Abstract
The vascular ectonucleotidases CD39 [ENTPD1 (ec-tonucleoside triphosphate diphosphohydrolase-1), EC 3.6.1.5] and CD73 [EC 3.1.3.5] generate adenosine from extracellular nucleotides. CD39 activity is critical in determining the response to ischemia-reperfusion injury (IRI), and CD39 null mice exhibit heightened sensitivity to renal IRI. Adenosine has multiple mechanisms of action in the vasculature including direct endothelial protection, antiinflammatory and antithrombotic effects and is protective in several models of IRI. Mice transgenic for human CD39 (hCD39) have increased capacity to generate adenosine. We therefore hypothesized that hCD39 transgenic mice would be protected from renal IRI. The overexpression of hCD39 conferred protection in a model of warm renal IRI, with reduced histological injury, less apoptosis and preserved serum creatinine and urea levels. Benefit was abrogated by pretreatment with an adenosine A2A receptor antagonist. Adoptive transfer experiments showed that expression of hCD39 on either the vasculature or circulating cells mitigated IRI. Furthermore, hCD39 transgenic kidneys transplanted into syngeneic recipients after prolonged cold storage performed significantly better and exhibited less histological injury than wild-type control grafts. Thus, systemic or local strategies to promote adenosine generation and signaling may have beneficial effects on warm and cold renal IRI, with implications for therapeutic application in clinical renal transplantation.
Keywords: Adenosine, cold ischemia, organ protection and preservation, renal ischemia reperfusion injury, transplant, transgenic, warm ischemia
Introduction
The success of transplantation is dependent on the state of the donor organ, which is directly impacted by ischemia reperfusion injury (IRI). IRI occurs at the time of organ retrieval, storage and engraftment and outcomes vary as to the extent of both warm and cold ischemia. Warm ischemia is generally brief with brain dead, heart-beating donors, although it may be quite prolonged with donors following cardiac arrest (1). Cold ischemic times can be prolonged with engraftment occurring up to 24 h after procurement.
Cold storage and reperfusion lead to loss of cell polarity, disruption of the cytoskeleton and perturbations in polarized membrane transport proteins (2). Reestablishment of blood flow is essential to halt ongoing ischemia, but paradoxically this initiates a cascade of metabolic and inflammatory events, amplifying the immunogenicity of the donor organ and increasing the risk of delayed graft function and acute rejection (3). Donor organs are perfused with preservation solution while stored at 4°C as a means of reducing ongoing organ damage. Three preservation solutions are currently used clinically and differ with respect to active constituents. University of Wisconsin (UW) solution contains adenosine at 5 μM, whereas Celsior and histidine-tryptophan-ketoglutarate solution (HTK) do not (4). Although adenosine is a potent antiinflammatory agent (5,6), this mediator is highly labile in vivo with a half-life of seconds in the vasculature (7). Certain adenosine agonists have been shown to protect against IRI in a number of small animal models (8–12).
Under basal conditions the pericellular concentration of adenosine is low. However, in states of inflammation the concentration increases dramatically, primarily as a consequence of the hydrolysis of ATP released from injured and dying cells. ATP is sequentially hydrolyzed to ADP and AMP by CD39 [ENTPD1 (ectonucleoside triphosphate diphosphohydrolase-1), EC 3.6.1.5]. AMP is converted to adenosine by CD73 [EC 3.1.3.5]. Adenosine signals via four G-protein coupled receptors of which the antiinflammatory A2A is the most extensively characterized. These nucleotidases thus provide an innate mechanism in which the local adenosine concentration is augmented in response to inflammation. Indeed, recent studies employing mice with genetic deletions of Cd39 or Cd73, both of which have reduced adenosine generating capacity, are more prone to renal IRI compared with littermate controls (13,14).
CD39 is transcriptionally upregulated in vitro (15) and in vivo mouse models (16,17), an effect that is dependent on the transcription factor Sp1. The Sp1 binding sites within the CD39 promoter are highly conserved between mice and humans (16). Therefore mice expressing human CD39 (hCD39) (18) were generated as a means of augmenting ENTPDase ectoenzymatic activity and downstream adenosine generation and the effect was examined in two models of renal IRI. Herein, we demonstrate that mice transgenic for hCD39 are protected in a model of renal warm IRI and a more clinically relevant kidney transplant model characterized by extended cold preservation. These results suggest that strategies to promote adenosine generation and signaling locally in the kidney may have beneficial effects in warm and cold IRI in clinical renal transplantation.
Materials and Methods
All studies using animals were approved by the Animal Ethics Committee, St. Vincent’s Hospital (Melbourne) Ltd, and were carried out in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 7th edition 2004, and the Prevention of Cruelty to Animals Act 1986, Victoria, Australia.
Mice
Transgenic mice expressing hCD39 under the mouse H-2Kb (MHC class I) promoter (18) and Cd39 null mice (19) were maintained in individually ventilated cages in a specific pathogen-free facility. Transgenic and null mice were backcrossed at least 10 and 7 generations, respectively, onto the C57/BL6 background. Wild-type (WT) littermates (from hCD39 +/− × WT crosses) were used as controls.
