ABSTRACT
p53 is a central regulator that turns on vast gene networks to maintain cellular integrity in the presence of various stimuli. p53 activates transcription initiation in part by aiding recruitment of TFIID to the promoter. However, the precise means by which p53 dynamically interacts with TFIID to facilitate assembly on target gene promoters remains elusive. To address this key issue, we have undertaken an integrated approach involving single-molecule fluorescence microscopy, single-particle cryo-electron microscopy, and biochemistry. Our real-time single-molecule imaging data demonstrate that TFIID alone binds poorly to native p53 target promoters. p53 unlocks TFIID's ability to bind DNA by stabilizing TFIID contacts with both the core promoter and a region within p53's response element. Analysis of single-molecule dissociation kinetics reveals that TFIID interacts with promoters via transient and prolonged DNA binding modes that are each regulated by p53. Importantly, our structural work reveals that TFIID's conversion to a rearranged DNA binding conformation is enhanced in the presence of DNA and p53. Notably, TFIID's interaction with DNA induces p53 to rapidly dissociate, which likely leads to additional rounds of p53-mediated recruitment of other basal factors. Collectively, these findings indicate that p53 dynamically escorts and loads TFIID onto its target promoters.
KEYWORDS: p53, TFIID, transcription, structure, single-molecule microscopy
INTRODUCTION
More than 50% of cancer patients harbor p53 mutations, highlighting the essential role of this protein in tumor suppression (1). To properly maintain genomic stability, p53 induces vast gene networks involved in diverse cellular pathways, including the cell cycle arrest, apoptosis, and DNA repair pathways (2). In response to various stress stimuli, p53 acts as a transcriptional activator that specifically binds consensus response elements (REs; two 10-base-pair half-sites [RRRCWWGYYY]) within its target genes to directly stimulate gene expression (2). p53 utilizes its ability to nonspecifically bind and slide along the DNA to expedite the search for target REs (3). Upon recognition of its response genes, p53 facilitates transcription at least in part by targeting the TFIID-mediated transcription machinery to the promoter (4–7). TFIID is composed of TATA-binding protein (TBP) and 14 TBP-associated factors (TAFs). TFIID recognizes and binds multiple core promoter elements (e.g., the TATA box, the initiator, and downstream core promoter elements [DPE]) surrounding the transcription start site (TSS) (8). Once bound, TFIID serves as a central scaffold for six basal factors, including RNA polymerase II, to form a preinitiation complex (PIC) directing transcription initiation. Importantly, without the assistance of activators such as p53, TFIID's core promoter recognition is weak and rate limiting for transcription initiation due to inefficient PIC assembly (9–16). As TFIID is responsible for transcription of at least ∼90% of protein-coding genes (17), unraveling how p53 intervenes in this central step of the PIC assembly (i.e., TFIID's binding of promoter DNA) is essential for understanding mechanisms controlling gene-specific transcription initiation throughout the cell.
Previous biochemical studies of different activators (e.g., p53 and Zta) suggested a conserved albeit poorly characterized mechanism for facilitating transcription (10, 18, 19). It has been shown that p53 binds to its REs and enhances TFIID promoter recruitment via direct contact with TBP and selected TAFs (19, 20). However, p53 target genes have diverse spatial arrangements of core promoter elements and p53 REs. Each target promoter also exhibits varied affinities for p53 and TFIID (1). Therefore, it is unclear how distinct combinations of p53 REs and core promoter elements affect the dynamics and the global architecture of TFIID assemblies on target genes. Additional evidence has indicated that p53 and other activators introduce structural changes within TFIID when associated with TFIIA and DNA (10, 18, 19). This activator-induced isomerization leads to enhanced TFIID binding to core promoter elements downstream of the TSS (10, 18, 19). However, the structural mechanism of p53-mediated isomerization of TFIID and its impact on PIC assembly are currently unknown.
Single-molecule-based imaging has emerged as an advanced tool to discern the complex dynamic behavior of large multisubunit protein assemblies involved in regulating gene expression. A recent study utilizing real-time single-molecule fluorescence microscopy provided the insight that TFIID's interaction with a synthetic core promoter DNA tightly correlates with productive transcription (21). Another single-molecule report uncovered TFIIB's dynamic binding to TFIID at the promoter (22). Additional live-cell studies also suggested that the PIC composition is extraordinarily dynamic, with the loading and promoter escape of RNA polymerase II occurring approximately every 4 to 9 s during bursts of transcription (23). An explanation of how activators such as p53 regulate TFIID's dynamic binding to native target DNA remains elusive.
Advanced single-particle transmission electron microscopy (TEM) has unraveled the three-dimensional (3D) structures of TFIID and its cocomplexes. Recent EM studies unmasked a structural plasticity of TFIID, likely attributable to its promoter recognition activity (20, 24–27). In particular, human TFIID features a “horseshoe” shape containing three major lobes (A, B, and C) and a well-defined central cavity (28, 29). Interestingly, we demonstrated that, in the absence of DNA, three different activators, including p53, introduce a common set of local structural changes in TFIID, such as movement of lobe A toward lobe B (20). Intriguingly, human TFIID undergoes a significant structural rearrangement involving movement of lobe A when bound to a specific promoter DNA optimized for TFIID recognition (26, 27). It remains to be determined if the p53-facilitated movement of lobe A results in a rearranged conformation of TFIID that contributes to stable interaction between TFIID and DNA.
To uncover the mechanistic underpinnings of how p53 facilitates TFIID assembly on promoter DNA, we exploited a combination of single-molecule fluorescence microscopy, biochemistry, and single-particle cryo-EM analysis. These studies illuminated dynamic interactions and static global molecular architectures of p53-induced TFIID assemblies on two representative target genes (i.e., hdm2 and bax). The data from our real-time single-molecule studies show that TFIID alone infrequently assembles onto these native p53 target promoters. TFIID/DNA binding primarily occurs within seconds. p53 facilitates TFIID's promoter recruitment by enhancing multiple TFIID contacts throughout the core promoter and RE. Notably, the presence of p53 also increases the percentage of TFIID molecules displaying long-lived DNA binding. Consistent with our single-molecule studies, EM structural analysis revealed that conversion of canonical TFIID to a rearranged DNA binding conformation is enhanced in the presence of p53 and DNA. TFIID/DNA binding induces p53 dissociation, which may potentially aid subsequent recruitment of p53 cocomplexes (e.g., p53/Mediator [30], p53/TFIIH [31], and p53/RNA polymerase II [32]) involved in PIC formation. Therefore, proper responses to divergent stress signals involve conserved structural mechanisms relating to p53's ability to dynamically stimulate TFIID's interaction with various target promoters.
RESULTS
TFIID association with native target genes in the presence of p53.
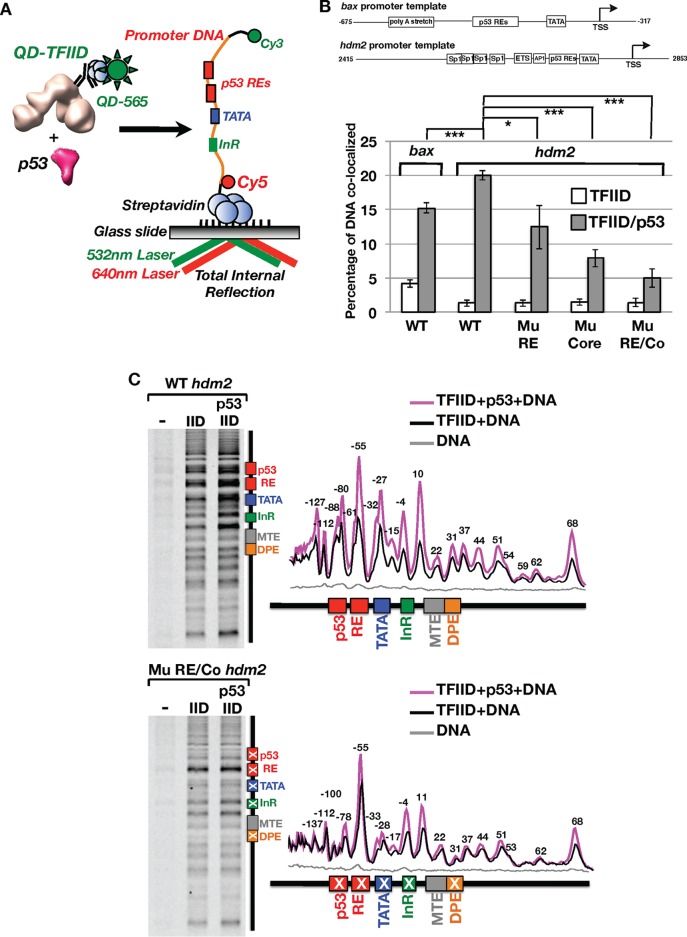
We sought to further understand the molecular mechanism pertaining to activator-mediated enhancement of TFIID's ability to bind DNA. Thus, dynamic interactions between p53, TFIID, and promoter DNA were characterized via a single-molecule colocalization assay using total internal reflection fluorescence (TIRF) microscopy (Fig. 1A) (21, 22). Over the first 15 min of binding, quantum dot (Qdot)-labeled TFIID (QD-TFIID) associated with only 1.5% of the Cy3/Cy5-labeled native wild-type hdm2 promoter DNA in the absence of p53 (Fig. 1B; see also the left panel in Fig. S1 in the supplemental material), revealing a low level of intrinsic affinity of TFIID for DNA. Increasing the time between addition of TFIID to the DNA and the imaging of binding did not improve colocalization efficiencies, suggesting that the reactions were at equilibrium (data not shown). This finding indicates that, at low (∼1 nM) concentrations, TFIID inefficiently recognizes DNA. On the other hand, increased binding of TFIID to DNA was observed in our bulk biochemical assembly assays when much higher concentrations (100 to 600 nM) were used as described for Fig. S6B.
FIG 1.
Single-molecule total internal reflection fluorescence (TIRF) microscopy analysis of quantum dot-labeled TFIID binding of promoter DNA in vitro. (A) Schematic representation of an in vitro single-molecule TIRF assay to monitor binding of quantum dot (QD)-labeled TFIID to fluorescently labeled bax or hdm2 promoter DNA in real time. (B) Dependence of TFIID/promoter DNA colocalization on p53, the p53 RE, and core promoter elements. Promoter DNA binding is plotted as a percentage of total DNA molecules in a field of view that are colocalized with TFIID over 15 min of binding based on TFIID-promoter DNA displacement histograms. A colocalization threshold of 84 nm was set on the basis of data from our previous study (21). Levels of TFIID binding to wild-type bax DNA with and without p53 are compared. Additional comparisons were made for TFIID binding to wild-type (WT), mutant p53 RE (Mu RE), mutant Core (Mu Core), or combined mutant RE and core (Mu RE/Co) hdm2 DNA templates. *, P < 0.05; ***, P < 0.001. (C) xlink-Exo analysis of TFIID binding to wild-type (top panel) and Mu RE/Co (bottom panel) hdm2 DNA. Base pair annotations (±2 bp) above individual peaks in the line traces (right panel) represent a −4-bp adjustment to reflect the known inability of exonuclease to cut at locations immediately adjacent to protein/DNA cross-link sites (35).
