Abstract
We assessed antimicrobial susceptibility against 211 Streptococcus pyogenes strains isolated from patients with severe invasive group A streptococcal infections. Overall, 3.8, 1.4, 1.4, and 0.5% of the isolates were resistant to erythromycin, clindamycin, telithromycin, and ciprofloxacin, respectively, and 10.4% had intermediate resistance to ciprofloxacin. All isolates were susceptible to ampicillin and cefotaxime.
Streptococcus pyogenes (group A streptococcus) is one of the most common human pathogens. It causes a wide array of infections, the most frequent of which is acute pharyngitis (strep throat). From the late 1980s, streptococcal toxic shock-like syndrome caused by S. pyogenes became a serious problem in both developed and developing countries. Symptoms such as pharyngitis, fever, and pain may suddenly develop, and the disease may progress very rapidly in some patients to soft-tissue necrosis, acute kidney failure, adult respiratory distress syndrome, disseminated intravascular coagulopathy, and multiorgan failure, leading to shock and death. One approach to treat severe invasive S. pyogenes infections has been to utilize a combination of penicillin and clindamycin. The rationale is that penicillin provides coverage against 100% of S. pyogenes strains and clindamycin has demonstrated greater efficiency in experimental models of necrotizing fasciitis (8). In this study, we tested the antimicrobial susceptibility of 211 S. pyogenes strains isolated from patients with severe invasive group A streptococcal infections.
The activity of the Working Group for Group A Streptococci in Japan is based on a network between the National Institute of Infectious Diseases (NIID) and prefectural public health institutes (PHIs); seven branch offices of the reference center are located in the PHIs of Fukushima, Kanagawa, Toyama, Osaka, Yamaguchi, Oita, and Tokyo. Information on streptococcal infections and clinical isolates is sent to the PHIs from 3,041 cooperative hospitals located all over Japan. All of them are collected by NIID. A total of 211 S. pyogenes isolates from 1992 to 2003 were cultured predominantly from sterile body sites of patients with severe invasive group A streptococcal infections (5, 6). All isolates were stored at −80°C until tests for susceptibility. We analyzed the antimicrobial susceptibility of the isolates by the Etest method for five drugs, ampicillin, cefotaxime, ciprofloxacin, clindamycin, and erythromycin, and by the broth microdilution method as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (7) for telithromycin. A 0.5 McFarland solution was used to inoculate a Mueller-Hinton agar plate containing 5% sheep blood (Becton Dickinson, Tokyo, Japan) for the Etest method or to inoculate Mueller-Hinton broth containing 5% lysed horse blood (Becton Dickinson) for the broth microdilution method, and the plates were incubated at 37°C in a CO2 atmosphere. The ATCC 49619 strain of Streptococcus pneumoniae was used as a quality control. The breakpoints of resistance to the drugs, except for ciprofloxacin and telithromycin, were those recommended by the NCCLS (7). An arbitrary breakpoint for resistance to ciprofloxacin of ≥4 μg/ml was used (as it has not been established for S. pyogenes by NCCLS) as a marker to indicate the development of quinolone resistance. Strains for which the ciprofloxacin MIC was 1 to less than 4 μg/ml were designated intermediate (1). The breakpoint for telithromycin was that recommended by NCCLS for S. pneumoniae. The following breakpoints for resistance were used: ampicillin, ≥0.25 μg/ml; cefotaxime, ≥0.5 μg/ml; ciprofloxacin, ≥4 μg/ml; clindamycin, ≥1 μg/ml; erythromycin, ≥1 μg/ml; telithromycin, ≥4 μg/ml. SmaI digestion combined with pulsed-field gel electrophoresis (PFGE) was performed as described previously (4). The resistance genes (ermA, ermB, and mefA) of erythromycin-resistant strains were screened by PCR with published primer sequences (2, 9). We investigated mutational alterations in the quinolone resistance-determining regions of the gyrA and parC genes of these isolates according to the methods described by Yan et al. (11) and translated their nucleotide sequences into amino acid sequences.
