Abstract
Tenofovir disoproxil fumarate (tenofovir DF) was studied in combination with rifampin in 24 healthy subjects in a multiple-dose, open-label, single-group, two-period study. All subjects were given tenofovir DF at 300 mg once a day (QD) from days 1 to 10 (period 1). From days 11 to 20 the subjects received tenofovir DF at 300 mg combined with rifampin at 600 mg QD (period 2). The multiple-dose pharmacokinetics of tenofovir (day 10 and 20) and rifampin (day 20) were assessed. The drug-related adverse events (AEs) experienced during this study were mostly mild. Only one grade 3 AE possibly or probably related to the treatment (raised liver enzyme levels) occurred during period 2; the subject was withdrawn from the study. Pharmacokinetic data for 23 subjects were thus evaluable. Point estimates for the mean ratios of tenofovir with rifampin versus tenofovir alone for the area under the concentration-time curve from time zero to 24 h (AUC0-24), the maximum concentration of drug in plasma (Cmax), and the minimum concentration of drug in plasma (Cmin) were 0.88, 0.84, and 0.85, respectively. The 90% classical confidence intervals for AUC0-24, Cmax, and Cmin were 0.84 to 0.92, 0.78 to 0.90, and 0.80 to 0.91, respectively, thus suggesting pharmacokinetic equivalence. Similarly, coadministration of rifampin and tenofovir DF did not result in changes in the values of the tenofovir pharmacokinetic parameters. For rifampin, the values of the pharmacokinetic parameters found in this study were comparable to those found in the literature, indicating that tenofovir DF has no effect on the pharmacokinetics of rifampin. In conclusion, adaptation of either the rifampin or the tenofovir DF dose for the simultaneous treatment of tuberculosis and human immunodeficiency virus (HIV) infection in HIV-infected patients is probably not required.
Coinfection with Mycobacterium tuberculosis and human immunodeficiency virus (HIV) is frequent, particularly in Africa and Asia (3, 14, 18). Simultaneous treatment of tuberculosis and HIV infection may lead to complex combination therapy. Rifampin is a drug of choice for the treatment of tuberculosis. Rifampin is known to have major pharmacokinetic interactions with HIV protease inhibitors and nonnucleoside reverse transcriptase inhibitors (8, 10, 12, 13, 16, 17). Tenofovir disoproxil fumarate (tenofovir DF) is the first drug from a new class of anti-HIV agents (nucleotide reverse transcriptase inhibitors) that has been recently approved for use for the treatment of HIV infections in adults. However, no data are available regarding its pharmacokinetics in combination with tuberculostatic drugs, in particular, rifampin. No influence of rifampin on the pharmacokinetics of tenofovir is expected, because both drugs are metabolized and eliminated in different ways. Tenofovir is eliminated unchanged by glomerular filtration and active tubular secretion (1, 6), while rifampin is extensively metabolized by intestinal and hepatic metabolism (4). However, a pharmacokinetic interaction cannot be excluded.
The clinical trial described here was designed to explore the pharmacokinetics of tenofovir DF with and without rifampin in an effort to establish whether there is a need to adjust the dosage of either medication when the two medications are used for the treatment of patients coinfected with M. tuberculosis and HIV.
MATERIALS AND METHODS
Study design.