Upon addition of p53, a clear enhancement of TFIID's association with the bax or hdm2 promoter DNA was observed (Fig. 1B). This result is consistent with a general increase in the level of DNA retained by TFIID in the presence of p53 in our bulk biochemical assays (Fig. S6B). A control experiment detected no p53-mediated colocalization of DNA with the Qdot-labeled TAF1 antibody (Ab) (0.2% ± 0.07% colocalization) in the absence of TFIID (data not shown). Therefore, p53's significant enhancement of the TFIID/DNA association was likely due to the direct interaction between p53 and TFIID.
To directly address the role of the p53 RE and core promoter elements in p53-mediated TFIID/DNA binding, three mutant hdm2 DNA templates were analyzed with the same real-time colocalization assay (Fig. 1B). The first mutant hdm2 DNA template (i.e., the mutant response element [Mu RE]) harbors mutations in the response elements known to impair p53's sequence-specific interactions (33). A mild but significant decrease of TFIID's association with DNA was observed on the mutant p53 RE template (Fig. 1B). This result suggests that enhancement of TFIID/DNA binding can occur via a mechanism that is independent of p53/RE contacts.
To assess the impact of the core promoter in p53-mediated TFIID/DNA binding, mutations (i.e., the mutant core) known to weaken TFIID binding and inhibit transcription (22, 34) were exploited. Upon addition of p53, TFIID's association with the mutant core template was further reduced compared to that seen with the wild-type promoter (Fig. 1B). As expected, when both the p53 RE and core promoter elements (i.e., combined mutant RE and core [Mu RE/Co]) were altered, TFIID/DNA colocalization was further decreased (Fig. 1B). These results indicate that both the p53 RE and core promoter elements are required for optimal association of TFIID with p53 target gene promoters.
p53 regulates TFIID/DNA contacts within the core promoter and p53 response elements.
As an alternative approach to studying p53-mediated TFIID/DNA binding, we developed an in vitro formaldehyde-cross-linking/exonuclease mapping assay (xlink-Exo). Our strategy was adapted from a well-established strategy (chromatin immunoprecipitation [ChIP]-exo) to map out protein/DNA contacts of transcription factors in vivo at near-base-pair resolution (35). On the basis of this analysis, TFIID makes extensive contacts with well-characterized core promoter elements (i.e., TATA, InR, MTE, and DPE) on the wild-type hdm2 DNA (Fig. 1C, top panels). Addition of p53 led to elevated cross-linking of TFIID to the wild-type core promoter, with some elements (i.e., TATA and InR) showing much larger changes than others (i.e., MTE/DPE) (Fig. 1C, top panels). Consistent with our single-molecule data, p53 increased the specific binding of TFIID to the core promoter. Importantly, there was a clear reduction in p53's ability to enhance contacts within mutated core promoter elements (Fig. 1C, bottom panels, and Fig. S2A). This finding is consistent with our single-molecule assay showing reduced TFIID/DNA colocalization on the mutant core promoter (Fig. 1B). Interestingly, p53 slightly increased TFIID's contacts within the −4 and +11 regions on these mutant core promoters (Fig. 1C, bottom panel, and Fig. S2A). Thus, our mutations did not completely eliminate TFIID/core promoter contacts and may explain why a small percentage of TFIID was still bound to the mutant promoters in our single-molecule assays (Fig. 1B).
In addition to these well-established contacts, our xlink-Exo assays also mapped TFIID/DNA interactions far upstream of TATA (−55 to −137) and downstream of the DPE (approximately +37 to +68) (Fig. 1C, top panel). This result is consistent with previous DNase I footprinting studies reporting TFIID/DNA interactions upstream of TATA (−40 to −140) and downstream of the DPE (+32 to +68) at similar positions on different promoters (26, 36, 37). Many of these upstream and downstream TFIID contacts were enhanced when p53 was copresent on the wild-type hdm2 promoter (Fig. 1C). Moreover, some of TFIID's upstream contacts (i.e., −55 to −88) reside within the p53 RE on the wild-type promoter even in the absence of p53 (Fig. 1C, top panels). Mutations of the p53 RE led to a loss of TFIID's contacts at −61 and −88, suggesting that TFIID recognizes specific sequences within the p53 RE (Fig. 1C, bottom panels, and Fig. S2B). Furthermore, a number of upstream TFIID contacts (−61, −79, and −88/−90) were also abrogated on the mutant core promoter (Fig. S2). This suggests that these novel upstream DNA contacts arise from TFIID's interaction with the TATA, InR, and DPE sequences. Therefore, TFIID may utilize additional uncharacterized elements to recognize promoters.
p53 modulates the prolonged association of TFIID with DNA.
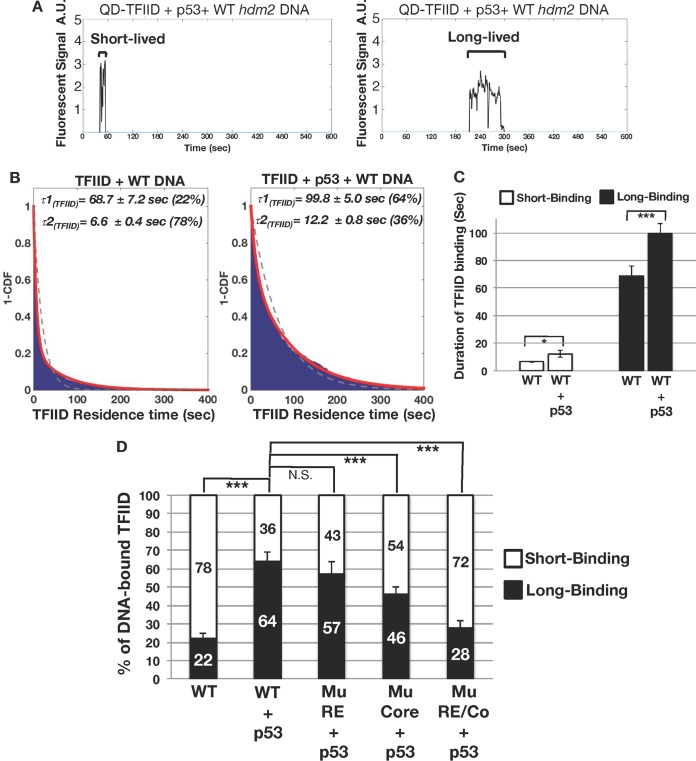
In addition to p53's ability to stimulate TFIID/DNA association, we sought to test if p53 could also stabilize TFIID's binding to DNA. Therefore, the amount of time that QD-TFIID remains bound to a single promoter DNA before dissociating (i.e., the residence time) with and without p53 was examined (Fig. 2A and B). Two populations of DNA-bound TFIID complexes displaying distinct residence times were detected on the wild-type hdm2 template in both the absence and presence of p53 (Fig. 2B). The majority (78%) of TFIID/DNA binding events were short-lived (average residence time of 6.6 ± 0.4 s) in the absence of p53 (Fig. 2B, left panel). Notably, p53 significantly increased the proportion of long-lived TFIID/DNA bound complexes from 22% to 64% (Fig. 2B, left versus right panel). Furthermore, p53 prolonged the residence time of both the short-lived and long-lived TFIID/DNA binding events (Fig. 2C). TFIID displayed a similar stability profile on the bax promoter in the presence of p53 (Fig. S3). These results suggest that TFIID interacts with DNA via two intrinsic binding modes that can be modulated by p53.
FIG 2.
Residence time analysis of TFIID bound to promoter DNA. (A) Traces of individual colocalized TFIID/promoter DNA binding events were further analyzed to determine TFIID's average residence time. TFIID displays a mixture of brief (left panel) and long-lived (right panel) binding events. A.U., arbitrary units. (B) 1-Cumulative distribution function (1-CDF) plots of TFIID bound to DNA in the absence (left panel) and presence (right panel) of p53 were fitted to a single-component (dashed line) or two-component (red line) exponential decay model. Fitting analysis reveals that TFIID complexes bound to promoter DNA display residence times comprised of a long-lived component (τ1) and a short-lived component (τ2). Percentages of the long-lived component (τ1) and short-lived component (τ2) are listed next to the residence time values. (C) Bar graph comparing TFIID's average levels of short-lived and long-lived binding in the absence and presence of p53. (D) Percentages of short-lived and long-lived TFIID binding events on the wild-type (RE), mutant RE (Mu RE), mutant Core (Mu Core), and mutant RE/Core (Mu RE/Co) hdm2 promoter DNA. *, P < 0.05; ***, P < 0.001; N.S., not significant.
We next defined how the RE and core promoter elements might regulate the relative proportions of these two TFIID/DNA binding populations in the presence of p53. Disruption of the p53 binding site on the hdm2 promoter (Mu RE) had little effect on the distributions of these two populations (Fig. 2D). This indicates that a p53-mediated increase in the percentage of TFIID molecules displaying long-lived DNA binding does not solely require contacts with the RE. In contrast, mutation of the core promoter region (Mu Core) reduced the percentage of TFIID molecules in the long-lived DNA binding population (Fig. 2D). Alteration of the p53 binding site and the core promoter elements (Mu RE/Co) further lowered the percentage of TFIID's long-lived DNA binding population (Fig. 2D). These data suggest that TFIID's long-lived DNA binding mode was related primarily to recognition of the core promoter, with p53/RE interactions providing additional stability postrecruitment to the DNA.
Our results also reveal that p53 RE and core promoter mutations also impacted TFIID's residence time on DNA (Fig. S4). Mutation of the p53 RE (Mu RE) predominantly decreased the residence time of TFIID's long-lived DNA binding without significantly affecting the short-lived events (Fig. S4B). Therefore, interactions between p53 and its RE are required for maximal stability of TFIID/DNA contacts on native target promoters. On the mutant core promoter (Mu Core), residence times for both the long-lived and short-lived TFIID/DNA binding events were also significantly reduced (Fig. S4B). Thus, both DNA binding modes were related to core promoter recognition. Mutation of both the p53 RE and core promoter (Mu RE/Co) had little effect on TFIID's residence time on DNA (Fig. S4B). These results suggest that mutation of both the RE and core promoter elements primarily effects TFIID's distribution between short-lived and long-lived DNA binding modes (Fig. 2D). Together with the single-molecule data, our xlink-Exo studies suggested that TFIID's remaining minimal contacts on our mutant promoter were sufficient to anchor short-lived TFIID binding to DNA.
p53/target promoter interactions in the presence of TFIID.