Warm renal ischemia-reperfusion injury (IRI)
Weight- and age-matched (23–33g, 8–10 weeks) male mice were anesthetized with intraperitoneal chloral hydrate and inhaled penthrane. The kidneys were exposed by a midline abdominal incision and ischemia was induced by occlusion of both renal pedicles for 30 min, followed by reperfusion. All mice received 500 μL of saline into the abdominal cavity prior to the wound being sutured. Mice were maintained on a heat pad at 37°C.
Sham surgery was performed as detailed above but without occlusion of the renal pedicles. At 24 and 48 h the mice were sacrificed by exsanguination via the IVC and the blood obtained was analyzed for creatinine and urea (Olympus AU 2700, Integrated Science, Chatswood, Australia). The kidneys were removed for histology. Kidneys were also procured after 24 or 48 h of reperfusion for histological analysis.
Murine renal transplant model
The donor kidney was excised either from a wild-type or hCD39 transgenic mouse, flushed and stored in Ringers solution for 5 h at 4°C before engraftment. The recipient mouse was anesthetised and a bilateral nephrectomy performed. The donor aorta and inferior vena cava (IVC) were anastomosed end-to-side to the recipient’s aorta and IVC. Bladder-to-bladder anastomosis was performed. The mice were monitored for up to 72 h.
Histology
0.5-μm 10% formalin-fixed and paraffin-embedded kidney sections were stained with H&E and PAS stains. Tissue sections were scored in a blinded fashion using the following semiquantitative scale designed to evaluate the degree of tubular necrosis: 0, normal kidney; 1, minimal necrosis (<10% involvement); 2, mild necrosis (10–<25% involvement); 3, moderate necrosis (25–75% involvement); and 4, severe necrosis (>75% involvement). TUNEL staining was performed with a FragELDNA fragmentation detection kit (Merck, Kilsyth, Australia) as per the manufacturer’s instructions. Whole coronal kidney sections were scanned at a magnification of 200× using a computer-assisted color video image analysis system (Video-Pro 32, Leading Edge, Adelaide, Australia). Images were captured using an Olympus H2B microscope and video camera (Olympus Optical Co. Ltd., Tokyo, Japan). Video image analysis measurements were made of the total area of green counter-stained nuclei (methyl green) and of brown nuclei (TUNEL stain). Twenty visual fields were analyzed per kidney sample, and the total number of TUNEL-stained cell nuclei (representing apoptotic cells) in each field was determined using feature counting (Video-Pro 32 software).
Immunohistochemistry
Fresh-frozen tissue sections were incubated with a fluorescein isothiocyanate-(FITC-) conjugated mouse anti-human CD39 monoclonal antibody, clone BU-61 (Ancell, Bayport, MN, USA) followed by peroxidase-conjugated sheep antifluorescein polyclonal antibody. Staining was developed using 3,3′-diaminobenzidine tetrahydrochloride and sections were counterstained with hematoxylin.
Adenosine antagonist treatment
(3-chlorostyryl) caffeine (CSC) (Sigma-Aldrich, Castle Hill, Australia), an A2A receptor antagonist, was given at 1-mg/kg body weight intraperitoneally 15 min prior to surgery (20).
Leukocyte collection
Kidneys were subjected to 30 min of warm ischemia followed by 3 h of reperfusion. The kidneys were then flushed with ice-cold phosphate-buffered saline (PBS) solution and digested with 2-mg/mL type V collagenase and 0.1 μg/mL of DNAse (both from Sigma-Aldrich, St. Louis MO, USA) for 30 min at 37°C. The reaction was terminated with 10% heat-inactivated fetal calf serum and the digested suspension was passed through a metal sieve, washed twice in Roswell Park Memorial Institute medium at 500g for 5 min and subjected to a Percoll gradient. The collected cells were stained with anti-CD4-PeCy5, anti-CD19-FITC, anti-Gr1-FITC, anti-CD45-PE (BD Pharmingen, Franklin Lakes, NJ), anti-CD8a-APC, F4/80-PE and NK1.1-APC (eBiosciences, San Diego, CA, USA), and analyzed on a FACScalibur flow cytometer (BD Pharmingen).
MPO activity assays
Following 30 min of IRI and 3 h of reperfusion, kidneys were flushed and then recovered for the measurement of MPO activity as previously described (21).
Adoptive transfer experiments
Following cervical dislocation, bone marrow was recovered from donor wild type and mutant mice by flushing the long bones with mouse tonicity PBS. The marrow was filtered and centrifuged, and the cells were counted. Transgenic mice received total myeloablative irradiation (two courses of 5.5 Gy, 2 h apart) before reconstitution with 0.8 × 106 WT bone marrow cells (200 μL volume) administered by tail vein injection. WT recipient mice were reconstituted with hCD39 transgenic bone marrow cells. Reconstitution was confirmed 4 weeks after transfer with a full blood count and flow cytometric analysis of hCD39 expression on peripheral blood leukocytes.
Data analysis
Statistical analysis was carried out using the Minitab 15 software program (Minitab Inc., Pennsylvania, PA, USA). The assumption of normal distribution of the data was tested using the test of equal variance. Unpaired sample data were then analyzed using the Student’s t-test or Mann–Whitney test. Statistical significance was assumed if the two-tailed p value was <0.05.
