical assistance and Elisabeth Mack, Philipps-University Marburg, for critically reading the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
H.I.D.M. planned and performed all experiments, analyzed data and wrote the manuscript. P.Z. constructed Pges-1::gfp::daf-16 strains. B.R.F. and J.R.Y. performed mass spectrometry analyses. All authors commented on the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest relating to this manuscript.
FUNDING
This study was funded by a postdoctoral fellowship from the German Academic Exchange Service to H.I.D.M, NIH Grant R01 AG032435 to Cynthia Kenyon, and start-up funds from the University of Innsbruck to H.I.D.M. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs Grant P40 OD010440.
REFERENCES
- 1.Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 2.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura T. The role of FOXO1 in β-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9:615–23. doi: 10.1038/nrendo.2013.157. [DOI] [PubMed] [Google Scholar]
- 4.Kloet DE, Burgering BM. The PKB/FOXO switch in aging and cancer. Biochim Biophys Acta. 2011;1813:1926–37. doi: 10.1016/j.bbamcr.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Shimokawa I, Komatsu T, Hayashi N, Kim SE, Kawata T, Park S, Hayashi H, Yamaza H, Chiba T, Mori R. The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell. 2015;14:707–09. doi: 10.1111/acel.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris BJ, Willcox DC, Donlon TA, Willcox BJ. FOXO3: A Major Gene for Human Longevity--A Mini-Review. Gerontology. 2015;61:515–25. doi: 10.1159/000375235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins R, Lithgow GJ, Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15:196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Wang WJ, Cao H, Lu J, Wu C, Hu FY, Guo J, Zhao L, Yang F, Zhang YX, Li W, Zheng GY, Cui H, et al. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet. 2009;18:4897–904. doi: 10.1093/hmg/ddp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidler T, Hartwig K, Daniel H, Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2010;11:183–95. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- 10.Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–77. doi: 10.1016/j.cell.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–27. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–64. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 13.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–66. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 14.Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–57. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- 15.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–88. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 16.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–83. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 17.Antebi A. Regulation of longevity by the reproductive system. Exp Gerontol. 2013;48:596–602. doi: 10.1016/j.exger.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–45. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 19.Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–57. doi: 10.1016/S0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- 20.Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–80. doi: 10.1016/S0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 21.Berman JR, Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124:1055–68. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y, Wollam J, Magner D, Karalay O, Antebi A. A steroid receptor-microRNA switch regulates life span in response to signals from the gonad. Science. 2012;338:1472–76. doi: 10.1126/science.1228967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams TW, Dumas KJ, Hu PJ. EAK proteins: novel conserved regulators of C. elegans lifespan. Aging (Albany NY) 2010;2:742–47. doi: 10.18632/aging.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aranda S, Laguna A, de la Luna S. DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2011;25:449–62. doi: 10.1096/fj.10-165837. [DOI] [PubMed] [Google Scholar]
- 25.Abbassi R, Johns TG, Kassiou M, Munoz L. DYRK1A in neurodegeneration and cancer: molecular basis and clinical implications. Pharmacol Ther. 2015;151:87–98. doi: 10.1016/j.pharmthera.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Raich WB, Moorman C, Lacefield CO, Lehrer J, Bartsch D, Plasterk RH, Kandel ER, Hobert O. Characterization of Caenorhabditis elegans homologs of the Down syndrome candidate gene DYRK1A. Genetics. 2003;163:571–80. doi: 10.1093/genetics/163.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao Z, Zhang Y, Ye Q, Saldanha JN, Powell-Coffman JA. C. elegans SWAN-1 Binds to EGL-9 and regulates HIF-1-mediated resistance to the bacterial pathogen Pseudomonas aeruginosa PAO1. PLoS Pathog. 2010;6:e1001075. doi: 10.1371/journal.ppat.1001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuelson AV, Carr CE, Ruvkun G. Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Dev. 2007;21:2976–94. doi: 10.1101/gad.1588907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–05. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 30.Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, Unterman TG, Cohen P. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J. 2001;355:597–607. doi: 10.1042/bj3550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Kim Y, Lee J, Chung J. Regulation of FOXO1 by TAK1-Nemo-like kinase pathway. J Biol Chem. 2010;285:8122–29. doi: 10.1074/jbc.M110.101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell LE, Proud CG. Differing substrate specificities of members of the DYRK family of arginine-directed protein kinases. FEBS Lett. 2002;510:31–36. doi: 10.1016/S0014-5793(01)03221-5. [DOI] [PubMed] [Google Scholar]
- 33.Soppa U, Becker W. DYRK protein kinases. Curr Biol. 2015;25:R488–89. doi: 10.1016/j.cub.2015.02.067. [DOI] [PubMed] [Google Scholar]
