Abstract
The in vivo efficacy of vancomycin and teicoplanin against five Staphylococcus aureus strains with different susceptibilities to them and methicillin was studied. Rabbits were allocated at random to groups for endocarditis induction with one of these five strains and then treated for 2 days with vancomycin or teicoplanin. Each treated group was compared with a control group infected with the same strain. Vancomycin and teicoplanin showed similar activities. Low MICs did not predict better in vivo results.
Glycopeptides constitute the drugs of reference for treating infections caused by methicillin-resistant Staphylococcus aureus (MRSA) strains, particularly severe septicemia or endocarditis (18). Two factors limit the clinical usefulness of glycopeptides. First, S. aureus strains with reduced susceptibility to glycopeptides have emerged. The first vancomycin-resistant strain was identified in Japan (11), and since then vancomycin resistance has been documented in Europe (3, 6, 20). Second, treatment failures despite in vitro susceptibility have been reported, most notably for patients with endocarditis and other severe infections (7, 19). Furthermore, new agents have been introduced recently for the treatment of staphylococcal infections. These facts warrant a reappraisal of the role for glycopeptides in the first-line treatment of severe S. aureus infections.
The objective of the study reported here was to evaluate the in vivo efficacy of glycopeptides in animals with severe staphylococcal infections due to strains with various patterns of susceptibility to methicillin and glycopeptides.
We studied five S. aureus strains, of which two strains were susceptible to both methicillin and glycopeptides (methicillin-susceptible S. aureus [MSSA] strains MSSA 1 and MSSA 2), two strains were resistant to methicillin but susceptible to glycopeptides (MRSA 3 and MRSA 4), and one strain was resistant to methicillin and exhibited heterogeneous reduced susceptibility to glycopeptides (glycopeptide-intermediate S. aureus [GISA] strain GISA 5). The four glycopeptide-susceptible strains were isolated from blood cultures, and the GISA strain was isolated from sputum of a cystic fibrosis patient. The mecA gene was detected by PCR in strains MRSA 3, MRSA 4, and GISA 5. MICs of vancomycin (Dakota pharm, Le Plessis-Robinson, France) and teicoplanin (Aventis, Paris, France) were determined by using the broth and agar dilution methods, with inoculum sizes ranging from 106 to 109 CFU/ml to look for a potential inoculum effect. Bactericidal activity was assessed based on the determination of minimal bactericidal concentrations (MBCs) by microdilution method and on the killing kinetics with an inoculum of 107 CFU/ml and 0, 1, 4, 8, and 20 mg of vancomycin or teicoplanin/liter; bacteria were counted after 0, 6, 24, and 48 h.
The rabbit aortic valve endocarditis model was used for the in vivo studies (16). Endocarditis of the aortic valve and left ventricle was induced by introduction of a polyethylene catheter followed 24 h later by an intravenous injection of 108 CFU of S. aureus. For each S. aureus strain, there were three groups, namely, vancomycin, teicoplanin, and control, for a total of 15 groups of animals. On day 3, the controls were killed, and the animals in the two other groups were started on a 48-h course of vancomycin or teicoplanin. Vancomycin was given as a continuous infusion in a dose of 100 mg/kg of body weight/day so that the steady-state serum level was equivalent to the usual target in humans (at least 20 mg/liter). Teicoplanin was given as an intravenous infusion in a dose of 18 mg/kg/day in order to produce steady-state serum levels similar to that of vancomycin. Because teicoplanin has a long half-life, a bolus of 3 mg/kg was given before the continuous infusion. Serum assays of vancomycin and teicoplanin were performed during treatment with an immunoenzymetric method. The treated animals were killed on day 5.
The aortic vegetations were harvested, weighed, and used for quantitative cultures on agar for 24 h at 37°C. Bacterial counts were expressed as log10 CFU per gram of vegetation.
Statistics
The primary evaluation criterion was the bacterial count in vegetation cultures (log10 CFU per gram of vegetation). The mean ± standard deviation count was determined for each group of animals. Analysis of variance (ANOVA) was used first to evaluate counts for each S. aureus strain. When ANOVA showed a significant difference, Scheffe's test was used for pairwise comparisons. All statistical tests were run on Statview (Abacus Concepts, Berkeley, Calif.).