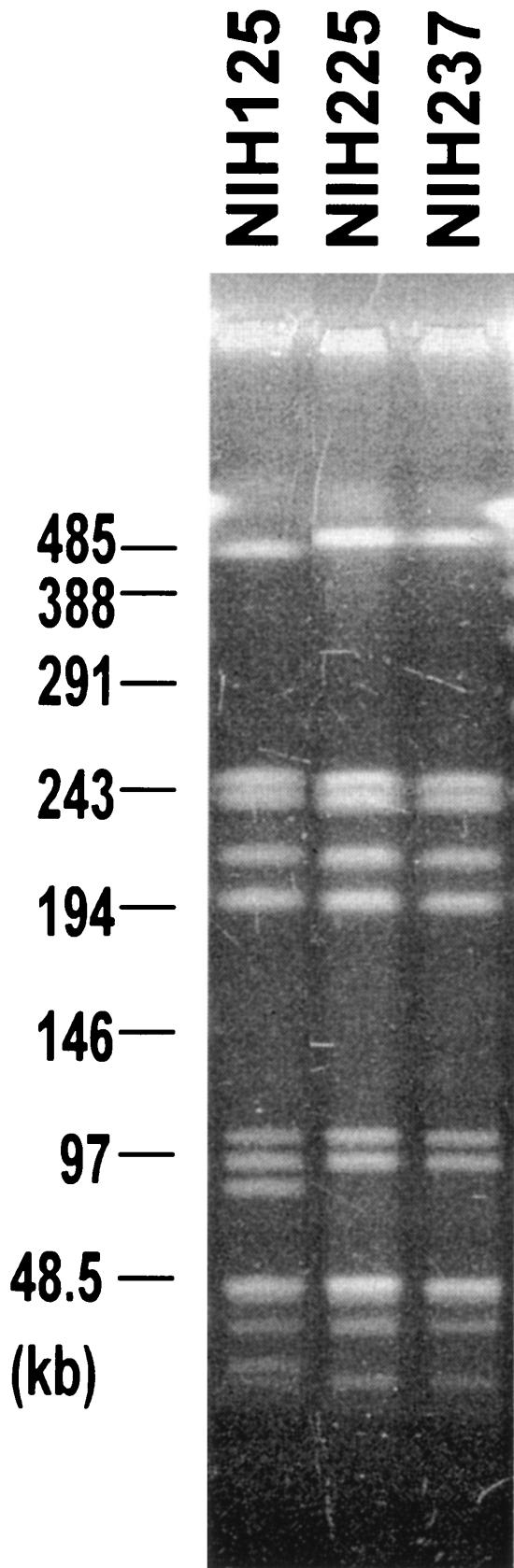
All 211 S. pyogenes isolates were susceptible to ampicillin and cefotaxime. We could divide the years covered by the study into two periods, 1993 to 1997 and 1998 to 2003. We had 3 to 12 isolates per year in the first period and 20 to 38 per year in the second period. Fourteen of 50 strains (28%) were resistant to ciprofloxacin in the first period, while only 2% were resistant in the second period. None of the isolates were resistant to erythromycin, clindamycin, and telithromycin in the first period, while resistant strains were observed in the second period. Overall, 3.8% of the isolates were resistant to erythromycin, 1.4% were resistant to clindamycin, and 1.4% were resistant to telithromycin. Three isolates were resistant to clindamycin, erythromycin, and telithromycin. Five isolates were resistant to erythromycin but not to clindamycin and telithromycin. These isolates were observed in 1998 to 2003 (Table 1). MICs of erythromycin for all three clindamycin- and telithromycin-resistant strains were more than 256 μg/ml. A strain resistant to erythromycin, clindamycin, and telithromycin was first isolated in 2000. In 2003, 10.0% (2 of 20) of the isolates were resistant to all three drugs, though there were no resistant isolates in 2001 and 2002. The three strains were isolated in different regions in Japan, but the emm genotypes were the same (emm1). Though two strains (NIH225 and NIH237) isolated in 2003 gave indistinguishable PFGE patterns, the profile of one strain (NIH125) isolated in 2000 differed in four bands from those of NIH225 and NIH237 (Fig. 1). From the criteria of Tenover et al. (10), NIH125 seems to be possibly related to strains NIH225 and NIH237. These results suggest that clonal or possibly related strains of erythromycin-, clindamycin-, and telithromycin-resistant S. pyogenes have emerged and have been spreading to cause severe invasive diseases. All of the erythromycin-, clindamycin-, and telithromycin-resistant strains carried the ermB gene. Five strains carrying the mefA or ermA gene were resistant to erythromycin but not to clindamycin and telithromycin (Table 1). Two strains carrying the ermA gene had the emm58 genotype. These two strains gave indistinguishable PFGE patterns. On the other hand, three strains carrying the mefA gene had the emm1 or emm12 genotype (Table 1).
TABLE 1.
Characteristics of erythromycin-resistant strains
| No. of isolates | Yr isolated | Resistance toa
|
Presence ofb:
|
emm allele | ||||
|---|---|---|---|---|---|---|---|---|
| EM | CLDM | TEL | ermA | ermB | mefA | |||
| 61 | 1998 | R | S | S | − | − | + | 12 |
| 125 | 2000 | R | R | R | − | + | − | 1 |
| 162 | 2001 | R | S | S | − | − | + | 1 |
| 170 | 2001 | R | S | S | + | − | − | 58 |
| 184 | 2001 | R | S | S | + | − | − | 58 |
| 220 | 2002 | R | S | S | − | − | + | 1 |
| 225 | 2003 | R | R | R | − | + | − | 1 |
| 237 | 2003 | R | R | R | − | + | − | 1 |
EM, erythromycin; CLDM, clindamycin; TEL, telithromycin. R, resistant; S, susceptible.
