Abstract
Purinergic signaling has been recognized as playing an important role in inflammation, angiogenesis, malignancy, diabetes and neural transmission. Activation of signaling pathways downstream from purinergic receptors may also be implicated in transplantation and related vascular injury. Following transplantation, the proinflammatory “danger signal” adenosine triphosphate (ATP) is released from damaged cells and promotes proliferation and activation of a variety of immune cells. Targeting purinergic signaling pathways may promote immunosuppression and ameliorate inflammation. Under pathophysiological conditions, nucleotide-scavenging ectonucleotidases CD39 and CD73 hydrolyze ATP, ultimately, to the anti-inflammatory mediator adenosine. Adenosine suppresses proinflammatory cytokine production and is associated with improved graft survival and decreased severity of graft-versus-host disease. Furthermore, purinergic signaling is involved both directly and indirectly in the mechanism of action of several existing immunosuppressive drugs, such as calcineurin inhibitors and mammalian target of rapamycin inhibitors. Targeting of purinergic receptor pathways, particularly in the setting of combination therapies, could become a valuable immunosuppressive strategy in transplantation. This review focuses on the role of the purinergic signaling pathway in transplantation and immunosuppression and explores possible future applications in clinical practice.
Introduction
Organ, tissue, and hematopoietic cell transplantation are often end-stage treatment strategies considered as salvage therapy. In the past decade, increasing public awareness and the subsequent increase in the number of donors have resulted in higher transplant frequencies with generally excellent outcomes (reviewed by Saidi and Hejazii Kenari [1]). Nevertheless, the long-term success of solid organ and hematopoietic cell transplantation, which is characterized by unfettered inflammation and immunological complications associated with ischemia–reperfusion injury (IRI), delayed graft function or altered engraftment, rejection, and graft-versus-host disease (GvHD), remains a major challenge. Transplantation-related metabolic changes may be associated with the activation of the purinergic signaling pathway, which has important metabolic and regulatory roles in multiple areas, such as inflammation, angiogenesis, malignancy, diabetes, and neural transmissions (2–6). Following transplantation, damaged or ischemic cells release the nucleotide adenosine triphosphate (ATP), which promotes inflammation, T cell activation, and proliferation of immune cells, leading to further cell damage.
The purinergic signaling pathway has been covered in detail in many reviews over the past decade (reviewed by Burnstock [7]); briefly, it involves the binding of extracellular ATP (eATP) to ion channel P2X receptors (P2X1–7) and G protein–coupled P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14). The distribution of receptor subtypes and their selective agonists and antagonists are summarized in Table 1. Receptor binding, in either an autocrine or paracrine fashion, promotes the proliferation of immune cells, T cell activation and inflammation (8–10). Consequently, targeting the purinergic signaling pathway to alleviate inflammation and promote immunosuppression offers a promising experimental strategy for improved outcomes in both solid organ grafting and hematopoietic stem cell transplantation. Such a target is the ectonucleotidases (CD39 and CD73) that hydrolyze eATP to adenosine diphosphate (ADP) and subsequently to adenosine monophosphate (AMP) and adenosine. ADP can activate P2Y receptors (9–12), whereas AMP and adenosine bind G protein–coupled P1 receptors (P1A1, P1A2a, P1A2b, P1A3) to promote regulatory T cell (Treg) proliferation and immunosuppression (8,9) (Figure 1).
Table 1.
Purinergic receptor subtype properties
| Receptor | Main distribution | Agonists | Antagonists | Transduction mechanisms |
|---|---|---|---|---|
| P1 | ||||
| P1A1 | Brain, spinal cord, testis, heart, autonomic nerve terminals | CCPA>R-PIA = S-ENBA, CVT-510, GR79236, 2′-MeCCPA, SDZ WAG 994, INO-8875, MRS 5474 | DPCPX, N-0840, MRS1754, WRC-0571, PSB36, SLV320, CGS 16943 | Gi/Go ↓cAMP |
| P1A2a | Brain, heart, lungs, spleen | HENECA>CGS 21680 = CVT-3146, ATL-146e, Regadenoson | KF17837, SCH58261, ZM241385, KW 6002 | GS ↑cAMP |
| P1A2b | Large intestine, bladder | Bay60-6583, NECA | PSB603, MRE-2029-F20, MRS1754, PSB0788 MRS1706, PSB1115, Alloxazine, GS-6201 | GS ↑cAMP, Gq/G11 |
| P1A3 | Lung, liver, brain, testis, heart | IB-MECA>MRS5698 > MRS5168 > 2-Cl-IB-MECA, DBXRM, VT160, HEMADO | MRS1220, L-268605, MRS1191, MRS1523(rat), VUF8504, VUF5574, MRS1334(human), PSB10 | Gi/Go, Gq/G11, ↓cAMP, PLC-β activation |
| P2 | ||||
| P2X1 | Smooth muscle, platelets, cerebellum, dorsal horn spinal neurons | BzATP > ATP = 2-MeSATP≥α,β-meATP=L-β,γ-meATP (rapid desensitization), PAPET-ATP | NF864 > NF449 > IP5I≥TNP-ATP> RO 0437626 > NF279, NF023, RO1, MRS2159 | Intrinsic cation channel (Ca2+ and Na+) |
| P2X2 | Smooth muscle, CNS, retina, chromaffin cells, autonomic and sensory ganglia, pancreas | ATP≥ATPγS≥2-MeSATP≫α,β-meATP (pH + zinc sensitive), β,γ-CF2ATP | PSB-1011 > RB2, isoPPADS>PPADS>Suramin, NF770, NF778, Aminoglycoside | Intrinsic ion channel (particularly Ca2+) |
| P2X3 | Sensory neurons, NTS, some sympathetic neurons | 2-MeSATP≥ATP≥Ap4A≥α,β-meATP (rapid desensitization), PAPET-ATP, BzATP | TNP-ATP, AF353, A317491, RO3, isoPPADS > NF110 > PPADS, Ip5I, phenol red, RN-1838, Spinorphin | Intrinsic cation channel |
| P2X4 | CNS, testis, colon, endothelial cells, microglia | ATP≫α,β-meATP≫ CTP, 2-MeSATP Ivermectin potentiation | 5-BDBD≫ TNP-ATP, PPADS>BBG, Paroxetine, phenolphthalein, CO donor (CORM 2), 5MPTP | Intrinsic ion channel (especially Ca2+) |
| P2X5 | Proliferating cells in skin, gut, bladder, thymus, spinal cord, heart, adrenal medulla | ATP=2-MeSATP=ATPγS≫α,β-meATP>AP4A | BBG>PPADS, Suramin | Intrinsic ion channel |
| P2X6 | CNS, motor neurons in spinal cord | - (only functions as a heteromultimer) | – | Intrinsic ion channel |
| P2X7 | Immune cells including dendritic cells (mast cells, macrophages), pancreas, skin, microglia | BzATP>ATP ≥2-MeSATP ≫α,β-meATP (clemastine potentiates) | KN62, BBG, KN04, MRS2427, O-ATP, RN-6189, Perazine, AZ10606120, A740003, A-438079, A-804598, GSK-1370319, Comp 31 (GSK), AZD-9056, CE-224,535, JNJ-47965567, JNJ-42253432 (penetrates BBB), decavanadate | Intrinsic cation channel and a large pore with prolonged activation |
| P2Y1 | Epithelial and endothelial cells, platelets, immune cells, osteoclasts, brain | MRS2365 > 2-MeSADP=Ap5(γB)≫ ADPβS>ATP >2-MeSATP=ADP | MRS2500 > MRS2279 > MRS2179, PIT, A3P5P | Gq/G11, PLC-β activation |
| P2Y2 | Immune cells, epithelial and endothelial cells, kidney tubules, osteoblasts | 2-thio-UTP>UTP, MRS2698 ≥ ATP, INS 365 > INS 37217, UTPγS>Ap4A>MRS 2768, Up4-phenyl ester | AR-C126313 > Suramin> RB2, PSB-716, MRS2576, PSB-0402 | Gq/G11 and possibly Gi/Go, PLC-β activation |
| P2Y4 | Endothelial cells, placenta, spleen, thymus | 2′-azido-dUTP> UTPγS, UTP≥ATP≥ Ap4A Up4U MRS4062 | ATP (human)>Reactive Blue 2 > Suramin, MRS2577, PPADS | Gq/G11 and possibly Gi, PLC-β activation |
| P2Y6 | Airway and intestinal epithelial cells, placenta, T cells, thymus, microglia (activated) | MRS2693 > UDPβS, PSB0474 > INS48823, Up3U, 3-phenacyl-UDP≫ UDP>UTP≫ ATP, α,β-meUDP, MRS2957, MRS4129, 5-OMe-UDP αB | MRS2578 > Reactive Blue 2, PPADS, MRS2567, MRS2575 (human) | Gq/G11, PLC-β activation |
| P2Y11 | Spleen, intestine, granulocytes | ATPγS>AR-C67085>BzATP≥ATP, NF546, NAD+, NAADP+, Sp-2-propylthio-ATP-α-B | NF157 > Suramin>RB2, 5′-AMPS, NF340, AMP-α-5, | Gq/G11 and GS, PLC-β activation |
| P2Y12 | Platelets, glial cells | 2-MeSADP≥ADP>ATP, ADP-β-S | AR-C69931MX> AZD6140 (Ticagrelor), INS50589 > RB2 > 2-MeSAMP AR-C66096, CT50547,PSB-0413, Carba-nucleosides, MRS2395, AR-C67085, [3H]PSB-0413, clopidogrel, AZD1283 | GαI, inhibition of adenylate cyclase |
| P2Y13 | Spleen, brain, lymph nodes, bone marrow, erythrocytes | ADP=2-MeSADP>2-MeSATP, ATP | AR-C69931MX>AR-C67085 > MRS2211, 2-MeSAMP | Gi/Go |
| P2Y14 | Placenta, adipose tissue, stomach, intestine, discrete brain regions, mast cells | MRS2690 > UDP>UDP glucose≥UDP-galactose, UDP-glucosamine, MRS2905 | PPTN | Gq/G11 |
| GPR17 | Oligodendrocytes | Uracil nucleotides/cysteinyl-leukotrienes, MDL29,951 | PZB01415033 | Gi, adenylate cyclase inhibition |
P2X receptor subtype agonist potencies are based on rat preparations, whereas P1 and P2Y receptor subtype agonist potencies are based on human preparations. Modified with permission from Burnstock (115).
