Abstract
Rhizopus oryzae is the most common cause of zygomycosis, a life-threatening infection that usually occurs in patients with diabetic ketoacidosis. Despite standard therapy, the overall rate of mortality from zygomycosis remains >50%, and new strategies for treatment are urgently needed. The activities of caspofungin acetate (CAS) and other echinocandins (antifungal inhibitors of the synthesis of 1,3-β-d-glucan synthase [GS]) against the agents of zygomycosis have remained relatively unexplored, especially in animal models of infection. We found that R. oryzae has both an FKS gene, which in other fungi encodes a subunit of the GS synthesis complex, and CAS-susceptible, membrane-associated GS activity. Low-dose but not high-dose CAS improved the survival of mice with diabetic ketoacidosis infected with a small inoculum but not a large inoculum of R. oryzae. Fungal burden, assessed by a novel quantitative PCR assay, correlated with increasing inocula and progression of disease, particularly later in the infection, when CFU counts did not. CAS decreased the brain burden of R. oryzae when it was given prophylactically but not when therapy was started after infection. These results indicate that CAS has significant but limited activity against R. oryzae in vivo and demonstrates an inverse dose-response effect. The potential for CAS to play a role in combination therapy against zygomycosis merits further investigation.
Zygomycosis is an infection caused by fungi of the class Zygomycetes, order Mucorales. Members of this order cause progressive, necrotic, and generally fatal infections in immunocompromised hosts, such as diabetics with ketoacidosis, neutropenic patients, patients taking corticosteroids, and patients with elevated available serum iron levels (12, 22, 28, 35). Rhizopus oryzae is the organism that is the most frequently isolated from patients with zygomycosis (22, 31).
The standard therapy for invasive zygomycosis consists of reversal of the underlying predisposing factors, widespread surgical debridement, and aggressive antifungal medication (12, 22, 35). Amphotericin B deoxycholate (AMB) remains the only antifungal agent approved for the therapy of invasive zygomycosis (12, 22, 35). Unfortunately, despite disfiguring surgical debridement and aggressive therapy with AMB, the overall rate of mortality from zygomycosis remains >50% (35), and it approaches 100% in patients with disseminated disease (20). New strategies for the treatment of zygomycosis are urgently needed.
The echinocandin class of antifungal antibiotics targets the synthesis of 1,3-β-d-glucan synthase (GS), the activity of which is essential for the assembly of a functional cell wall in many fungi (4, 8). The enzyme is a multisubunit complex, which includes an integral membrane protein and a regulatory subunit, encoded by members of the FKS and RHO1 gene families, respectively. Echinocandins such as caspofungin acetate (CAS) have activity against important fungal pathogens, including Candida and Aspergillus spp. (1, 4, 8). In contrast, limited in vitro studies have reported that echinocandins have high MICs for zygomycetes, Fusarium spp., and Cryptococcus neoformans (26, 29). Subsequent studies found that when CAS is combined with other drugs, such as AMB or calcineurin inhibitors, CAS demonstrated substantial in vitro activity against Fusarium solani and C. neoformans (2, 7). These studies prompted us to further investigate the in vitro and in vivo activities of CAS against R. oryzae. We report that R. oryzae has an FKS gene and that CAS inhibits GS activity in crude R. oryzae membrane preparations. Additionally, CAS demonstrates efficacy in vivo during disseminated R. oryzae infection in diabetic ketoacidotic mice.
(This work was presented in part at the 42nd and 43rd Interscience Conferences on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002, and Chicago, Ill., 14 to 17 September 2003, respectively.)
MATERIALS AND METHODS
Organism.
The R. oryzae 99-880 isolate and conditions for growth were described previously (21). Spores were counted and adjusted to the desired concentration in endotoxin-free phosphate-buffered saline (PBS). The viability of the spores was >95%, as determined by CFU quantitation. Susceptibility testing was performed in RPMI 1640 buffered with morpholinepropanesulfonic acid (0.165 M; pH 7.0), and the result was read after 24 h at 35°C. The minimum effective concentration of CAS was determined in this medium with the endpoint described previously (27).
Genomic DNA isolation, PCR, and Southern hybridization.
Approximately 105 R. oryzae spores were inoculated into 5 ml of GYEP medium (5% glucose, 0.1% yeast extract, 0.1% peptone) and incubated overnight at 37°C, and the genomic DNA was purified from the mycelium, as described previously (37).