The present study was designed to evaluate the effect of 600 mg of rifampin on the pharmacokinetics of 300 mg of tenofovir DF and also to assess whether tenofovir DF has a substantial impact on steady-state exposure to rifampin. This study was a multiple-dose, open-label, single-group, two-period study with 24 healthy volunteers. First, the subjects received tenofovir DF at 300 mg once daily (QD) for 10 days (period 1). At study day 10, a steady-state 24-h pharmacokinetic curve was obtained for tenofovir. During the second period of the study (period 2), tenofovir DF at 300 mg was combined with rifampin at 600 mg QD, again for 10 days. At study day 20, 24-h steady-state pharmacokinetic curves were obtained for tenofovir and rifampin. During the study both tenofovir DF and rifampin had to be taken with breakfast. On the days prior to study days 9 and 19, the subjects reported to the study center for direct observation of dosing with the medications with a standardized breakfast. Subsequently, on the evenings of study days 9 and 19 the subjects remained at the study center for two overnight stays and remained at the study center until the mornings of study days 11 and 21, respectively. On days 9, 10, 11, 19, and 20 the subjects received a standardized breakfast of 550 kcal (two slices of white bread, 15 g of low-fat margarine, 14 g of jelly, 150 ml of orange juice, and 150 ml of skim milk). The medication was administered immediately after breakfast with 200 ml of tap water. All other meals and snacks on the pharmacokinetic study days were also standardized. When the subjects took the medication at home, study drugs were administered with breakfast (at least two and at most three slices of wheat bread).
No crossover design was used in this study because rifampin could lead to considerable carryover effects, due to its long-lasting cytochrome P450-inducing effect. To eliminate this effect a longer washout period would be necessary, but this would have significantly prolonged the duration of the study and would have led to difficulties with subject recruitment and retention. This study was reviewed and approved by the independent ethics committee Arnhem-Nijmegen. Written informed consent was obtained from each study subject prior to the conduct of any study-related activity.
Study subjects.
Twenty-four healthy male and female subjects were eligible for inclusion in the study. The subjects could be between 18 and 65 years of age with a body weight of at least 50 kg and in good age-appropriate health condition, as established by the individual's medical history; a physical examination; electrocardiography; and the results of biochemistry, hematology, and urine analyses within the 3 weeks prior to administration of the first dose. Other inclusion criteria were an ability to sign informed consent voluntarily and a willingness to refrain from the use of contact lenses during the treatment with rifampin. Exclusion criteria were as follows: positive tests for HIV, hepatitis B virus, or hepatitis C virus; a tuberculin skin test reaction of more than 15 mm or a tuberculin skin test reaction of 1 to 15 mm with a chest X ray with abnormalities consistent with tuberculosis; pregnancy; breast-feeding; the lack of adequate contraception (e.g., hysterectomy; bilateral tubal ligation; the use of an intrauterine device, total abstinence, or double-barrier methods; or a postmenopausal state for 2 years) among female subjects of childbearing potential; a creatinine clearance rate <60 ml/min; or a serum creatinine level above 133 μmol/liter.
Sampling for pharmacokinetic studies.
For determination of the tenofovir and rifampin concentrations in blood plasma, samples of 5 ml of blood, recovered to obtain at least 2 ml of plasma, were collected in heparinized hard plastic tubes at the following times: just before drug intake (predosing); on day 10 and day 20; and at 1, 2, 3, 4, 6, 8, 10, 12, 16, 20, and 24 h after drug intake. The blood samples were centrifuged at 2,500 × g for 10 min at 4°C. The plasma was divided into equal portions, transferred to polypropylene tubes, and stored at ≤−18°C for samples containing tenofovir and ≤−70°C for samples containing rifampin.
Safety.
Blood samples for serum biochemistry analyses, including tests for glucose and hematologic analyses, and urine samples for urinalysis were taken on study days 1, 4, 9, 11, 15, 19, and 21. These samples were taken while the subjects were in a fasting condition. In females of childbearing potential, testing of blood for human chorionic gonadotropin was performed at the screening visit and on study days 11 and 21. An instant test of urine for human chorionic gonadotropin was performed on study day −1. To avoid possible interactions between drugs of abuse and study drugs, a urine drug screen was performed at the screening visit and on study days −1, 9, and 19 with the Instacheck Multi-Drug Screen panel (Forefront Diagnostics, San Diego, Calif.). Vital signs for cardiovascular safety (systolic and diastolic blood pressures and heart rate) were monitored, and an electrocardiogram was recorded at the screening visit. The medical and nursing staff of the trial center monitored the subjects for adverse events (AEs) throughout their confinement. Subjects voluntarily reported any AE or reported AEs in response to general questioning. All AEs occurring between the first intake of the trial medication(s) and the end of the trial were reported. The relationship of the trial drug(s) was not related or unlikely to be related to the trial drug(s) if evidence existed that the AE had a source other than the trial drug(s). AEs were recorded as possibly or probably related to the trial drug(s) if a temporal relationship existed between the event onset and administration of the trial drug(s) and there was no evidence of an alternative cause for the event.
