Abstract
Background
Adenosine agonists are protective in numerous models of ischemia-reperfusion injury (IRI). Pericellular adenosine is generated by the hydrolysis of extracellular adenosine triphosphate and adenosine diphosphate by the ectonucleotidase CD39 and the subsequent hydrolysis of adenosine monophosphate (AMP) by the ectonucleotidase CD73. CD39 activity is protective in kidney IRI, whereas the role of CD73 remains unclear.
Methods
Wild-type (WT), CD73-deficient (CD73KO), CD39-transgenic (CD39tg), and hybrid CD39tg.CD73KO mice underwent right nephrectomy and unilateral renal ischemia (18-min ischemia by microvascular pedicle clamp). Renal function (serum creatinine [SCr], micromolar per liter) and histologic renal injury (score 0–9) were assessed after 24-hr reperfusion. Treatments included a CD73 inhibitor and soluble CD73.
Results
Compared with WT mice (n=33, SCr 81.0, score 4.1), (1) CD73KO mice were protected (n=17, SCr 48.9, score 2.0, P<0.05), (2) CD39tg mice were protected (n=11, SCr 45.6, score 1.3, P<0.05), (3) WT mice treated with CD73 inhibitor were protected (n=9, SCr 43.3, score 1.2, P<0.05), (4) CD73KO mice reconstituted with soluble CD73 lost their protection (n=10, SCr 63.8, score 3.1, P=ns), (5) WT mice treated with soluble CD73 were not protected (n=7, SCr 78.0, score 4.1), and (6) CD39tg.CD73KO mice were protected (n=8, SCr 55.5, score 0.7, P<0.05).
Conclusions
Deficiency or inhibition of CD73 protects in kidney IRI, and CD39-mediated protection does not seem to be dependent on adenosine generation. These findings suggest that AMP may play a direct protective role in kidney IRI, which could be used in therapeutic development and organ preservation. Investigating the mechanisms by which AMP mediates protection may lead to new targets for research in kidney IRI.
Keywords: Kidney ischemia-reperfusion injury, CD73, CD39, Adenosine, Adenosine monophosphate
Ischemia-reperfusion injury (IRI) is inherent to organ transplantation and is associated with both acute and long-term graft dysfunction (1). Although the pathophysiology of IRI is incompletely understood, inflammation and the innate immune response are key components (2), and an unequivocal role for T lymphocytes has been defined in both liver (3) and kidney small animal models (4).
Adenosine is an innate antiinflammatory metabolite, and several studies support the role of adenosine as a regulator of the inflammatory response in IRI (5–7). Adenosine acts at four widely expressed G protein-coupled receptors—A1R, A2AR, A2BR, and A3R. In basal states, low concentrations of adenosine are present in the extracellular space. In ischemia, the extracellular concentration of adenosine triphosphate (ATP) rises significantly as it is released from the intracellular space (8). ATP is hydrolyzed to adenosine diphosphate and then adenosine monophosphate (AMP) by the ectoenzyme CD39 (ectonucleoside triphosphate diphosphohydrolase 1). AMP is hydrolyzed by the ectoenzyme CD73 (ecto-5-nucleotidase) to generate adenosine. Adenosine agonists are protective in several models of IRI including kidney IRI (9). The protection observed is mediated by the A2AR on circulating CD4+ T cells (10) and the A2BR within the renal parenchyma (6).
CD39 is expressed on the endothelium and vascular smooth muscle cells in many organs including the kidney (11) and various cells of the immune system including regulatory T cells (12). We have demonstrated that transgenic overexpression of CD39 protects in a models of renal IRI and kidney transplantation (13), as does treatment with soluble CD39. CD39-null mice demonstrate severe tubular injury after ischemia-reperfusion (14), and it has been shown that the protective effect of ischemic preconditioning in the kidney is dependent on CD39 expression (15). Although not confirmed, the principal mechanism involved in CD39-mediated protection in IRI of the kidney, liver (16), lung (17), and bowel (18) is generally considered to be the downstream generation of adenosine.