A pileup of amino acid sequences predicted from the FKS genes of Aspergillus fumigatus, Candida albicans, C. neoformans, and Saccharomyces cerevisiae was performed; and degenerate primers homologous to conserved regions in the pileup were designed. The primer sequences are as follows: sense primer, 5′-AAYCAIGAYAAITAIYTIGA-3′; antisense primer, 5′-TTICCRCAITGITAITAYTC-3′ (where I is inosine, Y is C or T, and R is A or G). PCR products were cloned with the pCR2.1 TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.) and sequenced with a Big Dye Terminator (version 3.0) cycle sequencing kit (Applied Biosystems, Foster City, Calif.). Sequencing reactions were run on an ABI PRISM 3100 genetic analyzer (Applied Biosystems), according to the instructions of the manufacturer.
For Southern blot analysis, approximately 15 μg of R. oryzae genomic DNA was digested with restriction enzymes, subjected to gel electrophoresis, and transferred to a Nytran membrane (Schleicher & Schuell, Keene, N.H.). The R. oryzae PCR product was radiolabeled with random primers (Invitrogen); hybridization and washing of the blot were performed at high stringency, and the signals were visualized by autoradiography.
Characterization of R. oryzae GS activity.
Crude R. oryzae membranes containing GS activity were isolated from mycelia grown in liquid culture. YME medium (0.4% yeast extract, 1.0% malt extract, 0.4% dextrose) was inoculated with 106 R. oryzae 99-880 spores per ml of culture, and the spores were incubated for 18 h at 37°C with shaking at 220 rpm. The mycelia were harvested by filtration over a Miracloth and rinsed several times with water. Excess water was squeezed from the fungal mass, and the wet weight was determined. The mycelia were resuspended in 3 ml of extraction buffer (50 mM HEPES [pH 7.5], 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, complete protease inhibitor [Roche, Indianapolis, Ind.]) per g (wet weight) and disrupted by passage (two times) through a prechilled French press at 12,000 lb/in2. Unbroken cells were removed by centrifugation at 2,500 × g for 10 min, and the crude membranes were collected by centrifugation at 100,000 × g for 1 h. The membrane pellet was resuspended in storage buffer (50 mM HEPES [pH 7.5], 1 mM EDTA, 1 mM dithiothreitol, and 20% glycerol containing protease inhibitors) by using a Dounce homogenizer, and the suspension was stored at −80°C. The membrane protein concentration was determined from a sample solubilized in detergent (0.03% sodium deoxycholate) and precipitated with trichloroacetic acid (TCA) (11) by using a Micro-BCA protein assay kit (Pierce, Rockford, Ill.).
GS activity was measured as described previously (11). Each 100-μl reaction mixture contained 30 μg of crude membranes, 50 mM HEPES (pH 7.5), 10% glycerol, 3.5 mg of bovine serum albumin per ml, 25 mM KF, 1 mM EDTA, 25 μM guanosine 5′-[gamma-thio]triphosphate (GTPγS), 0.5 mM UDP-glucose, and 0.3 μCi of [3H]UDP-glucose (34 Ci/mmol; Amersham, Piscataway, N.J.). The reaction mixtures were incubated at 30°C for 4 h with gentle rocking, the reactions were stopped by the addition of 100 μl of ice-cold 20% TCA, and the product was collected on glass-fiber filters (Whatman; Fisher Scientific) and washed three times with water. The radiolabeled product was measured by liquid scintillation counting. For product characterization, the reactions were terminated by boiling for 2 min, and solubility in either 0.1 M acetic acid or 0.1 M NaOH or susceptibility to digestion with 1,3-β-d-glucanase (0.25 U of Zymolyase T-100; Seikagaku America, E. Falmouth, Mass.) was measured as described previously (11, 27). The 50% inhibitory concentration (IC50) of CAS for GS was determined from a drug titration (100 to 0.002 μg of CAS per ml from a 10-mg/ml stock solution prepared in water) with Prism software (version 3.0; GraphPad, San Diego, Calif.).
Animal model.