The severities of the AEs were recorded and graded according to the common toxicity criteria (grades 1, 2, 3, and 4) of the National Institute of Allergy and Infectious Diseases.
Bioanalysis.
Tenofovir concentrations were determined by using a validated high-performance liquid chromatography assay with a fluorimetric detector by a modified method (5). A Symmetry Shield RP18 analytical column (3.5 μm; 150 by 4.6 mm; Waters, Etten-Leur, The Netherlands) was used. The method involved extraction of the drug and the internal standard, adefovir (Gilead Sciences, Foster City, Calif.), from 100 μl of human plasma by adding 200 μl of acetonitrile. The supernatant was evaporated, and 200 μl of 0.34% chloroacetaldehyde in 50 mM acetate buffer (pH 4.5) was added. Fluorescent compounds were obtained by 40 min of incubation at 90°C. After the samples were cooled at −20°C for 5 min, 10 μl was injected onto the column. The flow rate was 1 ml/min. The mobile phase consisted of a mixture of phosphate buffer (50 mM; pH 6.8) and acetonitrile (96:4; vol/vol) that resolved the drug and the internal standard from endogenous matrix components and other drugs that were possibly present. Chromatographic analysis was performed at 30°C under isocratic conditions with extinction and emission wavelengths of 232 and 420 nm, respectively. The retention times of tenofovir and the internal standard, adefovir, were 6.34 and 3.90 min, respectively. The concentrations of the quality controls used were 0.03, 0.21, and 1.05 mg/liter. The intra- and interassay coefficients of variation were less than 4% for all quality controls. The lower limit of quantification was 0.0045 mg/liter. The rate of recovery of tenofovir from human plasma was 86%.
Rifampin concentrations were determined by using a previously described (12) high-performance liquid chromatography method. The concentrations of the quality controls used were 2.85, 9.5, and 24 mg/liter. The intra- and interassay coefficients of variation were less than 1.1% for all quality controls. The lower limit of quantification was 0.50 mg/liter. Samples from the same subject were analyzed by use of the same standard curve.
Pharmacokinetic analysis.
Pharmacokinetic parameters for tenofovir and rifampin were calculated by noncompartmental methods by use of the WinNonlin software package (version 4.1; Pharsight Corporation, Mountain View, Calif.) and the log/linear trapezoidal rule. On the basis of the individual plasma concentration-time data, the following pharmacokinetic parameters were determined: the area under the plasma concentration-time curve (AUC) from time zero to 24 h (AUC0-24; in milligram · hour per liter), the maximum concentration of drug in plasma (Cmax; in milligrams per liter), the time to reach Cmax (Tmax; in hours), the minimum concentration drug in plasma (Cmin; in milligrams per liter), the apparent elimination half-life (t1/2; in hours), and the apparent oral clearance (CL/F; in liters per hour). AUC0-C*, where C* is the last quantifiable concentration, was calculated for rifampin. Cmin and CL/F were not calculated for rifampin.
Statistical analysis.
