Abstract
Background
In beef cattle, changes in the periovulatory endocrine milieu are associated with fertility and conceptus growth. A large preovulatory follicle (POF) and the resulting elevated concentrations of progesterone (P4) during diestrus positively affect pregnancy rates. Amino acids (AA) are important components of maternally derived secretions that are crucial for embryonic survival before implantation. The hypothesis is that the size of the POF and the concentration of P4 in early diestrus modulate the endometrial abundance of SLC transcripts related to AA transport and metabolism and subsequently impact luminal concentrations of AA. The follicle growth of Nelore cows was manipulated to produce two experimental groups: large POF and CL (LF-LCL group) and small POF and CL (SF-SCL group). On Day 4 (D4; Experiment 1) and Day 7 (D7; Experiment 2) after GnRH-induced ovulation (GnRH treatment = D0), the animals were slaughtered and uterine tissues and uterine washings were collected. qRT-PCR was used to evaluate the expression levels of AA transporters in D4 and D7 endometrial tissues. The concentrations of AA were quantified in D4 and D7 uterine washings by HPLC.
Results
Transcript results show that, on D4, SLC6A6, SLC7A4, SLC17A5, SLC38A1, SLC38A7 and SCLY and on D7 SLC1A4, SLC6A1, SLC6A14, SLC7A4, SLC7A7, SLC7A8, SLC17A5, SLC38A1, SLC38A7, SLC43A2 and DDO were more abundant in the endometria of cows from the LF-LCL group (P < 0.05). In addition, concentrations of AA in the uterine lumen were influenced by the endocrine profiles of the mother. In this context, D4 uterine washings revealed that greater concentrations of taurine, alanine and α-aminobutyric acid were present in SF-SCL (P < 0.05). In contrast, lower concentrations of valine and cystathionine were quantified on D7 uterine washings from SF-SCL cows (P < 0.05).
Conclusion
The present study revealed an association between the abundance of transcripts related to AA transport and metabolism in the endometrium and specific periovulatory endocrine profiles related to the receptive status of the mother. Such insights suggest that AAs are involved in uterine function to support embryo development.
Keywords: Amino acids, Beef cattle, Sex steroids, Uterus
Background
A profitable beef cattle production system requires high reproductive efficiency. Acceptable female fertility rates depend on an in-depth understanding of endocrine, cellular and molecular mechanisms regulating pregnancy. Hormonal variations during each bovine estrous cycle induce uterine changes that are crucial for uterine receptivity for conceptus development and implantation. Elevated levels of plasmatic progesterone (P4) immediately after conception are related to advanced conceptus elongation [1–3]. Moreover, recent studies have shown that low plasmatic concentrations of P4 are associated with a suboptimal uterine environment for conceptus (or blastocyst) development [3–5]. Conversely, a positive association exists between the probability of a successful pregnancy and plasma concentrations of P4 at D7 after estrus in both dairy and beef cows [4, 6]. However, the identities of molecules and mechanisms responsible for triggering the latter phenomena need further investigation.
In this context, information on the involvement of amino acid (AA) transport and metabolic pathways and availability to the embryo during early diestrus remains limited. During the first two weeks of pregnancy, before implantation, the conceptus depends exclusively on the intrauterine milieu created by the endometrial secretions or molecules transported into the lumen by the uterine endometrium prior to implantation and placentation [7]. Amino acids are important components of these maternally derived secretions that are crucial for embryonic survival mainly during early pregnancy [8–12]. Optimal amounts of essential and non-essential AAs are important for embryonic development, which is altered in case of suboptimal amounts of these molecules [13–15]. The regulation of AA transport and concentration in cells and tissues, including the placenta and endometrium, depends on specific transport proteins [16]. There is no consistent information regarding the regulation of protein synthesis and the activity of AA transporters; however, changes in transcriptional profiles of AA transporters reveal changes in AA availability in the uterine lumen [12]. Interestingly, AA transporter gene expression increases simultaneously in maternal endometrium and conceptus cells. This indicates transport of AAs from the maternal circulation to the endometrial lumen exclusively for embryonic development.
Amino acids seem to be indispensable for embryonic survival and development. When non-essential AAs were completely removed from the medium, blastocyst growth drastically decreased [14]; the same was observed in medium with any combination of glucose and phosphate without AAs [15]. More interestingly, P4 infusion in animals with no CL and no follicle increased AA availability in the uterus and maternal plasma of cows [17] and gene expression of AA transporters in ovine and bovine endometria [8, 12, 18].
During early embryonic development in vitro, AA requirements seem to change [19]. Until the blastocyst stage, an excess of essential AAs seems to be detrimental for embryonic development, at least in vitro [13]; however, the absence of non-essential AAs in the culture medium is unfavorable for embryonic development [14]. During the blastocyst expansion stage, few changes were observed in AA turnover during embryo culture [20], when patterns of AA depletion and release were similar to those observed in vitro for pre-elongation embryos [21]. More interestingly, AA turnover has been identified as an indicator of embryonic viability in humans and cattle [22, 23]. Another relevant finding is that enzymes present in endometrial tissue can regulate AA synthesis or degradation. These enzymes are not characterized in the bovine endometrium. In other tissues, DDO (D-aspartate oxidase) catalyzes the deamination of alanine and aspartate [24]. SCLY (selenocysteine lyase) is involved in the production of alanine and elemental selenium from selenocysteine [25].
In this context, AA uterine transport and luminal availability emerge as important variables that might play a role in maternal and embryonic well-being. However, there is limited information available on how changes in the pre- and post-ovulatory endocrine milieu affect the transport of AAs from the maternal circulation to endometrial cells and finally to the uterine lumen. We hypothesize that the size of the POF and subsequent physiological circulating concentrations of P4 in early diestrus modulate endometrial expression of AA transporter protein pathways.
We recently described a model to manipulate preovulatory follicle growth to produce groups of cyclic beef cows with distinctly different circulating preovulatory concentrations of E2 and early diestrus concentrations of P4 [26]. Based on the contrasting ovarian and endocrine characteristics of these two groups of animals, we studied the following variables on both D4 and D7 of the estrous cycle: (1) transcript abundance of AA transporters in endometrial tissues; (2) transcript abundance of enzymes related to AA metabolism in endometrial tissues; and (3) uterine luminal concentrations of AA. Previous studies indicated that the phenotype of smaller follicles and short proestrus was associated with lower receptivity and capacity to support conceptus development in comparison with a group manipulated to have a longer proestrus and ovulate a larger follicle [27]. We focused on D4 and D7 for our investigation because it is around D4-5 that the embryo moves from the oviduct to the uterus; thus, from this moment until implantation (i.e., D20 in cattle) the embryo depends exclusively on endometrial secretions for its development [28–30]. Moreover, most embryonic losses occur during the two first weeks of pregnancy [31]. Therefore, mechanistic insights retrieved from this animal model could serve as a potential basis for future strategies to fine-tune maternal receptivity towards the embryo.
Methods
Animals and reproductive management
Eighty-six Nelore (Bos indicus) cows started in the experiment. The females selected were cycling, pluriparous, and non-lactating and did not present any detectable reproductive disorder. To form two distinct groups of females with different POF sizes and subsequent CL volumes and plasmatic concentrations of P4 (Fig. 1), a hormonal protocol was used as described previously [26, 27, 32].
Fig. 1.
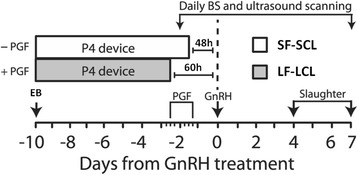
Experimental model and hormonal treatments. Growth of the pre-ovulatory follicle (POF) of beef cows was programmed to generate two groups of cows, the large follicle-large CL group (LF-LCL; associated with greater receptivity to the embryo and greater fertility) and small follicle-small CL group (SF-SCL). To decrease exposure to P4 and thereby stimulate growth of the POF, animals from LF-LCL group received an injection of PGF at the moment of intravaginal P4-releasing device insertion vs. no injections in the animals from SF-SCL group. Also, removal of the P4-releasing device was 12 h earlier in the LF-LCL group. Follicle size, ovulation and CL size were accessed by ultrasound scanning of the ovaries. Blood samples were collected for P4 assay. Ovulation was induced by GnRH on D0. On D4 (Experiment 1) and D7 (Experiment 2) animal were slaughtered for samples collection. BS, blood sampling; GnRH, 1 μg of busereline acetate im; P4, P4 progesterone-releasing device containing 1 g of P4; +PGF, cows received 0.5 mg of sodium cloprostenol on D-10; -PGF, cows did not receive Cloprostenol on D-10; EB, 2 mg of estradiol benzoate; Slaughter, endpoint for endometrial tissue and uterine washings collection (Adapted from Reference 26)
Briefly, the cows received two injections of prostaglandin F2α (PGF; 0.5 mg; Cloprostenol; Sincrocio®; Ourofino, Cravinhos, SP, Brazil) 14 d apart. Next, the ovaries were examined using transrectal ultrasonography (US) to confirm the presence of a PGF-induced CL 10 d after the second PGF administration, (D − 10; D0 = day of induction of ovulation by GnRH injection). On D − 10, each female was treated with 2 mg of estradiol benzoate (Sincrodiol®, Ourofino, Cravinhos, SP, Brazil) to stimulate the emergence of a follicular wave and received an intravaginal P4-releasing device (1 mg; Sincrogest®; Ourofino, Cravinhos, SP, Brazil). Additionally, females assigned to the large POF followed by large CL (LF-LCL) group also received a PGF injection (0.5 mg; Cloprostenol; Sincrocio®) on D-10 to induce CL regression during follicle development, whereas cows assigned to the small POF followed by small CL (SF-SCL) group did not. Sixty hours prior to the induction of ovulation, the P4 devices were removed and an injection of PGF was administered to the LF-LCL females, whereas cows in the SF-SCL received PGF administration 12 h later (D − 2.5 and D − 2, respectively). Ovulation was induced on D0 by administration of a gonadotrophin-releasing hormone agonist (GnRH; 1 μg buserelin; Sincroforte®; Ourofino, Cravinhos, SP, Brazil).