CD73 is a glycosyl phosphatidylinositol-linked glycoprotein ectoenzyme critical in the generation of extracellular adenosine. CD73 enzymatic activity is highest in the colon, kidney, and brain (19), and the expression of CD73 is up-regulated in hypoxic states by hypoxia-inducible factor 1 (20). CD73 has been implicated in the regulation of tissue endothelial and epithelial barrier function, which has been most clearly elucidated in vivo in the intestine and the lung (19). Postulated nonenzymatic functions of CD73 include as a signaling molecule (21) and an adhesion molecule (22). A role for CD73 in neutrophil adhesion and accumulation (23) and lymphocyte migration and activation (24, 25) has been demonstrated.
Ischemic preconditioning, in which short periods of ischemia protect an organ from a subsequent ischemic insult, is dependent on CD73 activity in both heart and kidney models (26, 27). The role of CD73 in a renal ischemic insult without preconditioning is less clear. We have previously shown that CD73 deficiency in mice results in protection from renal IRI (14), consistent with an earlier study in rats showing that treatment with a CD73 inhibitor led to renal protection, adenine nucleotide accumulation, and reduced adenosine generation (28). Conversely, attenuated damage in a mouse model of renal IRI with soluble CD73 treatment has also been reported (26).
To further investigate the role of CD73 in renal IRI, we performed a series of experiments using various genetically modified mice and treatments. After confirming our earlier finding that CD73 deficiency is protective in renal IRI, we showed that (1) treatment with a CD73 inhibitor also protects; (2) treatment with soluble CD73 did not protect wild-type (WT) mice and may have reversed the protection seen in CD73-deficient mice; and (3) the protection conferred by CD39 overexpression was not lost in the setting of CD73 deficiency. Together these findings suggest that AMP may mediate protection in renal IRI.
RESULTS
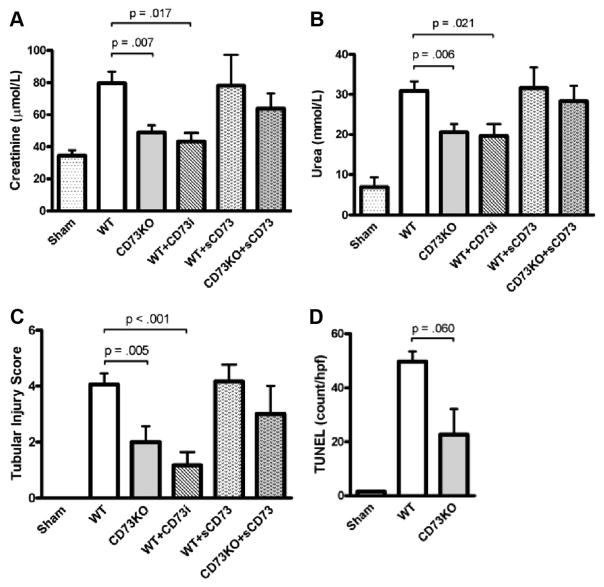
CD73 is critical in the downstream extracellular generation of adenosine, and adenosine agonists are protective in renal IRI. WT and CD73-deficient (CD73KO) mice underwent 18-min unilateral kidney ischemia followed by 24-hr reperfusion. In this model of IRI, WT mice exhibited significant renal injury and dysfunction (n=33, serum creatinine [SCr] 81.0 μmol/L, serum urea 30.9 mmol/L, tubular injury score 4.1) compared with sham-operated mice (n=19, SCr 34.4 μmol/L, serum urea 11.4 mmol/L, score 0, P<0.01; Fig. 1). Compared with WT mice, CD73KO mice had reduced renal tubular injury, necrosis, dilatation, and cast formation after IRI. This was reflected in the significantly lower histologic score (score 2.0, P<0.01) and correlated with improved renal function (n=17, SCr 48.9 μmol/L, serum urea 20.6 mmol/L, P<0.01; Fig. 1) and confirmed our previous study (14). Treatment of WT mice with soluble CD73 (200 U/kg intraperitoneally) 30 min before IRI did not alter the degree of renal injury or dysfunction (n=7, SCr 78.0 μmol/L, serum urea 31.6 mmol/L, score 4.1). There was, however, a trend toward restoration of injury in CD73-deficient mice (n=10, SCr 63.8 μmol/L, serum urea 28.3 mmol/L, score 3.1, P=ns; Fig. 1).