Statistical analyses were performed with SPSS software (version 11.0; SPSS Inc., 1989 to 1999). Descriptive statistics were calculated with Excel 2000 software (Microsoft Corporation, 1985 to 1999). Evaluation of the AUC0-24 and the Cmax of tenofovir was the main objective of this trial. These parameters are considered the primary characteristics for the extent and the rate of drug absorption, respectively. The bioequivalence of tenofovir was determined by comparing the values of the relevant pharmacokinetic parameters obtained with the test treatment (tenofovir DF and rifampin on study day 20) to those obtained with the reference treatment (tenofovir DF alone on study day 10) by using the following statistical methods. The AUC0-24, Cmax, and Cmin of tenofovir were reported for study day 10 and study day 20 together by use of the ratios of the values on study day 20/values on study day 10. The arithmetic means and standard deviations are given for study day 20 and study day 10. The geometric mean ratios and 90% classical confidence intervals for AUC0-24, Cmax, and Cmin were calculated. Treatments were considered bioequivalent if the respective 90% classical confidence intervals for AUC0-24 and Cmax were included within a bioequivalence range of 80 to 125% (20). The values of the pharmacokinetic parameters for rifampin were compared with data from the literature by the use of descriptive statistics. The study was powered for the tenofovir Cmax by using nQuery software, and a sample size of 15 was required to achieve an 80% power to reject the null hypothesis that the two treatments are not equivalent in favor of the alternative hypothesis that the means of the two treatments are equivalent when the expected difference is 0.000. By this approach, a sample size of 15 would provide a 93% power for AUC0-24. By considering the possibility that the subjects would drop out and/or that some difficulties with sample or pharmacokinetic analysis with some subjects would occur, 24 subjects were enrolled in this study.
RESULTS
Demographics.
Twenty-four subjects (13 males, 11 females) were enrolled in this trial. One male subject was black; all other subjects were Caucasian. The mean age of the subjects was 41 years (range, 20 to 63 years). The mean body weight was 77 kg (range, 58 to 97 kg), and the mean height was 1.75 m (range, 1.59 to 1.88 m).
Pharmacokinetics.
The pharmacokinetic evaluation was based on data sets for subjects that completed the study on both study days (study days 10 and 20). Data for 23 subjects were included in the pharmacokinetic analysis of tenofovir and rifampin. Table 1 provides a summary of the values of the pharmacokinetic parameters for tenofovir, including the arithmetic means, geometric mean ratios, and 90% confidence interval estimates for the pharmacokinetic parameters for tenofovir alone (study day 10) and tenofovir in combination with rifampin (study day 20). The tenofovir AUC0-24, Cmax, and Cmin were lower in period 2 when tenofovir DF was coadministered with rifampin. However, the magnitudes of these differences were small, with geometric mean ratios (90% confidence intervals) of 0.88 (0.84 to 0.92), 0.84 (0.78 to 0.90), and 0.85 (0.80 to 0.91) for AUC0-24, Cmax, and Cmin, respectively, suggesting pharmacokinetic equivalence when tenofovir DF was dosed with or without rifampin.
TABLE 1.
Pharmacokinetics of tenofovir
| Study day and statisticsa | AUC0-24 (mg · h/liter) | Cmax (mg/liter) | Cmin (mg/liter) | Tmax (h)c | t1/2 (h) | CL/F |
|---|---|---|---|---|---|---|
| Day 10 | 3.56 ± 0.77 (3.48)b | 0.36 ± 0.080 (0.36) | 0.071 ± 0.016 (0.069) | 1.0 (1.0-3.0) | 13.8 ± 4.53 (13.2) | 88.1 ± 19.0 (86.2) |
| Day 20 | 3.11 ± 0.57 (3.06) | 0.30 ± 0.060 (0.30) | 0.060 ± 0.011 (0.059) | 1.0 (1.0-2.0) | 11.6 ± 2.77 (11.2) | 99.8 ± 20.3 (98.0) |
| Geometric mean ratio for day 20/day 10 (90% CI) | 0.88 (0.84-0.92) | 0.84 (0.78-0.90) | 0.85 (0.80-0.91) |
n = 23. CI, confidence interval.
Values are arithmetic means ± standard deviations (geometric means), unless indicated otherwise.
Values are medians (ranges).