Follicular growth, ovulation and CL diameter were monitored by transrectal ultrasound. Only cows that ovulated in response to the GnRH (i.e., between 24 and 36 h after GnRH injection; N = 57) were included in the data analysis. The cows were slaughtered and their reproductive tracts collected for further analysis on D4 (Experiment 1; LF-LCL n = 16; SF-SCL n = 8) or D7 (Experiment 2; LF-LCL n = 18 SF-SCL n = 18).
Ultrasound Exams and CL Measurements
Transrectal ultrasound exams were performed using an Aloka SSD-500 device attached to a 5 MHz linear probe. The presence of an active CL on D-10, the time of ovulation, the size of the CL and the properties of the dominant follicle and POF were noted. Postmortem CLs were dissected and weighed separately, and the length, width and height were recorded. A calculated diameter for each CL was estimated by the mean of these three values. The CL volume was estimated using the formula for the volume of a sphere (V = 4/3πR3), where R was equal to half the mean diameter.
Plasma Progesterone Measurement
Blood was collected from the jugular vein on D4 and D7. The blood samples were centrifuged at 1500 × g for 30 min at 4 °C. Plasma aliquots were stored at −20 °C until analysis. Plasmatic concentrations of P4 were measured by radioimmunoassay analysis (Coat-A-Count®; Siemens Medical Solutions Diagnostics, Munich, Germany) as described previously [33].
Endometrial tissue and uterine fluid collection
Postmortem uteri were collected, and the uterine horns ipsilateral to the CL were washed with 20 mL PBS. Subsequently, the washings were centrifuged (1000 × g) for 30 min at 4 °C, and the supernatant was collected, snap frozen in liquid nitrogen and stored at -80 °C. Subsequently, endometrial tissue from the washed uteri was dissected from the intercaruncular region of the ipsilateral uterine horn, frozen in liquid nitrogen and stored at -80 °C.
Quantification of amino acids
The concentrations of free AA in uterine flushings were determined by high-performance liquid chromatography (HPLC) using an HPLC Luna Column 3u C18(2) 100A 250 × 4.6 mm (00G-4251-E0 - Phenomenex, Torrance, CA, USA). The protocol for AA quantification was adapted from [34]. Briefly, 100 μL of internal standards of free AAs and methanol were added to 200 μg of uterine flushing samples (n = 12 LF-LCL, n = 7 SF-SCL for Experiment 1; n = 9 LF-LCL, n = 10 SF-SCL for Experiment 2). The dissolved samples were deproteinized using Vivaspin 500 (MWCO 3000 Da, Sartorius, Goettingen, Germany) followed by centrifugation (15,000×g for 45 min at 4 °C) and derivatization and were subsequently analyzed by programmed chromatography. The samples were analyzed in simplicate. The results were obtained by comparing each AA peak to its corresponding peak on a multilevel (3 levels) standard curve, based on certified standards.
Analysis of gene expression levels in endometrial tissue
Total RNA was extracted from endometrial samples using the RNeasy Mini Kit (Qiagen Laboratories, Germantown, MD, USA) following manufacturer instructions. The concentration and purity of mRNA were estimated using NanoVue (GE Healthcare Life Sciences, Buckinghamshire, England). Total RNA extracts were stored at −80 °C until cDNA synthesis. One microgram of total RNA was used for reverse transcription using the High-Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad, CA, USA), according to manufacturer’s instructions.
Quantification of endometrial gene expression was obtained by qPCR analysis using a StepOne Plus® apparatus from Applied Biosystems. Transcript abundance was determined for several AA-transport-related genes. The genes tested were SLC1A1, SLC1A4, SLC1A5, SLC6A1, SLC6A6, SLC6A14, SLC7A2, SLC7A4, SLC7A5, SLC7A7, SLC7A8, SLC7A11, SLC17A5, SLC17A9, SLC36A2, SLC38A1, SLC38A4, SLC38A6, SLC38A7, SLC43A2, SCLY, and DDO. Each primer pair was analyzed, considering the probabilities of hairpin, homodimer and heterodimer formation, using Oligo Analyzer 3.1 software (IDT®; http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/). Sequence specificities were tested using the software Basic Local Alignment Search Tool (Blast) (http://blast.ncbi.nlm.nih.gov). PCR product identity was confirmed by sequencing (Table 1). Transcript abundance was compared between tissues from animals in the LF-LCL and SF-SCL groups from Experiment 1 (n = 8 per group) and Experiment 2 (n = 8 for LF-LCL and n = 9 for SF-SCL group). The selection of reference genes was performed using geNorm software (www.qbaseplus.com) [35]. After selection performed by the software, RPS18 was selected as an endogenous control for endometrium on D4, and cyclophilin, β-actin and GAPDH were chosen for endometrium on D7. Transcript abundance was calculated as the relative abundance between the target gene and the geometric average of selected housekeeping genes and given as an arbitrary value.
Table 1.
Target genes, primer sequence and amplicon information
| Gene | Gene Symbol | Representative ID | Sequence | Amplicon size, bp |
|---|---|---|---|---|
| Solute Carrier Family 1 member 1 | SLC1A1 a | NM_174599.2 | F: AAGGAGTTGGAGCAAATGGA | 152 |
| R: AACGAGATGGTATCGGACTTG | ||||
| Solute Carrier Family 1 member 4 | SLC1A4 a | NM_001081577.1 | F: ATCTTGATAGGCGTGGTTTC | 132 |
| R: GCAACACTGGTTCTCTCTATAA | ||||
| Solute Carrier Family 1 member 5 | SLC1A5 a | NM_174601.2 | F: TCCAAATCTGCCCAGTCCTCAACT | 141 |
| R: TTCCCATGATTCCCTATGCCCTGA | ||||
| Solute Carrier Family 6 member 1 | SLC6A1 a | NM_001077836.1 | F: GTGTCTCCATTTCCTGGTTT | 150 |
| R: GCACAGCACTGAAGATGAA | ||||
| Solute Carrier Family 6 member 14 | SLC6A14 a | NM_001098461.1 | F: GATGCTGCCACACAGATATT | 154 |
| R: TAGCAAATCCAGCGAACAC | ||||
| Solute Carrier Family 6 member 6 | SLC6A6 a | NM_174610.2 | F: GAAAGCCGTGACGATGAT | 158 |
| R: GGTGAAAGCCCTTCCTTAG | ||||
| Solute Carrier Family 7 member 2 | SLC7A2 a | XM_010820288.1 | F: GATGCTGGAGGGACTAGAT | 146 |
| R: AGATACCCAGGCACAGAA | ||||
| Solute Carrier Family 7 member 4 | SLC7A4 a | NM_001192042.1 | F: GACCCACGGACTCTAGTTTA R: GGTTGAGCGATACCTATTGTG |
156 |
| Solute Carrier Family 7 member 5 | SLC7A5 a | NM_174613.2 | F: TGTGGTCCGATAGGCATAGA | 151 |
| R: ACAACGGTGGATGCTGTT | ||||
| Solute Carrier Family 7 member 7 | SLC7A7 a | NM_001075151.1 | F: CCTCCAGGTCCTATGTATGT | 144 |
| R: CAGCCAGCAGGAGATAGA | ||||
| Solute Carrier Family 7 member 8 | SLC7A8 a | NM_001192889.1 | F: GAGATTGGATTGGTCAGTGG | 155 |
| R: GCTCCCACAACTGTGATAAG | ||||
| Solute Carrier Family 7 member 11 | SLC7A11 a | XM_010826337.1 | F: GTACAGGGATTGGCTTCATC | 144 |
| R: CTGGCACAACTTCCAGTATT | ||||