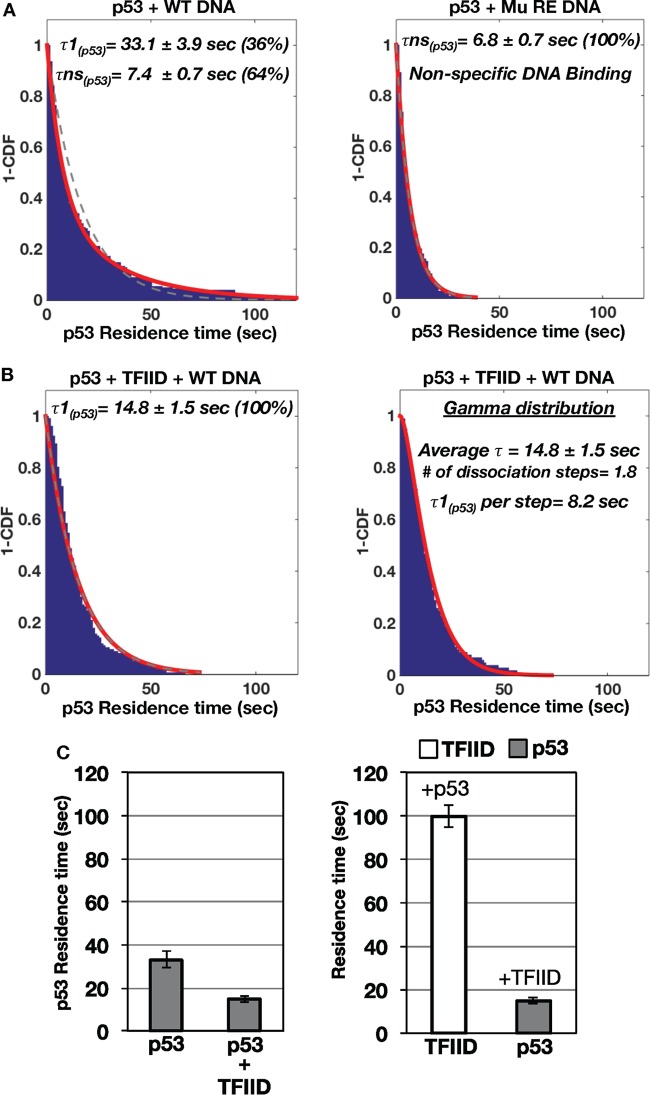
Our previous report documented that a TFIID variant could cooperatively increase the binding of the activator c-Jun to its binding site on promoter DNA (24). To quantitatively assess if this was the case for p53, the dissociation dynamics of fluorescently labeled p53/DNA interactions with or without TFIID were examined (Fig. 3). In the absence of TFIID, p53 primarily displayed two populations of distinct binding events on the wild-type hdm2 template (i.e., a long-lived [average = 33.1 ± 3.9 s] population and a short-lived [average = 7.4 ± 0.7 s] population) (Fig. 3A, left panel). Since earlier reports showed that p53 can bind DNA nonspecifically (38, 39), we hypothesized that these short-lived p53/DNA binding events were nonspecific interactions. To test our hypothesis, the same experiments were performed using the mutant p53 RE template (right panel). Indeed, disruption of p53's sequence-specific binding sites on DNA led to the presence of a single short-lived population (average = 6.8 ± 0.7 s). Based on this result, the long-lived p53/DNA binding events most likely represented sequence-specific interactions (Fig. 3A, left panel), whereas the short-lived p53/DNA binding events were nonspecific in nature. Alternatively, these short-lived events could represent interactions with an unknown lower-affinity p53 binding site in the promoter or binding of dimeric p53 to consensus half-sites within the mutant RE. It is of note that photobleaching of the fluorescently labeled p53 did not affect our results, since similar residence times were observed with multiple dyes with differing levels of photostability (data not shown). In addition, a previous study detected similar short residence times of approximately 10 to 12 s for p53 bound to a consensus site (40). Collectively, these findings indicate that p53 dynamically interacts with DNA via both specific and nonspecific interactions.
FIG 3.
Residence time analysis of p53 bound to promoter DNA with and without TFIID. (A) Individual colocalized p53/promoter DNA binding events were further analyzed to determine the average residence time for p53 bound to wild-type (left panel) and mutant p53 RE (right panel) hdm2 promoter DNA. One-cumulative distribution function (1-CDF) plots of p53 bound to promoter DNA were fitted to a single-component (dashed line) or two-component (red line) exponential decay model. Based on comparisons of the hdm2 promoter DNAs containing wild-type and mutant p53 REs, τ1 represents specific binding of p53 to the wild-type hdm2 DNA and τns represents nonspecific interactions between p53 and promoter DNA. (B) 1-CDF plots of p53 bound to the wild-type hdm2 DNA template in the presence of TFIID were fitted to a single-component (dashed line) and two-component (red line) exponential decay (left panel) or gamma distribution (right panel) model. (C) Bar graph of p53's residence time in the absence and presence of TFIID bound to the wild-type hdm2 DNA (left panel). Results of residence time analysis of TFIID and p53 bound to the wild-type hdm2 DNA are presented (right panel).
Importantly, addition of TFIID resulted in a single population of p53 bound to wild-type DNA with an “intermediate” residence time distinct from those exhibited by the two populations observed with p53 alone (Fig. 3B). Furthermore, we noticed that fitting of the data showed slight deviations from an exponential decay model. A previous study indicated that the existence of complex multistep dissociation events could be discerned using a gamma distribution model to fit single-molecule data (41). Indeed, we found that a gamma distribution model better represents the data (Fig. 3B, right panel). This analysis showed that p53 dissociates from the DNA in the presence of TFIID in ∼2 steps. The two steps correspond to roughly equivalent residence times of 8.2 s per step (average total time = 14.8 ± 1.5 s). Moreover, single-step dissociation kinetics was observed for p53 alone on the basis of gamma distribution fitting (data not shown). Therefore, TFIID alters both the rate and number of kinetic steps involved in p53's dissociation from promoter DNA.
Mutation of the p53 RE further reduces p53's residence time on DNA in the presence of TFIID (Fig. S5A, left panel, versus Fig. 3B, right panel). Disruption of TFIID's interaction with the TATA, InR, and DPE elements on the mutant p53 RE/Co DNA template does not lead to a further decrease in p53's residence time (Fig. S5A versus B). Fitting of the dissociation data on the mutant templates to a gamma distribution yielded poor fits, suggesting single-step dissociation kinetics for p53 on those mutant promoters (Fig. S5A and B, right panels).
Overall, these data suggest that TFIID binding to DNA can influence p53's stability at the RE. TFIID's interaction with p53 likely promotes the dissociation of p53 bound to specific sequences in the promoter (Fig. 3C, left panel). Importantly, the average duration of binding of p53 to the wild-type hdm2 template (∼15 s) is significantly shorter than TFIID's long-lived DNA binding time (∼100 s) (Fig. 3C, right panel). These dynamic studies strongly suggest that p53 transiently escorts TFIID to the promoter DNA. Importantly, p53 is not required to remain bound for TFIID's maximum stability on promoter DNA.
The conformational status of TFIID copresent with p53 and promoter DNA.
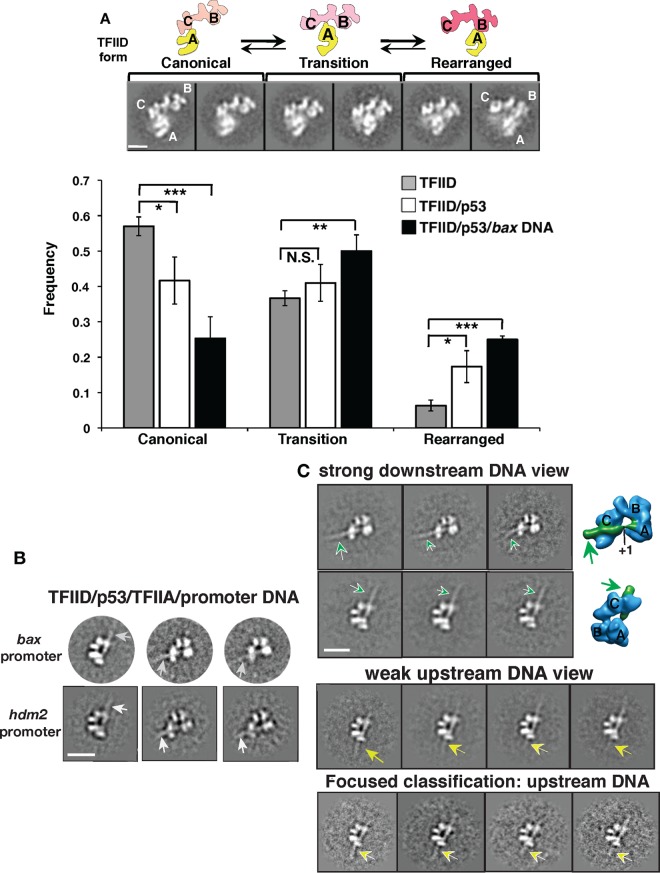
A recent cryo-EM study discovered that human TFIID switches from a canonical to a rearranged conformation upon binding to the supercore promoter (SCP), a DNA fragment optimized for interaction with TFIID (26, 34). Thus far, our single-molecule data indicated that p53 stimulates binding of TFIID to DNA. Therefore, we hypothesized that TFIID could form a rearranged DNA binding conformation with p53 and promoter DNA. Therefore, we set out to determine TFIID's global structural architecture when p53 and target promoter DNA were present. As the first step, unbiased reference-free 2D classification experiments were performed to examine conformational states of TFIID in the presence of p53 or p53/bax DNA (Fig. 4A). The movement of lobe A from lobe C to lobe B is the hallmark of the rearranged DNA binding conformation of TFIID (26). Therefore, three conformational states of TFIID represented by corresponding views of 2D class averages were defined based upon the position of lobe A relative to those of lobes C and B (top panels [i.e., canonical, transition, and rearranged states]). We found that addition of p53 led to an elevated percentage of particles within the corresponding view displaying the rearranged TFIID form compared to the results seen with TFIID alone (Fig. 4A, bottom panel). This finding implies that the formation of TFIID's rearranged state is affected by p53. This result is consistent with conformational changes in our previously observed negatively stained structure of a TFIID/p53 cocomplex (20). Furthermore, the 2D classification analysis showed that the populations of both the transition and rearranged forms increased when p53 and DNA were added (bottom panel). Therefore, these findings suggest that TFIID's conformational switches can be regulated via target gene promoters and/or its interaction with p53.