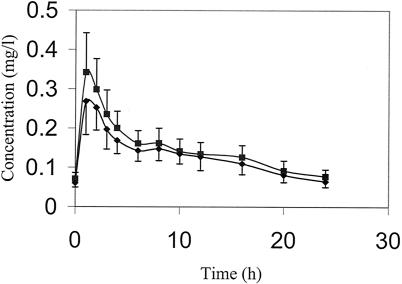
Figure 1 illustrates the effects of rifampin on the mean concentration-time profiles of tenofovir. Table 2 presents the values of the pharmacokinetic parameters for rifampin when it was combined with tenofovir and the values of the pharmacokinetic parameters of rifampin from the literature (4, 15). The values of the pharmacokinetic parameters for rifampin when it was combined with tenofovir are comparable to those in the literature when rifampin is administered with food, suggesting that tenofovir has no influence on rifampin exposure.
FIG. 1.
Plasma tenofovir concentrations. ▪, tenofovir concentration on study day 10 (n = 23) after administration of 300 mg QD; ♦, tenofovir concentration on study day 20 (n = 23) after administration of 300 mg combined with rifampin at 600 mg QD. Data are presented as means, and error bars indicate standard deviations.
TABLE 2.
Pharmacokinetics of rifampin
| Parameter | Value on day 20 (this study) (n = 23) | Value in the literature (n = 14)a |
|---|---|---|
| Tmax (h) | 2.4 (0.6)b | 4.43 (1.11) |
| Cmax (mg/liter) | 10.9 (3.0) | 7.27 (2.25) |
| AUC0-12 (mg · h/liter) | 43.27 (15.28) | 50.97 (14.27) |
| t1/2 (h) | 1.5 (0.3) |
The data are from reference 15 and are for subjects who received rifampin with breakfast.
Values are means (standard deviations).
Safety.
All 24 subjects reported one or more AEs at some time during the study. No subject experienced a grade 4 AE or a serious AE. In total, 160 grade 1 or grade 2 AEs were reported. A total of 102 AEs were judged to be possibly or probably related to a study drug(s). During treatment with tenofovir DF (period 1), 33 possibly or probably related AEs were reported, while during period 2 (tenofovir DF combined with rifampin), 69 possibly or probably related AEs were reported. Of the 69 AEs reported during period 2, 24 AEs were related only to rifampin. Each subject reported discoloration of the urine. Most of the study drug-related AEs were mild (85% were grade 1 in severity). All AEs resolved after the withdrawal of treatment.
All 24 subjects completed treatment period 1 (tenofovir DF alone). The most common AEs that were reported during treatment with tenofovir DF were fatigue, headache, and gastrointestinal disorders.
During period 2 one subject was withdrawn from the study due to several complaints, which were rash, headaches, abdominal disorders, fatigue, somnolence, and dizziness. The study medications were stopped on study day 15. At the follow-up visit, 5 days later, the subject developed elevated liver enzyme levels, which were judged to be a grade 3 AE. Nine days after the first follow-up visit the liver enzyme levels returned to normal. The AEs that occurred during the combination treatment with tenofovir DF and rifampin consisted mainly of flu-like symptoms (e.g., fatigue, headache, and gastrointestinal disorders) and urine discoloration, which are well-known AEs of rifampin (7).
No clinically significant hematology or urinalysis values were observed in this study.
DISCUSSION
This study was designed to investigate whether rifampin influences the pharmacokinetics of tenofovir. The study showed that bioequivalence could be suggested for tenofovir DF combined with rifampin and tenofovir DF given alone and that the combination of tenofovir DF with rifampin was generally well tolerated, as only one patient prematurely discontinued from study.
The confidence intervals for AUC and Cmin were 0.84 to 0.92 and 0.80 to 0.91, respectively, while the confidence interval was 0.78 to 0.90 for Cmax. By definition, bioequivalence was proven for AUC and Cmin but was only suggested for Cmax (20).
The tenofovir DF dose used in this study (300 mg QD) is the dose recommended for the treatment of HIV infection in adults (11). The rifampin dose used (600 mg QD) is an accepted regimen for the treatment of tuberculosis in patients weighing more than 50 kg (7). A previous study (2) has shown that steady-state conditions for rifampin are generally achieved after the sixth daily dose of rifampin at 600 mg. To ensure the achievement of steady-state pharmacokinetics, subjects were given tenofovir DF combined with rifampin for 10 days before pharmacokinetic assessment.