Results
Transgenic expression of human CD39 confers protection in a mouse model of renal warm IRI
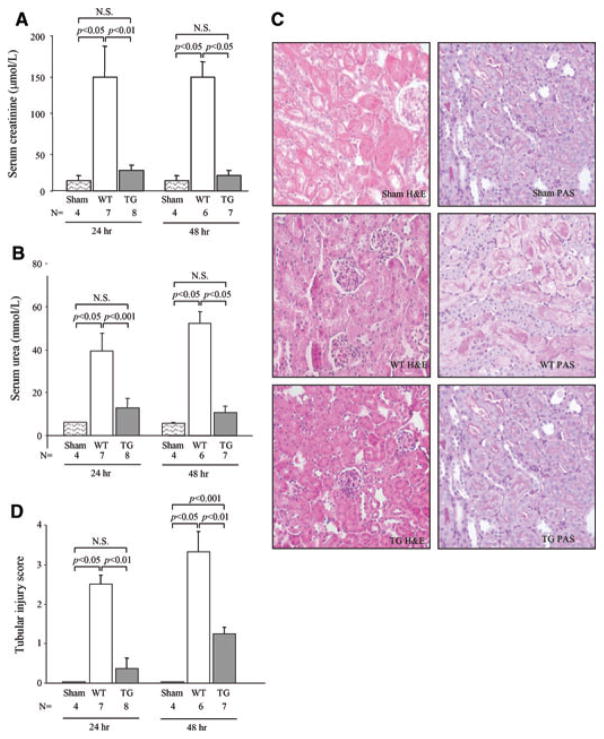
hCD39 transgenic and littermate WT mice were subjected to 30 min of bilateral renal ischemia followed by 24 or 48 h of reperfusion. WT mice exhibited an approximate six–sevenfold increase in serum creatinine (Figure 1A) and a seven–eightfold increase in urea (Figure 1B) at both 24 and 48 h when compared directly to sham-operated mice. In contrast, the serum creatinine and urea in hCD39 transgenic mice did not differ significantly from sham-operated animals at either 24 or 48 h reperfusion (Figure 1A and B).
Figure 1. hCD39 transgenic mice are protected from renal IRI.
Wild-type (WT) and hCD39 transgenic (TG) mice were subjected to 30 min of ischemia by bilateral occlusion of the renal pedicles, followed by reperfusion for 24 or 48 h. (A) Serum creatinine and (B) urea at 24 and 48 h. (C) Representative morphology of kidney sections after 24 h of reperfusion. Left column: H&E; right column: PAS. Magnification 400x. (D) Semiquantitative scoring of tubular damage. Results in A, B and D are presented as mean ± SEM. N.S: not significant.
Gross morphological changes were evident histologically with extensive acute tubular necrosis (ATN), which invariably extended beyond the corticomedullary junction into the renal cortex in WT mice (Figure 1C,D). Damage was minimal and restricted to the corticomedullary junction in kidneys from hCD39 transgenic mice. PAS-stained sections showed areas of complete disruption of the tubular basement membrane in WT kidneys but not in hCD39 transgenic kidneys (Figure 1C). Unwitnessed mortality totaling 22% was noted in the WT group between 24 and 48 h of reperfusion, presumably a consequence of renal failure; no deaths were observed in the hCD39 transgenic group and their condition remained stable.
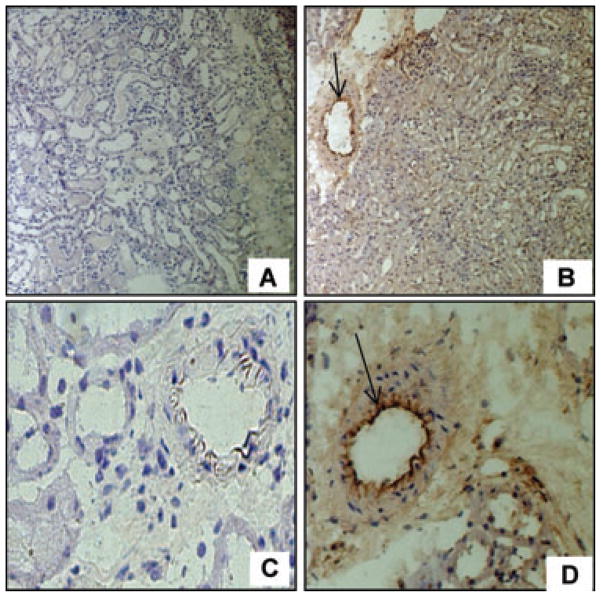
hCD39 expression was maintained after IRI as determined by immunohistochemistry (Figure 2). This suggests that the transgenic expression of hCD39 was able to mitigate the early loss of endogenous ENTPDase biological activity, providing a mechanism for the protection against renal IRI.
Figure 2. hCD39 expression in kidneys 24 h after IRI.
Immunohistochemistry on frozen sections showing that hCD39 expression is maintained in TG renal tissue post-IRI, particularly on the endothelium (arrows) Magnification: A&B 200×, C&D 400×.