≫, much greater affinity than; >, greater affinity than; ≥, greater or equal affinity than.
A317491, 5-[[[(3-phenoxyphenyl)methyl][(1S)-1,2,3,4-tetrahydro-1-naphthalenyl]amino]carbonyl]-1,2,4-benzenetricarboxylic acid; A3P5P, adenosine-3′-5′-bisphosphate; A-438079, 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride; A740003, N-[1-[[(cyanoamino)(5-quinolinylamino)methylene]amino]-2,2-dimethylpropyl]-3,4-dimethoxybenze-neacetamide; A-804598, N-cyano-N″-[(1S)-1-phenylethyl]-N′-5-quinolinyl-guanidine; ADP, adenosine 5′-diphosphte; ADPβS, adenosine-5′-(β-thio)-diphosphate; AF353, 5-(5-iodo-2-isopropyl-4-methoxy-phenoxy)-pyrimidine-2,4-diamine; 5′-AMPS, 5′-O-thiomnophosphate; Ap4A, diadenosine tetraphosphate; Ap5(γβ), adenosine pentaphosphate (βγ); ATL-146e, 4-{3-[6-Amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester; Ap4A, diadenosine tetraphosphate; ATPγS, adenosine-5′-(γ-thio)-triphosphate; AR-C126313, 2′-amino-2′-deoxy-2-thiouridine 5′-triphosphate; AR-C66096, 2-(propylthio)adenosine-5′-O-(β,γ-difluoromethylene)triphosphate; AR-C67085, [[[[(2R,3S,4R,5R)-5-(6-amino-2-propylsulfanylpurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]-dichloromethyl]phosphonic acid; AR-C69931MX, [dichloro-[[[(2R,3S,4R,5R)-3,4-dihydroxy-5-[6-(2-methylsulfanylethylamino)-2-(3,3,3-trifluoropropylsulfanyl)purin-9-yl]oxolan-2-yl]methoxy-hydroxyphosphoryl] oxy-hydroxyphosphoryl]methyl]phosphonic acid; ATP, adenosine 5′-triphosphte; ATPγS, adenosine-5′-(γ-thio)-triphosphate; AZ10606120, N-[2-[[2-[(2-hydroxyethyl)amino]ethyl] amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride; AZD1283, ethyl 6-(4-((benzylsulfonyl)carbamoyl)piperidin-1-yl)-5-cyano-2-methylnicotinate; ZD6140, 3-[7-[[2-(3,4-difluorophenyl)cyclopropyl]amino]-5-propylsulfanyltriazolo[5,4-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol; 2′-azido-dUTP, 2′-azido-deoxyuridine-5′-triphosphate; Bay 60-6583, 2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin-2-ylsulfanyl]acetamide; BBG, brilliant blue green; 5-BDBD, 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one; BzATP, 2′ (3′)-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate; β,γ-CF2ATP, α,β-difluoromethylene-ATP; cAMP, cyclic AMP; CCPA, chlorocyclopentyl adenosine; CGS 21680, 2-[p-(2-carboxyethyl)phenethylamino]-5¢-N-ethylcarboxamido adenosine; 2-Cl-IB-MECA, 2-Chloro-N6-(3-iodobenzyl)-9-[5-(methylcarbamoyl)-β-D-ribofuranosyl]adenine; CNS, central nervous system; CORM 2, carbon monoxide donor 2; CT50547, N1-(6-ethoxy-1,3-benzothiazol-2-yl-2-(7-ethoxy-4-hydroxy-2,2-dioxo-2H-2] 6benzo-[4,5][1,3]thiazolo[2,3-c][1,2,4] thiadiazin-3-yl)-2-oxo-1-ethanesulfonamide; CTP, cytosine triphosphate; CVT-3146, 2-(N-pyrazolyl) adenosine; CVT-510, N-(3(R)-tetrahydrofura-nyl)-6-aminopurine riboside; DBXRM, 1,3-Dibutylxanthine 7-riboside 5′-N-methylcarboxamide; DPCPX, 1,3-dipropyl-8-cyclopentylxanthine; GR79236, N-[(1S,trans)-2-hydroxycyclo-pentyl]adenosine; GS-6201, 3-Ethyl-3,9-dihydro-1-propyl-8-[1-[[3-(trifluoromethyl)phenyl]methyl]-1H-pyrazol-4-yl]-1H-purine-2,6-dione; GSK-1370319, N-[(2,4-dichlorophenyl)methyl]-1-methyl-5-oxo-L-prolinamide; GTP, guanosine-5′-triphosphate; HEMADO, 2-(1-Hexynyl)-N-methyl adenosine; HENECA, 2-hexynyladenosine-59-N-ethylcarboxamide; IB-MECA, N6-(3-Iodobenzyl)-9-[5-(methylcarbamoyl)-β-D-ribofuranosyl]adenine; INS 365, [[[[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]; INS 37217, P(1)-(uridine 5′)-P(4)- (2′-deoxycytidine 5′)tetraphosphate; INS48823, {[(3aR,4R,6R,6aR)-2-benzyl-6-(2,4-dioxo-1,2,3,4-tetra-hydropyrimidin-1-yl)-tetrahydro-2H-furo[3,4-d][1,3]dioxol-4-yl]methoxy}({[({[(2S,3R,4S,5S)-5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy) phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphinic acid; INS50589, [(2S,3aR,4R,6R,6aR)-6-[6-(ethylcarbamoylamino)purin-9-yl]-2-[(E)-2-phenylethenyl]-3a,4,6,6a-tetrahydrofuro[4,3-d][1,3]dioxol-4-yl]methyl dihydrogen phosphate; IP5I, di-inosine pentaphosphate; isoPPADS, iso-pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid; JNJ-42253432, 2-methyl-N-([1-(4-phenylpiperazin-1-yl)cyclohexyl]methyl)-1,2,3,4-tetrahydroisoquinoline-5-carboxamide; JNJ-47965567, 2-(Phenylthio)-N-[[tetrahydro-4-(4-phenyl-1-piperazinyl)-2H-pyran-4-yl] methyl-3-pyridinecarboxamide; KF17837, (E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine; KN04, N-[1-[N-methyl-p-(5-isoquinolinesulphonyl)benzyl]-2-(4-phenylpiperazine) ethyl]-5-isoquinoline-sulfonamide; KN62, 1-[N,O-bis(5-Isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine; KW 6002, (E)-1,3-Diethyl-8-(3,4-dimethoxyphenylethyl)-7-methyl-3,7-dihydro-1H-purine-2,6-dione; L-α,β-meATP, L-α,β-methylene ATP; L-β,γ-meATP, L-β,γ-methylene ATP; 2′-MeCCPA, 2-chloro-N-cyclopentyl-2′-methyladenosine; 2-MeSADP, 2-methylthio ADP; 2-MeSAMP, 2-methylthio AMP; 2-MeSATP, 2-methylthio ATP; α,β-meUDP, α,β-methylene UDP; ≫, much greater affinity than; >, greater affinity than; ≥, greater or equal affinity than.