FIG 4.
Structural EM analysis of p53's impact on TFIID bound to native target gene promoters. (A) TFIID's structural plasticity as defined by the position of its A lobe (26) (top panels; the major lobes of TFIID are labeled A, B, and C). TFIID's conformations are classified into three groups, representing the canonical, transition, and rearranged states, as shown. Data represent results of reference-free 2D classification analysis of negative-stain EM data sets showing populations of different TFIID conformations in the absence of p53 and in the presence of p53 only or p53 plus bax DNA. Bar, 100 Å. *, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S., not significant. (B) Three representative reference-free 2D class averages of p53/TFIID/TFIIA bound to either the bax or hdm2 DNA (arrows) are shown that are based on cryo-EM particle data sets. Bar, 200 Å. (C) Distinct 2D class averages showing views of the assemblies bound to the bax promoter DNA fragment downstream (top panel; DNA highlighted by arrows) and upstream (middle panel; DNA highlighted by arrows) of the transcription start site (TSS; +1) are presented. The orientations of the core promoter elements bound by TFIID were previously mapped in the 3D structure of TFIID/TFIIA bound to the super-core promoter (SCP) DNA (26). The corresponding views of the 3D structure of TFIID/TFIIA/SCP DNA are shown (far right) to indicate the DNA orientation. The strong 3D density highlighted in green represents the promoter DNA comprising the initiator, TSS, and downstream promoter elements. Since the upstream promoter DNA was weakly visible, focused classification analyses (bottom panel) were performed using the corresponding class averages (middle panel) to clarify the presence of the bax promoter DNA upstream of the TATA box (highlighted by arrows). Bar, 200 Å.
A previous biochemical study reported that p53 stimulated the assembly of TFIIA and TFIID on a synthetic promoter DNA (19). Interestingly, unlike p53, TFIIA alone promotes the canonical form of TFIID (26). Thus, to assess the structural framework of TFIID in the presence of p53 and TFIIA, two TFIID/p53/TFIIA assemblies on the native hdm2 and bax promoter fragments (488 bp and 500 bp) were obtained (Fig. S6). To visualize both conformations and the DNA, cryo-EM data of these assemblies were obtained for reference-free 2D classification analysis. Some class averages showing the presence of promoter DNA spanning the central cavity of TFIID were observed (Fig. 4B). Moreover, the assemblies displayed similar rearranged TFIID architectures, despite the different organizations of their promoters. Each template harbored nonconsensus core promoter elements with different spatial arrangements between TATA and the initiator (Fig. 1B, top panel) (8, 42, 43). Yet these structural studies suggest that TFIID binds different promoters in common rearranged conformations via a conserved mechanism.
Next, to identify the relative DNA orientation and any distinct structural features, we compared the class averages of our two assemblies with the previous structural analysis of TFIID/TFIIA/SCP DNA containing a mapped DNA orientation (26). Our cocomplexes displayed clear views of TFIID stably bound to the bax DNA downstream of the TSS (Fig. 4C, top two rows). In contrast, upstream promoter DNA was weakly visible in our averages (middle panel). These results suggest that the p53 RE upstream of the TATA box element is flexible within the cocomplex. To define promoter DNA upstream of TATA, focused classification was performed on a class average displaying flexible upstream DNA (middle panel) as previously described (26). This analysis revealed a clear density for the upstream promoter DNA (highlighted by yellow arrows in the bottom panel). Given the strong density detected for downstream DNA within the assembly, these results imply that TFIID serves as a strong anchor for downstream promoter DNA during PIC assembly.
The 2D class averages of TFIID/DNA/p53/TFIIA assemblies exhibit a topology similar to that determined for the averages from TFIID/TFIIA bound to the SCP DNA (refer to Fig. 3 in reference 26), raising two major points. First, TFIID adapts a common DNA binding conformation to bind various p53 target gene promoters. Second, structural rearrangements within TFIID related to DNA binding may cause p53 to dissociate, as supported by our single-molecule dynamics results (Fig. 3). To test these hypotheses, single-particle 3D reconstruction was conducted to determine the 3D structure of the TFIID/TFIIA/p53/bax DNA cocomplex (Fig. S7A). The overall structural architecture of our cocomplex is comparable to the 3D structure of TFIID/TFIIA/SCP DNA. Our 3D reconstruction was also verified by analyzing its 3D projections with their matching reference-free 2D averages (Fig. S7B). The same observation was also obtained with the 3D structure of the TFIID/TFIIA/p53/hdm2 promoter DNA (Fig. S8). These results suggest that TFIID utilizes one common rearranged form to bind various promoters.
Our 3D reconstruction did not reveal any prominent extra densities that would most likely represent the p53 protein. This indeed suggests that p53 dissociates from the TFIID/TFIIA/bax DNA cocomplex. Alternatively, p53 could have a transient interaction with the assembly, which led to the EM density being averaged out during analysis. Since we had previously determined the 3D structure of the p53 bound TFIID cocomplex without DNA (20), we attempted to capture the p53-bound state of TFIID in the cryo-EM data set of our TFIID/TFIIA/p53/bax DNA assembly. Thus, 3D reconstructions were performed using the structure of p53/TFIID as an initial reference volume. A poor-resolution 3D reconstruction resembling our previously published p53/TFIID cocomplex (20) was obtained, suggesting that a small subset of the single cryo-EM particles might have contained p53-bound TFIID without DNA (Fig. S9). This observation suggests that the majority of TFIID complexes had switched to the p53-free, DNA-bound state when p53 and DNA were copresent. Collectively, these structural studies support the observations from our single-molecule analyses, illustrating that p53 dissociates from TFIID and DNA when recruited to target promoters. Our work also implies that when p53 stimulates TFIID binding to DNA, a common rearranged DNA binding scaffold is formed on various target genes.
DISCUSSION
TFIID infrequently interacts with native p53 target genes in the absence of additional factors.
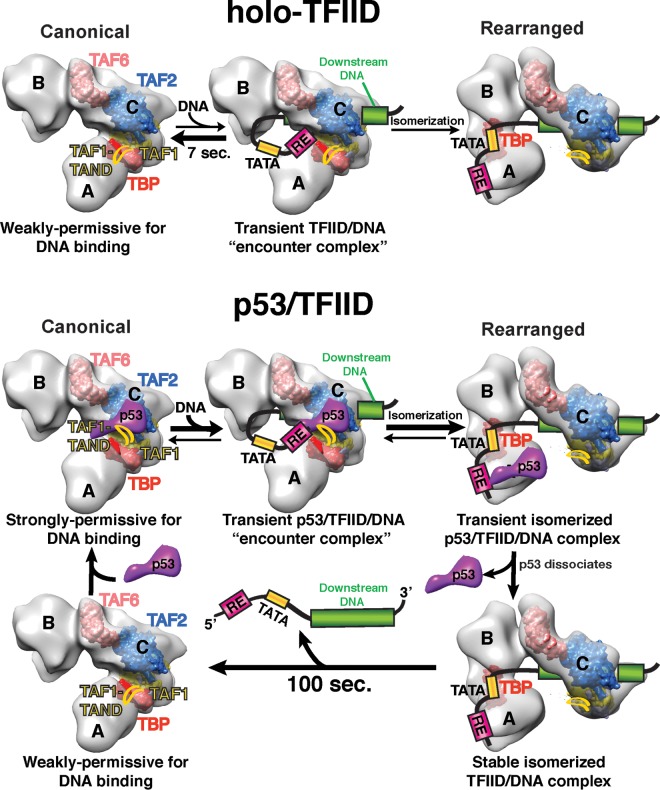
Our single-molecule assays revealed that TFIID poorly associates with native p53 target promoters in the absence of p53 (Fig. 1B). TFIID's inefficient DNA binding activity may be one way to prevent spurious basal expression of these stress response genes. TFIID/promoter interactions are likely repressed in part due to the presence of the TAF1 N-terminal domain (TAND), which occupies TBP's concave DNA binding surface (44–47). This TAF1/TBP interaction is also thought to control the TFIID's global conformation to regulate promoter interactions. TFIID's canonical conformation is nonpermissive to DNA binding (26, 27) (Fig. 5, top panel, left side). Researchers postulate that the TAF1 TAND acts to tether TBP to TFIID's lobe C in the canonical form (27). Large-scale structural rearrangement of TFIID dramatically shifts TBP's position within TFIID to allow simultaneous contacts of TBP with the upstream TATA and TAF1 with downstream core promoter elements (Fig. 5, top panel, right side). Thus, TAF1/TBP interactions repress the large-scale structural rearrangement within TFIID required for TBP to bind the TATA element in a core promoter.
FIG 5.
A representative model of how TFIID engages the core promoter at various p53-responsive promoters. A proposed model of how p53 aids TFIID in core promoter recognition based on our current and previous (20) studies is presented. EM structures of canonical and rearranged forms of TFIID are shown in light gray. Space fill models of the TFIID subunits, TBP (dark red), TAF1 (yellow), TAF2 (blue), and TAF6 (light red) are positioned in the rearranged form as shown in reference 27. In the canonical form of TFIID, TBP is roughly positioned based on the location of TFIIA as shown in reference 26. In the absence of p53, TFIID primarily exists in the canonical conformation which is weakly permissive for DNA binding due in part to interactions between the TAF1 TAND and TBP. The canonical form of TFIID can infrequently and transiently interact with DNA. In rare occurrences, canonical TFIID isomerizes to a structurally rearranged form that involves severance of TAF1 TAND/TBP contacts and movement of TBP from lobe A or C to lobe B. In the presence of p53, the TBP/TAF1 TAND interaction is disrupted, leading to formation of a TFIID complex that is highly permissive with respect to isomerization and stable DNA binding. Once TFIID structurally rearranges and binds DNA via movement of TBP from lobe A or C to lobe B, p53 disengages from TFIID through loss of its contacts with lobe A and C. Dissociation of p53 from the TFIID/promoter DNA scaffold results in “repurposing” of the p53 RE for further assembly of the PIC components.
TFIID's infrequent association with p53 target gene promoters occurs via distinct short-lived and long-lived DNA binding modes (Fig. 1B and 2B and C; see also Fig. S4B in the supplemental material). The majority (78%) of these TFIID/DNA binding events last just a few seconds (∼7 s) (Fig. 2B and D). This short-lived DNA binding population may represent a transient nonproductive TFIID encounter complex in the canonical form where TAF1 prevents stable TBP/TATA interactions (Fig. 5). In such a scenario, the TAFs may make multiple contacts with the initiator and/or DPE to temporarily anchor TFIID to DNA. It is likely that this transient dynamic interaction behavior explains why we were unable to obtain the 3D structure of those short-lived DNA-bound TFIID complexes via cryo-EM.