FIGURE 1.
Wild-type (WT) mice (n=33), mice deficient in CD73 (CD73KO; n=17), WT mice treated with CD73 inhibitor (WT+CD73i; n=9), WT mice treated with soluble CD73 (WT+sCD73; n=7), and CD73KO mice treated with soluble CD73 (CD73KO+sCD73; n=10) were subjected to 18-min unilateral renal ischemia followed by 24 hr reperfusion and compared with sham-operated mice (n=19). Serum creatinine (A), serum urea (B), tubular injury score (C), and degree of apoptosis with terminal deoxynucleotide transferase-mediated dUTP nick-end labeling staining (D) are presented as mean±SEM.
The extent of apoptosis in the renal parenchyma, as quantified by terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) staining, was reduced in CD73KO mice compared with WT mice (n=5, 49.7 vs. 22.7 TUNEL+ nuclei per hpf, P=0.06; Fig. 1).
WT mice were treated with a CD73 inhibitor (adenosine 5′-(α,β-methylene)diphosphate, 2 mg/kg intraperitoneally) 30 min before IRI. This conferred significant histologic and functional protection (n=9, SCr 43.3 μmol/L, serum urea 19.6 mmol/L, score 1.2, P<0.05) to a level observed in CD73KO mice (Fig. 1).
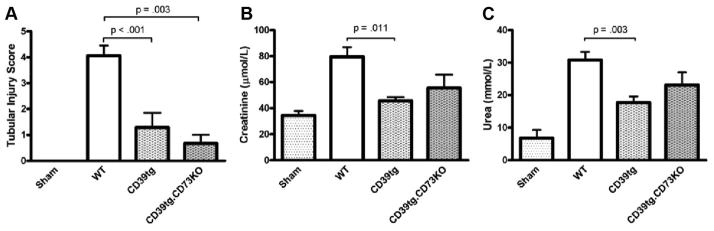
Kidney injury and dysfunction in mice overexpressing CD39 were significantly attenuated compared with WT mice (n=11, SCr 45.6 μmol/L, serum urea 17.7 mmol/L, score 1.3, P<0.05) and validates our previous work (13). Mice overexpressing CD39 and deficient in CD73 had significantly less tubular injury that WT mice (score 0.7, P<0.01) and a trend toward protected renal function in kidney IRI (SCr 55.5 μmol/L, serum urea 23.1 mmol/L; Fig. 2).
FIGURE 2.
Wild-type (WT) mice (n=33), mice overexpressing CD39 (CD39tg; n=11), hybrid CD39tg.CD73KO mice (n=8), and sham-operated mice (n=19) were subjected to renal ischemia-reperfusion injury. Tubular injury score (A), serum creatinine (B), and serum urea (C) are presented as mean±SEM.
DISCUSSION
The protective role of adenosine signaling in kidney IRI is well established. This is based on the observed effects of adenosine receptor agonists and antagonists, and the consequence of genetic alteration of adenosine receptors in several IRI models (6, 7, 9). Despite this, the precise role of CD73, which is an important generator of extracellular adenosine, remains equivocal. CD73 is necessary for the protective phenomenon of ischemic preconditioning in the kidney (26). In the same study, no effect of CD73 deficiency was demonstrated in IRI, but treatment of WT mice with soluble CD73 conferred protection. Conversely, we have previously demonstrated protection in CD73-deficient mice (14), and an earlier study of kidney IRI in rats demonstrated attenuated renal injury in rats treated with a CD73 inhibitor (28).
Given these discordant results, we sought to consolidate and extend our preliminary findings in CD73-deficient mice in a unilateral model of kidney IRI (14). In this study, we confirm that CD73 deficiency does confer protection in terms of renal function and tubular damage. Treatment with soluble CD73 did not protect WT mice in IRI. In fact, reconstitution of CD73-deficient mice with soluble CD73 exacerbated renal injury. Furthermore, the degree of apoptosis in renal tissue was diminished in CD73-deficient mice after IRI.