| Solute Carrier Family 17 member 5 | SLC17A5 a | NM_001205974.1 | F: CTGCAGTCCCTTATTTAGGC | 144 |
| R: GGAATATCGCAGGTCCAATC | ||||
| Solute Carrier Family 17 member 9 | SLC17A9 a | NM_001100378.2 | F: GTCTAGACACACCAAGG | 141 |
| R: GGGAAGGTCTCCTTAAA | ||||
| Solute Carrier Family 36 member 2 | SLC36A2 a | NM_001206180.1 | F: GACGACCAAGGGATAAC | 157 |
| R: AGAGTGGAGGAGATGAA | ||||
| Solute Carrier Family 38 member 1 | SLC38A1 a | XM_002687321.3 | F: TCACGGTTCGATCTTCTTTATT | 131 |
| R: ATCCTTCATGGAGGGTATGA | ||||
| Solute Carrier Family 38 member 4 | SLC38A4 a | NM_001205943.1 | F: CTGTGCCCATAGTGCTATTC | 139 |
| R: GGCACAAGGATGACCAAA | ||||
| Solute Carrier Family 38 member 6 | SLC38A6 a | XM_010823227.1 | F: CACCTCAATACTGCCCATATAC | 150 |
| R: GACGCCACACTGTCATAAA | ||||
| Solute Carrier Family 38 member 7 | SLC38A7 a | NM_001100355.1 | F: TATAGCTGTGATGGCAAAGG | 153 |
| R: CTCAGGAAGCTGGATATTT | ||||
| Solute Carrier Family 43 member 2 | SLC43A2 a | NM_001075546.1 | F: AAGGCCCAGGATGAGAT | 149 |
| R: AAGCAGGAGACAGCAAAG | ||||
| Cyclophilin | PPIA b | NM_178320.2 | F: GCCATGGAGCGCTTTGG | 65 |
| R: CCACAGTCAGCAATGGTGATCT | ||||
| β-actin | ACTB # | NM_173979.3 | F: GGATGAGGCTCAGAGCAAGAGA | 78 |
| R: TCGTCCCAGTTGGTGACGAT | ||||
| D-aspartate oxidase | DDO a | NM_173908.2 | F: CTGGCGTATCCAATGTAACC | 158 |
| R: CCTCAAACCCACTTTCTCTC | ||||
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH b | NM_001034034.2 | F: GCCATCAATGACCCCTTCAT | 70 |
| R: TGCCGTGGGTGGAATCA | ||||
| Ribosomal protein S18 Selenocysteine lyase |
RPS18
b
SCLY a |
NM_001033614.2 NM_001083804.1 |
F: GCCTGAGAAACGGCTACCAC R: CACCAGACTTGCCCTCCAAT F: AGGAAGGCCAAGGAGATTAT |
171 151 |
| R: CGTGGACTTTGTGGAAGTG |
(F) Primer forward sequence; (R) Primer reverse sequence. aindicates primer sequences obtained by PrimerQuestQM software (IDT technologies).b[64]
Statistical analysis
Cows from each group were ranked according to the plasmatic concentration of P4 at D7, P4 at D7/P4 at D2 ratio, CL size at D7, CL weight, follicle size at D2, D1 and D0 and preovulatory follicle size as previously described [36]. The samples selected for analysis were according to this ranking. Further analyses were conducted in 8 animals per group (D4) and 8 and 9 animals (D7) from the LF-LCL and SF-SCL groups, respectively. The data were tested for normality of residuals using the Shapiro-Wilk test and for homogeneity of variance using the F-max text (SAS; Version 9.2; SAS Institute). The data were analyzed independently for Experiments 1 and 2, as they were not conducted contemporaneously. Discrete dependent variables (diameter of the dominant follicle on D2 and D0, POF diameter, CL volume and weight measured postmortem and plasmatic concentrations of P4 on D4 or D7) were analyzed by one-way ANOVA for the effect of group using the PROC GLM procedure (SAS; Version 9.2; SAS Institute). The amino acid quantification data were tested for the presence of outliers by Dixon’s test, and outliers were removed before the normality of residuals test. Uterine flushing concentrations of amino acid values that did not follow the normality of residuals were transformed for analysis. On D4, uterine flushing concentrations of threonine, alanine, methionine sulfone internal standard, tyrosine, leucine and lysine were transformed to natural log and isoleucine, ornithine and lysine were transformed to rank. On D7, taurine, proline, α-aminobutyric acid, valine, cysteine, isoleucine and lysine were transformed to natural log and β-alanine was transformed to rank. Concentrations of amino acid in uterine flushings and relative gene expression were analyzed by Student’s t-test. Means were considered significantly different when they presented a P value of 0.05 or less. Means with P values between 0.06 and 0.1 were considered as approaching significance.
Results
Animal Model
The hormonal strategy that we employed successfully produced groups of cows presenting distinctly different periovulatory ovarian and endocrine characteristics, as expected and described earlier [26, 32, 36–38]. Specifically, for Experiment 1, cows assigned to the LF-LCL group had larger follicle diameters on D2, D1 and D0 compared to animals from the SF-SCL group (P < 0.01; Table 2). Pre-ovulatory follicles were also larger for animals from the LF-LCL group (P < 0.01; Table 2), but plasmatic concentrations of P4 on D4 were similar for animals from both groups (P > 0.05; Table 2). For Experiment 2, cows assigned to the LF-LCL group had larger POF diameters than animals from the SF-SCL group (P < 0.01; Table 2). Furthermore, the larger POFs resulted in larger and heavier CLs on D7 (P < 0.05; Table 2). Moreover, plasmatic concentrations of P4 on D7 were also greater in cows from the LF-LCL group. More details on ovarian and endocrine responses from the LF-LCL and SF-SCL groups on D4 and D7 were published elsewhere [26, 39].
Table 2.
Follicle, CL and P4 measurements
| End-points | LF-LCL | SF-SCL | P < F |
|---|---|---|---|
| Experiment 1 (D4) Follicle diameter, mm | |||
| D2 | 12.66 ± 0.4 | 8.84 ± 0.6 | 0.00006 |
| D1 | 13.93 ± 0.6 | 9.58 ± 0.3 | 0.00003 |
| D0 | 15.64 ± 0.6 | 11.52 ± 0.4 | 0.00005 |
| Pre-ovulatory follicle, mm | 15.99 ± 0.3 | 11,32 ± 0.2 | 0.00000002 |
| CL volume on D4, cm3 | 2.66 ± 1.2 | 2.3 ± 1 | 0.03 |
| CL weight on D4, g | 1.06 ± 0.06 | 0.68 ± 0.08 | 0.002 |
| Plasma P4 concentrations on D4, ng/mL | 1.17 ± 0.3 | 0.8 ± 0.1 | 0.21 |
| Experiment 2 (D7) Follicle diameter, mm | |||
| D2 | 10.73 ± 0.7 | 7.09 ± 0.3 | 0.0002 |
| D1 | 11.79 ± 0.6 | 7.73 ± 0.4 | 0.000001 |
| D0 | 13.04 ± 0.5 | 9.68 ± 0.4 | 0.00002 |
| Pre-ovulatory follicle, mm | 13.63 ± 0.5 | 10.09 ± 0.3 | 0.000005 |
| CL volume on D7, cm3 | 2.29 ± 0.2 | 1.53 ± 0.2 | 0.03 |
| CL weight on D7, g | 2.83 ± 0.2 | 1.53 ± 0.2 | 0.0001 |
| Plasma P4 concentrations,ng/mL | |||
| on D7 | 3.68 ± 0.2 | 2.1 ± 0.2 | 0.008 |
Cows were synchronized to have larger (LF-LCL; Exp 1 n = 8; Exp 2 n = 8) or smaller (SF-SCL; Exp 1 n = 8; Exp 2 n = 9) pre-ovulatory follicles and corpora lutea. Mean ± SEM
Gene Expression
On D4, the abundances of SLC6A6, SLC7A4, SLC17A5, SLC38A1, SLC38A7 and SCLY were on average 64.34, 70.64, 42.36, 56.24, 35,54 and 41.45% greater, respectively, in the endometrium of LF-LCL cows than in the SF-SCL endometrial tissue (P ≤ 0.05; Table 3; Fig. 7). These solute carriers are responsible for alanine, serine, proline, taurine, β-alanine, aspartate, glutamate, histidine, ornithine, lysine and glutamine transport. The enzyme SCLY is responsible for alanine synthesis from selenocysteine.
Table 3.