A potential mechanism for p53 regulation of TFIID/DNA binding.
A number of factors, including activators (i.e., c-Jun and VP16) and TFIIA, can stimulate TFIID/promoter binding by derepressing the TAF1-mediated inhibition of TBP (19, 48–51). TFIIA has also been found to aid conversion of canonical TFIID to the rearranged DNA binding form, likely via disruption of TBP/TAF1 TAND interactions (26, 27). It was previously unclear whether activators could also promote structural rearrangement of TFIID to stimulate promoter association. Our past work has shown that p53 binds stably to TFIID and targets TAF1 in the absence of DNA (20). We also found that p53's interaction with TFIID also results in movement of lobe A toward lobe B in a manner analogous to that seen with a structurally rearranged DNA binding form of TFIID (20). Therefore, we postulated that the level of DNA binding may be increased in this structurally rearranged preassembled p53/TFIID complex. Indeed, our single-molecule data, along with data from previous bulk biochemical experiments, indicate that p53 can dramatically stimulate the binding of TFIID to DNA (Fig. 1B) (19). Our results demonstrate that the conversion of canonical TFIID to the structurally rearranged form is enhanced in the presence of p53 and DNA (Fig. 4A). Thus, we speculate that p53, whose acidic activation domain is highly homologous to VP16 (52), may potentially disrupt TAF1/TBP interactions to aid in TFIID structural rearrangement and DNA binding (Fig. 5, bottom panel). Future high-resolution cryo-EM studies of a p53/TFIID complex will help provide better mechanistic insights into p53's ability to structurally regulate TBP/TAF1 contacts or to induce additional global changes in TFIID's conformation.
In the presence of p53, TFIID can still colocalize with a small percentage of the mutant core promoter DNA even in the absence of a p53 RE (Fig. 1B). In such cases, p53 may be disrupting the TAF1/TBP interaction to facilitate TBP's known ability to bind nonspecific DNA (53). However, at this time, our single-molecule assays cannot discriminate between TFIID's recognition of a secondary lower-affinity site and nonspecific DNA binding throughout the mutant core promoter template.
TFIID isomerization in the presence of p53 may be linked to long-lived DNA binding.
Cryo-EM of a TFIID/TFIIA/DNA complex has revealed a number of TBP/TAF contacts with core promoter elements spanning a region from approximately bp −33 to +32 surrounding the transcription start site (27). Many of these contacts, especially TBP/TATA, are possible only due to TFIID's structural rearrangement or isomerization upon DNA binding. Therefore, we anticipate that the p53-mediated binding of TFIID to DNA is also likely a multistep isomerization process involving contacts with multiple core promoter elements (Fig. 5, bottom panel). Indeed, our xlink-Exo analysis revealed numerous TFIID cross-links throughout the promoter that are enhanced by p53 (Fig. 1C). The locations of many of these TFIID/hdm2 DNA contacts in the core promoter were strictly conserved in the TFIID/TFIIA/supercore promoter DNA cryo-EM structure (27). Consistently, the architectures of TFIID bound to the hdm2 and supercore promoters were equivalent (Fig. S7 and S8). This suggests that TFIID can specifically bind our native hdm2 promoter in the presence of p53 to adopt a common DNA binding conformation.
In the presence of p53, TFIID associates with DNA robustly. Analysis of single-molecule dissociation kinetics also revealed two TFIID/core promoter DNA binding modes, with the interactions being predominantly long-lived (∼100 s) when p53 was present (Fig. 2B and D). Therefore, p53 shifts the TFIID population from short-lived to long-lived DNA binding, likely acting to facilitate TFIID's known isomerization on promoters (Fig. 5, bottom panel). This isomerization step may permit stable interaction of TFIID with the upstream DNA, TATA box, and downstream DNA, resulting in long-lived DNA binding (Fig. 5, bottom panel). Due to p53's dynamic interaction with DNA, we cannot directly pinpoint p53's structural impact on TFIID/promoter isomerization. Thus, currently we speculate that the p53-mediated long-lived DNA-bound TFIID complex is structurally related to the stable TFIID/TFIIA/promoter DNA complex depicted in our cryo-EM studies. In support of this model, previous biochemical assays have shown that activators induce conformational changes within TFIID that enhance multiple contacts with promoter sequences downstream of the TSS (10).
Mutation of the p53 RE affected only the residence time of TFIID's long-lived DNA binding population (Fig. S4). Importantly, the percentage of long-lived DNA binding events was essentially unchanged on the mutant RE template (Fig. 2D). This suggests that isomerization of TFIID does not depend on p53 contacts with the RE. Given that distinct TFIID contacts surrounding the p53 RE were identified via our xlink-Exo assays (Fig. 1C), we speculate that this upstream DNA is involved in both binding p53 and stabilizing interaction of an isomerized form of TFIID with the promoter. These upstream contacts may be mediated by the TAF4 subunit of TFIID, which was previously shown to bind ∼70 bp of DNA with a weak sequence preference (54).
TFIID isomerization as a mechanism to increase dissociation of p53 from the promoter.
Our single-molecule data indicate that TFIID reduces p53's residence time on DNA (Fig. 3A and B). This result is consistent with previous DNase I footprinting studies showing decreased binding of p53 to REs in the presence of TFIID (19). More importantly, p53's residence time on DNA is 6.7-fold shorter (∼15 s) than TFIID's (∼100 s) (Fig. 3C, right panel). Therefore, p53 disengages the promoter before TFIID during long-lived DNA binding events. In our cryo-EM structures, we are also unable to detect density associated with p53 in the rearranged DNA-bound TFIID, further confirming our single-molecule findings (Fig. S7, S8, and S9). Previous structural analysis showed that p53 bridges lobes A and C of TFIID in the absence of DNA (20). Thus, we speculate that lobe A's movement away from lobe C upon DNA binding disrupts p53/TFIID contacts, inducing p53's dissociation from the assembly (Fig. 5, bottom panel). In our current studies, the dynamic nature of p53's interaction prevented structural visualization of its location on the TFIID/DNA complex. To capture structural intermediates where p53 remains bound to the TFIID/DNA scaffold, future cryo-EM studies will be performed on p53/TFIID/DNA assemblies that are gently chemically cross-linked.
While p53 dissociates from the TFIID/DNA scaffold, it is possible that p53 reassociates with the complex, particularly at the high concentrations (600 nM) used in the experiments whose results are shown in Fig. S6. We suspect that p53's rapid removal could potentially lead to faster “repurposing” of the RE for additional rounds of p53-mediated recruitment of other basal transcription factors such as TFIIB (55, 56), RNA polymerase II (32), and TFIIH (31, 52, 57). Stable prolonged binding of p53 to a TFIID/DNA complex can potentially negatively regulate PIC formation and squelch transcription. In such a scenario, p53 would occupy the RE, which may inhibit subsequent recruitment of a p53/basal factor cocomplex. Alternatively, p53 transiently bound to TFIID and the RE at the promoter could still have an activation domain free to recruit other interacting basal factors. These two mechanisms are not mutually exclusionary. Future work will test these two possibilities and determine whether TFIID-mediated dissociation of p53 from the promoter is required for enhanced activator-dependent PIC formation.
TFIID/promoter binding dynamics in relation to transcriptional burst kinetics.
Once p53 loaded TFIID onto our native p53 target gene promoters, TFIID remained bound for only approximately 100 s, likely due to interactions with suboptimal or nonconsensus core promoter elements (Fig. 2). Notably, our residence times determined for TFIID at the promoter were measured in the absence of additional PIC components. Most likely, additional factors such as TFIIA, TFIIF, and RNA polymerase II alter the stability of TFIID and p53 as the PIC forms on promoter DNA. However, recent in vivo work suggests that transcription complex formation is highly dynamic. Consistent with our data, yeast TBP occupies the HSC82 heat shock promoter for 60 s in vivo (58). In human tissue culture cells, RNA polymerase II loads onto promoters approximately every 4 to 9 s to form convoys on an active gene (23). These bursts of RNA polymerase II convoys last for approximately 100 s before the promoter becomes inactive again (23). Therefore, it is tempting to speculate that the long-lived TFIID residency (∼100 s) on our native target promoters could be related to RNA polymerase II convoy duration (∼100 s) and transcriptional bursting. TFIID's dynamic binding to promoters could be a mechanism to allow rapid shutdown of stress response gene expression after reestablishment of cellular homeostasis.
TFIID can also direct infrequent loading of RNA polymerase II convoys on mutant TATA-containing promoters, leading to bursts of transcription (23). Interestingly, the duration of RNA polymerase II convoys on the mutant TATA promoter is only slightly less than that on a wild-type TATA template (∼60 to 80 s) (23). Our study also revealed infrequent p53-mediated binding of TFIID on our mutant core promoter DNA lasting for a significantly long time (∼64 s) (Fig. 2D and S4). Therefore, we suspect that TFIID stably loaded onto our mutant core DNA is fully competent to direct low levels of infrequent transcription. Overall, our studies indicated that p53 and, potentially, activators in general serve as escorts to dynamically recruit and load the basal transcription machinery onto DNA via complex interactions among the RE, the core promoter, and bound basal factors (i.e., TFIID). Our work also revealed how p53 stimulates TFIID binding to different promoters that comprise variable arrangements of p53 binding sites and core promoter elements. More importantly, our combined functional and structural studies are crucial for understanding how eukaryotic transcription complexes dynamically assemble on different protein-coding gene promoters.
MATERIALS AND METHODS
Reagents and protein purification.
Experimental details, including reagents, protein purification of the p53/TFIID/TFIIA/DNA cocomplexes, and the fluorescence-labeled proteins/DNA used in this study, along with our custom-built TIRF microscope setup, are described in the supplemental material.
In vitro immunoassembly assay.