A2A adenosine receptor antagonist treatment attenuates the protective effect of CD39 overexpression
ATP is a proinflammatory danger signal, whereas adenosine has many beneficial antiinflammatory effects (22). The relative contributions of ATP hydrolysis and enhanced adenosine generation in mediating protection against IRI were examined by treating hCD39 transgenic mice with an A2A receptor antagonist (CSC) prior to ischemia. CSC treatment abrogated the protective effect of hCD39 expression both functionally and morphologically (Figure 3A, B), suggesting that adenosine signaling is integral to this model. Interestingly, the histological injury score was significantly greater in CSC-treated WT mice than in CSC-treated transgenic mice.
Figure 3. Signaling through the A2A receptor mediates protection.
Mice were treated with 1mg/kg CSC (A2A receptor antagonist) 15 min prior to induction of ischemia followed by 24 h of reperfusion. (A) Serum creatinine, (B) semiquantitative tubular injury score in mice pretreated (+) with CSC. (C) Representative histology showing evidence of cast deposition in Cd39 null kidney (arrow); magnification 200×. Results are presented as mean ± SEM. N.S.: not significant.
Further definition of the protective role of adenosine was sought by subjecting Cd39 null mice to renal IRI. These mice lack endogenous Cd39 and have a reduced capacity to hydrolyze ATP and consequently to generate adenosine (19). Cd39 null mice exhibited a significantly greater histological injury score than WT mice (Figure 3B) with prominent tubular cast deposition in the renal parenchyma (Figure 3C). Together, these data suggest that the CD39-adenosinergic axis confers protection against IRI. Disruption of this mechanism by either deleting Cd39 (Cd39 null) or blocking A2A receptor signaling (CSC treatment) exacerbates renal injury, implicating effects of ATP accumulation and/or lack of adenosine generation in the mechanism of injury.
Transgenic expression of hCD39 decreases apoptosis of tubular cells and CD4+ T cell infiltration
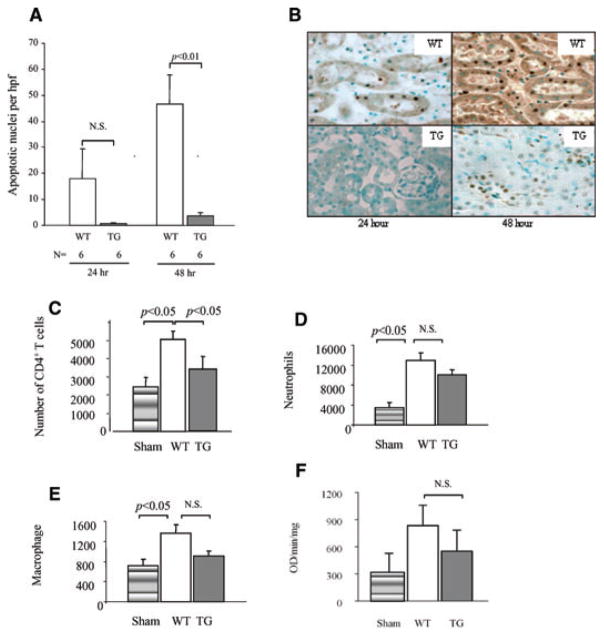
Both apoptotic and necrotic cell death occur during IRI (23). Under physiological conditions, apoptosis is a protective mechanism that can occur without evoking an immune response (24). In IRI, apoptosis incites inflammation during reperfusion amplifying ischemic injury (24). Therapies aimed at inhibiting apotosis have been demonstrated to result in improved graft outcome (25). TUNEL staining showed that apoptosis was present after 4 h of reperfusion in WT mice (data not shown) and increased with longer reperfusion times. There were significantly more apoptotic nuclei in WT kidneys than in hCD39 transgenic kidneys by 48 h (Figure 4A,B) correlating with other parameters of injury and with mortality.
Figure 4. Apoptosis and CD4+ T-cell infiltrate are reduced in hCD39 kidneys after IRI.
(A) Sections of renal tissue were analyzed by TUNEL-stain and the number of apoptotic nuclei per high power field (hpf) were counted. Results are presented as mean ± SEM. N.S (not significant). (B) Representative sections of TUNEL-stained renal cortex at 24 and 48 h. Magnification 400×. (C–F) Mice were subjected to 30 min of ischemia and 3 h of reperfusion. Leukocytes were extracted from the kidneys and analyzed by flow cytometry. (C) CD4+ T cells. (D) Neutrophils. (E) Macrophages. (F) MPO activity assay was performed following 3 h of reperfusion. Data are representative of two independent experiments; n = 4 for each group in each experiment.
Leukocyte subsets are recognized as putative mediators in the pathogenesis of renal IRI (26). Kidneys were recovered after 30 min of ischemia and 3 h of reperfusion, a time deemed optimal to examine initial cellular infiltration (27,28), and leukocytes were extracted and analyzed by flow cytometry. There was a significantly greater number of CD4+ T cells, macrophages and neutrophils in WT kidneys subjected to IRI than in sham-operated controls (Figure 4C) consistent with the role of these cells in the injury process (29). In contrast, CD4+ T cell infiltration in hCD39 transgenic mice was comparable to sham-operated controls and significantly less than in WT mice (Figure 4C). There was no significant difference in the number or activity of infiltrating neutrophils or macrophages in transgenic mice compared to WT mice as determined by flow cytometry (Figure 4C–E) or myeloperoxidase activity assay (Figure 4F). CD8+ T cell infiltration was similar in kidneys from WT and hCD39 transgenic mice following IRI (data not shown).