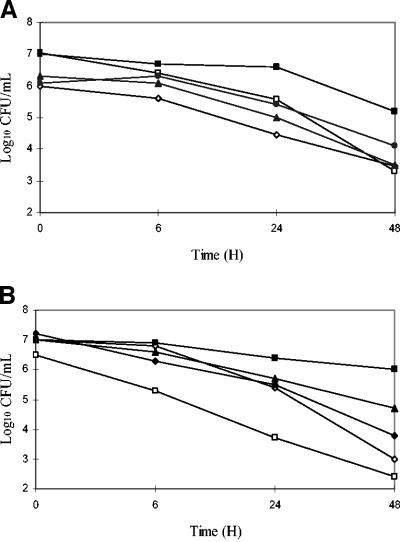
Vancomycin and teicoplanin had MICs and MBCs (Table 1) ranging from 0.25 to 1 mg/liter for MSSA 1, MSSA 2, MRSA 3, and MRSA 4, indicating good in vitro susceptibility of these four strains to glycopeptides (NCCLS recommendations). No inoculum effect was found. For GISA 5, the MICs of vancomycin and teicoplanin were 4 and 8 mg/liter, respectively, confirming the reduced susceptibility of this strain to glycopeptides. Killing curves showed that vancomycin exhibited similar activities against all four glycopeptide-susceptible strains, whatever the concentrations used, contrasting with a marked decrease in bactericidal activity against the GISA 5 strain (Fig. 1A). With teicoplanin at the same concentrations, bactericidal activity against the two MRSA strains was decreased compared to that against the two MSSA strains, and an even greater decrease was noted with the GISA 5 strain (Fig. 1B).
TABLE 1.
MICs and MBCs of the five studied strains
| Drug | MIC/MBC (mg/liter) for:
|
||||
|---|---|---|---|---|---|
| MSSA 1 | MSSA 2 | MRSA 3 | MRSA 4 | GISA 5 | |
| Vancomycin | 0.5/0.5 | 1/1 | 1/1 | 0.5/1 | 4/8 |
| Teicoplanin | 0.25/0.25 | 0.5/1 | 1/1 | 0.5/1 | 8/12 |
FIG. 1.
Kill curves of vancomycin (A) and teicoplanin (B) at 20 mg/liter. ◊, MSSA 1; □, MSSA 2; ▴, MRSA 3; •, MRSA 4; and ▪, GISA 5.
The in vivo study (Table 2) showed that the two glycopeptides were active against only two of the five strains, namely, MSSA 1 and MRSA 3. With MSSA 2, a small but significant difference was noted compared to the control group. With MRSA 4 and GISA 5, no significant differences were found with the control group. For none of these five strains was a significant difference in activity noted between vancomycin and teicoplanin. Levels of both glycopeptides in serum reached 20 mg/liter after 4 h and 30 mg/liter after 24 h.
TABLE 2.
In vivo results after 48-h treatment of the five studied strains
| Regimen | Mean log10 CFU/g of vegetation ± SD (no. of rabbits)
|
||||
|---|---|---|---|---|---|
| MSSA 1 | MSSA 2 | MRSA 3 | MRSA 4 | GISA 5 | |
| Controls | 7.3 ± 1.7 (6) | 9.7 ± 0.9 (5) | 8.7 ± 0.9 (5) | 8.4 ± 1.4 (8) | 8.2 ± 1.1 (4) |
| Vancomycin | 3.0 ± 1.3 (7)a | 8.3 ± 1.5 (5) | 3.0 ± 0.9 (4)a | 6.6 ± 1.6 (6) | 6.7 ± 1.9 (6) |
| Teicoplanin | 2.6 ± 0.2 (4)a | 7.6 ± 0.5 (5)a | 4.3 ± 1.6 (7)a | 7.8 ± 0.8 (5) | 5.8 ± 1.9 (6) |
The P value versus results from control rabbits was < 0.05 by Scheffe's test after ANOVA.