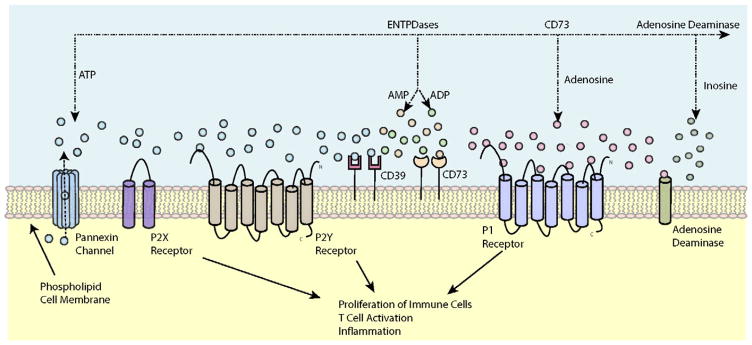
Figure 1. Overview of purinergic signaling.
Intracellular ATP is released from cells through the opening of pannexin hemi channels or via P2X7 receptors and can serve as an extracellular signaling molecule. ATP subsequently signals with various P2 receptors, both ligand-gated P2X and G protein–coupled P2Y receptors, in an autocrine manner, which has been implicated in a wide variety of physiological processes. Eventually, ATP is hydrolyzed by ENTPDases expressed on cell surfaces, including CD39 and the ecto-5′-nucleotidase CD73, which promote the generation of adenosine. Adenosine signals on G protein–coupled P1 receptors and is metabolized to inosine by the enzyme adenosine deaminase. ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine triphosphate.
In 2011, ≈170 000 persons in the United States were living with a transplanted organ, and the majority required continual immunosuppressive therapies to maintain tolerance and to prevent organ rejection (reviewed by Newell [13]). Existing immunosuppressive regimens affect the regulation of purinergic responses at the level of agonist/mediator release, receptor functionality and the nucleotide-scavenging ectonucleotidases that ultimately generate adenosine (reviewed by Burnstock [14]). Current immunosuppressive drugs, such as mammalian target of rapamycin (mTOR) inhibitors, mycophenolic acid (MPA) or calcineurin inhibitors (CNIs), are closely linked to cell metabolism and might affect, and simultaneously be affected by, purinergic signals. The purpose of this review was to explore the role of purinergic signaling in transplantation and immunosuppression and the implications for clinical practice.
Purinergic Signaling in Specific Organs, Tissues, and Cells
Liver
Purinergic signaling is implicated in a variety of liver diseases and posttransplantation outcomes such as inflammation, rejection, hepatic IRI, hepatic regeneration, steatohepatitis, fibrosis, and cancer (15–20). The liver produces the majority of the nucleotides in the body and concurrently has high ATPase and ADPase activities. Mechanical stress during transplantation results in the secretion of ATP or ADP from hepatocytes and Kupffer cells, which subsequently bind P2X and P2Y receptors expressed on hepatocytes, hepatic sinusoids and other resident cells or bind P1 receptors following hydrolysis to adenosine by selected ectonucleotidases (Figure 2) (reviewed by Burnstock et al [21]). Although adenosine can promote immunosuppression through the proliferation and activation of Tregs via activation of the P1A2a receptor (reviewed by Roberts et al [22]), protracted ectonucleotidase-dependent adenosine production following ethanol or fructose ingestion can lead to hepatic steatosis, fibrosis and, ultimately, cirrhosis. These diverse biological effects of adenosine have important implications for the development of purinergic therapies in liver transplantation (21). CD39 is important for rapid posttransplant hepatic regeneration and subsequent graft outcomes, as shown by the reduced regenerative capacity of liver transplanted into CD39-deficient mice (reviewed by Beldi et al [19]). Transgenic CD39 has also been shown to be beneficial in protecting liver grafts from early IRI in mice after 18 h of cold ischemia, an effect attributed to the depletion of hepatic CD4+ T cells but not NK T cells that may have a greater role in warm IRI (23). Monitoring hepatic ATP levels in conjunction with serum hyaluronic acid has been suggested as an approach to assess graft viability and posttransplant instability (reviewed by Vaughn et al [24]). The Immu-Know assay (Cylex, Inc., Columbia, MD), which measures peripheral blood CD4+ total ATP levels, has shown promise in determining immunosuppression and diagnosing early rejection (21).
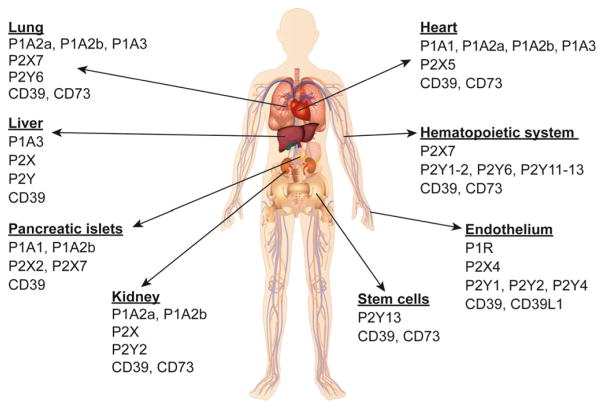
Figure 2. Distribution of purinergic receptors.
An overview of purinergic receptor CD39 and CD73 expression is shown in selected organs, tissues, and cells. Further information on the distribution of purinergic receptors can be found in Table 1.
Kidney
Purinergic P2X and P2Y receptors are expressed on a variety of renal cells, including mesangial cells, podocytes and cells of the parietal sheets (Figure 2), and all renal cells can produce ATP under conditions such as mechanical or osmotic stress, fluid flow or agonist stimulation (25,26). Furthermore, overexpression of the ectonucleotidase CD39 protected against renal IRI in a mouse model, raising the possibility that soluble CD39 (e.g. apyrase) could be used therapeutically to prevent organ damage in kidney transplantation (8,27).
In a renal transplantation model characterized by 5 h of cold preservation, mice receiving a CD39-overexpressing graft had better renal function and remained healthy for a longer duration compared with the control recipients. This effect was attributed to the increased production of adenosine in these mice, and the P1A2a receptor may also be involved because blocking this receptor with a specific antagonist reversed the observed benefits (28). In the same study, transgenic expression of human CD39 conferred renal protection in a mouse model of warm IRI, and together with results from the transplantation model, the authors concluded that CD39 expression had beneficial effects on both warm and cold renal IRI (28).
Other studies have shown that P1A2a receptor activation considerably reduces T cell–mediated renal injury (22). The P1A2b receptor was shown to protect against tissue injury and to improve kidney function in a mouse model of renal ischemia using ischemic preconditioning, an effect attributed to the inhibition of neutrophil-dependent production of tumor necrosis factor α (TNF-α) (29,30). A recent review by Solini et al gives a more detailed overview of the involvement of ATP and P2 receptors in both physiological and pathological signaling in the kidney (31).
Heart
Some of the earliest work on the extracellular action of purines was performed on heart and blood vessels (32–34), and it is known that cardiac cells express purinergic receptors and ectonucleotidases (Figure 2) (22). An in vitro cardiac cell ischemia model showed that adenosine prevented necrosis and promoted cardioprotection through P1A2a/2b receptor activation (35). Furthermore, adenosine-treated grafts in a rat heterotopic heart transplant model showed less inflammation and fewer infiltrating cells, with reduced subsequent IRI and myocardial injury compared with the control grafts (36). This effect suggests that storing and transporting grafts in the presence of adenosine, as in UW solution, may reduce the impact of IRI and improve graft outcomes (22). Furthermore, cardiac xenograft rejection and vascular thrombosis observed in mice was reversed when CD39 was introduced either by adenovirus-mediated overexpression (37) or administration of apyrase (38). Similarly, CD39 has been shown to be cardioprotective by reducing myocardial infarction following P1A2b-mediated coronary artery occlusion (22). Studies in a cardiac allotransplantation model demonstrated that reduced graft expression of P1A2b increased inflammatory and immune responses and ultimately reduced allograft survival when either the donor or recipient was CD73 deficient, demonstrating the protective effect of CD73 (39).
