Abstract
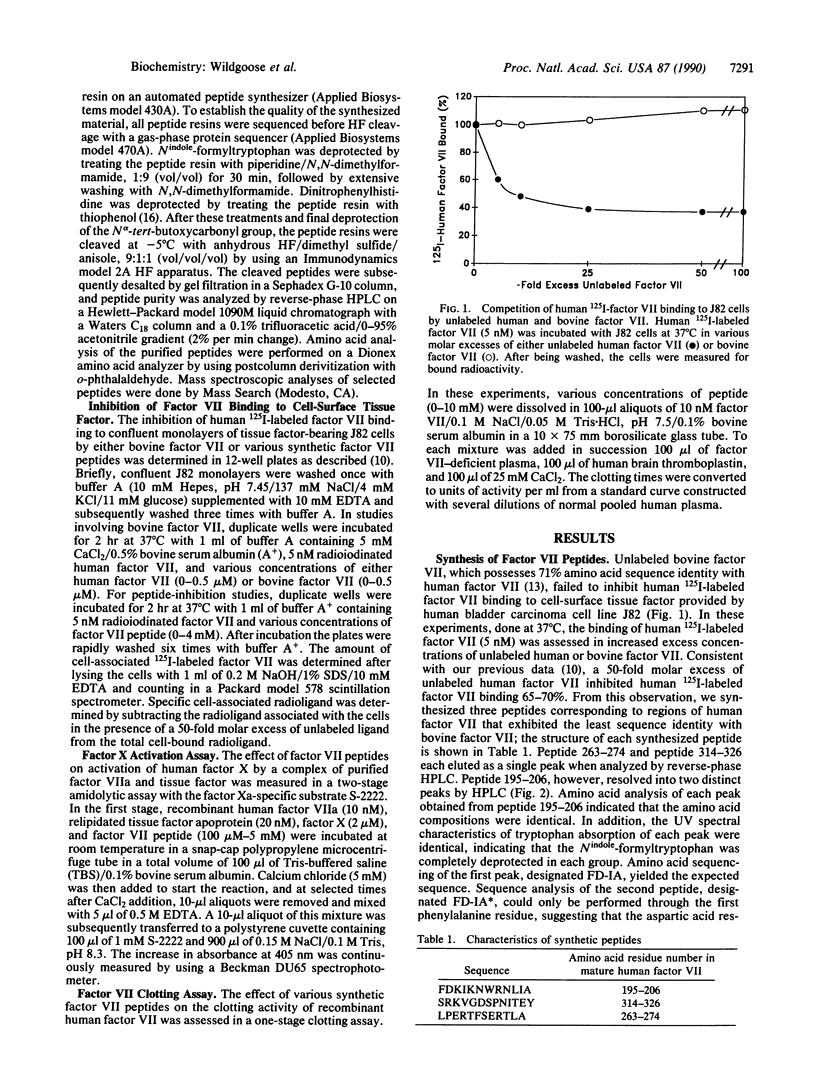
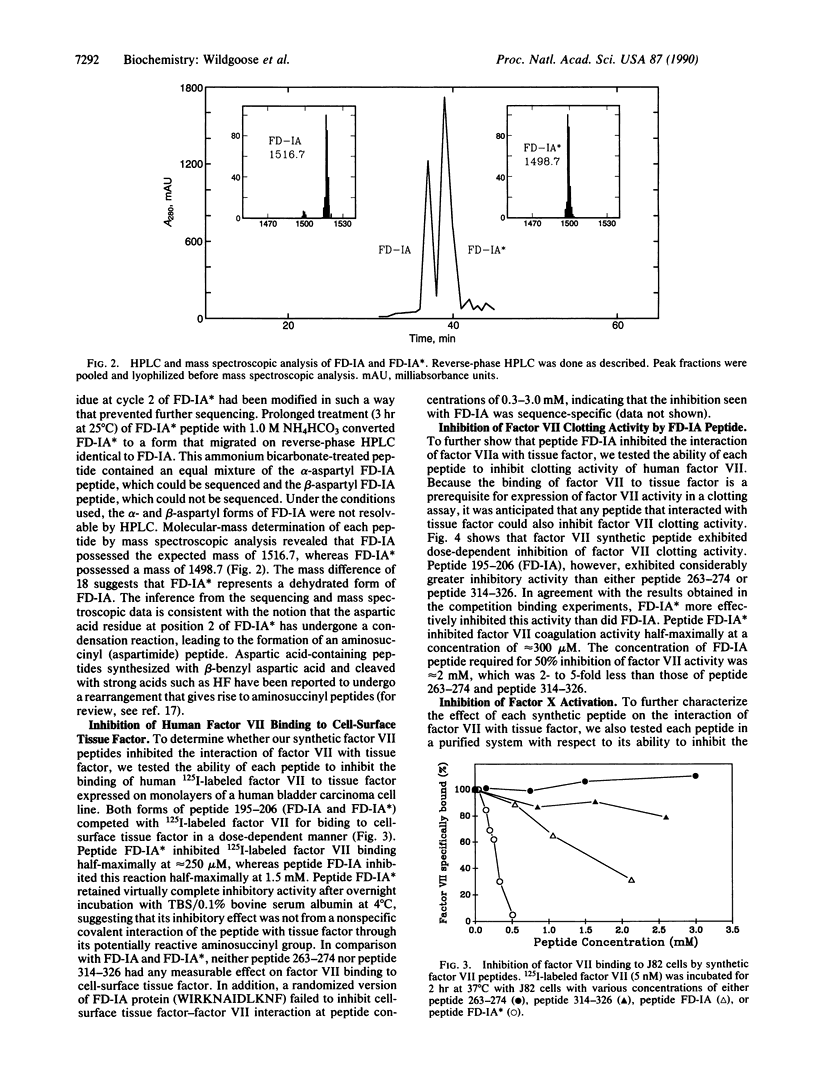
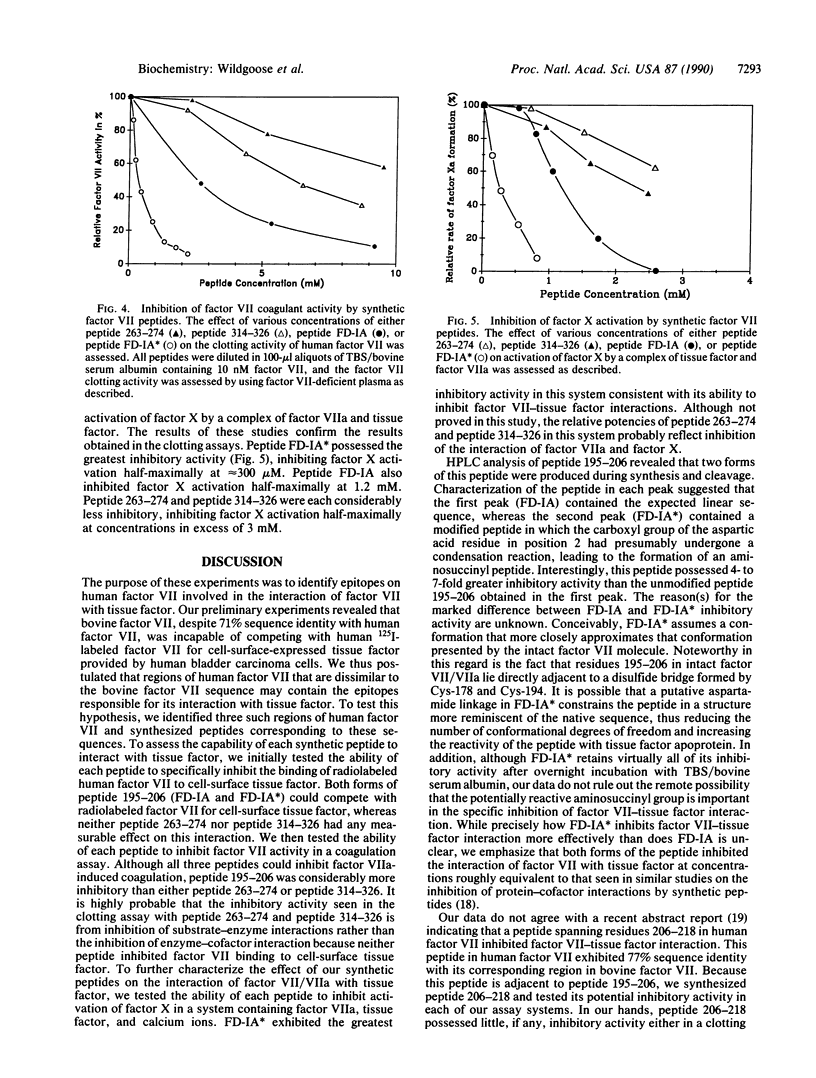
Previous studies indicated that human and bovine factor VII exhibit 71% amino acid sequence identity. In the present study, competition binding experiments revealed that the interaction of human factor VII with cell-surface human tissue factor was not inhibited by 100-fold molar excess of bovine factor VII. This finding indicated that bovine and human factor VII are not structurally homologous in the region(s) where human factor VII interacts with human tissue factor. On this premise, we synthesized three peptides corresponding to regions of human factor VII that exhibited marked structural dissimilarity to bovine factor VII; these regions of dissimilarity included residues 195-206, 263-274, and 314-326. Peptide 195-206 inhibited the interaction of factor VII with cell-surface tissue factor and the activation of factor X by a complex of factor VIIa and tissue factor half-maximally at concentrations of 1-2 mM. A structurally rearranged form of peptide 195-206 containing an aspartimide residue inhibited these reactions half-maximally at concentrations of 250-300 microM. In contrast, neither peptide 263-274 nor peptide 314-326, at 2 mM concentration, significantly affected either factor VIIa interaction with tissue factor or factor VIIa-mediated activation of factor X. Our data provide presumptive evidence that residues 195-206 of human factor VII are involved in the interaction of human factor VII with the extracellular domain of human tissue factor apoprotein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL W. N., ALTON H. G. A brain extract as a substitute for platelet suspensions in the thromboplastin generation test. Nature. 1954 Nov 6;174(4436):880–881. doi: 10.1038/174880a0. [DOI] [PubMed] [Google Scholar]