Transgenic expression of hCD39 on either the vasculature or blood constituents mitigates IRI
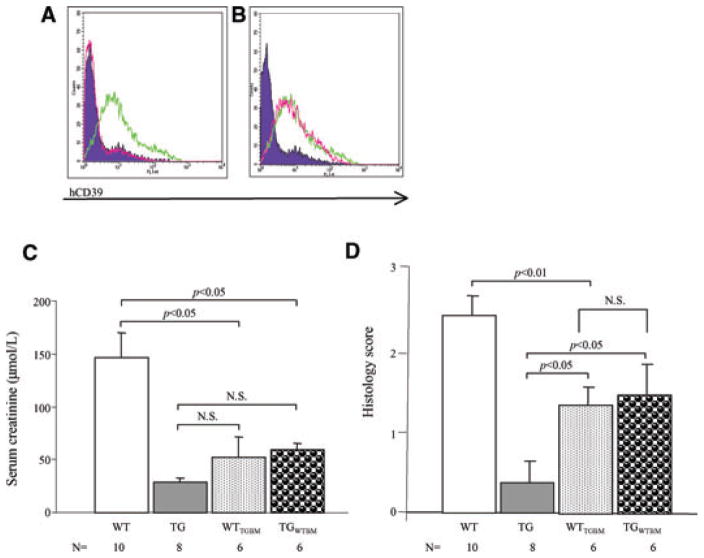
The human CD39 transgene is under the control of the H2Kb promoter, driving ubiquitous expression (18). The relative contributions of hCD39 expression in the tissue and blood compartments to the protection against IRI were assessed by adoptive transfer experiments. Transgenic mice received WT bone marrow following sublethal irradiation, resulting in expression of the transgene being limited to the parenchyma (TGWTBM). The converse experiment (WT mice reconstituted with transgenic bone marrow) confined transgene expression to the circulating cells (WTTGBM). Full reconstitution was confirmed by flow cytometric analysis (Figure 5A,B).
Figure 5. Expression of hCD39 on either bone marrow-derived cells or tissues affords protection against renal IRI.
(A) Flow-cytometric analysis of leukocytes and platelets from WT (purple-filled curve), hCD39 transgenic (green line) and hCD39 transgenic mice reconstituted with WT bone marrow (TGWTBM) (pink line). (B) Flow-cytometric analysis of leukocytes and platelets from WT (purple-filled curve), hCD39 transgenic (green line) and WT mice reconstituted with hCD39 transgenic bone marrow (WTTGBM) (pink line). Chimeric mice expressing hCD39 only on bone marrow-derived cells (WTTGBM) or vasculature (TGWTBM) were subjected to 30 min of ischemia followed by 24 h of reperfusion. (C) Serum creatinine. (D) Semiquantitative tubular injury score. Results are presented as mean ± SEM. N.S., not significant.
The expression of hCD39 on either the bone marrow-derived cells (WTTGBM) or vasculature (TGWTBM) was sufficient to reduce the impact of IRI on renal function as compared to WT mice (Figure 5C). Further, in WTTGBM mice there was reduced ATN compared to WT mice (p < 0.01) and a trend towards protection of renal architecture with less tubular necrosis when hCD39 expression was limited to the vasculature (TGWTBM), but this did not reach statistical significance (p = 0.08) (Figure 5D). Tubular injury, but not serum creatinine, was greater in both adoptively transferred groups compared to nonmanipulated transgenic mice (Figure 5C,D). These results suggest that expression of hCD39 either on bone marrow-derived cells or the renal parenchyma mitigate IRI. However, maximum protection appears to require expression on both renal tissue and circulating cellular components.
hCD39 expression in the donor kidney reduces ATN in a renal transplant model
Although the cold storage of cadaveric donor organs adversely impacts short-term graft function (30,31) it is unavoidable in the process of organ allocation. A syngeneic murine renal transplant model characterised by prolonged cold ischemia was developed to examine the effect of hCD39 transgene expression in a setting that simulated aspects of clinical renal transplantation. Donor kidneys from either WT or hCD39 transgenic mice were transplanted into nephrectomised WT recipients after storage for 5 h at 4°C. Warm ischemia was limited to 30 min. Severe ATN was induced in WT donor kidneys at 24 h of reperfusion (Figure 6A), reflected in a high tubular injury score (Figure 6B) and elevated serum creatinine (Figure 6C).
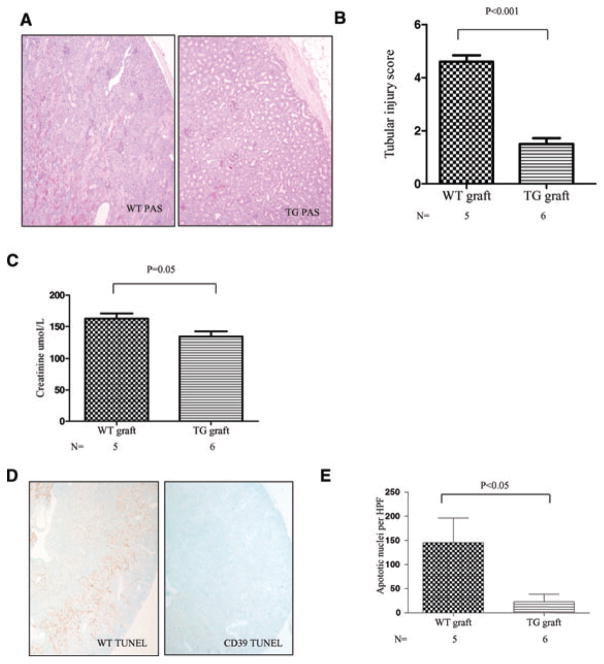
Figure 6. Expression of hCD39 on the donor kidney reduces cold ischemic ATN.