Targeting of the P2X7R receptor with oxidized ATP (oATP) was shown to promote cardiac transplant survival in 80% of murine recipients of a fully mismatched allograft, an effect that correlated with the inhibition of T cell activation and differentiation (40).
Lung
Pulmonary and alveolar epithelial cells express purinergic receptors and ectonucleotidases (Figure 2) (22,41,42), and purinergic signaling is known to contribute to the pathophysiology of asthma and chronic obstructive pulmonary disease (reviewed by Adriaensen and Timmermans [43]). Inhibition of P2X7 signaling through the use of oATP, a specific P2X7 inhibitor, resulted in fewer inflammatory cells, less rejection, improved lung function, and prolonged survival of lung allografts in mice (44). The suppression of inflammation by CD73-mediated adenosine production, particularly through the P1A2a receptor, is known to be crucial in graft survival and lung function (22). In a porcine model of lung transplantation, activation of the P1A2a receptor during early reperfusion limited inflammation and preserved lung function (45). Conversely, it has been suggested that activation of the P1A2b receptor may promote bronchiolitis obliterans syndrome, a manifestation of chronic pulmonary allograft dysfunction (46). These findings suggest that a P1A2a receptor agonist, possibly coupled with a P1A2b receptor antagonist, might be beneficial in limiting acute pulmonary IRI and subsequently improving graft survival and lung function following transplantation.
Pancreatic islets
Expression of purinergic receptors on islet beta cells has been documented (Figure 2) and plays a definitive role in insulin secretion (47,48), whereby elevated extracellular ATP levels have been associated with hyperinsulinemia (49). A recent study by Vergani and colleagues reported the expression of P2X7 receptors on islets and subsequent inhibition of this purinergic receptor using oxidized ATP in mouse models of islet allograft rejection (50). This intervention resulted in significant improvement in graft survival, with 30% of the mice maintaining good graft function at 100 days. Furthermore, when combined with sirolimus, the intervention resulted in favorable long-term graft outcomes at 100 days in 7 of the 10 mice. In another transplantation model, human CD39 overexpression in transgenic islets significantly delayed clot formation time in the presence of human blood, prolonging graft islet viability (51).
The immunosuppressive effect of adenosine in inhibiting interferon γ production from neutrophils has been shown to prevent early loss of transplanted islets in the livers of mice (52). Another study using a multiple low-dose streptozotocin mouse model reported that overexpression of CD39, which promotes the production of adenosine, protected islet grafts from T cell–mediated injury and reduced susceptibility to diabetes (53).
Endothelium
Extracellular ATP is rapidly broken down by ectoenzymes (54), such as the nucleoside triphosphate diphospho-hydrolase family, including NTPDase 1 (CD39), the nucleotide pyrophosphatase/phosphodiesterase family, ecto-5′-nucleotidase (CD73), and alkaline phosphatase (9,55–57). CD39 is expressed on quiescent endothelial cells playing a critical role in thromboregulation (58), whereas NTPDase 2 is widely expressed on vascular adventitial cells (Figure 2) (59).
In episodes of chronic rejection, the endothelial layer is invariably damaged, triggering a collateral pathway of coagulation mediated primarily through thrombin, a potent platelet agonist. The downstream effects of thrombin are mediated through protease-activated receptors (reviewed by Shrivastava et al [60]) and lead to the activation of platelets, endothelial cells and a wide array of immune cells. This response is synergistically augmented through the release of ADP by activated platelets and endothelial cells from dense intracellular granules, resulting in ectonucleotidase-mediated production of AMP and adenosine.
Bearing in mind the destructive effects of thrombus formation in mediating transplant rejection and its biological pathways of formation, one can appreciate the role of ectonucleotidases in modulating allograft responses. The activity of CD39 has been reported to be significantly decreased in chronic rejecting grafts, highlighting a potentially therapeutic avenue of complementation. The viability of this prospect was confirmed in mouse models of rejection in conjunction with transgenic expression of human CD39. In this study, mice overexpressing human CD39 had increased cardiac allograft survival along with protection from thrombosis compared with wild type (WT) mice, serving only to further highlight the previously untapped antithrombotic therapeutic potential of ectonucleotidases in transplantation (61).
Stem cells: mesenchymal and hematopoietic
Allogeneic hematopoietic stem cell transplantation (allo-HSCT), a curative therapeutic option for patients with hematological cancers, is performed >27 000 times globally per year (62); however, GvHD remains a major obstacle for a broader application of allo-HSCT. Extracellular nucleotides and ectonucleotidases are involved in inflammation (reviewed by Deaglio and Robson [12]) and can be particularly implicated in GvHD (63,64). Interestingly, multiple studies show that mesenchymal stromal/stem cells (MSCs), which have the potential to upregulate CD39 and CD73 (Figure 2) and to increase adenosine production to suppress activated T cells (65), promote tolerance following allo-HSCT (66–70), diabetes (71), and solid organ transplantation (72).
A recent publication has shown that suppressive-type MSCs can be induced by IL-17A and promote the generation of CD4+CD25highCD127lowFoxP3+ Tregs (iTregs) from activated CD4+CD25− T cells in vitro. These induced iTregs express both CD39 and CD73 as well as CD69, OX40, cytotoxic T lymphocyte–associated antigen 4, and glucocorticoid-induced TNF receptor–related protein. The generated, suppressive MSCs are thus able to boost iTreg functionality to potently suppress T cell activation and appear to have minimal immunogenicity. These MSCs and other regulatory cells might be considered a potential option for patients with corticosteroid-refractory GvHD (73).
Impact of Purinergic Signaling on Immunosuppressive Drugs
Calcineurin inhibitors
The serine/threonine phosphatase calcineurin has a role in the activation of T cells, and inhibition of this protein by CNIs is a widely used treatment strategy to promote immunosuppression following transplantation. Cyclosporine forms a complex with the cytosolic protein cyclophilin, which binds and inactivates calcineurin and ultimately inhibits the transcription of IL-2 and other proinflammatory cytokines by preventing calcineurin-dependent dephosphorylation of the transcription factor nuclear factor of activated T cells (NFAT) (Figure 3) (74,75). Tacrolimus, also known as FK506, inhibits NFAT dephosphorylation by calcineurin, although the precise mechanism of action differs from that of cyclosporine. Tacrolimus binds to the immunophilin FKBP12, forming an FKBP12–FK506 complex that inactivates calcineurin (74,75).
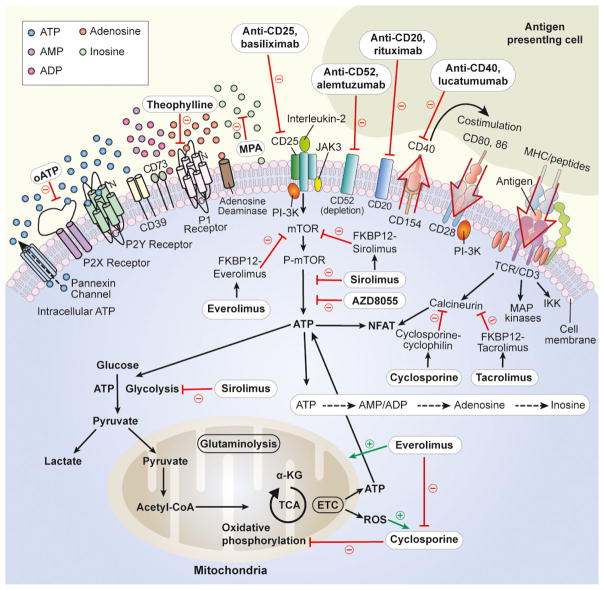
Figure 3. Immunosuppressive drugs and purinergic signaling.
The intracellular or extracellular site of action of immunosuppressive drugs is shown. Green arrows indicate a stimulatory effect. Red lines indicate an inhibitory effect. ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine triphosphate; CoA, coenzyme A; CD, cluster of differentiation; ETC, electron transport chain; FKBP, FK506 binding protein; IKK, I-kappa-B kinase; JAK, janus kinase; KG, ketoglutarate; MAP, mitogen-activated protein; MHC, major histocompatibility complex; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; NFAT, nuclear factor of activated T cells; oATP, oxidized adenosine triphosphate; P1/2, purinergic 1/2; P-, phospho-; PI-3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; TCA, tricarboxylic acid; TCR, T cell receptor.