Relative quantification of transcript abundance by qPCR
| Gene | LF-LCL | SF-SCL | P < F |
|---|---|---|---|
| SLC1A1 | |||
| D4 | 0.1215 ± 0.0126 | 0.1662 ± 0.0339 | 0.2371 |
| D7 | 0.0051 ± 0.0004 | 0.0044 ± 0.0005 | 0.2830 |
| SLC1A4 | |||
| D4 | 0.0107 ± 0.0014 | 0.0073 | 0.08 |
| D7 | 0.0391 ± 0.004 | 0.0262 ± 0.004 | 0.05 |
| SLC1A5 | |||
| D4 | 1.25 ± 0.08 | 1.35 ± 0.1 | 0.45 |
| D7 | 0.7 ± 0.07 | 0.9 ± 0.01 | 0.51 |
| SLC6A1 | |||
| D4 | 0.0006 ± 0.0001 | 0.0005 ± 0.0001 | 0.5506 |
| D7 | 0.4561 ± 0.0350 | 0.2633 ± 0.0282 | 0.0006 |
| SLC6A6 | |||
| D4 | 0.0181 ± 0.0017 | 0.0110 ± 0.0007 | 0.0020 |
| D7 | 0.0682 ± 0.0068 | 0.0711 ± 0.0090 | 0.8064 |
| SLC6A14 | |||
| D4 | 0.021 ± 0.004 | 0.025 ± 0.006 | 0.58 |
| D7 | 0.0286 ± 0.0028 | 0.0169 ± 0.0011 | 0.001 |
| SLC7A2 | |||
| D4 | 0.0067 ± 0.0014 | 0.0068 ± 0.0012 | 0.9437 |
| D7 | 0.0047 ± 0.0007 | 0.0045 ± 0.0009 | 0.8666 |
| SLC7A4 | |||
| D4 | 0.0012 ± 0.0002 | 0.0007 ± 0.0002 | 0.052 |
| D7 | 0.0077 ± 0.0011 | 0.0034 ± 0.0006 | 0.0036 |
| SLC7A5 | |||
| D4 | 0.0522 ± 0.008 | 0.0549 ± 0.005 | 0.78 |
| D7 | 0.0598 ± 0.01 | 0.0599 ± 0.007 | 0.99 |
| SLC7A7 | |||
| D4 | 0.0167 ± 0.0012 | 0.0159 ± 0.0015 | 0.6886 |
| D7 | 0.0579 ± 0.0031 | 0.0434 ± 0.0025 | 0.0021 |
| SLC7A8 | |||
| D4 | 0.0153 ± 0.0021 | 0.0180 0.0051 | 0.6238 |
| D7 | 0.1068 ± 0.0157 | 0.0373 ± 0.0071 | 0.0012 |
| SLC7A11 | |||
| D4 | 0.0004 ± 0.0001 | 0.0004 ± 0.0001 | 0.7969 |
| D7 | 0.0004 ± 0.0001 | 0.0005 ± 0.0001 | 0.8120 |
| SLC17A5 | |||
| D4 | 0.0193 ± 0.0012 | 0.0135 ± 0.0015 | 0.0089 |
| D7 | 0.0629 ± 0.0074 | 0.0362 ± 0.0024 | 0.0039 |
| SLC17A9 | |||
| D4 | 0.0033 ± 0.0005 | 0.0028 ± 0.0007 | 0.6243 |
| D7 | 0.0054 ± 0.0008 | 0.0048 ± 0.0009 | 0.6354 |
| SLC36A2 | |||
| D4 | 0.0005 ± 0.0001 | 0.0006 ± 0.0002 | 0.55 |
| D7 | 0.0035 ± 0.0013 | 0.0025 ± 0.001 | 0.56 |
| SLC38A1 | |||
| D4 | 0.066 ± 0.008 | 0.042 ± 0.004 | 0.019 |
| D7 | 0.63 ± 0.08 | 0.38 ± 0.04 | 0.01 |
| SLC38A4 | |||
| D4 | 0.0072 ± 0.0009 | 0.0086 ± 0.004 | 0.19 |
| D7 | 0.0158 ± 0.009 | 0.01978 ± 0.002 | 0.12 |
| SLC38A6 | |||
| D4 | 0.0044 ± 0.0001 | 0.0047 ± 0.0003 | 0.3745 |
| D7 | 0.0077 ± 0.0005 | 0.0066 ± 0.0004 | 0.1304 |
| SLC38A7 | |||
| D4 | 0.0404 ± 0.004 | 0.0302 ± 0.0014 | 0.02 |
| D7 | 0.9601 ± 0.009 | 0.7069 ± 0.008 | 0.05 |
| SLC43A2 | |||
| D4 | 0.6539 ± 0.0217 | 0.6357 ± 0.1383 | 0.8983 |
| D7 | 0.0065 ± 0.0006 | 0.0044 ± 0.0006 | 0.0326 |
| DDO | |||
| D4 | 0.0113 ± 0.0021 | 0.0119 ± 0.0023 | 0.8501 |
| D7 | 0.0208 ± 0.0028 | 0.0123 ± 0.0011 | 0.0123 |
| SCLY | |||
| D4 | 0.0090 ± 0.0006 | 0.0064 ± 0.0003 | 0.0009 |
| D7 | 0.0122 ± 0.0011 | 0.0110 ± 0.0005 | 0.3279 |
Mean ± standard error of the mean of the relative abundance of target genes and endogenous controls for large follicle-large CL group (LF-LCL; Exp 1 n = 8; Exp 2 n = 8) and small follicle-small CL group (SF-SCL; Exp 1 n = 8; Exp 2 n = 9)
Fig. 7.
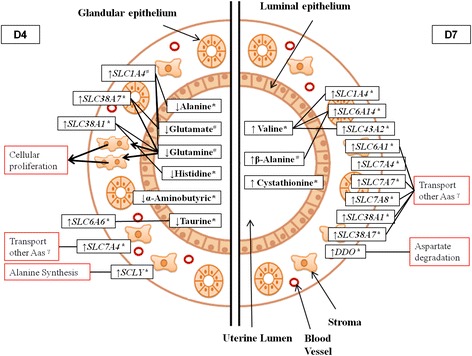
Amino acid transport in the uterus of cows on D4 and D7 of diestrus. This figure shows the comparative abundance of transcripts related to amino acid (AA) transport and metabolism and the luminal concentration of AA between more receptive endometrium (Large Follicle-Large Corpus Luteum group) and less receptive endometrium (Small Follicle-Small Corpus Luteum group) on D4 and D7 after estrus. On D4, the transport of AA seems to occur preferentially from the uterine lumen towards endometrial cells, because despite elevated expression of genes related to AA transporters in endometrium there is lower availability of AA in uterine washings. Such direction of transport benefit events such as cell proliferation, which requires AA. On D7, AA availability in uterine lumen and abundance of genes related to AA transport are both stimulated in the more receptive endometrium. This phenotype is consistent with a greater provision of substrates to support embryonic needs for growth. ↑, up-regulated in LF-LCL group in comparison to SF-SCL group; ↓ down-regulated in LF-LCL group in comparison to SF-SCL group; γ, carriers related to transport of AAs similarly abundant in the lumen of both groups. solid lines connect a transporter with its cognate substrate(s); *, P ≤ 0.05; #, P < 0.1; SLC, Solute carrier protein; SCLY, Selenocysteine Lyase; DDO, D-aspartate Oxidase
On D7, the transcript abundances of SLC1A4, SLC6A1, SLC6A14, SLC7A4, SLC7A7, SLC7A8, SLC17A5, SLC38A1, SLC38A7, SLC43A2 and DDO were 49.62, 73.26, 68.98, 126.14, 33.32, 186.4, 73.88, 67.93, 35.91, 46.28, and 69.64% greater in the endometrium of the LF-LCL group (P ≤ 0.05; Table 3; Fig. 7).
Amino Acid Quantification in the Uterine Washings
Glutamate, aminoadipic acid, asparagine, serine, glutamine, glycine, histidine, β-alanine, taurine, β-aminoisobutyric acid, threonine, alanine, proline, α-aminobutyric acid, tyrosine, valine, methionine, cystathionine, cysteine, isoleucine, leucine, phenylalanine, tryptophan, ornithine and lysine were the AAs detected in uterine flushings on D4 and D7 (Table 4).
Table 4.