Bilateral nephrectomized wild-type (WT) mice received a renal transplant from either a WT or hCD39 transgenic donor. Kidney grafts were subjected to 5 h of cold (storage) ischemia followed by reperfusion for 24 h. (A) Representative morphology (PAS) of kidney sections after 24 h of reperfusion. Magnification 40×. (B) Semiquantitative tubular injury score. (C) Serum creatinine. (D) Representative sections of TUNEL-stained renal cortex post transplantation. Magnification 40×. (E) Quantitation of TUNEL-stained apoptotic nuclei per high power field were counted. Results are presented as mean ± SEM.
ATN was also evident in hCD39 transgenic donor kidneys but was significantly less than in WT donor kidneys and restricted to a band at the corticomedullary junction (Figure 6A). The tubular injury score and serum creatinine were significantly lower in recipients of transgenic grafts (Figure 6B,C). TUNEL staining demonstrated extensive apoptosis in all WT grafts, in contrast to minimal apoptosis in hCD39 transgenic grafts (Figure 6D,E). The reperfusion period was extended to up to 72 h in the next set of experiments. All recipients of WT donor kidneys died within 48 h (median 45 h, Table 1). In contrast, only one recipient in the hCD39 transgenic donor group died before 48 h, and the remaining recipients exhibited normal behavior patterns and no sign of ill health when sacrificed at 57.5–72 h (median 69 hours, p < 0.01). Histological examination of transgenic kidney grafts revealed minimal tubular damage with less than 20% cortical involvement, whereas examination of a WT kidney graft recovered just after death revealed extensive (>70%) cortical damage (data not shown).
Table 1.
Survival of WT mice receiving WT or hCD39 transgenic renal grafts.
| Donor kidney | Recipient | Termination of experiment (hours) |
|---|---|---|
| WT | WT | 33.5*, 36*, 45*, 45*, 46*, 47* |
| hCD39 | WT | 46.5*, 57.5, 57.5, 69, 69, 72 |
Recipient mouse dead.
Together these data show that the expression of hCD39 on the renal tissue itself imparts robust protection against both warm and cold IRI encountered in transplantation, improving both immediate and short-term graft function.
Discussion
Expression of hCD39 in transgenic mice enhances the conversion of extracellular purinergic nucleotides to adenosine (18). Here, we demonstrate that transgenic expression of hCD39 is protective in a murine model of warm renal IRI and is associated with inhibition of renal injury and decreased tubular apoptosis. The overexpression of hCD39 potentially serves dual purposes. The first is the hydrolysis of extracellular ATP, a putative danger signal that promotes inflammation (32). The second is increased generation of adenosine and consequent antiinflammatory signaling.
The technique employed to induce renal ischemia in this study was that of clamping of the renal pedicle. Inherent to this method are the potential problems of venous congestion or outflow obstruction during ischemia. Others have induced IRI through selective occlusion of the renal artery (13) that negates these potential confounders. Despite these putative confounders the results from this study were reproducible and there was no evidence of venous congestion or thrombosis within the renal parenchyma during histological examination.
The severe tubular injury observed in Cd39 null mice is consistent with previous reports (33) and suggests the involvement of excessive ATP as a mediator of this injury. However, the finding that protection by hCD39 transgene expression was abrogated by pretreatment with an A2A receptor antagonist also implicates protective signaling by adenosine and is consistent with studies in A2A receptor knock-out mice (34). The poorer histological features of the null mice compared to the WT mice treated with the A2A receptor antagonist imply a role for other adenosine receptors. Indeed, the endothelial A2B receptor is induced by hypoxia (35) and a pivotal role for the A2B receptor in renal protection in vivo has recently been described (36). Using mutant mice the source of A2B receptor protection has been localized to the renovasculature (36), whereas protection attributable to the A2A receptor is a function of circulating CD4+ T cells (8).