Male BALB/c mice (weight, ≥24 g; Harlan, San Diego, Calif.) were housed in groups of five mice each and were given irradiated feed and sterile water ad libitum. Animal studies were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Mice were rendered diabetic by administering streptozotocin (210 mg/kg of body weight), as described previously (21). This dose of streptozotocin caused diabetes in 80 to 90% of the injected mice (data not shown). Glycosuria and ketonuria were determined with keto-Diastix reagent strips (Bayer, Elkhart, Ind.) 7 days after streptozotocin treatment. Only mice that developed diabetes with mild ketoacidosis (level of ketonuria, ≤5 mg/dl) were included in the study (39). A suspension of 0.2 ml of PBS containing R. oryzae spores in endotoxin-free PBS was injected into the lateral tail vein. To confirm the inoculum, dilutions were streaked on potato dextrose agar (PDA) plates, and the colonies were counted following 24 h of incubation at room temperature. After infection, the mice were randomly sorted into different treatment groups.
Drugs and therapy.
AMB (Fungizone; Bristol-Myers Squibb) was given in 5% glucose at a dose of 1 mg/kg/day. CAS powder (Merck Research Laboratories) was dissolved daily in sterile water and was given at a total dose of 1, 5, or 10 mg/kg/day.
Drugs were administered intravenously via the lateral tail vein. Because intravenous injection of diabetic mice with a 1-mg/kg dose of AMB proved to be toxic (21), we chose to administer both drugs twice daily (b.i.d.) at the same total dose (e.g., AMB and CAS at 1 mg/kg/day were given as 0.5 mg/kg b.i.d.). This dosing regimen eliminated the infusion toxicity associated with AMB and showed no toxicity of CAS (21). Therapy was initiated either 24 h after infection and continued for 4 days (delayed therapy) or 24 h prior to infection with a repeat dose given immediately prior to infection (prophylaxis). In both therapy regimens, infected untreated control mice received vehicle alone. The primary end point for efficacy was the time of survival, and tissue fungal burden was assessed as a secondary end point.
Quantitation of tissue fungal burden in target organs and histopathological examination.
At selected times after infection, mice were euthanized by pentobarbital administration (300 mg/kg intraperitoneally) and the organs were removed and weighed. To directly compare the CFU counts with the values obtained by quantitative PCR (qPCR), the kidneys were cut in half and the fungal burden was quantified by either CFU or qPCR analysis. For all other tissue fungal burden studies, the organs were evaluated solely by qPCR. Additionally, for histopathological analysis, the kidneys and brains were collected from infected mice at 48 h postinfection and fixed in 10% zinc formalin. Fixed tissues were embedded in paraffin, and 5-μm sections were stained with periodic acid-Schiff for light microscopy examination.
To measure the CFU in infected tissues, the harvested organs were homogenized with a Pro 250 handheld tissue homogenizer (Pro Scientific, Inc., Monroe, Conn.) on setting 5. Homogenized tissues were serially diluted in 0.85% saline, and homogenates were spread on PDA plates containing 50 μg of chloramphenicol per ml. The colonies present after 24 h at room temperature were counted, and values were expressed as log10 CFU per gram of tissue. For qPCR analysis, the samples were homogenized as described previously (4), except that the tissue and hyphae were mechanically disrupted by vigorous agitation in a Mixer Mill 300 instrument (QIAGEN, Valencia, Calif.) at 1,800 oscillations per min (three times for 30 s each time), with 1-min pauses between bursts. DNA, which was extracted as described previously (4), was stored at −20°C. Oligonucleotide amplification primers and a dual-labeled fluorogenic oligonucleotide hybridization probe complementary to the R. oryzae 18S rRNA gene (GenBank accession no. AF113440) were designed with Primer Express software (version 1.5; Applied Biosystems) and synthesized by Applied Biosystems or Integrated DNA Technologies (Coralville, Iowa). The sequences of these oligonucleotides are as follows: (i) sense amplification primer, 5′-GCGGATCGCATGGCC-3′; (ii) antisense amplification primer, 5′-CCATGATAGGGCAGAAAATCG-3′; and (iii) hybridization probe, 5′-FAM-TGTGCCGGCGACGGTCCAC-TAMRA-3′ (where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). The qPCRs were performed as described previously (4).