Concentrations of amino acids in uterine washings
| Amino acid | LF-LCL, μmol/g | SF-SCL, μmol/g | P < F |
|---|---|---|---|
| GLU | |||
| D4 | 115.30 ± 9.23 | 157.40 ± 23.49 | 0.066 |
| D7 | 127.93 ± 13.97 | 153.13 ± 21.92 | 0.38 |
| AAD | |||
| D4 | 91.52 ± 0.60 | 91.63 ± 1.19 | 0.93 |
| D7 | 91.54 ± 0.82 | 92.71 ± 0.93 | 0.22 |
| ASN | |||
| D4 | 115.63 ± 2.80 | 126.66 ± 7.56 | 0.12 |
| D7 | 139.83 ± 6.55 | 141.68 ± 6.43 | 0.84 |
| SER | |||
| D4 | 6.76 ± 1.03 | 11.04 ± 2.80 | 0.19 |
| D7 | 17.34 ± 1.96 | 15.15 ± 3.12 | 0.63 |
| GLN | |||
| D4 | 95.74 ± 2.51 | 108.58 ± 7.08 | 0.06 |
| D7 | 120.05 ± 6.16 | 122.52 ± 7.91 | 0.81 |
| GLY | |||
| D4 | 119.36 ± 12.39 | 164.37 ± 31.04 | 0.13 |
| D7 | 339.70 ± 41.51 | 352.53 ± 43.62 | 0.84 |
| HYS | |||
| D4 | 11.21 ± 0.51 | 12.81 ± 0.61 | 0.08 |
| D7 | 12.42 ± 0.74 | 12.75 ± 0.82 | 0.80 |
| B-ALA | |||
| D4 | 70.44 ± 0.60 | 71.18 ± 0.82 | 0.58 |
| D7 | 80.95 ± 3.88 | 73.78 ± 1.06 | 0.08 |
| TAU | |||
| D4 | 117.20 ± 9.57 | 197.90 ± 32.56 | 0.01 |
| D7 | 460.47 ± 106.32 | 334.93 ± 41.22 | 0.69 |
| THR | |||
| D4 | 6.88 ± 2.00 | 8.77 ± 2.79 | 0.66 |
| D7 | 10.10 ± 1.65 | 9.83 ± 1.73 | 0.92 |
| ALA | |||
| D4 | 55.59 ± 5.42 | 95.70 ± 21.07 | 0.04 |
| D7 | 76.94 ± 8.97 | 91.15 ± 11.40 | 0.36 |
| PRO | |||
| D4 | 6.27 ± 0.98 | 9.72 ± 2.21 | 0.13 |
| D7 | 29.23 ± 7.78 | 23.63 ± 3.93 | 0.81 |
| AAAB | |||
| D4 | 19.82 ± 1.38 | 28.71 ± 2.85 | 0.03 |
| D7 | 32.48 ± 7.75 | 23.29 ± 2.38 | 0.82 |
| TYR | |||
| D4 | 6.22 ± 0.99 | 8.37 ± 1.99 | 0.16 |
| D7 | 6.20 ± 0.74 | 6.77 ± 0.78 | 0.62 |
| VAL | |||
| D4 | 19.11 ± 1.91 | 23.90 ± 3.56 | 0.23 |
| D7 | 33.11 ± 7.81 | 16.73 ± 2.27 | 0.03 |
| MET | |||
| D4 | 22.16 ± 0.26 | 22.36 ± 0.29 | 0.64 |
| D7 | 22.73 ± 0.56 | 22.70 ± 0.26 | 0.96 |
| CYS | |||
| D4 | 127.83 ± 6.44 | 142.07 ± 10.47 | 0.27 |
| D7 | 309.37 ± 49.54 | 177.92 ± 17.55 | 0.02 |
| ILE | |||
| D4 | 18.09 ± 2.25 | 21.58 ± 3.25 | 0.21 |
| D7 | 15.40 ± 0.95 | 15.80 ± 1.13 | 0.93 |
| PHE | |||
| D4 | 120.92 ± 16.94 | 120.33 ± 25.84 | 0.98 |
| D7 | 117.49 ± 23.60 | 142.63 ± 16.80 | 0.39 |
| TRP | |||
| D4 | 388.73 ± 35.18 | 433.83 ± 76.04 | 0.54 |
| D7 | 403.82 ± 32.67 | 398.52 ± 33.38 | 0.91 |
| ORN | |||
| D4 | 98.07 ± 1.86 | 96.62 ± 2.29 | 0.51 |
| D7 | 97.36 ± 2.33 | 96.71 ± 1.83 | 0.82 |
| LYS | |||
| D4 | 4.61 ± 1.04 | 4.90 ± 1.58 | 0.65 |
| D7 | 13.44 ± 3.69 | 6.73 ± 0.42 | 0.70 |
Mean ± standard error of the mean of the concentration of amino acid in μmol/g of uterine washings for large follicle-large CL group (LF-LCL; Exp 1 n = 12; Exp 2 n = 9) and small follicle-small CL group (SF-SCL; Exp 1 n = 7; Exp 2 n = 10)
When the levels in the uterine washings were compared between groups, D4 data showed that concentrations of taurine, alanine and α-aminobutyric acid in uterine flushings were higher (68.71, 70 and 44.81%, respectively) in SF-SCL washings compared to their LF-LCL counterparts (P ≤ 0.05; Table 4; Figs. 2, 3, 4 and 7).
Fig. 2.
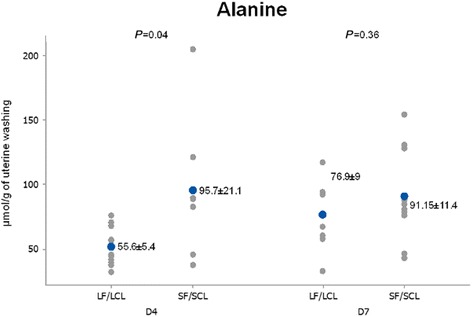
Individual and mean concentrations of alanine in uterine washings from D4 and D7 of diestrus. Each gray dot indicates an individual animal. Blue dots indicate mean ± sem. LF-LCL indicates Large Follicle-Large CL group and SF-SCL indicates Small Follicle-Small CL group
Fig. 3.
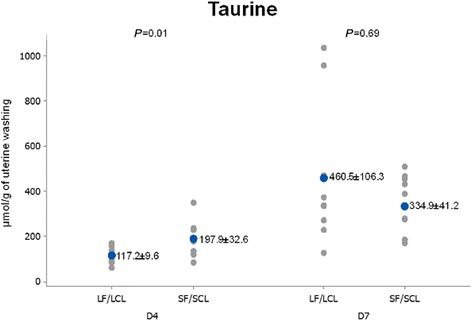
Individual and mean concentrations of taurine in uterine washings from D4 and D7 of diestrus. Each gray dot indicates an individual animal. Blue dots indicate mean ± sem. LF-LCL indicates Large Follicle-Large CL group and SF-SCL indicates Small Follicle-Small CL group
Fig. 4.
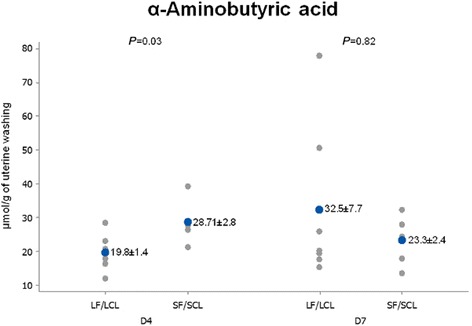
Individual and mean concentrations of α-aminobutyric acid in uterine washings from D4 and D7 of diestrus. Each gray dot indicates an individual animal. Blue dots indicate mean ± sem. LF-LCL indicates Large Follicle-Large CL group and SF-SCL indicates Small Follicle-Small CL group
Conversely, on D7, the concentrations of valine and cystathionine in uterine flushings were greater (95 and 74.18%, respectively) in the LF-LCL uterine washings (Table 4; Figs. 5, 6 and 7).
Fig. 5.
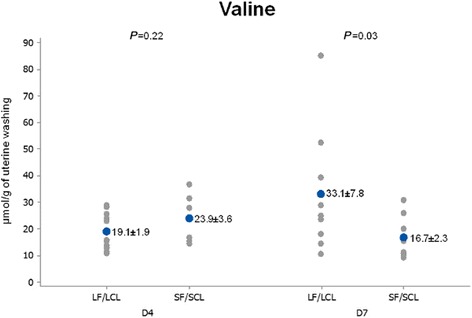
Individual and mean concentrations of valine in uterine washings from D4 and D7 of diestrus. Each gray dot indicates an individual animal. Blue dots indicate mean ± sem. LF-LCL indicates Large Follicle-Large CL group and SF-SCL indicates Small Follicle-Small CL group
Fig. 6.
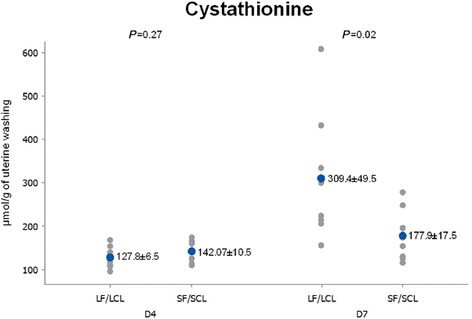
Individual and mean concentrations of cystathionine in uterine washings from D4 and D7 of diestrus. Each gray dot indicates an individual animal. Blue dots indicate mean ± sem. LF-LCL indicates Large Follicle-Large CL group and SF-SCL indicates Small Follicle-Small CL group
Discussion
Disappointing fertility success in the beef cow industry is mainly caused by excessive rates of embryonic mortality during early pregnancy, which has been linked to inadequate endometrial receptivity [40, 41]. During early pregnancy, the mother needs to provide the optimal uterine microenvironment for the embryo in order to facilitate initial embryonic-maternal interactions leading to subsequent implantation [42]. However, the exact definition of a microenvironment optimal for the proper development of embryos during early pregnancy remains to be elucidated. As previous studies pointed towards the importance of greater concentrations of AA in the uterus to increase fertility outcome in dairy cows [9], this study attempted to investigate the link between AA metabolic pathways and uterine function to support embryo growth during early diestrus in beef cows. Therefore, we characterized AA transport and metabolic pathways in the endometrium and AA levels in the uterine lumen on D4 and D7 after induction of ovulation in response to different endocrine profiles. Furthermore, the study was conducted in cyclic, non-inseminated cows based on the previous report by Forde et al. [43]. In that report, the authors indicated that from the first week of diestrus until after D13, the pregnant cow endometrium undergoes molecular changes similar to those of the cyclic cow endometrium. Thus, because sample collection was conducted on D4 and D7, it is reasonable to assume that the treatment effects observed in the present report would be similar were the animals pregnant.
In this context, a previously described in vivo receptivity model was used here [26, 32, 36, 38], aiming to define the AA signatures of the receptive endometrial tissues and histotroph. Using the same model, we were able to manipulate two fundamental aspects of receptivity: compared to the LF-LCL group, fertility was lower in the SF-SCL group (i.e., the low receptivity group) [27], and the concentration of PGR in the endometrium was greater in the same group on D7 of the estrous cycle [37]. More specifically, data on the bovine endometrial tissue transcriptome associated with AA transport have been integrated with information on uterine flushing AA profiles from highly receptive (LF-LCL) versus low-receptive (SF-SCL) groups of cows. In beef cattle, altered endocrine patterns during follicle growth are known to influence the follicular size and steroidogenic capacity before ovulation [44]. Thereby, corpora lutea originating from larger follicles are characterized by larger sizes, resulting in greater circulating concentrations of P4 compared to those originating from smaller follicles [45, 46]. In our study, POF influenced CL size and plasmatic levels of P4 on D7 but not on D4. It is important to mention that CL is a transient endocrine organ that starts to develop after ovulation, when theca and granulosa cells differentiate into luteal cells [47]. The D4 and D7 CL are considered to be in different stages of development [48, 49]. Specifically, at D4, the CL is under early development and did not achieve a large enough area to show differences in P4 production as it did on D7. Interestingly, gene expression related to P4 production indicated a greater capacity for P4 production at D7 than at D4 [49]. In cattle, POF size is directly related to E2 production [50, 51], suggesting that POF size and probably circulating concentrations of E2 influence the uterine environment and endometrial gene expression on D4 and D7.