Leucocyte subsets are recognized as putative mediators in the pathogenesis of renal IRI (37). Our finding of a significant CD4+ T cell infiltrate in the kidneys of WT mice subjected to IRI implicates a pathogenic role for T cells in this model, which is supported by data from others (8,29). Recently regulatory T cells, a subset of CD4+ T cells, have been shown to contribute to the protective effect of the phenomenon of ischemic preconditioning (38) and limit the fibrotic response following IRI (39). Examination of renal parenchymal cells by flow cytometry revealed only low numbers of CD4+ T cells in kidneys from hCD39 transgenic mice following IRI, at a level comparable to sham-operated controls, the latter probably reflective of T cell aggregates normally present in kidneys (28). Confounding the interpretation of these results is the finding that hCD39 transgenic mice have a peripheral CD4+ T cell lymphopenia, although regulatory T cell number and function is preserved (40), both of which are inherently protective in IRI. It is interesting, however, that the neutrophilic infiltrate within the parenchyma was similar in WT and hCD39 transgenic kidneys given the proposed pathogenic role of neutrophils in IRI (10) and the recent demonstration of an important role for the A2B receptor in limiting neutrophil migration in hypoxic-induced inflammation (41). It is striking that tissue-restricted expression of hCD39 confers a similar degree of protection as blood borne expression, given the latter group of chimeric mice are immunodeficient with a peripheral CD4+ T cell lymphopenia. Putative mechanisms of tissue-derived protection include a direct cytoprotective effect of CD39 expression on the endothelium (19,22), an antiapoptotic effect of adenosine on A1 receptors on renal tubular cells (10), or the generation of an adenosine-rich milieu that bathes infiltrating T cells (9), which is both suppressive and antiproliferative (42). It would be interesting to determine the cellular specificity of the protective effect by generating mice with targeted overexpression of CD39 in particular renal or circulating cells, using tissue-restricted promoters.
The utilization of a pure warm ischemia model to define the pathophysiology of IRI carries with it the inherent flaws and fails to replicate the true sequence of events during organ transplantation. A renal transplant model has important advantages as it is known that different pathogenetic mechanisms are involved in warm and cold ischemia (43). Moreover, hypothermia may modify the organ’s subsequent response to reperfusion. The murine renal transplantation model used in this study more closely mimics the clinical situation by encompassing both warm and cold ischemia. Kidneys were stored for 5 h at 4°C in Ringers solution, which lacks the active constituents of most preservation solutions, such as adenosine in UW solution, that are aimed at reducing ongoing organ injury (4) thus minimizing potential confounding factors. The most compelling finding of this study was that kidneys that over expressed hCD39 were protected in the renal transplant model, which was most evident histopathologically. Whereas WT donor kidneys had evidence of extensive ATN extending into the renal cortex accompanied by significant apoptosis, damage in the hCD39 transgenic grafts was limited to a band at the corticomedullary junction, an area of marginal oxygenation and high basal metabolic rate that is particularly vulnerable to hypoxic states (44). The extent of renal injury in WT donor kidneys was not compatible with short-term survival with all recipients dead within 2 days. In contrast, all but one recipient of hCD39 transgenic donor kidneys were well at 48–72 h correlating with minimal histological damage. The putative clinical flow on effect is improved graft function and survival.
In conclusion, increasing experimental evidence supports a role for early nonspecific inflammatory events occurring in the peritransplant period, such as IRI, in the pathogenesis of delayed graft function, acute rejection and chronic allograft dysfunction. We have shown that the transgenic expression of hCD39 mitigates tubular cell apoptosis and necrosis, which are features of IRI, in part by the generation of adenosine. The delivery of CD39 in the clinic may be achieved with modification of soluble CD39 such that it is targeted to sites of endothelial injury (45) thereby providing putative anticoagulant and antiinflammatory effects that are localized to the graft with minimal systemic effect.
Acknowledgments
Funding Sources: This work was supported by the NHMRC (Australia) and Genzyme Renal Innovations Program (KMD) and NIH (SCR).
The authors thank the staff of the Bioresources Centre, St. Vincent’s Hospital Melbourne, for animal breeding and maintenance, Professor Prue Hill and the Department of Anatomical Pathology St. Vincent’s Health for invaluable guidance and assistance and Adam Winterhalter for assistance with FACS analysis (Figure 4).
Abbreviations
- ADP
adenosine di-phosphate
- AMP
adenosinemono-phosphate
- ATN
acute tubular necrosis
- ATP
adenosine tri-phosphate
- CSC
(3-chlorostyryl) caffeine
- ENTPD1
ectonucleosidetriphosphate diphosphohydrolase-1
- FITC
fluorescein isothiocyanate
- hCD39
humanCD39
- H&E
hematoxylin and eosin
- HTK
histidine-tryptophan-ketoglutarate solution
- IRI
ischemia-reperfusion injury
- MPO
myeloperoxidase
- PAS
periodic acid Schiff
- PBS
phosphate-buffered saline solution
- TG
hCD39 transgenic mice
- TGWTBM
TG mice adoptively transferred with WT bone marrow
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labelling
- UW
University of Wisconsin
- WT
Wild-type
- WTTGBM
WT mice adoptively transferred with TG bone marrow
Footnotes
SC was the recipient of an NHMRC postgraduate research scholarship.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Foley DP, Fernandez LA, Leverson G, et al. Donation after cardiac death: The University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242:724–731. doi: 10.1097/01.sla.0000186178.07110.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stubenitsky BM, Brasile L, Booster MH, Haisch CE, Kootstra G. Deletrious effect of prolonged cold ischemia on renal function. Transpl Int. 2001;14:256–260. doi: 10.1007/s001470100326. [DOI] [PubMed] [Google Scholar]