HeLa cells (32 liters) were grown in suspension culture with 1× Dulbecco's modified Eagle's medium (DMEM) plus 5% newborn calf serum for each assay. Nuclear extract was prepared and fractionated with phosphocellulose P11 (P-Cell) resins exactly as previously described (20). P-Cell column fractions eluting at 1 M KCl–HEMG buffer (20 mM HEPES, 0.2 mM EDTA, 2 mM MgCl2, 10% glycerol, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) (pH 7.9) were pooled, dialyzed to 0.3 M KCl–HEMG buffer, combined with final concentration of 0.1% NP-40, and then immunoprecipitated overnight at 4°C with an anti-TAF4 monoclonal antibody (MAb) covalently conjugated to protein G-Sepharose beads (GE Healthcare Life Sciences). TAF4 immunoprecipitates were extensively washed with 0.65 M KCl–HEMG buffer, 0.3 M KCl–HEMG buffer, and 0.1 M KCl–HEMG buffer (containing 0.05% NP-40 buffer and 10 μM leupeptin) and split into four assembly reaction mixtures as indicated in Fig. 1. Three reaction mixtures containing TFIID-bound resins were incubated with a 6-fold molar excess of promoter DNA. Next, two TFIID/DNA reaction mixtures were further combined with a 10-fold molar excess of p53 alone and/or with TFIIA added. In total, four reaction mixtures were incubated at 4°C for 2 h prior to elution of the cocomplex from the TAF4 MAb affinity resin. TFIID/factor/DNA-bound resins were washed 5 times with 0.1 M KCl–HEMG buffer (containing 0.1% NP-40 and 10 μM leupeptin) followed by elution with a peptide (1 mg/ml) in 0.1 M KCl–HEMG buffer (plus 0.1% NP-40 and 10 μM leupeptin) recognized by the TAF4 MAb. Eluates were analyzed with 4% to 12% SDS-PAGE (NuPage gel system; Thermo Fisher) and visualized by silver staining analysis.
Xlink-Exo assay.
Exonuclease assays were carried out in 10-μl reaction mixtures containing a modified 50 mM KCl–HEMG buffer (pH 7.9) (HEMG buffer [12.5 mM HEPES, 0.05 mM EDTA, 6.25 mM MgCl2, 5% glycerol] plus 1% DTT, 0.05 M PMSF, and 0.01% NP-40), IRdye800 (IDT) antisense strand-labeled hdm2 promoter DNA (9 nM), p53 (90 nM, monomer), and TFIID (4 nM). After incubation at room temperature for 20 min, the reaction mixtures were cross-linked with formaldehyde (1% final) for 5 min and stopped by addition of Tris-HCl (100 mM final concentration, pH 8.0). Next, each reaction mixture was mixed with exonuclease III (NEB) (50 units diluted in a buffer containing MgCl2 [16.25 mM final concentration]) and incubated at 37°C for 1 h. Proteinase K (10 μg) was then added into each reaction, and the reaction mixture was incubated at 70°C for 3 h. Reaction mixtures were mixed in formamide loading dye, heated at 95°C, and resolved on a 8% polyacrylamide–Tris-borate-EDTA (TBE) gel containing 7 M urea. A series of known 5′ truncated IRdye800 (IDT) antisense strand-labeled wild-type hdm2 DNA fragments were generated and used as molecular standards to determine the size of exonuclease-digested bands seen on our mapping gels.
TIRF single-molecule imaging.
Passivated glass coverslips (see the supplemental material) were assembled into a multichannel flow cell using double-sided tape (3M). Flow cells were mounted on the microscope, and streptavidin was added to the flow cell to coat surfaces. Each flow cell held ∼50 μl of sample. Reference beads that served as fiducial marks were immobilized on the bottom surface of the flow cell. Biotinylated Cy3/Cy5 promoter DNA in 1× phosphate-buffered saline (PBS) was then loaded into the flow cell and bound over time specifically to the streptavidin that was noncovalently attached through biotin linkages on the top and bottom surfaces. Promoter DNA templates were briefly imaged at 3 Hz to determine surface-attached DNA densities that were sufficient for localization of single DNA molecules. Unbound biotinylated Cy3/Cy5 promoter DNA was washed away. Promoter DNA templates were then imaged at 3 Hz. The surface-attached Cy3/Cy5 promoter DNA was then photobleached. Reactivation of the Cy3/Cy5-labeled promoter DNA was not evident over the time scale of our experiments (typically 15 to 20 min). Qdot 565-labeled TFIID (1 nM) was then added in the absence and presence of unlabeled p53 (8 nM, monomer) to the flow cell in modified 50 mM KCl–HEMG buffer (pH 7.9) (HEMG buffer [12.5 mM HEPES, 0.05 mM EDTA, 6.25 mM MgCl2, 5% glycerol] plus 1% DTT, 0.05 M PMSF, and 0.01% NP-40) and immediately imaged at 3 Hz at 23°C. An oxygen-scavenging system consisting of 0.4% b-d-glucose, 1 mg/ml glucose oxidase (Sigma), and 0.4 mg/ml catalase (Sigma) was used for imaging. For experiments designed to measure p53's interaction with promoter DNA, Alexa Fluor 555- or Cy3- or Cy5-labeled p53 (8 nM, monomer) was added in the absence or presence of unlabeled TFIID (1 nM) to the flow cell containing surfaced-attached mapped promoter DNA and immediately imaged at 3 Hz. Alexa Fluor 555-labeled p53, and Cy3-labeled p53, and Cy5-labeled p53 had equivalent residence times on promoter DNA, indicating that the photobleaching of the molecules did not significantly affect the results.
Single-molecule colocalization analysis.
Fluorescent spots in each frame for the movie corresponding to the promoter DNA, TFIID, or p53 were mapped using 2D Gaussian fitting methods (21, 59, 60) to determine locations (x, y) at subpixel resolution. Colocalization analysis was performed as described in reference 21 with the following modifications. Coordinates of fluorescent spots were adjusted on the basis of the movement of the fiducial marks (i.e., reference beads) over time. Stage movement or drift was typically less than 200 nm over the time of imaging (∼20 min). Fluorescent spots that were located within 84 nm (1 pixel) throughout the movie were grouped in a cluster. The positions of all spots within a cluster were averaged to obtain the overall location of an individual cluster of molecules. Position maps of the clustered surface-attached DNA molecules were then compared with position maps of either the TFIID clusters or the p53 clusters. Clusters of TFIID or p53 molecules were found within 3 pixels (252 nm) of a DNA cluster. Displacements of TFIID or p53 clustered molecules with a maximum equal to (0, 0) in the x and y positions are indicative of nonrandom colocalization between DNA and TFIID or p53 clusters. Displacements between the x and y positions of the TFIID or p53 clusters and those of the DNA clusters were then plotted as a 2D histogram with a bin size of 50 nm. 2D Gaussian fitting of the 2D histogram yields σ, which is equivalent to the variation in the displacement of TFIID or p53 within 252 nm of a DNA molecule. A colocalization threshold was set to 1.8 σ (84 nm).
Single-molecule residence time analysis of TFIID and p53 on promoter DNA.
For each TFIID or p53 cluster that colocalized with a DNA cluster (i.e., within 84 nm), we then plotted the amplitude from the 2D Gaussian fit of every fluorescent spot within the TFIID or p53 cluster as a function of time. In-house-customized MATLAB software was then used to analyze the rise and the fall of the amplitude of the fluorescent signal to determine the appearance and corresponding disappearance of each spot over time. The residence time for each DNA binding event is defined as the difference in time between the appearance and disappearance of a TFIID or p53 fluorescent spot. The fluorescent signal from TFIID or p53 had to last for at least 1.5 s to be considered a discrete binding event in our analysis. Due to rapid stochastic blinking of the quantum dot, a dissociation event was considered to have occurred only if the quantum dot signal remained in the off state longer than 4.5 s. At least 300 binding events of colocalized TFIID or 100 binding events of p53 clusters were analyzed for each particular promoter DNA construct. For a subset of molecules (∼100), the data were processed by hand to determine the appearance and disappearance of fluorescent spots. Comparison of the residence times generated by our automated software and by hand yielded negligible differences. Histograms of residence times for TFIID or p53 bound to promoter DNA were fitted to a two-component exponential decay model to determine the average residence time of TFIID or p53 bound to promoter DNA (60). If the difference between the fitted residence times for each individual component in the two-component model was less than 20%, histograms were refitted using a single-component exponential decay model (60). For Fig. 3 and Fig. S5 in the supplemental material, data were additionally fitted to a gamma distribution (41) via the use of custom MATLAB software. A two-sided Student's t test was used to determine statistical significance.
2D classification analysis of TFIID cocomplexes via negative-stain EM.
A 4-μl volume (total amount, 10 to 20 ng) of fresh TFIID/p53/bax DNA assemblies in 0.1 M KCl–HEMG buffer (20 mM HEPES, 0.2 mM EDTA, 2 mM MgCl2, 10% glucose [final pH 7.9]) was applied directly onto a thin carbon film supported by holey carbon on a 400-mesh copper grid (Pacific Grid Tech), which was freshly glow discharged, for 3 min. After incubation of the sample on the grid, the sample grid was stained with five successive 75-μl drops of 1% uranyl formate.
The negative-stain image data were collected with a Tecnai F20 Twin transmission electron microscope operating at 120 KeV at a calibrated magnification of ×62,000 with a defocus range of −0.5 μm to −2.5 μm using a Tietz F416 (4,000- by 4,000-pixel) charge-coupled-device (CCD) camera (resulting in 2.82 Å/pixel) at a dosage of 39.98 e−/Å. Totals of 14,000 particles (for TFIID alone), 13,855 particles (for TFIID/p53), and 19,262 particles (for TFIID/p53/bax DNA) were manually picked using boxer (EMAN [61]). These particles were aligned and classified in a reference-free fashion as previously described (24) for 8 iterations. The similarity of the measurement of lobe's A position to those of lobes B and C was employed as described in reference 26. On the basis of the position of lobe A in relation to that of lobe C versus that of lobe B (26), three conformational groups of TFIID (i.e., canonical, transition, and rearranged states) were defined in this study. The canonical form is represented by lobe A retaining its attachment to lobe C. The transition state is represented by lobe A beginning to detach and move between lobe C and B. The rearranged form is represented by lobe A reattaching to lobe B. Next, all 2D class averages yielded from iterations 5, 6, 7, and 8 for each TFIID/p53 preparation with or without the bax DNA were examined carefully to select 2D class averages displaying views of these three conformational groups. The number of 2D averages in each conformational form versus the total averages was calculated to yield the percentages of TFIID's three conformations in each TFIID/p53 preparation with or without the bax DNA. Three independent protein purifications and EM data acquisition/analysis were performed to obtain the standard deviation for the figure. A two-sided Student's t test was used to determine statistical significance.
Cryo-EM data collection and image analysis.
For cryo-EM sample grid preparation, a thin carbon film supported by a 400-mesh carbon-thickened C-flat holey grid with hole diameters of 1.2 μm and spacing of 1.3 μm (CF-1.2/1.3-4C; Protochips) was subjected to plasma cleaning for 2 min using a Denton high-vacuum evaporator. The grid was loaded into a Vitrobot (FEI) that was preset at 100% humidity at 4°C for vitrification of our samples. A 3-μl volume (total amount, approximately 504 to 900 ng) of the p53/TFIID/TFIIA/DNA assemblies was applied directly onto the grid, incubated for 2 min, and then blotted for 6.5 s prior to being plunge-frozen in liquid ethane.