The uterine flushing AA signatures show that on D4, concentrations of taurine, alanine and α-aminobutyric acid in uterine luminal flushings were greater in the SF-SCL group, which in this animal model represents the low-receptive uterus. The mRNA of SCLY, the enzyme related to alanine production, was up-regulated in the LF-LCL group. Moreover, SLC6A6 mRNA, which transports β-alanine and mainly taurine, was more abundant in the endometrium of cows from the LF-LCL group. A collective interpretation of these data suggests that (1) alanine biosynthesis was stimulated in the LF-LCL group and that (2) alanine transport was stimulated in the LF-LCL group, mainly in a lumen-to-endometrium direction. Stimulating accumulation of alanine in the endometrium may be important for endometrial cellular functions, such as proliferation, to guarantee endometrial receptivity. Alternatively, in accordance with data on cardiomyocytes, excess supplementation of AA may downregulate their transporters [52]. This relationship could explain the findings in the SF-SCL group.
Although early bovine embryos are purported to use all available AAs, alanine is unique in that it is not used and is even secreted by the embryo [53]. This suggests that regulation of this AA, which is available in greater concentrations in low-receptive than in high-receptive uteri, plays no major role in early embryonic development. Taurine, which was more abundant in the SF-SCL group, seems to be beneficial for embryonic development [54]; however, the addition of an elevated dose of cysteamine, a taurine precursor, to embryo culture media was toxic to the bovine embryo [55]. Supplementation of α-aminobutyric acid in the embryo culture medium did not affect porcine embryo cell viability [56].
Similar to the alanine transporter, SLC38A1 is a transporter with high affinity for glutamine and is more abundant in high-receptive endometrial tissue than in low-receptive tissue, whereas glutamine tended to be more abundant in uterine washings of the low-receptive group. These data can be associated with the major proliferative activity found in the LF-LCL endometrium on D4, which was related to adequate uterus conditioning for receiving the embryo [39]. This proliferative activity may also be responsible for the observation of an increase in transporter abundance without an associated increase in the concentration of the target substrate in the uterine lumen. Indeed, glutamine is essential for cell proliferation in other tissues [57].
On D7, regulation of AA transporter transcript abundance and cognate substrates is complex, and several scenarios were observed. For example, SLC1A4 was more abundant in the endometrium of LF-LCL cows, although its direct targets, serine, tyrosine, threonine, were present in similar concentrations in the uterine lumen of cows of both groups. Likewise, SLC38A1 and SLC38A7 transcripts were up-regulated in the endometrium of the LF-LCL group on D7. These transporters have high affinity for glutamine transport, but this AA was present in similar concentrations in the uterine lumen of both groups. In contrast, regardless of a similar abundance of the transporter SLC7A11 between groups on D4 and D7, cystathionine, a product of serine and homocysteine condensation by cystathionine β-synthase [58], and valine were present in greater concentrations in the uterine washings of cows from the LF-LCL group compared to cows from the SF-SLC group. In a third scenario, SLC6A14 and one of its transporting targets, valine, were stimulated in the LF-LCL group. Correspondingly, in the human cervix, an increase in SLC6A14 mRNA was associated with a parallel increase in protein [59]. Interestingly, after the 4-cell stage, valine, along with leucine, isoleucine, and methionine, was favorable for the cleavage rates of the diploids after compaction and increased the total number of cells in the blastocyst and inner cell mass [60]. SLC7A8 transcript was up-regulated in the LF-LCL endometrium on D7, and this carrier transports neutral AAs such as valine, which is also up-regulated in the LF-LCL group. SLC7A8 is also responsible for alanine transport. Despite the fact that the concentration of alanine in uterine washings is similar for both groups, the transcript abundance of DDO, an enzyme related to alanine degradation, is up-regulated in LF-LCL group. Perhaps there is a requirement to strictly control luminal concentrations of alanine on D7.
In this study, AA concentrations were quantified using uterine washings, composed of histotroph diluted in 20 mL of PBS. Such a method of quantification is appropriate for estimating AA availability in the uterus.
Collectively, the present data suggest that in the more receptive uterine phenotype, on D4, AA transport and metabolic pathways are directed to supply endometrial requirements for growth and function. Coincidentally, around D4, embryos have a limited demand for AAs, and they are in transit from the oviduct to the uterus. Thus, the AA composition of the histotroph reflects the endometrial demands for proper function more than a milieu for embryonic development. More interestingly, on D7, when the embryo is expected to be in the uterus, AAs are more abundant in the uterine secretions of the LF-LCL group, probably to serve as a supply to meet embryonic requirements. Correspondingly, transcript abundance data show a greater number of AA transporters that are up-regulated in the more receptive endometrium on D7. This phenotype is probably associated with the greater AA requirement for the subsequent stages of embryonic development (i.e., hatching and elongation). In terms of sex steroid regulation of such processes, the phenotype on D4 reflects classical actions of estradiol that include stimulation of endometrial cell proliferation, while the phenotype on D7 is associated with actions of progesterone, such as glandular secretory activity and histotroph formation to support embryonic growth [4, 39, 61–63]. A summarized view of the AA transport systems in the endometrium in association with receptivity during early diestrus is presented in Fig. 7.
To the best of our knowledge, this is the first investigation that relates early diestrus temporal changes in AA transport and availability in the uterine lumen to different periovulatory endocrine patterns that characterize fertility phenotypes. This study points to an important link between AA metabolic pathways in the endometrium and uterine receptivity. In addition, these data can serve as a basis for novel studies in bovine reproduction biotechnology with the aim of developing new tools to improve beef cattle fertility and profitability.
Conclusions
This study showed that the transcript abundance of AA transporters in the endometrium is linked with the receptive state of the endometrial tissue at both D4 and D7. Additionally, the AA patterns in uterine flushings differ between contrasting receptivity status of cows, both at D4 and at D7. The latter data indicate that AA metabolism and transport may be a potential key that needs to be regulated in order to fine-tune the maternal receptive state during early diestrus.
Acknowledgements
Authors wish to thank FAPESP, CNPq and CAPES for financial support and scholarships, Ourofino, Cravinhos, SP, Brazil for providing drugs and hormones, Dr. Messias Alves da Trindade Neto, faculty, post-doctoral fellows, students and staff of the School of Veterinary Medicine and Animal Science of the University of São Paulo for technical support.
Funding
FAPESP 2014/01727-4 to MISS
CNPq- 481199/2012-8 and FAPESP- 2011/03226-4 to MB
CNPq 140527/2013-3 to MRF
The funding bodies had no participation on the study, collection, analysis, interpretation of data nor in writing the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Authors’ contributions
MFR contributed to animal management, qPCR analysis, statistical analysis of transcripts and amino acids data and was a major contributor in writing the manuscript. MISS contributed to animal management, performed samples preparation for qPCR analysis and performed qPCR analysis. GP performed animals and reproductive management, statistical analysis of follicle, corpus luteum and progesterone data and contributed in writing the manuscript. VVH contributed in writing the manuscript. MB was the PI and contributed to experiment design and writing the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not Applicable.
Ethics approval
Animal procedures were approved by the Ethics and Animal Handling Committee of the University of São Paulo (protocol 2280/2011). The experiments were conducted at the University of São Paulo, Pirassununga, Brazil.
Abbreviations
- AA
Amino acid
- AAAB
α-amino butyric acid
- AAD
α-amino adipic acid
- ALA
Alanine
- ANOVA
Analysis of variance
- ASN
Asparagine
- ASNS
Asparagine synthase
- B-ALA
β-alanine
- cDNA
Complementar deoxyribonucleic acid
- CL
Corpus luteum
- CYS
Cysteine
- DDO
D-aspartate oxidade
- E2
Estradiol
- GLN
Glutamine
- GLU
Glutamate
- GLY
Glycine
- GnRH
Gonadotrophin releasing hormone
- GPT
Glutamic-pyruvate transaminase
- HPLC
High capacity liquid chromatography
- HYS
Histidine
- ILE
Isoleucine
- LF-LCL
Large follicle-large CL group
- LYS
Lysine
- MET
Methionine
- ORN
Ornithine
- P4
Progesterone
- PBS
Phosphate buffer solution
- PHE
Phenylalanine
- POF
Pre-ovulatory follicle
- PRO
Proline
- PROC GLM
General linear models procedure
- qRT-PCR
Real-time reverse transcriptase-polymerase chain reaction
- RNA
Ribonucleic acid
- SCLY
Selenocysteine lyase
- SER
Serine
- SF-SCL
Small follicle-small CL group
- SLC
Solute carrier protein
- TAU
Taurine
- THR
Threonine
- TRP
Tryptophan
- TYR
Tyrosine
- VAL
Valine
Contributor Information
Moana Rodrigues França, Email: mfranca@usp.br.