The variability in glycopeptide activity noted in the rabbit endocarditis model is probably relevant to published reports of failed glycopeptide therapy in humans with staphylococcal infections. In these patients (7, 19), the absence of a therapeutic effect was not correlated with MIC elevation. Other agents or combinations of agents have been introduced recently for the treatment of staphylococcal infections, including those due to MRSA strains. Studies of the same rabbit model have shown early and reproducible activity of these new agents against several S. aureus strains, some of which were MRSA strains (1, 12).
The two glycopeptides used in our study were similar to each other regarding activity against the five S. aureus strains tested. Steady-state serum teicoplanin levels were far greater than 10 times the MIC for susceptible strains. This condition would be expected to ensure optimal efficacy, according to relevant data in the literature (2, 10, 14). The poor diffusion of teicoplanin within vegetations (4) and the high rate of protein binding (2) do not seem to have noticeably affected the level of activity against S. aureus in comparison with vancomycin.
Our in vivo data show that a low MIC does not always predict a better response to glycopeptides over the first 2 days of treatment. Early in vivo effects do not seem to be influenced by in vitro parameters.
Tolerance, defined as a loss of bactericidal activity (7, 13, 21), has been reported. We found no evidence of tolerance, as the in vitro bactericidal effect of vancomycin on the glycopeptide-susceptible strains was unimpaired. In contrast, the high MIC for the GISA 5 strain, classified as having intermediate susceptibility to glycopeptides, was strongly correlated with the loss of bactericidal activity in vitro and in vivo. This finding is in agreement with a study comparing two isogenic S. aureus strains with different glycopeptide susceptibility patterns in an endocarditis model (15).
Although the treatment period was brief (48 h) and the antibiotics had slow killing kinetics, we found noticeable differences in activity across strains that were not predicted by the in vitro data. The source of these differences must therefore be sought elsewhere than in the intrinsic in vitro activity of the antibiotics. Host-related factors can influence in vivo activity. Studies have investigated the bactericidal effects of endogenous peptides produced by neutrophils or platelets that seem to act by causing lysis of the bacterial wall (23, 24). Their activity may be influenced by exposure to some antibiotics, most notably those with effects on the bacterial wall, such as penicillins and vancomycin (22). Conceivably, an interaction between these peptides and the glycopeptides located within endocarditis vegetations may explain these findings.
Decreased susceptibility of S. aureus strains to vancomycin may be related to a change in the bacterial target. Studies have documented thickening of the bacterial cell wall that traps the vancomycin molecules (5, 8, 9, 17). However, these findings were obtained in vitro. To date, there are no in vivo data on cell wall thickness and structure of bacteria located within sites of infection.
In conclusion, for patients with severe infections requiring immediately effective antibiotic treatment, the possibility that glycopeptides may have limited activity should be borne in mind when selecting antistaphylococcal agents. Given the variability in the in vivo activities of glycopeptides, even against strains with in vitro susceptibility, the place for new antistaphylococcal agents, or new combinations of antistaphylococcal agents, with proven early and consistent efficacy needs to be determined.
REFERENCES
- 1.Batard, E., C. Jacqueline, D. Boutoille, A. Hamel, H. B. Drugeon, N. Asseray, R. Leclercq, J. Caillon, G. Potel, and D. Bugnon. 2002. Combination of quinupristin-dalfopristin and gentamicin against methicillin-resistant Staphylococcus aureus: experimental rabbit endocarditis study. Antimicrob. Agents Chemother. 46:2174-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers, H., and S. Kennedy. 1990. Effects of dosage, peak and trough concentrations in serum, protein binding, and bactericidal rate on efficacy of teicoplanin in a rabbit model of endocarditis. Antimicrob. Agents Chemother. 34:510-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesneau, O., A. Morvan, and N. E. Solh. 2000. Retrospective screening for heterogeneous vancomycin resistance in diverse Staphylococcus aureus clones disseminated in French hospitals. J. Antimicrob. Chemother. 45:887-890. [DOI] [PubMed] [Google Scholar]