- 34.Berber S, Wood M, Llamosas E, Thaivalappil P, Lee K, Liao BM, Chew YL, Rhodes A, Yucel D, Crossley M, Nicholas HR. Homeodomain-Interacting Protein Kinase (HPK-1) regulates stress responses and ageing in C. elegans. Sci Rep. 2016;6:19582. doi: 10.1038/srep19582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P, Judy M, Lee SJ, Kenyon C. Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab. 2013;17:85–100. doi: 10.1016/j.cmet.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghazi A, Henis-Korenblit S, Kenyon C. A transcription elongation factor that links signals from the reproductive system to lifespan extension in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000639. doi: 10.1371/journal.pgen.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–93. [PubMed] [Google Scholar]
- 38.Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/S0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 39.Pellettieri J, Reinke V, Kim SK, Seydoux G. Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans. Dev Cell. 2003;5:451–62. doi: 10.1016/S1534-5807(03)00231-4. [DOI] [PubMed] [Google Scholar]
- 40.Chen AT, Guo C, Itani OA, Budaitis BG, Williams TW, Hopkins CE, McEachin RC, Pande M, Grant AR, Yoshina S, Mitani S, Hu PJ. Longevity genes revealed by integrative analysis of isoform-specific daf-16/FoxO mutants of caenorhabditis elegans. Genetics. 2015;201:613–29. doi: 10.1534/genetics.115.177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon ES, Narasimhan SD, Yen K, Tissenbaum HA. A new DAF-16 isoform regulates longevity. Nature. 2010;466:498–502. doi: 10.1038/nature09184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heimbucher T, Liu Z, Bossard C, McCloskey R, Carrano AC, Riedel CG, Tanasa B, Klammt C, Fonslow BR, Riera CE, Lillemeier BF, Kemphues K, Yates JR, 3rd, et al. The Deubiquitylase MATH-33 Controls DAF-16 Stability and Function in Metabolism and Longevity. Cell Metab. 2015;22:151–63. doi: 10.1016/j.cmet.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall JA, Tabata M, Rodgers JT, Puigserver P. USP7 attenuates hepatic gluconeogenesis through modulation of FoxO1 gene promoter occupancy. Mol Endocrinol. 2014;28:912–24. doi: 10.1210/me.2013-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burgering BM. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 2006;8:1064–73. doi: 10.1038/ncb1469. [DOI] [PubMed] [Google Scholar]
- 45.Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci USA. 2001;98:7916–21. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SB, Frattini V, Bansal M, Castano AM, Sherman D, Hutchinson K, Bruce JN, Califano A, Liu G, Cardozo T, Iavarone A, Lasorella A. An ID2-dependent mechanism for VHL inactivation in cancer. Nature. 2016;529:172–77. doi: 10.1038/nature16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell. 1999;97:717–26. doi: 10.1016/S0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- 48.Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, Ruvkun G, Ausubel FM. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 49.Miyata S, Begun J, Troemel ER, Ausubel FM. DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics. 2008;178:903–18. doi: 10.1534/genetics.107.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budde MW, Roth MB. The response of Caenorhabditis elegans to hydrogen sulfide and hydrogen cyanide. Genetics. 2011;189:521–32. doi: 10.1534/genetics.111.129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei Y, Kenyon C. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2016;113:E2832–41. doi: 10.1073/pnas.1524727113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Treviño-Villarreal JH, Mejia P, Ozaki CK, Wang R, Gladyshev VN, Madeo F, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–44. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budde MW, Roth MB. Hydrogen Sulfide Increases HIF-1 Activity Independent of VHL-1 in C. elegans. Mol Biol Cell. 2009;21:212–17. doi: 10.1091/mbc.E09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–36. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–98. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolters DA, Washburn MP, Yates JR., 3rd An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem. 2001;73:5683–90. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- 57.McDonald WH, Tabb DL, Sadygov RG, MacCoss MJ, Venable J, Graumann J, Johnson JR, Cociorva D, Yates JR., 3rd MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun Mass Spectrom. 2004;18:2162–68. doi: 10.1002/rcm.1603. [DOI] [PubMed] [Google Scholar]
- 58.Xu T, Park SK, Venable JD, Wohlschlegel JA, Diedrich JK, Cociorva D, Lu B, Liao L, Hewel J, Han X, Wong CC, Fonslow B, Delahunty C, et al. ProLuCID: an improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J Proteomics. 2015;129:16–24. doi: 10.1016/j.jprot.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cociorva DL, Tabb D, Yates JR. Validation of tandem mass spectrometry database search results using DTASelect. Baxevanis Andreas D, et al., editors. Curr Protoc Bioinforma. 2007 doi: 10.1002/0471250953.bi1304s16. Chapter 13: Unit 13.4. [DOI] [PubMed] [Google Scholar]
- 60.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Chen D, Smith MA, Zhang B, Pan X. Selection of reliable reference genes in Caenorhabditis elegans for analysis of nanotoxicity. PLoS One. 2012;7:e31849. doi: 10.1371/journal.pone.0031849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoogewijs D, Houthoofd K, Matthijssens F, Vandesompele J, Vanfleteren JR. Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC Mol Biol. 2008;9:9. doi: 10.1186/1471-2199-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vilchez D, Morantte I, Liu Z, Douglas PM, Merkwirth C, Rodrigues AP, Manning G, Dillin A. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489:263–68. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]
- 64.Steinbaugh MJ, Narasimhan SD, Robida-Stubbs S, Moronetti Mazzeo LE, Dreyfuss JM, Hourihan JM, Raghavan P, Operaña TN, Esmaillie R, Blackwell TK. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife. 2015;4:e07836. doi: 10.7554/eLife.07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Himpel S, Tegge W, Frank R, Leder S, Joost HG, Becker W. Specificity determinants of substrate recognition by the protein kinase DYRK1A. J Biol Chem. 2000;275:2431–38. doi: 10.1074/jbc.275.4.2431. [DOI] [PubMed] [Google Scholar]
- 66.Riedel CG, Dowen RH, Lourenco GF, Kirienko NV, Heimbucher T, West JA, et al. DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat Cell Biol. 2013;15:491–501. doi: 10.1038/ncb2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.