Maressa Izabel Santos da Silva, Email: maressaisabel@hotmail.com.
Guilherme Pugliesi, Email: pugliesi_vet@hotmail.com.
Veerle Van Hoeck, Email: vvanhoeck@gmail.com.
Mario Binelli, Email: binelli@usp.br.
References
- 1.Garrett JE, Geisert RD, Zavy MT, Morgan GL. Evidence for maternal regulation of early conceptus growth and development in beef cattle. J Reprod Fertil. 1988;84:437–446. doi: 10.1530/jrf.0.0840437. [DOI] [PubMed] [Google Scholar]
- 2.Satterfield MC, Song G, Kochan KJ, Riggs PK, Simmons RM, Elsik CG, et al. Discovery of candidate genes and pathways in the endometrium regulating ovine blastocyst growth and conceptus elongation. Physiol Genomics. 2009;39:85–99. doi: 10.1152/physiolgenomics.00001.2009. [DOI] [PubMed] [Google Scholar]
- 3.Carter F, Forde N, Duffy P, Wade M, Fair T, Crowe MA, et al. Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod Fertil Dev. 2008;20:368–375. doi: 10.1071/RD07204. [DOI] [PubMed] [Google Scholar]
- 4.Forde N, Carter F, Fair T, Crowe MA, Evans AC, Spencer TE, et al. Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol Reprod. 2009;81:784–794. doi: 10.1095/biolreprod.108.074336. [DOI] [PubMed] [Google Scholar]
- 5.Clemente M, de La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, Roche JF, et al. Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction. 2009;138:507–517. doi: 10.1530/REP-09-0152. [DOI] [PubMed] [Google Scholar]
- 6.Demetrio DG, Santos RM, Demetrio CG, Vasconcelos JL. Factors affecting conception rates following artificial insemination or embryo transfer in lactating Holstein cows. J Dairy Sci. 2007;90:5073–5082. doi: 10.3168/jds.2007-0223. [DOI] [PubMed] [Google Scholar]
- 7.Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci. 2004;82(E-Suppl):E4–13. doi: 10.2527/2004.8213_supplE4x. [DOI] [PubMed] [Google Scholar]
- 8.Gao H, Wu G, Spencer TE, Johnson GA, Li X, Bazer FW. Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod. 2009;80:86–93. doi: 10.1095/biolreprod.108.071597. [DOI] [PubMed] [Google Scholar]
- 9.Meier S, Mitchell MD, Walker CG, Roche JR, Verkerk GA. Amino acid concentrations in uterine fluid during early pregnancy differ in fertile and subfertile dairy cow strains. J Dairy Sci. 2014;97:1364–1376. doi: 10.3168/jds.2013-6954. [DOI] [PubMed] [Google Scholar]
- 10.Hugentobler SA, Diskin MG, Leese HJ, Humpherson PG, Watson T, Sreenan JM, et al. Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol Reprod Dev. 2007;74:445–454. doi: 10.1002/mrd.20607. [DOI] [PubMed] [Google Scholar]
- 11.Hugentobler SA, Sreenan JM, Humpherson PG, Leese HJ, Diskin MG, Morris DG. Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod Fertil Dev. 2010;22:684–694. doi: 10.1071/RD09129. [DOI] [PubMed] [Google Scholar]
- 12.Forde N, Simintiras CA, Sturmey R, Mamo S, Kelly AK, Spencer TE, et al. Amino Acids in the Uterine Luminal Fluid Reflects the Temporal Changes in Transporter Expression in the Endometrium and Conceptus during Early Pregnancy in Cattle. PLoS One. 2014;9:e100010. doi: 10.1371/journal.pone.0100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steeves TE, Gardner DK. Temporal and differential effects of amino acids on bovine embryo development in culture. Biol Reprod. 1999;61:731–740. doi: 10.1095/biolreprod61.3.731. [DOI] [PubMed] [Google Scholar]
- 14.Daniel JC, Krishnan RS. Amino acid requirements for growth of the rabbit blastocyst in vitro. J Cell Physiol. 1967;70:155–160. doi: 10.1002/jcp.1040700204. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Niwa K, Lim JM, Okuda K. Effects of phosphate, energy substrates, and amino acids on development of in vitro-matured, in vitro-fertilized bovine oocytes in a chemically defined, protein-free culture medium. Biol Reprod. 1993;48:1320–1325. doi: 10.1095/biolreprod48.6.1320. [DOI] [PubMed] [Google Scholar]
- 16.Smith RJ, Dean W, Konfortova G, Kelsey G. Identification of novel imprinted genes in a genome-wide screen for maternal methylation. Genome Res. 2003;13:558–569. doi: 10.1101/gr.781503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groebner AE, Rubio-Aliaga I, Schulke K, Reichenbach HD, Daniel H, Wolf E, et al. Increase of essential amino acids in the bovine uterine lumen during preimplantation development. Reproduction. 2011;141:685–695. doi: 10.1530/REP-10-0533. [DOI] [PubMed] [Google Scholar]
- 18.Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen. ii. glucose transporters in the uterus and peri-implantation conceptuses. Biol Reprod. 2009;80:94–104. doi: 10.1095/biolreprod.108.071654. [DOI] [PubMed] [Google Scholar]
- 19.Partridge RJ, Leese HJ. Consumption of amino acids by bovine preimplantation embryos. Reprod Fertil Dev. 1996;8:945–950. doi: 10.1071/RD9960945. [DOI] [PubMed] [Google Scholar]
- 20.Donnay I, Leese HJ. Embryo metabolism during the expansion of the bovine blastocyst. Mol Reprod Dev. 1999;53:171–178. doi: 10.1002/(SICI)1098-2795(199906)53:2<171::AID-MRD6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Morris DG, Humpherson PG, Leese HJ, Sreenan JM. Amino acid turnover by elongating cattle blastocysts recovered on days 14-16 after insemination. Reproduction. 2002;124:667–673. doi: 10.1530/rep.0.1240667. [DOI] [PubMed] [Google Scholar]
- 22.Houghton FD, Hawkhead JA, Humpherson PG, Hogg JE, Balen AH, Rutherford AJ, et al. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum Reprod. 2002;17:999–1005. doi: 10.1093/humrep/17.4.999. [DOI] [PubMed] [Google Scholar]
- 23.Sturmey RG, Bermejo-Alvarez P, Gutierrez-Adan A, Rizos D, Leese HJ, Lonergan P. Amino acid metabolism of bovine blastocysts: a biomarker of sex and viability. Mol Reprod Dev. 2010;77:285–296. doi: 10.1002/mrd.21173. [DOI] [PubMed] [Google Scholar]
- 24.Setoyama C, Miura R. Structural and functional characterization of the human brain D-aspartate oxidase. J Biochem. 1997;121:798–803. doi: 10.1093/oxfordjournals.jbchem.a021655. [DOI] [PubMed] [Google Scholar]
- 25.Mihara H, Kurihara T, Watanabe T, Yoshimura T, Esaki N. cDNA cloning, purification, and characterization of mouse liver selenocysteine lyase. Candidate for selenium delivery protein in selenoprotein synthesis. J Biol Chem. 2000;275:6195–6200. doi: 10.1074/jbc.275.9.6195. [DOI] [PubMed] [Google Scholar]
- 26.Mesquita FS, Pugliesi G, Scolari SC, França MR, Ramos RdS, Oliveira ML, et al. Manipulation of the periovulatory sex-steroidal milieu affects endometrial but not luteal gene expression on early diestrus Nelore cows., vol. in press. Theriogenology: Theriogenology. 2014. [DOI] [PubMed]
- 27.Pugliesi G, Santos FB, Lopes E, Nogueira É, Maio JR, Binelli M. Improved fertility in suckled beef cows ovulating large follicles or supplemented with long-acting progesterone after timed-AI. Theriogenology. 2016;85:1239–1248. doi: 10.1016/j.theriogenology.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Bazer FW. Uterine protein secretions: Relationship to development of the conceptus. J Anim Sci. 1975;41:1376–1382. doi: 10.2527/jas1975.4151376x. [DOI] [PubMed] [Google Scholar]
- 29.Roberts RM, Bazer FW. The functions of uterine secretions. J Reprod Fertil. 1988;82:875–892. doi: 10.1530/jrf.0.0820875. [DOI] [PubMed] [Google Scholar]
- 30.Kane MT, Morgan PM, Coonan C. Peptide growth factors and preimplantation development. Hum Reprod Update. 1997;3:137–157. doi: 10.1093/humupd/3.2.137. [DOI] [PubMed] [Google Scholar]
- 31.Dunne LD, Diskin MG, Sreenan JM. Embryo and foetal loss in beef heifers between day 14 of gestation and full term. Anim Reprod Sci. 2000;58:39–44. doi: 10.1016/S0378-4320(99)00088-3. [DOI] [PubMed] [Google Scholar]
- 32.França MR, Mesquita FS, Lopes E, Pugliesi G, Van Hoeck V, Chiaratti MR, et al. Modulation of periovulatory endocrine profiles in beef cows: consequences for endometrial glucose transporters and uterine fluid glucose levels. Domest Anim Endocrinol. 2015;50:83–90. doi: 10.1016/j.domaniend.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Garbarino EJ, Hernandez JA, Shearer JK, Risco CA, Thatcher WW. Effect of lameness on ovarian activity in postpartum holstein cows. J Dairy Sci. 2004;87:4123–4131. doi: 10.3168/jds.S0022-0302(04)73555-9. [DOI] [PubMed] [Google Scholar]