The protection observed with pharmacologic inhibition of CD73 supports the work of others (28) and further substantiates the results obtained with CD73KO mice. Our findings, however, must be reconciled with the protective effect of soluble CD73 treatment found by Grenz et al. (26). The IRI models used are certainly different. Our model involves 18 min of unilateral kidney ischemia using a microvascular pedicle clamp in 10- to 14-week-old male mice, whereas Grenz et al. (29) used 30-min unilateral ischemia with a hanging weight system occluding the renal artery alone in 4- to 6-week-old mice. Isolated renal artery occlusion negates the potential confounders of venous congestion and outflow obstruction. The model used in this study leads to a less severe ischemic insult in our model as reflected in the lower SCr in WT mice after 24-hr reperfusion (81 vs. ~265 μmol/L [26]). The results from this study were reproducible, and there was no evidence of venous congestion or thrombosis within the renal parenchyma during histologic examination. This suggests that CD73 may play a complex and dynamic role in kidney IRI in which the overall effect of AMP hydrolysis and adenosine generation changes depending on the nature of the inciting insult and the severity of injury.
Although CD73 does have nonenzymatic functions (22, 24), its biologic activity has generally been attributed to its capacity to generate extracellular adenosine. Our findings suggest that AMP may be playing a protective role in kidney IRI. We have shown that overexpression of human CD39 confers protection in kidney IRI (13). Mice transgenic for human CD39 have an increased capacity to generate both AMP and adenosine after proinflammatory stimuli (30). To investigate whether CD39-mediated protection in renal IRI is dependent on adenosine generation, we examined mice over-expressing CD39 and deficient in CD73. Introduction of CD73 deficiency onto the CD39-transgenic (CD39tg) background did not cause loss of the protective phenotype and resulted in a trend toward further protection in terms of histologic damage. This suggests that CD39-mediated protection in kidney IRI is not dependent on adenosine generation. Taken together with the findings that CD73 deficiency and inhibition protect in IRI, this implies that extracellular AMP may play a direct protective role in IRI rather than to act merely as a substrate for the generation of adenosine.
The mechanism by which extracellular AMP may mediate protection is not known. No direct target receptors for AMP have been demonstrated. Although GPR80 was postulated to be an AMP receptor, this has since been refuted (31). The intracellular enzyme AMP-activated protein kinase is activated in IRI (32) and has been shown to mediate protection (33, 34). However, no mechanism for the transport of extra-cellular AMP into the cell has been identified. Alternatively, protection by AMP may relate to the capacity for tissue ATP regeneration after an ischemic insult as proposed by van Waarde et al. (28). Although purinergic signaling by ATP is known to have proinflammatory and prothrombotic consequences (35), ATP as a cellular energy source is required during recovery from ischemia.
We demonstrate here that treatment of CD73-deficient mice with soluble CD73 abrogates their protection from kidney IRI and that inhibition of CD73 in WT mice protects in this model. Furthermore, the combination of CD39 overexpression and CD73 deficiency is also protective. The precise role of CD73 in kidney IRI is likely complex and dynamic. Further studies are required to clarify how CD73 mediates damaging and protective effects during different phases of an ischemic insult, and whether these effects can be attributed to its enzymatic activity. Our findings suggest that AMP may play a protective role in IRI. Although further studies are needed to confirm this assertion, it has widespread implications in the field of transplantation. Pharmacologic therapies that increase AMP concentrations may be of value, and genetic manipulation to the same end may be beneficial in xenotransplantation. In addition, AMP may be of benefit in preservation fluid. Further research into the mechanisms behind AMP-mediated protection may lead to insights into the pathophysiology of IRI that identify novel targets for investigation and drug development.
MATERIALS AND METHODS
Mice
C57Bl/6 mice used in this study are as follows: CD73KO mice (19) (a kind gift from Dr Linda Thompson, Oklahoma Medical Research Foundation), human CD39tg mice (30), and CD39tg crossed with CD73KO (CD39tg.CD73KO), which were bred in-house. The mice were validated and characterized as described previously (19, 30). Mice were bred and housed in an approved animal facility at the Immunology Research Centre, Melbourne, Australia, and all experiments were carried out with the approval of the Animal Ethics Committee of St Vincent’s Hospital (Melbourne) Ltd. In this study, a total of 68 WT mice, 27 CD73KO mice, 11 CD39tg, and 8 CD39tg.CD73KO mice were used.