ATP signaling through the P2Y2 receptor dose-dependently increased NFAT activity and intracellular Ca2+ in rat neonatal cardiac fibroblasts, with an associated decrease in angiotensin type 1 receptor. This effect of ATP was inhibited by the addition of cyclosporine, indicating that ATP-mediated NFAT induction is achieved through the calcineurin–NFAT pathway and thus is inhibited by CNIs (76). Inhibition of the P1A3 adenosine receptor with theophylline improved glomerular filtration rate and renal blood flow in an acute tacrolimus toxicity model in rats, suggesting a role for pathological levels of adenosine in nephropathies associated with CNI treatment (77).
Mycophenolic acid
Mycophenolate mofetil is an antiproliferative agent that is metabolized to MPA and inhibits the synthesis of guanine monophosphate, thereby blocking the proliferation of B and T lymphocytes. MPA achieves this effect by binding and inhibiting the enzyme inosine monophosphate dehydrogenase, the crucial enzyme of de novo purine synthesis (Figure 3) (78,79). Because of this mechanism of action, MPA has a direct inhibitory effect on the synthesis of purines and thus can modify the amount of eATP that is available for purinergic signaling. MPA treatment in a primary human T lymphocyte model resulted in up to 50% reduction in ATP concentration in comparison to control cells (80).
mTOR inhibitors
The mTOR inhibitors sirolimus and everolimus block the activity of mTOR, a serine/threonine protein kinase with multifarious physiological roles in regulating cell metabolism, growth, proliferation, angiogenesis and immune responses. Both sirolimus and everolimus are similar to tacrolimus in that they bind to the immunophilin FKBP12; however, rather than inhibiting calcineurin, the FKBP12–sirolimus or FKBP12–everolimus complex inhibits the activity of mTOR (Figure 3) (reviewed by Klintmalm and Nashan [81]).
The relative levels of ATP and adenosine in the extracellular space can directly influence the activation or inhibition of mTOR and the subsequent induction of either proinflammatory or anti-inflammatory responses, respectively (reviewed by Cobbold [82]). mTOR is known to be an important downstream effector protein in purinergic signaling, and mTOR phosphorylation is a required step in ATP-induced cell proliferation, which can be inhibited by rapamycin/sirolimus and other mTOR inhibitors (e.g. AZD8055) that compete with ATP at the mTOR-active site (83,84). Treatment of mice with rapamycin/sirolimus in combination with oATP, an irreversible P2X7 purinergic receptor inhibitor, resulted in long-term islet function in 80% of transplanted mice, suggesting a synergistic effect between mTOR inhibitors and purinergic inhibitors that might be beneficial following transplantation in humans (50).
When combined with the CNI cyclosporine, sirolimus treatment enhanced cyclosporine-induced inhibition of purinergic metabolism compared with cyclosporine alone in perfused rat brain slices after 4 h of perfusion, suggesting synergistic immunosuppressive activity between the CNI and the mTOR inhibitor (85); however, this synergistic effect was not observed following similar combination treatment with cyclosporine and everolimus. Increasing blood concentrations of sirolimus decreased relative Krebs cycle urine metabolite levels in rats, but increasing everolimus concentrations did not have the same effect either alone or in combination with cyclosporine (86).
Other immunosuppressants
The anti-CD25 monoclonal antibody basiliximab binds to the IL-2 receptor α chain (Figure 3), thereby inhibiting purinergic signaling and other pathways leading to IL-2–mediated proliferation of T cells and allograft rejection (reviewed by McKeage and McCormack [87]). The anti-CD40 monoclonal antibody lucatumumab acts by preventing the interaction between CD40 and CD40 ligand and thus inhibiting the presentation of antigen to T cells. Interestingly, low concentrations of eATP have been shown to interfere with the same target as lucatumumab, the CD40–CD40 ligand complex and subsequently inhibit the activation of T cells by dendritic cells (88). Other antibody-based drugs used as immunosuppressants in transplantation include antithymocyte globulin (polyclonal), alemtuzumab (anti-CD52), and rituximab (anti-CD20) (reviewed by van Sandwijk et al [89]). A recent review by Cekic and Linden gives a detailed overview of purinergic signaling in specific immune cells following tissue injury (90).
Relevance in Preclinical Research and for Clinical Practice
Ectonucleotidases (CD39/CD73)
As described in the preceding sections, CD39 and CD73 are both abundantly expressed on Tregs (91,92), which allows them to hydrolyze the proinflammatory danger signal ATP to the anti-inflammatory mediator adenosine. Evidence of this protective role of CD39 was observed in a skin allograft rejection model in which the adoptive transfer of Tregs from CD39-deficient mice failed to prevent skin allograft rejection compared with the transfer of WT Tregs (92).
This protective effect of CD39 appears to involve adenosine production because activation of the adenosine 2A receptor has been shown to enhance survival of skin allografts in mice (93). Several immune cells express CD73, such as subsets of B and T lymphocytes (94), Tregs (92) and MSCs (95) but also intestinal epithelial cells (96) and endothelial cells of capillaries and venules (97). CD73 generates nucleosides for the purine salvage pathway; neighboring cells can take up the nucleosides via facilitated diffusion and use the molecules to recover nucleic acid bases and subsequently synthesize new nucleotides to meet critical metabolic needs of the cell. In addition, CD73 generates adenosine, which activates the seven-transmembrane domain G protein–coupled P1 purinergic (adenosine) receptors.
The P1A1 and P1A3 receptors bind to a Gi protein and cause a reduced intracellular concentration of cyclic AMP (cAMP) by inhibiting adenylyl cyclase, whereas the P1A2a and P1A2b receptors bind to a Gs protein and increase the intracellular concentration of cAMP by stimulating adenylyl cyclase. In addition, P1A2b and P1A3 receptors can interact with a Gq protein and stimulate phospholipase C (reviewed by Hasko et al [98]). Signaling cascades of adenosine receptors, however, are much more complex because they have also been shown to modulate protein kinase C, phosphoinositide 3 kinase and mitogen-activated protein kinases (reviewed by Jacobson and Gao [99]). P1 receptors have individual affinities toward their substrate. Although the half maximal effective concentration (EC50) of the P1A1, P1A2a, and P1A3 receptors is 0.01–1.00 μM, activation of the P1A2b receptor requires adenosine levels >10 μM (EC50 24 μM). This means that the P1A2b receptor is not activated under physiological conditions but rather plays a role only when adenosine concentration is increased because of cellular stress, such as in the case of inflammation (98).
In the majority of clinically relevant models, adenosine provides an anti-inflammatory signal and counteracts the proinflammatory reactions induced by the presence of ATP in the extracellular space. CD73 and its product adenosine have also been implicated as regulatory mechanisms in rejection of cardiac and tracheal allografts. In a model of heterotopic murine cardiac transplantation, CD73 deficiency of either the donor or the recipient resulted in significantly reduced allograft survival (39). These observations are consistent with results from earlier studies that showed involvement of adenosine in the suppression of proinflammatory cytokine production (100,101). In addition, 60 days after transplantation, CD73-deficient allografts showed more severe luminal occlusion in the graft coronary arteries correlating to cardiac allograft vasculopathy as well as significantly higher levels of donor-reactive alloantibodies in the chronic rejection phase. These effects were, at least in part, mediated by the P1A2b receptor.
The role of CD73 and endogenous adenosine was investigated in a study of murine acute GvHD with an MHC major mismatch between donor and recipient (64). A CD73 deficiency in either donor or recipient mice resulted in significantly more severe GvHD, with reduced survival of the recipient, increased GvHD histopathology scores and increased concentrations of IL-6 and interferon γ in the serum of recipient mice compared with WT mice. Furthermore, deletion of CD73 resulted in increased proliferation of alloreactive CD4+ and CD8+ T cells. These data are consistent with previous reports showing that even low concentrations of extracellular adenosine and other P1 receptor agonists inhibit T cell activation and expansion via binding to the P1A2a receptor (102). Furthermore, endogenous adenosine binding to the P1A2a receptor limited the expansion of alloreactive T cells and dampened the severity of acute GvHD. These findings extend those from previous studies (103) that suggested activation of the P1A2a receptor via the selective agonist ATL146e improved the survival of GvHD mice without affecting donor cell engraftment. Moreover, the treatment of T cells with ATL146e reduced in vitro migration toward the chemokines CCL20, CXCL12 and CXCL10 by at least 30%, whereas in vivo administration of ATL146e decreased the serum levels of various proinflammatory cytokines. P1A2a receptor activation also improved the clinical condition of mice with already established GvHD by reversing weight loss (103).