The reason for the lower observed tenofovir levels is unknown. Several mechanisms could contribute to this interaction. Because tenofovir is not metabolized and is eliminated unchanged by a combination of glomerular filtration and active tubular secretion (1, 6), it is unlikely that the inducing effect of rifampin on hepatic and intestinal cytochrome P450 enzymes (especially CYP3A4) (8) is the mechanism responsible for this effect. This is supported by no apparent changes in the tenofovir t1/2 and no clinically relevant effects of rifampin on the tenofovir Cmin.
Similarly, as tenofovir minimally binds to proteins in human plasma or serum (<0.7 and 7.2%, respectively) (11), altered distribution is also probably not the mechanism responsible for the pharmacokinetic differences observed. As the decrease in the tenofovir Cmax was 16% while the decrease in AUC0-24 was 12%, the cause may be in the process of tenofovir DF or tenofovir absorption. Rifampin has been shown to be an inducer of the efflux transporter P glycoprotein (9). No information exists in the literature that P glycoprotein plays a role in the process of absorption of tenofovir in vivo. However, van Gelder et al. (19) have described the transport of tenofovir DF by a P-glycoprotein-related efflux mechanism in the Caco-2 system.
AEs led to one discontinuation in this study; grade 3 elevations in hepatic enzyme levels were reported after the medication was stopped during period 2, when tenofovir DF was combined with rifampin. Liver disturbance is a well-known side effect of rifampin. Gastrointestinal disorders are well-known AEs of both tenofovir and rifampin and occurred in a total of 46% of the study subjects during both study periods. During period 2 all subjects reported discoloration of their urine, which is a well-known AE of rifampin (7).
Some additional considerations are important for the extrapolation of the results of this study to patients. First, it should be noted that all the participants in this study were healthy subjects. It cannot be excluded that the pharmacokinetics of tenofovir and rifampin are different in HIV-infected patients coinfected with M. tuberculosis due to one or both of the diseases. Second, 23 of the 24 subjects of this study were Caucasian. Race might have an effect on the values of the pharmacokinetic parameters for tenofovir, although the available pharmacokinetic data do not indicate substantial differences with regard to race (11). Finally, the subjects in this study were given tenofovir DF and rifampin only, while HIV-infected patients coinfected with M. tuberculosis are treated with other antiretroviral and tuberculostatic drugs, which can cause interactions.
In conclusion, the data from this study demonstrate that the addition of rifampin to tenofovir DF is well tolerated, and the small decrease in plasma tenofovir levels during combination treatment suggests that these drugs can be coadministered without the need for dose adjustments. This implies that standard doses should be a starting point for the use of these medications by HIV-infected patients. Additional pharmacokinetic studies in a clinical setting are warranted to confirm the findings of this study.
Acknowledgments
This work was supported by a grant from Gilead Sciences.
Marga de Graaff and Noor van Ewijk are thanked for analyzing the plasma samples used for pharmacokinetic analyses. Peter Koopmans is acknowledged for medical support. Frits Mulder is thanked for secretarial support. Diane Voncken and supportive personnel at Farma Research B.V. are acknowledged.