+, gene present; −, gene absent.
FIG. 1.
Ethidium bromide staining of SmaI-digested genomic DNAs of erythromycin-, clindamycin-, and telithromycin-resistant strains after separation by PFGE. Strain numbers are indicated. Sizes of lambda concatemers are shown on the left.
Among the isolates tested, 10.9% were not susceptible to ciprofloxacin; 10.4% had intermediate resistance to ciprofloxacin, and 0.5% were resistant. One resistant strain (MIC, ≥32 μg/ml) was isolated in 1995. The intermediate strains were isolated every year except for 1995 and 2003; the rates at which strains were isolated were high, especially in 1992 to 1994 and 1997: 1992, 33.3% (1 ciprofloxacin-intermediate isolate out of 3 isolates); 1993, 42.9% (3 of 7); 1994, 50.0% (6 of 12); 1997, 33.3% (2 of 6). In recent years, the rate of ciprofloxacin-intermediate isolates has decreased compared with rates for 1992 to 1997. One resistant strain had mutations in both GyrA and ParC in comparison with a ciprofloxacin-susceptible strain; Phe replaced Ser at position 81 of the GyrA protein, and Phe replaced Ser at position 79 of the ParC protein. The positions of these mutations in ParC and GyrA are consistent with those in a previous report (11). On the other hand, all the ciprofloxacin-intermediate isolates have a mutation in ParC, i.e., Phe or Ala replaced Ser at position 79, but not in GyrA. We determined the emm sequences of the strains that had intermediate resistance to ciprofloxacin. Of the 23 strains, 12 and 6 strains showed emm3 and emm6, respectively, and the remaining showed emm1, emm4, emm11, emm12, or emm75. In Japan, the isolation frequency of emm3 genotype strains from patients with severe invasive group A streptococcal infections rapidly increased during 1993 to 1994 (5, 6). At this time, all the ciprofloxacin-intermediate strains isolated from the patients with severe invasive infections had emm3. The sudden increase of ciprofloxacin-intermediate strains in this period was due to the increase of the isolation frequency of emm3 genotype strains.
Toxic shock-like syndrome is a fatal disease, and administration of appropriate antimicrobial drugs at the early stage of the disease is one of essential treatments. The use of ampicillin and clindamycin is the first choice. In this study, we found that all isolates from patients of severe invasive group A streptococcal infections were sensitive to ampicillin and cefotaxime, which have killing activity for the growing bacteria. Clindamycin is more effective for bacteria at the nonvegetative stage than ampicillin. We found that strains with the ermB gene have appeared and that isolates with this gene that cause severe invasive infections are resistant to clindamycin as well as to telithromycin and erythromycin. This is important information for the treatment of severe invasive group A streptococcal infections; examination of ermB in the isolates is critical for treatment.
Felmingham et al. (3) reported that 15.8% of S. pyogenes strains isolated from community-acquired respiratory tract infections in 1999 to 2000 in Japan were resistant to erythromycin. However, the rate (1 of 53; 1.9%) of erythromycin-resistant isolates from severe invasive group A streptococcal infections in 1999 to 2000 (Table 1) was less than that from community-acquired respiratory tract infections. This suggests that the rate of erythromycin-resistant isolates from community-acquired respiratory tract infections does not always correspond to that from severe invasive group A streptococcal infections. Or it may reflect the difference between major emm types among the isolates (5, 6).
The frequency of isolation of strains with intermediate resistance to ciprofloxacin was high before 1997, while it has been recently decreasing. The frequency of isolation of emm3 genotype strains from patients with severe invasive group A streptococcal infections rapidly increased during 1993 to 1994 and 2001 to 2002 (5, 6; T. Ikebe, unpublished result). Almost all of the emm3 strains isolated in 1993 to 1994 were ciprofloxacin-intermediate strains, while all the emm3 strains isolated in 2001 to 2002 were susceptible to ciprofloxacin. This may reflect the different origins of the strains (P < 0.0001). Further characterization will be required. However, the emergence of ciprofloxacin-susceptible strains may give us another choice of drug, ciprofloxacin, for treatment.
Acknowledgments
This work was supported by a grant from Ministry of Health, Labors and Welfare.