Induction of Renal IRI
Male, 10- to 14-week-old mice underwent unilateral kidney IRI surgery as described previously (14). Briefly, mice were anesthetized and placed on a heating pad to maintain their core body temperature at 37°C during the surgery. A midline abdominal incision was made, and the renal pedicles were bluntly dissected. A right nephrectomy was performed. A microvascular clamp (Roboz, Rockville, MD) was placed on the left renal pedicle for 18 min, whereas the animal was kept at 37°C in an incubator. After 18-min ischemia, the clamp was removed. The surgical wounds were sutured in two layers with 5-0 silk. The animals received 100 mL/kg warm normal saline instilled into the peritoneal cavity during the procedure. Sham-operated mice underwent a right nephrectomy alone. The mice were allowed to recover for 2 hr under a heating lamp and then kept on a heating pad. After 24 hr of reperfusion, the mice were killed, and blood and kidney samples were obtained.
Treatment
In the relevant groups, mice were administered pharmacologic agents by intraperitoneal injection 30 min before IRI. The total fluid volume administered to each mouse was maintained at 100 mL/kg. The CD73 inhibitor adenosine 5′-(α,β-methylene)diphosphate (Sigma-Aldrich, Castle Hill, NSW Australia) was given at 2 mg/kg. Soluble CD73 (5′nucleotidase from Crotalus atrox venom, Sigma Aldrich) was given at 200 U/kg.
Assessment of Renal Function
Whole blood was collected from an inferior vena cava puncture and sent for biochemical analysis. SCr concentration (modified Jaffe rate reaction) and serum urea concentration were measured by the Department of Pathology of St Vincent’s Hospital (Melbourne) Ltd (Olympus AU 2700, Integrated Science, Chatswood, NSW, Australia).
Renal Histology and Scoring
Ten percent formalin-fixed and paraffin-embedded kidney tissue sections (4 μm) were stained with hematoxylin-eosin. Three high-power fields in each of the cortex, cortical-medullary junction, and medulla in a minimum of two sections were assessed by a nephrologist blinded to the experimental group. The degree of injury was based on necrosis, cast formation, cell swelling, and dilatation. The percentage area of renal tissue involved in each field was assessed, and the average percentage noted for each sample was used to determine a semiquantitative score of 0 to 9—score 0: 0% to 10%, score 1: 11% to 20%, and score 2: 21% to 30%. This scoring system, although not validated against other measures of kidney damage, was found to be reproducible on repeated, blinded application.
TUNEL Assay
The TUNEL stain was used to detect DNA fragmentation that results from apoptosis as per the manufacturer’s instruction (FragELDNAFragmentation Detection Kit, EMD Biosciences, Germany). Slides treated with DNAse I were used as positive controls. Quantification was performed as described (36). The TUNEL-positive cells were counted by an investigator blinded to the experimental group at 100× magnification in 20 sequentially selected fields in the renal cortex and cortical-medullary junction for each section.
Data Analysis
Results are expressed as mean±SEM. Groups were compared using an unpaired Student’s t test (two tailed). A value of P less than 0.05 was considered significant.
Acknowledgments
This work was supported by the NHMRC (Australia), Genzyme Renal Innovations Program (K.M.D.), NIH (S.C.R.), and a Kidney Health Australia research scholarship (S.V.R.).
The authors thank the staff at the Immunology Research Centre and Bioresources center for the generation and maintenance of mice used in this study.