REFERENCES
- 1.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borin, M. T., J. H. Chambers, B. J. Carel, S. Gagnon, and W. W. Freimuth. 1997. Pharmacokinetic study of the interaction between rifampin and delavirdine mesylate. Clin. Pharmacol. Ther. 61:544-553. [DOI] [PubMed] [Google Scholar]
- 3.Bowen, E. F., P. S. Rice, N. T. Cooke, R. J. Whitfield, and C. F. Rayner. 2000. HIV seroprevalence by anonymous testing in patients with Mycobacterium tuberculosis and in tuberculosis contacts. Lancet 356:1488-1489. [DOI] [PubMed] [Google Scholar]
- 4.Burman, W. J., K. Gallicano, and C. Peloquin. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40:327-341. [DOI] [PubMed] [Google Scholar]
- 5.Cundy, K. C., C. Sueoka, G. R. Lynch, L. Griffin, W. A. Lee, and J. P. Shaw. 1998. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob. Agents Chemother. 42:687-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks, S. G., P. Barditch-Crovo, P. S. Lietman, F. Hwang, K. C. Cundy, J. F. Rooney, N. S. Hellmann, S. Safrin, and J. O. Kahn. 1998. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 42:2380-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas, J. G., and M. J. McLeod. 1999. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin. Pharmacokinet. 37:127-146. [DOI] [PubMed] [Google Scholar]
- 8.Finch, C. K., C. R. Chrisman, A. M. Baciewicz, and T. H. Self. 2002. Rifampin and rifabutin drug interactions: an update. Arch. Intern. Med. 162:985-992. [DOI] [PubMed] [Google Scholar]
- 9.Greiner, B., M. Eichelbaum, P. Fritz, H. P. Kreichgauer, O. von Richter, J. Zundler, and H. K. Kroemer. 1999. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J. Clin. Investig. 104:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Justesen, U. S., A. B. Andersen, N. A. Klitgaard, K. Brosen, J. Gerstoft, and C. Pedersen. 2004. Pharmacokinetic interaction between rifampin and the combination of indinavir and low-dose ritonavir in HIV-infected patients. Clin. Infect. Dis. 38:426-429. [DOI] [PubMed] [Google Scholar]
- 11.Kearney, B. P., J. F. Flaherty, and J. Shah. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 43:595-612. [DOI] [PubMed] [Google Scholar]
- 12.la Porte, C. J., E. P. Colbers, R. Bertz, D. S. Voncken, K. Wikstrom, M. J. Boeree, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2004. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob. Agents Chemother. 48:1553-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Cortes, L. F., R. Ruiz-Valderas, P. Viciana, A. Alarcon-Gonzalez, J. Gomez-Mateos, E. Leon-Jimenez, M. Sarasanacenta, Y. Lopez-Pua, and J. Pachon. 2002. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin. Pharmacokinet. 41:681-690. [DOI] [PubMed] [Google Scholar]
- 14.Msamanga, G. I., and W. W. Fawzi. 1997. The double burden of HIV infection and tuberculosis in sub-Saharan Africa. N. Engl. J. Med. 337:849-851. [DOI] [PubMed] [Google Scholar]
- 15.Peloquin, C. A., R. Namdar, M. D. Singleton, and D. E. Nix. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12-18. [DOI] [PubMed] [Google Scholar]
- 16.Polk, R. E., D. F. Brophy, D. S. Israel, R. Patron, B. M. Sadler, G. E. Chittick, W. S. Symonds, Y. Lou, D. Kristoff, and D. S. Stein. 2001. Pharmacokinetic interaction between amprenavir and rifabutin or rifampin in healthy males. Antimicrob. Agents Chemother. 45:502-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribera, E., L. Pou, R. M. Lopez, M. Crespo, V. Falco, I. Ocana, I. Ruiz, and A. Pahissa. 2001. Pharmacokinetic interaction between nevirapine and rifampicin in HIV-infected patients with tuberculosis. J. Acquir. Immune Defic. Syndr. 28:450-453. [DOI] [PubMed] [Google Scholar]
- 18.Schluger, N. W. 1999. Issues in the treatment of active tuberculosis in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 28:130-135. [DOI] [PubMed] [Google Scholar]
- 19.van Gelder, J., S. Deferme, L. Naesens, E. De Clercq, M. G. van den, R. Kinget, and P. Augustijns. 2002. Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug Metab. Dispos. 30:924-930. [DOI] [PubMed] [Google Scholar]
- 20.Williams, R. L., M. L. Chen, and W. W. Hauck. 2002. Equivalence approaches. Clin. Pharmacol. Ther. 72:229-237. [DOI] [PubMed] [Google Scholar]