- 3.Halloran P, Aprile M. Factors influencing early renal function in cadaver kidney transplants. A case-control study. Transplantation. 1988;45:122–127. doi: 10.1097/00007890-198801000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Southard JH, van Gulik TM, Ametani MS, et al. Important components of the UW solution. Transplantation. 1990;49:251–257. doi: 10.1097/00007890-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Linden J. Molecular approach to adenosine receptors: Receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 6.Linden J. New insights into the regulation of inflammation by adenosine. J Clin Invest. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi S, Hartl R, Wang M, et al. The acute cerebrovascular effects of intracarotid adenosine in nonhuman primates. Anesth Analg. 2003;97:231–237. doi: 10.1213/01.ane.0000065599.71629.91. table of contents. [DOI] [PubMed] [Google Scholar]
- 8.Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 9.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 11.Ely SW, Berne RM. Protective effects of adenosine in myocardial ischemia. Circulation. 1992;85:893–904. doi: 10.1161/01.cir.85.3.893. [DOI] [PubMed] [Google Scholar]
- 12.Ely SW, Mentzer RM, Jr, Lasley RD, Lee BK, Berne RM. Functional and metabolic evidence of enhanced myocardial tolerance to ischemia and reperfusion with adenosine. J Thorac Cardiovasc Surg. 1985;90:549–556. [PubMed] [Google Scholar]
- 13.Grenz A, Zhang H, Eckle T, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 14.Grenz A, Zhang H, Hermes M, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 15.Eltzschig HK, Ibla JC, Furuta GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dwyer KM, Robson SC, Nandurkar HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enjyoji K, Sevigny J, Lin Y, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson KA, Nikodijevic O, Padgett WL, Gallo-Rodriguez C, Maillard M, Daly JW. 8-(3-Chlorostyryl)caffeine (CSC) is a selective A2-adenosine antagonist in vitro and in vivo. FEBS Lett. 1993;323:141–144. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 22.Robson S. The therapeutic potential of CD39: Interview with Dr Simon Robson by Emma Quigley. Expert Opin Ther Targets. 2006;10:649–652. doi: 10.1517/14728222.10.5.649. [DOI] [PubMed] [Google Scholar]
- 23.Gobe G, Willgoss D, Hogg N, Schoch E, Endre Z. Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int. 1999;56:1299–1304. doi: 10.1046/j.1523-1755.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Daemen MA, van ‘t Veer C, Denecker G, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilbao G, Contreras JL, Eckhoff DE, et al. Reduction of ischemia-reperfusion injury of the liver by in vivo adenovirus-mediated gene transfer of the antiapoptotic Bcl-2 gene. Ann Surg. 1999;230:185–193. doi: 10.1097/00000658-199908000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalasani G, Li Q, Konieczny BT, et al. The allograft defines the type of rejection (acute versus chronic) in the face of an established effector immune response. J Immunol. 2004;172:7813–7820. doi: 10.4049/jimmunol.172.12.7813. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 28.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int. 2007;71:1223–1231. doi: 10.1038/sj.ki.5002203. [DOI] [PubMed] [Google Scholar]
- 29.Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koning OH, Ploeg RJ, van Bockel JH, et al. Risk factors for delayed graft function in cadaveric kidney transplantation: A prospective study of renal function and graft survival after preservation with University of Wisconsin solution in multi-organ donors. European Multicenter Study Group. Transplantation. 1997;63:1620–1628. doi: 10.1097/00007890-199706150-00015. [DOI] [PubMed] [Google Scholar]
- 31.Connolly JK, Dyer PA, Martin S, Parrott NR, Pearson RC, Johnson RW. Importance of minimizing HLA-DR mismatch and cold preservation time in cadaveric renal transplantation. Transplantation. 1996;61:709–714. doi: 10.1097/00007890-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 32.Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu B, Rajakumar SV, Robson SC, et al. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation. 2008;86:1707–1712. doi: 10.1097/TP.0b013e31819022bc. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z, Day YJ, Toufektsian MC, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Abdulla P, Hoffman E, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grenz A, Osswald H, Eckle T, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. doi: 10.1038/ki.1997.200. [DOI] [PubMed] [Google Scholar]
- 38.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77:771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ascon M, Ascon DB, Liu M, et al. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int. 2009;75:526–535. doi: 10.1038/ki.2008.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pommey S, Lu B, Robson S, d’Apice A, Cowan P, Dwyer K. Donor livers expressing human CD39 are less susceptible to ischemia reperfusion injury in a mouse liver transplant model. Xenotransplantation. 2009;16:8. [Google Scholar]
- 41.Rosenberger P, Schwab JM, Mirakaj V, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 42.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen TN, Haworth RA, Southard JH. Warm and cold ischemia result in different mechanisms of injury to the coronary vasculature during reperfusion of rat hearts. Transplant Proc. 2000;32:15–18. doi: 10.1016/s0041-1345(99)00855-6. [DOI] [PubMed] [Google Scholar]
- 44.Mason J, Torhorst J, Welsch J. Role of the medullary perfusion defect in the pathogenesis of ischemic renal failure. Kidney Int. 1984;26:283–293. doi: 10.1038/ki.1984.171. [DOI] [PubMed] [Google Scholar]
- 45.Dezfouli S, Crikis S, Dwyer K, et al. Development of RSOLCD39-PSGL as a novel therapeutic with anti-thrombotic and enti-inflammatory effects. XXII ISTH Congress 2009; Thursday July 16 2009; Boston USA. 2009. [Google Scholar]