- Beebe D. P., Miles L. A., Plow E. F. A linear amino acid sequence involved in the interaction of t-PA with its endothelial cell receptor. Blood. 1989 Nov 1;74(6):2034–2037. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Hagen F. S., Gray C. L., O'Hara P., Grant F. J., Saari G. C., Woodbury R. G., Hart C. E., Insley M., Kisiel W., Kurachi K. Characterization of a cDNA coding for human factor VII. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2412–2416. doi: 10.1073/pnas.83.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson T. L., Stormorken H., Prydz H. Species specificity of tissue thromboplastin. Haemostasis. 1984;14(5):440–444. doi: 10.1159/000215102. [DOI] [PubMed] [Google Scholar]
- Kondo S., Kisiel W. Evidence that plasma lipoproteins inhibit the factor VIIa-tissue factor complex by a different mechanism that extrinsic pathway inhibitor. Blood. 1987 Dec;70(6):1947–1954. [PubMed] [Google Scholar]
- Nawroth P. P., Kisiel W., Stern D. M. Anticoagulant and antithrombotic properties of a gamma-carboxyglutamic acid-rich peptide derived from the light chain of blood coagulation factor X. Thromb Res. 1986 Dec 1;44(5):625–637. doi: 10.1016/0049-3848(86)90164-7. [DOI] [PubMed] [Google Scholar]
- Nemerson Y., Bach R. Tissue factor revisited. Prog Hemost Thromb. 1982;6:237–261. [PubMed] [Google Scholar]
- Nemerson Y. Tissue factor and hemostasis. Blood. 1988 Jan;71(1):1–8. [PubMed] [Google Scholar]
- Paborsky L. R., Tate K. M., Harris R. J., Yansura D. G., Band L., McCray G., Gorman C. M., O'Brien D. P., Chang J. Y., Swartz J. R. Purification of recombinant human tissue factor. Biochemistry. 1989 Oct 3;28(20):8072–8077. doi: 10.1021/bi00446a016. [DOI] [PubMed] [Google Scholar]