Organ and graft heterogeneity
Transplant rejection due to organ and graft heterogeneity is a major obstacle in clinical practice, and strategies to improve tolerance include the suppression of T and B cell effector functions in addition to the activation of immunosuppressive Tregs (104). Lung, cardiac, renal and liver allografts (and xenografts) exhibit quite distinct patterns of vascular inflammation and thrombosis that may dictate differential outcomes following transplantation. In prior studies of pig-to-primate xenotransplantation, protective-type genes (heme oxygenase I, superoxide dismutases and CD39), together with thromboregulatory genes such as von Willebrand factor and P-selectin, were noted to be substantially upregulated in renal grafts that were associated with platelet consumption (105). In cardiac xenografts, fibrin deposition was quite marked and associated with increased plasminogen activator inhibitor 1 expression. The different outcomes observed in clinical practice might reflect overall differences in gene expression profiles, including those of purinergic signaling components, in renal and cardiac grafts (105,106).
Xenotransplantation
The use of xenotransplantation in clinical practice has the potential to overcome the shortfall of available human organs and tissues for patients that urgently require transplantation (107,108). In the United States alone, 18 potential transplant recipients die every day due to a lack of critical organs (reviewed by Michel et al [109]). The pig is often cited as the most suitable source of xenografts (reviewed by Ekser et al [110]). Research into the genetic manipulation of pigs to reduce xenograft rejection has focused largely on removing the Gal antigen from the surface of xenotransplant cells (109). Increasing the expression of human CD39 and CD73 is also considered a potential strategy to prevent xenograft rejection by increasing the hydrolysis of ATP to adenosine and promoting immunosuppression (107,111). In addition, CD39 is known to exert anticoagulant/antithrombotic properties that may further aid in preventing xenograft rejection.
The current model for the pathogenesis of acute GvHD suggests that the disease develops in three stages. The first phase in acute GvHD is triggered by the cytotoxic chemotherapy or irradiation therapy of the recipient. This treatment causes necrotic and apoptotic cell death, particularly in the intestinal tract, leading to intestinal barrier breakdown, with subsequent activation of a local immune response including macrophages and other antigen-presenting cells (APCs). Consequently, proinflammatory cytokines like TNF-α and IL-1β are released, and neutrophil granulocytes are recruited into the intestinal tract, a process driven by the translocation of pathogen-associated molecular patterns, such as bacterially derived lipopolysaccharide. In addition to these infectious stimuli, tissue destruction after the preconditioning treatment leads to release of specific noninfectious danger-associated molecular patterns, such as ATP (reviewed by Zeiser et al [112]). These signals activate the innate immune system of the host, especially the APCs, via interaction with purinergic, Toll-like or nucleotide oligomerization domain-like receptors.
In the second phase of GvHD, transplanted donor T cells interact with APCs, most importantly with dendritic cells (113). Local proinflammatory cytokines produced in the first GvHD phase serve as further stimuli for activation, differentiation and proliferation of the donor-derived T cells (reviewed by Zeiser et al [114]). In the third GvHD phase, after initial expansion the differentiated effector cells, mostly T cells but also NK cells, macrophages and neutrophils, migrate to the target tissues of GvHD (e.g. skin, gastrointestinal tract, and liver) and cause tissue destruction. ATP was recently shown to be released from necrotic cells after the preconditioning treatment prior to allo-HSCT, and that serves as a critical danger signal for the activation of the immune system (63). Results from in vivo and in vitro models have shown that CD73 and endogenous adenosine significantly reduce the severity of acute GvHD (64,102,103).
Conclusions
Purinergic signaling is closely associated with the regulation and direction of inflammation and local immune responses; therefore, targeting the purinergic signaling pathway by increasing ectonucleotidase activity and/or boosting short-term adenosine-mediated immunosuppression holds great potential for preventing allograft vascular injury, thereby ameliorating rejection and promoting tolerance. Existing immunosuppressive agents such as CNIs, MPA and mTOR inhibitors can interact with purinergic signaling pathways either directly or indirectly. These interactions provide great opportunities for the design of possible combination therapies with purinergic receptor agonists/antagonists to further manipulate and optimize immunosuppression in transplantation.
Based on studies in transplantation models, CD39 and other components of purinergic signaling pathways may be considered potentially crucial regulators of vascular inflammation, thrombosis and tolerance induction. Consequently, further studies to increase our understanding of purinergic signaling in transplantation are of vital importance to clinical practice.
Acknowledgments
Medical writing and editorial support in the development of this manuscript was provided by Rowan Higgs of Novartis Ireland Ltd. and Dhaval Gupta of Novartis Healthcare Pvt. Ltd.
Abbreviations
- 2-MeSADP
2-methylthio adenosine diphosphate
- 2-MeSATP
2-methylthio adenosine triphosphate
- 5-BDBD
5-(3-Bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one
- ADP
adenosine diphosphate
- allo-HSCT
allogeneic hematopoietic stem cell transplantation
- AMP
adenosine monophosphate
- Ap5(γB)
adenosine pentaphosphate γ-boranophosphate
- APC
antigen-presenting cell
- ATP
adenosine triphosphate
- BBG
brilliant blue green
- BzATP
2′- & 3′-O-(4-benzoyl-benzoyl) adenosine triphosphate
- cAMP
cyclic AMP
- CCPA
chlorocyclopentyl adenosine
- CNI
calcineurin inhibitor
- CNS
central nervous system
- CTP
cytosine triphosphate
- eATP
extracellular adenosine triphosphate
- EC50
half maximal effective concentration
- GvHD
graft-versus-host disease
- IP3
inosine triphosphate
- Ip5
Idi-inosine pentaphosphate
- IRI
ischemia–reperfusion injury
- iTreg
CD4+CD25highC-D127lowFoxP3+ regulatory T cell
- MPA
mycophenolic acid
- MSC
mesenchymal stromal/stem cell
- mTOR
mammalian target of rapamycin
- NECA
5′-N-ethylcarboxamido adenosine
- NFAT
nuclear factor of activated T cells
- NTS
nucleus tractus solitarius
- oATP
oxidized adenosine triphosphate
- PLC
phospholipase C
- RB2
reactive blue 2
- TNF-α
tumor necrosis factor α
- Treg
regulatory T cell
Footnotes
Search Strategy and Selection Criteria
Data for this review were identified by searches of PubMed and Medline and references from relevant articles, using the search terms purinergic, transplantation, and immunosuppression. Only full-length articles published in English were considered, with no date restriction. Additional articles were included at the discretion of the authors based on their experience in this field.
Disclosure
All authors contributed equally to manuscript conception, writing and review. R.Z., S.C.R., T.V., and G.B. have no conflicts of interest to disclose as described by the American Journal of Transplantation. T.V. acknowledges support from The Academy of Medical Sciences and the Wellcome Trust. M.D. is an employee of Novartis Pharma GmbH Germany.