Cryo-EM data were collected with a JEM-2100F transmission electron microscope (Jeol) operated at 120 KeV at a magnification of ×50,000 with a defocus range of −2.5 μm to −4.5 μm. Digital micrographs were collected using a TEM 2,048- by 2,048-pixel CCD camera (TemCam-F224HD; TVIPS) with a pixel size of 24 μm and calibrated magnification of ×84,037 (resulting in 2.86 Å/pixel) under low-dose (∼13 e−/Å) conditions via the use of semiautomatic image acquisition SerialEM software (62). Digital micrographs showed homogeneous particles in terms of a lack of aggregation and the apparent integrity of the intact complexes.
Particles were manually selected using boxer (EMAN) (61) for initial totals of 32,275 particles from the p53/TFIID/TFIIA/bax DNA data set and 22,070 particles from the p53/TFIID/TFIIA/hdm2 DNA data set. The particles were then phase flipped using contrast transfer function (CTF)-estimated values determined by CTFFIND3 (63) and extracted using SPIDER (system for processing of image data from electron microscopy and related fields [64]) to a particle window size of 128 by 128 pixels (5.72 Å/pixel), following the protocol described in reference 26. The particles were then normalized prior to the following data analyses. Reference-free 2D class averaging image analysis was performed using IMAGIC (65), precisely following the procedure described previously (20). Using these 2D class averages as the templates, we automatically picked particles from a larger cryo-EM data set using Signature (66) embedded in the Appion image processing platform (67). A final total of 59,632 particles for the p53/TFIID/TFIIA/bax DNA cocomplex was obtained, normalized, and low-pass band filtered to perform the 3D reconstruction using SPIDER's multireference projection-matching approach as previously described (29). Both rearranged and canonical forms of TFIID/TFIIA/SCP DNA from the Electron Microscopy Data Bank (accession numbers 2282 and 2283, respectively) were filtered to 60 Å as a reference for the first cycle of projection matching. Different initial reference models, including TFIID alone, p53-bound TFIID, and TFIIA-bound TFIID, were also tested for model bias refinement. New 3D volumes reconstructed from the data set were used as references for all subsequent cycles of alignment (using angular step sizes ranging from an initial 20° to a final 2°). The resolution throughout the refinement was determined by the 0.5 cutoff in the Fourier shell correlation (FSC) curve. The 3D structure of the p53/TFIID/TFIIA/bax assembly was filtered at a final resolution of ∼35 Å. All the 3D reconstructions were represented as isodensity surfaces using the UCSF Chimera package (68) developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco.
Supplementary Material
ACKNOWLEDGMENTS
We specifically thank R. Tjian, S. Chu, and E. Nogales for their initial support of this study, S. Zheng for generating TAF4 MAb supernatant and HeLa cells, D. King for providing the TAF4 MAb elution peptide, A. Revyakin, A. Pertsinidis, and S. R. Park for their help on initial stages of the single-molecule microscopy, and R. Henderson for his technical advice critical for the cryo-EM work. We also thank S. M. Shenoy, J. Hargitai, J. Wang, S. Lenny, J. Greally, and E. Eng for high-performance computing cluster and cryo-EM technical support. We appreciate assistance from Einstein's AIF facility, especially F. P. Macaluso, L. Cummins, and G. S. Perumal. We are grateful to M. Keogh, Charles Kenworthy, and E. Nogales for critical comments of the manuscript.
This study was supported by startup funds (Albert Einstein College of Medicine) and by NIH/NIBIB 5U01EB021236-02 (R.A.C.). Some of this work was performed at the Simons Electron Microscopy Center at the New York Structural Biology Center, which is supported by a grant from the Simons Foundation (grant no. 349247) with additional support from NIH S10 OD019994-01, the Agouron Institute (grant no. F00316), NIH S10 RR029300-01, NIH S10 RR017291-01, NYSTAR, and NIH C06 RR017528-01-CEM. The Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, is supported by NIH NIGMS P41-GM103311. W.L.L. is an affiliated member of the New York Structural Biology Center. M.C. is an HHMI fellow of the Damon Runyon Cancer Research Foundation.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00085-17.
REFERENCES
- 1.Menendez D, Inga A, Resnick MA. 2009. The expanding universe of p53 targets. Nat Rev Cancer 9:724–737. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- 2.Bieging KT, Mello SS, Attardi LD. 2014. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer 14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tafvizi A, Huang F, Fersht AR, Mirny LA, van Oijen AM. 2011. A single-molecule characterization of p53 search on DNA. Proc Natl Acad Sci U S A 108:563–568. doi: 10.1073/pnas.1016020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H, Levine AJ. 1995. Human TAFII31 protein is a transcriptional coactivator of the p53 protein. Proc Natl Acad Sci U S A 92:5154–5158. doi: 10.1073/pnas.92.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thut CJ, Chen JL, Klemm R, Tjian R. 1995. p53 transcriptional activation mediated by coactivators TAFII40 and TAFII60. Science 267:100–104. doi: 10.1126/science.7809597. [DOI] [PubMed] [Google Scholar]
- 6.Buschmann T, Lin Y, Aithmitti N, Fuchs SY, Lu H, Resnick-Silverman L, Manfredi JJ, Ronai Z, Wu X. 2001. Stabilization and activation of p53 by the coactivator protein TAFII31. J Biol Chem 276:13852–13857. doi: 10.1074/jbc.M103786200. [DOI] [PubMed] [Google Scholar]
- 7.Li AG, Piluso LG, Cai X, Gadd BJ, Ladurner AG, Liu X. 2007. An acetylation switch in p53 mediates holo-TFIID recruitment. Mol Cell 28:408–421. doi: 10.1016/j.molcel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Juven-Gershon T, Hsu JY, Kadonaga JT. 2006. Perspectives on the RNA polymerase II core promoter. Biochem Soc Trans 34:1047–1050. doi: 10.1042/BST0341047. [DOI] [PubMed] [Google Scholar]
- 9.Colgan J, Manley JL. 1992. TFIID can be rate limiting in vivo for TATA-containing, but not TATA-lacking, RNA polymerase II promoters. Genes Dev 6:304–315. doi: 10.1101/gad.6.2.304. [DOI] [PubMed] [Google Scholar]
- 10.Chi T, Carey M. 1996. Assembly of the isomerized TFIIA–TFIID–TATA ternary complex is necessary and sufficient for gene activation. Genes Dev 10:2540–2550. doi: 10.1101/gad.10.20.2540. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Gralla JD, Carey M. 1992. The acidic activator GAL4-AH can stimulate polymerase II transcription by promoting assembly of a closed complex requiring TFIID and TFIIA. Genes Dev 6:1716–1727. doi: 10.1101/gad.6.9.1716. [DOI] [PubMed] [Google Scholar]
- 12.White J, Brou C, Wu J, Lutz Y, Moncollin V, Chambon P. 1992. The acidic transcriptional activator GAL-VP16 acts on preformed template-committed complexes. EMBO J 11:2229–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi T, Carey M. 1993. The ZEBRA activation domain: modular organization and mechanism of action. Mol Cell Biol 13:7045–7055. doi: 10.1128/MCB.13.11.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieberman PM, Berk AJ. 1994. A mechanism for TAFs in transcriptional activation: activation domain enhancement of TFIID-TFIIA–promoter DNA complex formation. Genes Dev 8:995–1006. doi: 10.1101/gad.8.9.995. [DOI] [PubMed] [Google Scholar]
- 15.Chi T, Lieberman P, Ellwood K, Carey M. 1995. A general mechanism for transcriptional synergy by eukaryotic activators. Nature 377:254–257. doi: 10.1038/377254a0. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi N, Boyer TG, Berk AJ. 1995. A class of activation domains interacts directly with TFIIA and stimulates TFIIA-TFIID-promoter complex assembly. Mol Cell Biol 15:6465–6473. doi: 10.1128/MCB.15.11.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee HS, Pugh BF. 2012. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 483:295–301. doi: 10.1038/nature10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman PM, Ozer J, Gursel DB. 1997. Requirement for transcription factor IIA (TFIIA)-TFIID recruitment by an activator depends on promoter structure and template competition. Mol Cell Biol 17:6624–6632. doi: 10.1128/MCB.17.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing J, Sheppard HM, Corneillie SI, Liu X. 2001. p53 stimulates TFIID-TFIIA-promoter complex assembly, and p53-T antigen complex inhibits TATA binding protein-TATA interaction. Mol Cell Biol 21:3652–3661. doi: 10.1128/MCB.21.11.3652-3661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu WL, Coleman RA, Ma E, Grob P, Yang JL, Zhang Y, Dailey G, Nogales E, Tjian R. 2009. Structures of three distinct activator-TFIID complexes. Genes Dev 23:1510–1521. doi: 10.1101/gad.1790709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revyakin A, Zhang Z, Coleman RA, Li Y, Inouye C, Lucas JK, Park SR, Chu S, Tjian R. 2012. Transcription initiation by human RNA polymerase II visualized at single-molecule resolution. Genes Dev 26:1691–1702. doi: 10.1101/gad.194936.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, English BP, Grimm JB, Kazane SA, Hu W, Tsai A, Inouye C, You C, Piehler J, Schultz PG, Lavis LD, Revyakin A, Tjian R. 2016. Rapid dynamics of general transcription factor TFIIB binding during preinitiation complex assembly revealed by single-molecule analysis. Genes Dev 30:2106–2118. doi: 10.1101/gad.285395.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tantale K, Mueller F, Kozulic-Pirher A, Lesne A, Victor JM, Robert MC, Capozi S, Chouaib R, Backer V, Mateos-Langerak J, Darzacq X, Zimmer C, Basyuk E, Bertrand E. 2016. A single-molecule view of transcription reveals convoys of RNA polymerases and multi-scale bursting. Nat Commun 7:12248. doi: 10.1038/ncomms12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu WL, Coleman RA, Grob P, King DS, Florens L, Washburn MP, Geles KG, Yang JL, Ramey V, Nogales E, Tjian R. 2008. Structural changes in TAF4b-TFIID correlate with promoter selectivity. Mol Cell 29:81–91. doi: 10.1016/j.molcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papai G, Tripathi MK, Ruhlmann C, Layer JH, Weil PA, Schultz P. 2010. TFIIA and the transactivator Rap1 cooperate to commit TFIID for transcription initiation. Nature 465:956–960. doi: 10.1038/nature09080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cianfrocco MA, Kassavetis GA, Grob P, Fang J, Juven-Gershon T, Kadonaga JT, Nogales E. 2013. Human TFIID binds to core promoter DNA in a reorganized structural state. Cell 152:120–131. doi: 10.1016/j.cell.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louder RK, He Y, Lopez-Blanco JR, Fang J, Chacon P, Nogales E. 2016. Structure of promoter-bound TFIID and model of human pre-initiation complex assembly. Nature 531:604–609. doi: 10.1038/nature17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andel F III, Ladurner AG, Inouye C, Tjian R, Nogales E. 1999. Three-dimensional structure of the human TFIID-IIA-IIB complex. Science 286:2153–2156. doi: 10.1126/science.286.5447.2153. [DOI] [PubMed] [Google Scholar]