- Pedersen A. H., Lund-Hansen T., Bisgaard-Frantzen H., Olsen F., Petersen L. C. Autoactivation of human recombinant coagulation factor VII. Biochemistry. 1989 Nov 28;28(24):9331–9336. doi: 10.1021/bi00450a013. [DOI] [PubMed] [Google Scholar]
- Sakai T., Lund-Hansen T., Paborsky L., Pedersen A. H., Kisiel W. Binding of human factors VII and VIIa to a human bladder carcinoma cell line (J82). Implications for the initiation of the extrinsic pathway of blood coagulation. J Biol Chem. 1989 Jun 15;264(17):9980–9988. [PubMed] [Google Scholar]
- Sakai T., Lund-Hansen T., Thim L., Kisiel W. The gamma-carboxyglutamic acid domain of human factor VIIa is essential for its interaction with cell surface tissue factor. J Biol Chem. 1990 Feb 5;265(4):1890–1894. [PubMed] [Google Scholar]
- Takeya H., Kawabata S., Nakagawa K., Yamamichi Y., Miyata T., Iwanaga S., Takao T., Shimonishi Y. Bovine factor VII. Its purification and complete amino acid sequence. J Biol Chem. 1988 Oct 15;263(29):14868–14877. [PubMed] [Google Scholar]
- Tam J. P., Riemen M. W., Merrifield R. B. Mechanisms of aspartimide formation: the effects of protecting groups, acid, base, temperature and time. Pept Res. 1988 Sep-Oct;1(1):6–18. [PubMed] [Google Scholar]
- Thim L., Bjoern S., Christensen M., Nicolaisen E. M., Lund-Hansen T., Pedersen A. H., Hedner U. Amino acid sequence and posttranslational modifications of human factor VIIa from plasma and transfected baby hamster kidney cells. Biochemistry. 1988 Oct 4;27(20):7785–7793. doi: 10.1021/bi00420a030. [DOI] [PubMed] [Google Scholar]
- Wildgoose P., Kisiel W. Activation of human factor VII by factors IXa and Xa on human bladder carcinoma cells. Blood. 1989 May 15;73(7):1888–1895. [PubMed] [Google Scholar]