References
- 1.Saidi RF, Hejazii Kenari SK. Clinical transplantation and tolerance: Are we there yet? Int J Organ Transplant Med. 2014;5:137–145. [PMC free article] [PubMed] [Google Scholar]
- 2.Kunzli BM, Bernlochner MI, Rath S, et al. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal. 2011;7:231–241. doi: 10.1007/s11302-011-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G, Novak I. Purinergic signalling and diabetes. Purinergic Signal. 2013;9:307–324. doi: 10.1007/s11302-013-9359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013;9:491–540. doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fumagalli M, Lecca D, Abbracchio MP. Role of purinergic signalling in neuro-immune cells and adult neural progenitors. Front Biosci (Landmark Ed) 2011;16:2326–2341. doi: 10.2741/3856. [DOI] [PubMed] [Google Scholar]
- 6.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 8.Vergani A, Tezza S, Fotino C, et al. The purinergic system in allotransplantation. Am J Transplant. 2014;14:507–514. doi: 10.1111/ajt.12567. [DOI] [PubMed] [Google Scholar]
- 9.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chernogorova P, Zeiser R. Ectonucleotidases in solid organ and allogeneic hematopoietic cell transplantation. J Biomed Biotechnol. 2012;2012:208204. doi: 10.1155/2012/208204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newell KA. Clinical transplantation tolerance. Semin Immunopathol. 2011;33:91–104. doi: 10.1007/s00281-011-0255-y. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. BioEssays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 15.Beldi G, Banz Y, Kroemer A, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales E, Julien B, Serriere-Lanneau V, et al. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol. 2010;52:54–62. doi: 10.1016/j.jhep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmelzle M, Duhme C, Junger W, et al. CD39 modulates hematopoietic stem cell recruitment and promotes liver regeneration in mice and humans after partial hepatectomy. Ann Surg. 2013;257:693–701. doi: 10.1097/SLA.0b013e31826c3ec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Han L, Seth P, et al. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology. 2013;57:205–216. doi: 10.1002/hep.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beldi G, Enjyoji K, Wu Y, et al. The role of purinergic signaling in the liver and in transplantation: Effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn BP, Robson SC, Longhi MS. Purinergic signaling in liver disease. Dig Dis. 2014;32:516–524. doi: 10.1159/000360498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnstock G, Vaughn B, Robson SC. Purinergic signalling in the liver in health and disease. Purinergic Signal. 2014;10:51–70. doi: 10.1007/s11302-013-9398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts V, Stagg J, Dwyer KM. The role of ectonucleotidases CD39 and CD73 and adenosine signaling in solid organ transplantation. Front Immunol. 2014;5:64. doi: 10.3389/fimmu.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pommey S, Lu B, McRae J, et al. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4 + T cells. Hepatology. 2013;57:1597–1606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 24.Vaughn BP, Robson SC, Burnstock G. Pathological roles of purinergic signaling in the liver. J Hepatol. 2012;57:916–920. doi: 10.1016/j.jhep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth JW, Tam FW, Unwin RJ. P2 purinoceptors: Renal pathophysiology and therapeutic potential. Clin Nephrol. 2012;78:154–163. doi: 10.5414/cn107325. [DOI] [PubMed] [Google Scholar]
- 26.Burnstock G, Evans LC, Bailey MA. Purinergic signalling in the kidney in health and disease. Purinergic Signal. 2014;10:71–101. doi: 10.1007/s11302-013-9400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts V, Lu B, Rajakumar S, Cowan PJ, Dwyer KM. The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal. 2013;9:135–143. doi: 10.1007/s11302-012-9342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crikis S, Lu B, Murray-Segal LM, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10:2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grenz A, Kim JH, Bauerle JD, Tak E, Eltzschig HK, Clambey ET. Adora2b adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF-alpha release. J Immunol. 2012;189:4566–4573. doi: 10.4049/jimmunol.1201651. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Grenz A, Osswald H, Eckle T, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solini A, Usuelli V, Fiorina P. The dark side of extracellular ATP in kidney diseases. J Am Soc Nephrol. 2015;26:1007–1016. doi: 10.1681/ASN.2014070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnstock G, Pelleg A. Cardiac purinergic signalling in health and disease. Purinergic Signal. 2015;11:1–46. doi: 10.1007/s11302-014-9436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 35.Urmaliya VB, Church JE, Coupar IM, Rose’Meyer RB, Pouton CW, White PJ. Cardioprotection induced by adenosine A1 receptor agonists in a cardiac cell ischemia model involves cooperative activation of adenosine A2A and A2B receptors by endogenous adenosine. J Cardiovasc Pharmacol. 2009;53:424–433. doi: 10.1097/FJC.0b013e3181a443e2. [DOI] [PubMed] [Google Scholar]
- 36.Lim SH, Lee S, Noda K, et al. Adenosine injection prior to cardioplegia enhances preservation of senescent hearts in rat heterotopic heart transplantation. Eur J Cardiothorac Surg. 2013;43:1202–1208. doi: 10.1093/ejcts/ezs509. [DOI] [PubMed] [Google Scholar]
- 37.Imai M, Takigami K, Guckelberger O, et al. Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplantation. 2000;70:864–870. doi: 10.1097/00007890-200009270-00003. [DOI] [PubMed] [Google Scholar]
- 38.Koyamada N, Miyatake T, Candinas D, et al. Apyrase administration prolongs discordant xenograft survival. Transplantation. 1996;62:1739–1743. doi: 10.1097/00007890-199612270-00008. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa T, Bouis D, Liao H, Visovatti SH, Pinsky DJ. Ecto-5′ nucleotidase (CD73)-mediated adenosine generation and signaling in murine cardiac allograft vasculopathy. Circ Res. 2008;103:1410–1421. doi: 10.1161/CIRCRESAHA.108.180059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vergani A, Tezza S, D’Addio F, et al. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation. 2013;127:463–475. doi: 10.1161/CIRCULATIONAHA.112.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barth K, Kasper M. Membrane compartments and purinergic signalling: Occurrence and function of P2X receptors in lung. FEBS J. 2009;276:341–353. doi: 10.1111/j.1742-4658.2008.06795.x. [DOI] [PubMed] [Google Scholar]
- 42.Burnstock G, Brouns I, Adriaensen D, Timmermans JP. Purinergic signaling in the airways. Pharmacol Rev. 2012;64:834–868. doi: 10.1124/pr.111.005389. [DOI] [PubMed] [Google Scholar]
- 43.Adriaensen D, Timmermans JP. Purinergic signalling in the lung: Important in asthma and COPD? Curr Opin Pharmacol. 2004;4:207–214. doi: 10.1016/j.coph.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Liu K, Vergani A, Zhao P, et al. Inhibition of the purinergic pathway prolongs mouse lung allograft survival. Am J Respir Cell Mol Biol. 2014;51:300–310. doi: 10.1165/rcmb.2013-0362OC. [DOI] [PubMed] [Google Scholar]
- 45.Reece TB, Ellman PI, Maxey TS, et al. Adenosine A2A receptor activation reduces inflammation and preserves pulmonary function in an in vivo model of lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1137–1143. doi: 10.1016/j.jtcvs.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, LaPar DJ, Steidle J, et al. Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development. J Heart Lung Transplant. 2010;29:1405–1414. doi: 10.1016/j.healun.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillaire-Buys D, Chapal J, Bertrand G, Petit P, Loubatieres-Mariani MM. Purinergic receptors on insulin-secreting cells. Fundam Clin Pharmacol. 1994;8:117–127. doi: 10.1111/j.1472-8206.1994.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 48.Loubatieres-Mariani MM, Chapal J. Purinergic receptors involved in the stimulation of insulin and glucagon secretion. Diabete Metab. 1988;14:119–126. [PubMed] [Google Scholar]
- 49.Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 50.Vergani A, Fotino C, D’Addio F, et al. Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes. 2013;62:1665–1675. doi: 10.2337/db12-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dwyer KM, Mysore TB, Crikis S, et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006;82:428–432. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 52.Nitta T, Itoh T, Matsuoka N, et al. Prevention of early loss of transplanted islets in the liver of mice by adenosine. Transplantation. 2009;88:49–56. doi: 10.1097/TP.0b013e3181aa6c9b. [DOI] [PubMed] [Google Scholar]
- 53.Chia JS, McRae JL, Thomas HE, et al. The protective effects of CD39 overexpression in multiple low-dose streptozotocin-induced diabetes in mice. Diabetes. 2013;62:2026–2035. doi: 10.2337/db12-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmermann H. Ectonucleotidases: Some recent developments and a note on nomenclature. Drug Dev Res. 2001;52:44–56. [Google Scholar]
- 55.Knowles AF. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signalling. 2011;7:21–45. doi: 10.1007/s11302-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit Rev Biochem Mol Biol. 2014;49:473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 57.Zimmermann H. Nucleotide signaling in nervous system development. Pflugers Arch. 2006;452:573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]
- 58.Goepfert C, Imai M, Brouard S, Csizmadia E, Kaczmarek E, Robson SC. CD39 modulates endothelial cell activation and apoptosis. Mol Med. 2000;6:591–603. [PMC free article] [PubMed] [Google Scholar]