- 29.Grob P, Cruse MJ, Inouye C, Peris M, Penczek PA, Tjian R, Nogales E. 2006. Cryo-electron microscopy studies of human TFIID: conformational breathing in the integration of gene regulatory cues. Structure 14:511–520. doi: 10.1016/j.str.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Meyer KD, Lin SC, Bernecky C, Gao Y, Taatjes DJ. 2010. p53 activates transcription by directing structural shifts in Mediator. Nat Struct Mol Biol 17:753–760. doi: 10.1038/nsmb.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Léveillard T, Andera L, Bissonnette N, Schaeffer L, Bracco L, Egly JM, Wasylyk B. 1996. Functional interactions between p53 and the TFIIH complex are affected by tumour-associated mutations. EMBO J 15:1615–1624. [PMC free article] [PubMed] [Google Scholar]
- 32.Singh SK, Qiao Z, Song L, Jani V, Rice W, Eng E, Coleman RA, Liu WL. 2016. Structural visualization of the p53/RNA polymerase II assembly. Genes Dev 30:2527–2537. doi: 10.1101/gad.285692.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourdon JC, Deguin-Chambon V, Lelong JC, Dessen P, May P, Debuire B, May E. 1997. Further characterisation of the p53 responsive element–identification of new candidate genes for trans-activation by p53. Oncogene 14:85–94. doi: 10.1038/sj.onc.1200804. [DOI] [PubMed] [Google Scholar]
- 34.Juven-Gershon T, Cheng S, Kadonaga JT. 2006. Rational design of a super core promoter that enhances gene expression. Nat Methods 3:917–922. doi: 10.1038/nmeth937. [DOI] [PubMed] [Google Scholar]
- 35.Chang GS, Chen XA, Park B, Rhee HS, Li P, Han KH, Mishra T, Chan-Salis KY, Li Y, Hardison RC, Wang Y, Pugh BF. 2014. A comprehensive and high-resolution genome-wide response of p53 to stress. Cell Rep 8:514–527. doi: 10.1016/j.celrep.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galasinski SK, Lively TN, Grebe De Barron A, Goodrich JA. 2000. Acetyl coenzyme A stimulates RNA polymerase II transcription and promoter binding by transcription factor IID in the absence of histones. Mol Cell Biol 20:1923–1930. doi: 10.1128/MCB.20.6.1923-1930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Boskovic Z, Hussain MM, Hu W, Inouye C, Kim HJ, Abole AK, Doud MK, Lewis TA, Koehler AN, Schreiber SL, Tjian R. 28 August 2015. Chemical perturbation of an intrinsically disordered region of TFIID distinguishes two modes of transcription initiation. Elife doi: 10.7554/eLife.07777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazur SJ, Sakaguchi K, Appella E, Wang XW, Harris CC, Bohr VA. 1999. Preferential binding of tumor suppressor p53 to positively or negatively supercoiled DNA involves the C-terminal domain. J Mol Biol 292:241–249. doi: 10.1006/jmbi.1999.3064. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg RL, Freund SM, Veprintsev DB, Bycroft M, Fersht AR. 2004. Regulation of DNA binding of p53 by its C-terminal domain. J Mol Biol 342:801–811. doi: 10.1016/j.jmb.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 40.Emamzadah S, Tropia L, Vincenti I, Falquet B, Halazonetis TD. 2014. Reversal of the DNA-binding-induced loop L1 conformational switch in an engineered human p53 protein. J Mol Biol 426:936–944. doi: 10.1016/j.jmb.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 41.Floyd DL, Harrison SC, van Oijen AM. 2010. Analysis of kinetic intermediates in single-particle dwell-time distributions. Biophys J 99:360–366. doi: 10.1016/j.bpj.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyashita T, Reed JC. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 43.Phelps M, Darley M, Primrose JN, Blaydes JP. 2003. p53-independent activation of the hdm2-P2 promoter through multiple transcription factor response elements results in elevated hdm2 expression in estrogen receptor alpha-positive breast cancer cells. Cancer Res 63:2616–2623. [PubMed] [Google Scholar]
- 44.Liu D, Ishima R, Tong KI, Bagby S, Kokubo T, Muhandiram DR, Kay LE, Nakatani Y, Ikura M. 1998. Solution structure of a TBP-TAF(II)230 complex: protein mimicry of the minor groove surface of the TATA box unwound by TBP. Cell 94:573–583. doi: 10.1016/S0092-8674(00)81599-8. [DOI] [PubMed] [Google Scholar]
- 45.Anandapadamanaban M, Andresen C, Helander S, Ohyama Y, Siponen MI, Lundstrom P, Kokubo T, Ikura M, Moche M, Sunnerhagen M. 2013. High-resolution structure of TBP with TAF1 reveals anchoring patterns in transcriptional regulation. Nat Struct Mol Biol 20:1008–1014. doi: 10.1038/nsmb.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kokubo T, Gong DW, Yamashita S, Horikoshi M, Roeder RG, Nakatani Y. 1993. Drosophila 230-kD TFIID subunit, a functional homolog of the human cell cycle gene product, negatively regulates DNA binding of the TATA box-binding subunit of TFIID. Genes Dev 7:1033–1046. doi: 10.1101/gad.7.6.1033. [DOI] [PubMed] [Google Scholar]
- 47.Kokubo T, Yamashita S, Horikoshi M, Roeder RG, Nakatani Y. 1994. Interaction between the N-terminal domain of the 230-kDa subunit and the TATA box-binding subunit of TFIID negatively regulates TATA-box binding. Proc Natl Acad Sci U S A 91:3520–3524. doi: 10.1073/pnas.91.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lively TN, Ferguson HA, Galasinski SK, Seto AG, Goodrich JA. 2001. c-Jun binds the N terminus of human TAF(II)250 to derepress RNA polymerase II transcription in vitro. J Biol Chem 276:25582–25588. doi: 10.1074/jbc.M100278200. [DOI] [PubMed] [Google Scholar]
- 49.Lively TN, Nguyen TN, Galasinski SK, Goodrich JA. 2004. The basic leucine zipper domain of c-Jun functions in transcriptional activation through interaction with the N terminus of human TATA-binding protein-associated factor-1 (human TAF(II)250). J Biol Chem 279:26257–26265. doi: 10.1074/jbc.M400892200. [DOI] [PubMed] [Google Scholar]
- 50.Kokubo T, Swanson MJ, Nishikawa JI, Hinnebusch AG, Nakatani Y. 1998. The yeast TAF145 inhibitory domain and TFIIA competitively bind to TATA-binding protein. Mol Cell Biol 18:1003–1012. doi: 10.1128/MCB.18.2.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozer J, Mitsouras K, Zerby D, Carey M, Lieberman PM. 1998. Transcription factor IIA derepresses TATA-binding protein (TBP)-associated factor inhibition of TBP-DNA binding. J Biol Chem 273:14293–14300. doi: 10.1074/jbc.273.23.14293. [DOI] [PubMed] [Google Scholar]
- 52.Xiao H, Pearson A, Coulombe B, Truant R, Zhang S, Regier JL, Triezenberg SJ, Reinberg D, Flores O, Ingles CJ, Greenblatt J. 1994. Binding of basal transcription factor TFIIH to the acidic activation domains of VP16 and p53. Mol Cell Biol 14:7013–7024. doi: 10.1128/MCB.14.10.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coleman RA, Pugh BF. 1995. Evidence for functional binding and stable sliding of the TATA binding protein on nonspecific DNA. J Biol Chem 270:13850–13859. doi: 10.1074/jbc.270.23.13850. [DOI] [PubMed] [Google Scholar]
- 54.Gazit K, Moshonov S, Elfakess R, Sharon M, Mengus G, Davidson I, Dikstein R. 2009. TAF4/4b x TAF12 displays a unique mode of DNA binding and is required for core promoter function of a subset of genes. J Biol Chem 284:26286–26296. doi: 10.1074/jbc.M109.011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Berk AJ. 1995. Reversal of in vitro p53 squelching by both TFIIB and TFIID. Mol Cell Biol 15:6474–6478. doi: 10.1128/MCB.15.11.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Espinosa JM, Verdun RE, Emerson BM. 2003. p53 functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol Cell 12:1015–1027. doi: 10.1016/S1097-2765(03)00359-9. [DOI] [PubMed] [Google Scholar]
- 57.Okuda M, Nishimura Y. 2015. Real-time and simultaneous monitoring of the phosphorylation and enhanced interaction of p53 and XPC acidic domains with the TFIIH p62 subunit. Oncogenesis 4:e150. doi: 10.1038/oncsis.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poorey K, Viswanathan R, Carver MN, Karpova TS, Cirimotich SM, McNally JG, Bekiranov S, Auble DT. 2013. Measuring chromatin interaction dynamics on the second time scale at single-copy genes. Science 342:369–372. doi: 10.1126/science.1242369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang B, Jones SA, Brandenburg B, Zhuang X. 2008. Whole-cell 3D STORM reveals interactions between cellular structures with nanometer-scale resolution. Nat Methods 5:1047–1052. doi: 10.1038/nmeth.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Zhang Z, Li L, Chen BC, Revyakin A, Hajj B, Legant W, Dahan M, Lionnet T, Betzig E, Tjian R, Liu Z. 2014. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell 156:1274–1285. doi: 10.1016/j.cell.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ludtke SJ, Baldwin PR, Chiu W. 1999. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol 128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 62.Mastronarde DN. 2005. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Mindell JA, Grigorieff N. 2003. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol 142:334–347. doi: 10.1016/S1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 64.Shaikh TR, Gao H, Baxter WT, Asturias FJ, Boisset N, Leith A, Frank J. 2008. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat Protoc 3:1941–1974. doi: 10.1038/nprot.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M. 1996. A new generation of the IMAGIC image processing system. J Struct Biol 116:17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 66.Chen JZ, Grigorieff N. 2007. SIGNATURE: a single-particle selection system for molecular electron microscopy. J Struct Biol 157:168–173. doi: 10.1016/j.jsb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Lander GC, Stagg SM, Voss NR, Cheng A, Fellmann D, Pulokas J, Yoshioka C, Irving C, Mulder A, Lau PW, Lyumkis D, Potter CS, Carragher B. 2009. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J Struct Biol 166:95–102. doi: 10.1016/j.jsb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.