The following are members of the Working Group for Group A Streptococci in Japan: S. Saito (Akita Prefectural Institute of Public Health, Akita); K. Ootani (The Yamagata Prefectural Institute of Public Health, Yamagata); M. Oguro (Sendai City Institute of Public Health, Sendai); J. Fujisaki (Niigata Prefectural Research Institute for Health and Environmental Sciences, Niigata); K. Sugama and K. Hirasawa (Fukushima Prefectural Institute of Public Health, Fukushima); S. Hosorogi, D. Tanaka, and J. Isobe (Toyama Institute of Health, Toyama); M. Sakaki (Hiroshima Prefectural Institute of Public Health and Environment, Hiroshima); Y. Kasama (Hiroshima City Institute of Public Health, Hiroshima); H. Tanaka (Ehime Prefectural Institute of Public Health and Environmental Science, Ehime); C. Sunahara (Kagawa Prefectural Institute of Public Health, Kagawa); T. Yasuoka (Public Health Institute of Kochi Prefecture, Kochi); T. Shimizi (Tokushima Prefectural Institute of Public Health and Environmental Sciences, Tokushima); S. Moroishi (The Saga Prefectural Institute of Public Health, Saga); Y. Abe and K. Ogata (The Oita Prefectural Institute of Health and Environment, Oita); J. Kudaka (Okinawa Prefectural Institute of Health and Environment, Okinawa); T. Ikebe and H. Watanabe (National Institute of Infectious Diseases, Tokyo); M. Endoh and R. Okuno, (Tokyo Metropolitan Institute of Public Health, Tokyo); R. Suzuki (Kanagawa Prefectural Public Health Laboratory, Kanagawa); C. Katsukawa and R. Kawahara (Osaka Prefectural Institute of Public Health, Osaka); and M. Tomita (Yamaguchi Prefectural Research Institute of Public Health, Yamaguchi).
REFERENCES
- 1.Arvand, M., M. Hoeck, H. Hahn, and J. Wagner. 2000. Antimicrobial resistance in Streptococcus pyogenes isolates in Berlin. J. Antimicrob. Chemother. 46:621-624. [DOI] [PubMed] [Google Scholar]
- 2.De Azavedo, J. C. S., H. R. Yeung, J. D. Bast, L. C. Duncan, B. S. Borgia, and E. D. Low. 1999. Prevalence and mechanisms of macrolide resistance in clinical isolates of group A streptococci from Ontario, Canada. Antimicrob. Agents Chemother. 43:2144-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felmingham, D., J. D. Farrell, R. R. Reinert, and I. Morrissey. 2004. Antibacterial resistance among children with community-acquired respiratory tract infections (PROTEKT 1999-2000). J. Infect. 48:39-55. [DOI] [PubMed] [Google Scholar]
- 4.Ikebe T., A. Wada, Y. Inagaki, K. Sugama, R. Suzuki, D. Tanaka, A. Tamaru, Y. Fujinaga, Y. Abe, Y. Shimizu, H. Watanabe, and the Working Group for Group A Streptococci in Japan. 2002. Dissemination of the phage-associated novel superantigen gene speL in recent invasive and noninvasive Streptococcus pyogenes M3/T3 isolates in Japan. Infect. Immun. 70:3227-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikebe, T., N. Murai, M. Endo, R. Okuno, S. Murayama, K. Saitoh, S. Yamai, R. Suzuki, J. Isobe, D. Tanaka, C. Katsukawa, A. Tamaru, A. Katayama, Y. Fujinaga, K. Hoashi, J. Ishikawa, H. Watanabe, and the Working Group for Group A Streptococci in Japan. 2003. Changing prevalent T serotypes and emm genotypes of Streptococcus pyogenes isolates from streptococcal toxic shock-like syndrome (TSLS) patients in Japan. Epidemiol. Infect. 130:569-572. [PMC free article] [PubMed] [Google Scholar]
- 6.Inagaki, Y., T. Konda, S. Murayama, S. Yamai, A. Matsushima, Y. Gyobu, D. Tanaka, A. Tamaru, C. Katsukawa, A. Katayama, M. Tomita, Y. Fuchi, K. Hoashi, H. Watanabe, and the Working Group for Group A Streptococci in Japan. 1997. Serotyping of Streptococcus pyogenes isolated from common and severe invasive infections in Japan, 1990-5: implication of the T3 serotype strain-expansion in TSLS. Epidemiol. Infect. 119:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6, 4th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Stevens, D. L., E. A. Gibbons, R. Bergstrom, and V. Winn. 1988. The eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 158:23-28. [DOI] [PubMed] [Google Scholar]
- 9.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenover, F. C., D. R. Arbeit, V. R. Goering, A. P. Mickelsen, E. B. Murbara, H. D. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan, S. S., L. M. Fox, M. S. Holland, F. Stock, J. V. Gill, and P. D. Fedorko. 2000. Resistance to multiple fluoroquinolones in a clinical isolate of Streptococcus pyogenes: identification of gyrA and parC and specification of point mutations associated with resistance. Antimicrob. Agents Chemother. 44:3196-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]