- 59.Sevigny J, Sundberg C, Braun N, et al. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood. 2002;99:2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- 60.Shrivastava S, McVey JH, Dorling A. The interface between coagulation and immunity. Am J Transplant. 2007;7:499–506. doi: 10.1111/j.1600-6143.2006.01653.x. [DOI] [PubMed] [Google Scholar]
- 61.Dwyer KM, Robson SC, Nandurkar HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Center for International Blood and Marrow Transplant Research. 2014 [cited 20 Oct 2015]. Available from: http://www.cibmtr.org/
- 63.Wilhelm K, Ganesan J, Muller T, et al. Graft-versus-host disease enhanced by extracellular adenosine triphosphate activating P2X7R. Nat Med. 2010;12:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 64.Tsukamoto H, Chernogorova P, Ayata K, et al. Deficiency of CD73/ecto-5′-nucleotidase in mice enhances acute graft-versus-host disease. Blood. 2012;119:4554–4564. doi: 10.1182/blood-2011-09-375899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saldanha-Araujo F, Ferreira FI, Palma PV, et al. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7:66–74. doi: 10.1016/j.scr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Baron F, Lechanteur C, Willems E, et al. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2010;16:838–847. doi: 10.1016/j.bbmt.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Gregoire-Gauthier J, Selleri S, Fontaine F, et al. Therapeutic efficacy of cord blood-derived mesenchymal stromal cells for the prevention of acute graft-versus-host disease in a xeno-genic mouse model. Stem Cells Dev. 2012;21:1616–1626. doi: 10.1089/scd.2011.0413. [DOI] [PubMed] [Google Scholar]
- 68.Joo SY, Cho KA, Jung YJ, et al. Mesenchymal stromal cells inhibit graft-versus-host disease of mice in a dose-dependent manner. Cytotherapy. 2010;12:361–370. doi: 10.3109/14653240903502712. [DOI] [PubMed] [Google Scholar]
- 69.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 70.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 71.Jurewicz M, Yang S, Augello A, et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes. 2010;59:3139–3147. doi: 10.2337/db10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;72:1653–1655. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 73.Sivanathan KN, Rojas-Canales DM, Hope CM, et al. Interleukin-17A-induced human mesenchymal stem cells are superior modulators of immunological function. Stem Cells. 2015;33:2850–2863. doi: 10.1002/stem.2075. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 75.Knops N, Levtchenko E, van den Heuvel B, Kuypers D. From gut to kidney: Transporting and metabolizing calcineurin-inhibitors in solid organ transplantation. Int J Pharm. 2013;452(1–2):14–35. doi: 10.1016/j.ijpharm.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 76.Nishida M, Ogushi M, Suda R, et al. Heterologous down-regulation of angiotensin type 1 receptors by purinergic P2Y2 receptor stimulation through S-nitrosylation of NF-kappaB. Proc Natl Acad Sci U S A. 2011;108:6662–6667. doi: 10.1073/pnas.1017640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLaughlin GE, Alva MD, Egea M. Adenosine receptor antagonism in acute tacrolimus toxicity. Nephrol Dial Transplant. 2006;21:1961–1965. doi: 10.1093/ndt/gfl082. [DOI] [PubMed] [Google Scholar]
- 78.Kaltenborn A, Schrem H. Mycophenolate mofetil in liver transplantation: A review. Ann Transplant. 2013;18:685–696. doi: 10.12659/AOT.889299. [DOI] [PubMed] [Google Scholar]
- 79.Maripuri S, Kasiske BL. The role of mycophenolate mofetil in kidney transplantation revisited. Transplant Rev (Orlando) 2014;28:26–31. doi: 10.1016/j.trre.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Qiu Y, Fairbanks LD, Ruckermann K, et al. Mycophenolic acid-induced GTP depletion also affects ATP and pyrimidine synthesis in mitogen-stimulated primary human T-lymphocytes. Transplantation. 2000;69:890–897. doi: 10.1097/00007890-200003150-00038. [DOI] [PubMed] [Google Scholar]
- 81.Klintmalm GB, Nashan B. The role of mTOR inhibitors in liver transplantation: Reviewing the evidence. J Transplant. 2014;2014:845438. doi: 10.1155/2014/845438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cobbold SP. The mTOR pathway and integrating immune regulation. Immunology. 2013;140:391–398. doi: 10.1111/imm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerasimovskaya EV, Tucker DA, Weiser-Evans M, et al. Extra-cellular ATP-induced proliferation of adventitial fibroblasts requires phosphoinositide 3-kinase, Akt, mammalian target of rapamycin, and p70 S6 kinase signaling pathways. J Biol Chem. 2005;280:1838–1848. doi: 10.1074/jbc.M409466200. [DOI] [PubMed] [Google Scholar]
- 84.Rosborough BR, Raich-Regue D, Liu Q, Venkataramanan R, Turnquist HR, Thomson AW. Adenosine triphosphate-competitive mTOR inhibitors: A new class of immunosuppressive agents that inhibit allograft rejection. Am J Transplant. 2014;14:2173–2180. doi: 10.1111/ajt.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christians U, Gottschalk S, Miljus J, et al. Alterations in glucose metabolism by cyclosporine in rat brain slices link to oxidative stress: Interactions with mTOR inhibitors. Br J Pharmacol. 2004;143:388–396. doi: 10.1038/sj.bjp.0705939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bohra R, Schoning W, Klawitter J, et al. Everolimus and sirolimus in combination with cyclosporine have different effects on renal metabolism in the rat. PLoS ONE. 2012;7:e48063. doi: 10.1371/journal.pone.0048063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McKeage K, McCormack PL. Basiliximab: A review of its use as induction therapy in renal transplantation. BioDrugs. 2010;24:55–76. doi: 10.2165/11203990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 88.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 89.van Sandwijk MS, Bemelman FJ, Ten Berge IJ. Immunosuppressive drugs after solid organ transplantation. Neth J Med. 2013;71:281–289. [PubMed] [Google Scholar]
- 90.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 91.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3 + Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 92.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sevigny CP, Li L, Awad AS, et al. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol. 2007;178:4240–4249. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- 94.Thompson LF, Ruedi JM, Glass A, Low MG, Lucas AH. Antibodies to 5′-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J Immunol. 1989;143:1815–1821. [PubMed] [Google Scholar]
- 95.Sattler C, Steinsdoerfer M, Offers M, et al. Inhibition of T-cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39 expression and adenosine generation. Cell Transplant. 2011;20:1221–1230. doi: 10.3727/096368910X546553. [DOI] [PubMed] [Google Scholar]
- 96.Strohmeier GR, Lencer WI, Patapoff TW, et al. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thomson LF, Ruedi JM, Glass A, et al. Production and characterization of monoclonal antibodies to the glycosyl phosphatidylinositol-anchored lymphocyte differentiation antigen ecto-5′-nucleotidase (CD73) Tissue Antigens. 1990;35:9–19. doi: 10.1111/j.1399-0039.1990.tb01750.x. [DOI] [PubMed] [Google Scholar]
- 98.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discovery. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eigler A, Greten TF, Sinha B, Haslberger C, Sullivan GW, Endres S. Endogenous adenosine curtails lipopolysaccharide-stimulated tumour necrosis factor synthesis. Scand J Immunol. 1997;45:132–139. doi: 10.1046/j.1365-3083.1997.d01-377.x. [DOI] [PubMed] [Google Scholar]
- 101.Hasko G, Kuhel DG, Chen JF, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 102.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 103.Lappas CM, Liu PC, Linden J, Kang EM, Malech HL. Adenosine A2A receptor activation limits graft-versus-host disease after allogenic hematopoietic stem cell transplantation. J Leukoc Biol. 2010;87:345–354. doi: 10.1189/jlb.0609388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, et al. Fox-p3 + T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 105.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 107.Boksa M, Zeyland J, Slomski R, Lipinski D. Immune modulation in xenotransplantation. Arch Immunol Ther Exp (Warsz) 2015;63:181–192. doi: 10.1007/s00005-014-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 109.Michel SG, Madariaga ML, Villani V, Shanmugarajah K. Current progress in xenotransplantation and organ bioengineering. Int J Surg. 2014;13:239–244. doi: 10.1016/j.ijsu.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 110.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: The next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 111.Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2012;19:23–30. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeiser R, Penack O, Holler E, Idzko M. Danger signals activating innate immunity in graft-versus-host disease. J Mol Med (Berl) 2011;89:833–845. doi: 10.1007/s00109-011-0767-x. [DOI] [PubMed] [Google Scholar]
- 113.Shlomchik W, Couzens MS, Tang CB, et al. Prevention of graft-versus-host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 114.Zeiser R, Beilhack A, Negrin RS. Acute graft-versus-host disease-challenge for a broader application of allogeneic hematopoietic cell transplantation. Curr Stem Cell Res Ther. 2006;1:203–212. doi: 10.2174/157488806776956896. [DOI] [PubMed] [Google Scholar]
- 115.Burnstock G. Introduction: ATP and its metabolites as potent extracellular agonists. In: Schwiebert EM, editor. Current Topics in Membranes. San Diego: Academic Press; 2003. pp. 1–27. [Google Scholar]