- 34.White JA, Hart RJ, Fry JC. An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J Automat Chem. 1986;8:170–177. doi: 10.1155/S1463924686000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos RS, Oliveira ML, Izaguirry AP, Vargas LM, Soares MB, Mesquita FS, et al. The periovulatory endocrine milieu affects the uterine redox environment in beef cows. Reprod Biol Endocrinol. 2015;13:39. doi: 10.1186/s12958-015-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araújo ER, Sponchiado M, Pugliesi G, Van Hoeck V, Mesquita FS, Membrive CM, et al. Spatio-specific regulation of endocrine-responsive gene transcription by periovulatory endocrine profiles in the bovine reproductive tract. Reprod Fertil Dev. 2016;28:1533–44. [DOI] [PubMed]
- 38.Ramos RS, Mesquita FS, D’Alexandri FL, Gonella-Diaza AM, Papa PC, Binelli M. Regulation of the polyamine metabolic pathway in the endometrium of cows during early diestrus. Mol Reprod Dev. 2014;81:584–594. doi: 10.1002/mrd.22323. [DOI] [PubMed] [Google Scholar]
- 39.Mesquita FS, Ramos RS, Pugliesi G, Andrade SC, Van Hoeck V, Langbeen A, et al. The Receptive Endometrial Transcriptomic Signature Indicates an Earlier Shift from Proliferation to Metabolism at Early Diestrus in the Cow. Biol Reprod. 2015;93:52. doi: 10.1095/biolreprod.115.129031. [DOI] [PubMed] [Google Scholar]
- 40.Minten MA, Bilby TR, Bruno RG, Allen CC, Madsen CA, Wang Z, et al. Effects of fertility on gene expression and function of the bovine endometrium. PLoS One. 2013;8:e69444. doi: 10.1371/journal.pone.0069444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diskin MG, Murphy JJ, Sreenan JM. Embryo survival in dairy cows managed under pastoral conditions. Anim Reprod Sci. 2006;96:297–311. doi: 10.1016/j.anireprosci.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Cavagna M, Mantese JC. Biomarkers of endometrial receptivity--a review. Placenta. 2003;24(Suppl B):S39–47. doi: 10.1016/S0143-4004(03)00184-X. [DOI] [PubMed] [Google Scholar]
- 43.Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, et al. Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod. 2011;85:144–156. doi: 10.1095/biolreprod.110.090019. [DOI] [PubMed] [Google Scholar]
- 44.Stegner JE, Kojima FN, Bader JF, Lucy MC, Ellersieck MR, Smith MF, et al. Follicular dynamics and steroid profiles in cows during and after treatment with progestin-based protocols for synchronization of estrus. J Anim Sci. 2004;82:1022–1028. doi: 10.2527/2004.8241022x. [DOI] [PubMed] [Google Scholar]
- 45.Vasconcelos JL, Sartori R, Oliveira HN, Guenther JG, Wiltbank MC. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology. 2001;56:307–314. doi: 10.1016/S0093-691X(01)00565-9. [DOI] [PubMed] [Google Scholar]
- 46.Pfeifer LF, Mapletoft RJ, Kastelic JP, Small JA, Adams GP, Dionello NJ, et al. Effects of low versus physiologic plasma progesterone concentrations on ovarian follicular development and fertility in beef cattle. Theriogenology. 2009;72:1237–1250. doi: 10.1016/j.theriogenology.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN. Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol. 1998;145:47–54. doi: 10.1016/S0303-7207(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 48.Redmer DA, Grazul AT, Kirsch JD, Reynolds LP. Angiogenic activity of bovine corpora lutea at several stages of luteal development. J Reprod Fertil. 1988;82:627–634. doi: 10.1530/jrf.0.0820627. [DOI] [PubMed] [Google Scholar]
- 49.Park HJ, Park SJ, Koo DB, Kong IK, Kim MK, Kim JM, et al. Unfolding protein response signaling is involved in development, maintenance, and regression of the corpus luteum during the bovine estrous cycle. Biochem Biophys Res Commun. 2013;441:344–350. doi: 10.1016/j.bbrc.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 50.Lopes AS, Butler ST, Gilbert RO, Butler WR. Relationship of pre-ovulatory follicle size, estradiol concentrations and season to pregnancy outcome in dairy cows. Anim Reprod Sci. 2007;99:34–43. doi: 10.1016/j.anireprosci.2006.04.056. [DOI] [PubMed] [Google Scholar]
- 51.Nishimoto H, Hamano S, Hill GA, Miyamoto A, Tetsuka M. Classification of bovine follicles based on the concentrations of steroids, glucose and lactate in follicular fluid and the status of accompanying follicles. J Reprod Dev. 2009;55:219–224. doi: 10.1262/jrd.20114. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Yang L, Yang YJ, Liu XY, Jia JG, Qian JY, et al. Low-dose taurine upregulates taurine transporter expression in acute myocardial ischemia. Int J Mol Med. 2013;31:817–824. doi: 10.3892/ijmm.2013.1264. [DOI] [PubMed] [Google Scholar]
- 53.Kuran M, Robinson JJ, Brown DS, McEvoy TG. Development, amino acid utilization and cell allocation in bovine embryos after in vitro production in contrasting culture systems. Reproduction. 2002;124:155–165. doi: 10.1530/rep.0.1240155. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi Y, Kanagawa H. Effects of glutamine, glycine and taurine on the development of in vitro fertilized bovine zygotes in a chemically defined medium. J Vet Med Sci. 1998;60:433–437. doi: 10.1292/jvms.60.433. [DOI] [PubMed] [Google Scholar]
- 55.Guyader-Joly C, Guérin P, Renard JP, Guillaud J, Ponchon S, Ménézo Y. Precursors of taurine in female genital tract: effects on developmental capacity of bovine embryo produced in vitro. Amino Acids. 1998;15:27–42. doi: 10.1007/BF01345278. [DOI] [PubMed] [Google Scholar]
- 56.Whitaker BD, Knight JW. Exogenous gamma-glutamyl cycle compounds supplemented to in vitro maturation medium influence in vitro fertilization, culture, and viability parameters of porcine oocytes and embryos. Theriogenology. 2004;62:311–322. doi: 10.1016/j.theriogenology.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Ko TC, Beauchamp RD, Townsend CM, Thompson JC. Glutamine is essential for epidermal growth factor-stimulated intestinal cell proliferation. Surgery. 1993;114:147–153. [PubMed] [Google Scholar]
- 58.Mendes MI, Santos AS, Smith DE, Lino PR, Colaço HG, de Almeida IT, et al. Insights into the regulatory domain of cystathionine Beta-synthase: characterization of six variant proteins. Hum Mutat. 2014;35:1195–1202. doi: 10.1002/humu.22616. [DOI] [PubMed] [Google Scholar]
- 59.Gupta N, Prasad PD, Ghamande S, Moore-Martin P, Herdman AV, Martindale RG, et al. Up-regulation of the amino acid transporter ATB(0,+) (SLC6A14) in carcinoma of the cervix. Gynecol Oncol. 2006;100:8–13. doi: 10.1016/j.ygyno.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 60.Van Thuan N, Harayama H, Miyake M. Characteristics of preimplantational development of porcine parthenogenetic diploids relative to the existence of amino acids in vitro. Biol Reprod. 2002;67:1688–1698. doi: 10.1095/biolreprod.102.004812. [DOI] [PubMed] [Google Scholar]
- 61.Arai M, Yoshioka S, Tasaki Y, Okuda K. Remodeling of bovine endometrium throughout the estrous cycle. Anim Reprod Sci. 2013;142:1–9. doi: 10.1016/j.anireprosci.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Reynolds LP, Kirsch JD, Kraft KC, Knutson DL, McClaflin WJ, Redmer DA. Time-course of the uterine response to estradiol-17beta in ovariectomized ewes: uterine growth and microvascular development. Biol Reprod. 1998;59:606–612. doi: 10.1095/biolreprod59.3.606. [DOI] [PubMed] [Google Scholar]
- 63.Spencer TE, Johnson GA, Burghardt RC, Bazer FW. Progesterone and placental hormone actions on the uterus: insights from domestic animals. Biol Reprod. 2004;71:2–10. doi: 10.1095/biolreprod.103.024133. [DOI] [PubMed] [Google Scholar]
- 64.Bettegowda A, Patel OV, Ireland JJ, Smith GW. Quantitative analysis of messenger RNA abundance for ribosomal protein L-15, cyclophilin-A, phosphoglycerokinase, beta-glucuronidase, glyceraldehyde 3-phosphate dehydrogenase, beta-actin, and histone H2A during bovine oocyte maturation and early embryogenesis in vitro. Mol Reprod Dev. 2006;73:267–278. doi: 10.1002/mrd.20333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].