- 4.Cremieux, A. C., B. Maziere, J. M. Vallois, M. Ottoviani, A. Azancoi, H. Raffoui, A. Bouvet, J. J. Pocidalo, and C. Carbon. 1989. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J. Infect. Dis. 159:938-944. [DOI] [PubMed] [Google Scholar]
- 5.Cui, L., H. Murakami, K. Kuwahara-Arai, H. Hanaki, and K. Hiramatsu. 2000. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 44:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisel, R., F. J. Schmitz, A. C. Fluit, and H. Labischinski. 2001. Emergence, mechanism and clinical implications of reduced glycopeptide susceptibility in Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 20:685-697. [DOI] [PubMed] [Google Scholar]
- 7.Gopal, V., A. Bisno, and J. Silverblatt. 1976. Failure of treatment in Staphylococcus aureus endocarditis. In vivo and in vitro observations. JAMA 236:1604-1606. [PubMed] [Google Scholar]
- 8.Hanaki, H., K. Kuwahara-Arai, S. Boyle-Vavra, R. Daum, H. Labischinski, and K. Hiramatsu. 1998. Activated cell wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 42:199-209. [DOI] [PubMed] [Google Scholar]
- 9.Hanaki, H., H. Labischinski, Y. Inaba, N. Kondo, H. Murakami, and K. Hiramatsu. 1998. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J. Antimicrob. Chemother. 42:315-320. [DOI] [PubMed] [Google Scholar]
- 10.Harding, I., A. McGowan, L. White, E. Darley, and V. Reed. 2000. Teicoplanin therapy for Staphylococcus aureus septicemiae: relationship between pre-dose serum concentration and outcome. J. Antimicrob. Chemother. 45:835-841. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., et al. 1997. Methicillin-resistant S. aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 12.Jacqueline, C., J. Caillon, V. Le Mabecque, A. F. Miegeville, D. Bugnon, and G. Potel. 2003. Linezolid combined with imipenem: in vivo synergy in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Program Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstract no. B-1093.
- 13.May, J., K. Shannon, A. King, and G. French. 1998. Glycopeptide tolerance in Staphylococcus aureus. J. Antimicrob. Chemother. 42:189-197. [DOI] [PubMed] [Google Scholar]
- 14.McGrath, B. J., S. L. Kang, G. W. Kaatz, and M. J. Rybak. 1994. Bactericidal activities of teicoplanin, vancomycin, and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob. Agents Chemother. 38:2034-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavie, J., A. Lefort, M. C. Ploy, L. Massias, F. Chau, L. Garry, F. Denis, and B. Fantin. 2003. Influence of reduced susceptibility to glycopeptides on activities of vancomycin and teicoplanin against Staphylococcus aureus in experimental endocarditis. Antimicrob. Agents Chemother. 47:2018-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlman, B., and L. Freedman. 1971. Experimental endocarditis II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J. Biol. Med. 44:206-213. [PMC free article] [PubMed] [Google Scholar]
- 17.Reipert, A., K. Ehlert, T. Kast, and G. Bierbaum. 2003. Morphological and genetic differences in two isogenic Staphylococcus aureus strains with decreased susceptibilities to vancomycin. Antimicrob. Agents Chemother. 47:568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez, A., M. Vicente, and T. Olay. 1987. Single and combination antibiotic therapy for experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 31:1444-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small, P., and F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vedel, G., M. Leruez, F. Lemann, E. Hraoui, and D. Ratovohery. 1990. Prevalence of Staphylococcus aureus and coagulase-negative staphylococci with decreased sensitivity to glycopeptides as assessed by determination of MICs. Eur. J. Clin. Microbiol. Infect. Dis. 9:820-822. [DOI] [PubMed] [Google Scholar]
- 21.Voorn, G. P., J. Kuyvenhoven, W. H. F. Goessens, W. C. Schmal-Bauer, P. H. M. Broeders, J. Thompson, and M. F. Michel. 1994. Role of tolerance in treatment and prophylaxis of experimental Staphylococcus aureus endocarditis with vancomycin, teicoplanin, and daptomycin. Antimicrob. Agents Chemother. 38:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong, Y., M. Yeaman, and A. Bayer. 1999. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob. Agents Chemother. 43:1111-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeaman, M. R. 1997. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 25:951-970. [DOI] [PubMed] [Google Scholar]
- 24.Yeaman, M. R., A. S. Bayer, S. P. Koo, W. Foss, and P. M. Sullam. 1998. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Investig. 101:178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]