References
- 1.Halloran PF, Melk A, Barth C. Rethinking chronic allograft nephropathy: The concept of accelerated senescence. J Am Soc Nephrol. 1999;10:167. doi: 10.1681/ASN.V101167. [DOI] [PubMed] [Google Scholar]
- 2.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lappas CM, Day YJ, Marshall MA, et al. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabb H. The T cell as a bridge between innate and adaptive immune systems: Implications for the kidney. Kidney Int. 2002;61:1935. doi: 10.1046/j.1523-1755.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- 5.Day YJ, Huang L, McDuffie MJ, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grenz A, Osswald H, Eckle T, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HT, Gallos G, Nasr SH, et al. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 8.Weissmuller T, Eltzschig HK, Colgan SP. Dynamic purine signaling and metabolism during neutrophil-endothelial interactions. Purinergic Signal. 2005;1:229. doi: 10.1007/s11302-005-6323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HT, Emala CW. Systemic adenosine given after ischemia protects renal function via A(2a) adenosine receptor activation. Am J Kidney Dis. 2001;38:610. doi: 10.1053/ajkd.2001.26888. [DOI] [PubMed] [Google Scholar]
- 10.Day YJ, Huang L, Ye H, et al. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: The role of CD4+T cells and IFN-gamma. J Immunol. 2006;176:3108. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- 11.Kishore BK, Isaac J, Fausther M, et al. Expression of NTPDase1 and NTPDase2 in murine kidney: Relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol. 2005;288:F1032. doi: 10.1152/ajprenal.00108.2004. [DOI] [PubMed] [Google Scholar]
- 12.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crikis S, Lu B, Murray-Segal LM, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu B, Rajakumar SV, Robson SC, et al. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation. 2008;86:1707. doi: 10.1097/TP.0b013e31819022bc. [DOI] [PubMed] [Google Scholar]
- 15.Grenz A, Zhang H, Hermes M, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 16.Hart ML, Gorzolla IC, Schittenhelm J, et al. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckle T, Fullbier L, Wehrmann M, et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 18.Guckelberger O, Sun XF, Sevigny J, et al. Beneficial effects of CD39/ectonucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost. 2004;91:576. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- 19.Thompson LF, Eltzschig HK, Ibla JC, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 22.Airas L, Niemela J, Jalkanen S. CD73 engagement promotes lymphocyte binding to endothelial cells via a lymphocyte function-associated antigen-1-dependent mechanism. J Immunol. 2000;165:5411. doi: 10.4049/jimmunol.165.10.5411. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Thompson LF, Karhausen J, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: Coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 24.Dianzani U, Redoglia V, Bragardo M, et al. Co-stimulatory signal delivered by CD73 molecule to human CD45RAhiCD45ROlo (naive) CD8+ T lymphocytes. J Immunol. 1993;151:3961. [PubMed] [Google Scholar]
- 25.Airas L, Hellman J, Salmi M, et al. CD73 is involved in lymphocyte binding to the endothelium: Characterization of lymphocyte-vascular adhesion protein 2 identifies it as CD73. J Exp Med. 1995;182:1603. doi: 10.1084/jem.182.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grenz A, Zhang H, Eckle T, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 27.Eckle T, Krahn T, Grenz A, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 28.Van Waarde A, Stromski ME, Thulin G, et al. Protection of the kidney against ischemic injury by inhibition of 5′-nucleotidase. Am J Physiol. 1989;256(2 pt 2):F298. doi: 10.1152/ajprenal.1989.256.2.F298. [DOI] [PubMed] [Google Scholar]
- 29.Grenz A, Eckle T, Zhang H, et al. Use of a hanging-weight system for isolated renal artery occlusion during ischemic preconditioning in mice. Am J Physiol Renal Physiol. 2007;292:F475. doi: 10.1152/ajprenal.00275.2006. [DOI] [PubMed] [Google Scholar]
- 30.Dwyer KM, Robson SC, Nandurkar HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi AD, Harden TK, Nicholas RA. GPR80/99, proposed to be the P2Y(15) receptor activated by adenosine and AMP, is not a P2Y receptor. Purinergic Signal. 2004;1:67. doi: 10.1007/s11302-004-5069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young LH, Li J, Baron SJ, et al. AMP-activated protein kinase: A key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15:110. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Russell RR, III, Li J, Coven DL, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peralta C, Bartrons R, Serafin A, et al. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164. doi: 10.1053/jhep.2001.29197. [DOI] [PubMed] [Google Scholar]
- 35.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 36.Thomas SE, Andoh TF, Pichler RH, et al. Accelerated apoptosis characterizes cyclosporine-associated interstitial fibrosis. Kidney Int. 1998;53:897. doi: 10.1111/j.1523-1755.1998.00835.x. [DOI] [PubMed] [Google Scholar]