Differences in DNA recovery between samples were normalized by quantifying a nonmurine, nonfungal DNA sequence on a plasmid which was added to the saline used to homogenize all samples. The plasmid bears a 3-kb fragment containing the protein-coding region of Eimeria tenella PKG cDNA (GenBank accession no. AF411961). After homogenization and DNA isolation, the samples were analyzed by qPCR with the following primers and probe specific for the PKG gene sequence: (i) sense amplification primer, 5′-AGGGCTTTGCTGCACGAC-3′; (ii) antisense amplification primer, 5′-TCCACCTCGGGACTGTTTG-3′; and (iii) hybridization probe, 5′-FAM-TGCTACTGTTGCAGACCGCCGCT-TAMRA-3′.
qPCR quantitation of the PKG target sequence allows an estimate of the recovery of DNA from crude homogenate through the final DNA sample to be made. The percent recovery of PKG DNA is calculated for each experimental sample and is used to normalize each qPCR datum point for the R. oryzae 18S rRNA gene target.
Statistical analysis.
Survival data were analyzed by the nonparametric log-rank test. Differences in tissue fungal burdens in the infected organs were compared by the nonparametric Steel test for multiple comparisons. Correlations were calculated by the nonparametric Spearman rank sum test. P values of <0.05 were considered significant.
RESULTS
R. oryzae FKS gene.
In all fungi examined to date, the presence of an FKS gene has been predictive of GS activity, and there is a high degree of homology between FKS genes from diverse fungal genera (9). We used degenerate primers homologous to conserved residues in a number of known FKS sequences in an effort to identify an FKS homolog in R. oryzae. A fragment similar in size to the equivalent region of A. fumigatus FKS (∼400 bp) was amplified by PCR, cloned, and sequenced. The predicted amino acid sequence revealed a portion of a unique Fks protein with a high degree of conservation (64%) to other members of the Fksp family (Fig. 1). The R. oryzae FKS fragment was used as a probe for total R. oryzae genomic DNA on a Southern blot, and only a single FKS homolog was detected under conditions of high stringency (data not shown).
FIG. 1.
Amino acid sequences derived from the FKS genes of S. cerevisiae (FKS1, GenBank accession no. U12893; FKS2, GenBank accession no. U16783), C. albicans (GenBank accession no. D88815), C. neoformans (GenBank accession no. AF102882), and A. fumigatus (GenBank accession no. U79728) were aligned with the putative R. oryzae Fksp amino acid sequence. Gray shading represents amino acids that share identity among all Fks proteins shown. Asterisks denote positions whose sequences could not be determined due to the incorporation of inosine in the degenerate primers used to clone the R. oryzae FKS fragment.
R. oryzae membranes contain CAS-susceptible GS activity.
Fungal GS is an enzyme complex associated with the plasma membrane. To look for GS activity in R. oryzae, crude membranes prepared from growing mycelium were incubated with the GS substrate UDP-glucose and evaluated for the synthesis of a TCA-insoluble product. The activity in crude membranes was both time and protein dependent. As expected for authentic 1,3-β-d-glucan, the radiolabeled product of the R. oryzae GS reactions was 100% soluble in dilute base, partially soluble (30.5%) in dilute acid, and susceptible to digestion with exo-1,3-β-d-glucanase (data not shown). The GS activity in crude R. oryzae membranes was inhibited by CAS in a dose-dependent manner, with an estimated IC50 of 11.9 μg/ml.
In vitro inhibition of R. oryzae growth by CAS and AMB.
The susceptibility of R. oryzae strain 99-880 to CAS and AMB was measured in a liquid broth microdilution assay. By using the conditions defined in the NCCLS M38A document (28a), the MIC and the minimum effective concentration of CAS were >16 μg/ml for this isolate, and the AMB MIC was 0.25 μg/ml for this isolate.
CAS improves the survival of diabetic mice infected with a small inoculum of R. oryzae.
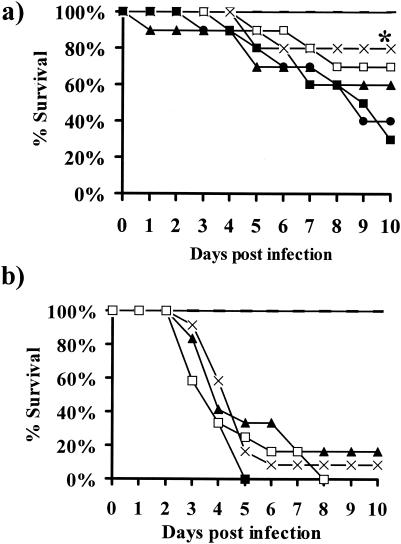
The inhibition of R. oryzae GS by CAS and the discovery of an FKS homolog demonstrate that the drug target is present in this organism. CAS might be effective against R. oryzae in vivo, despite the high MIC, especially given the known constraints of MIC testing with molds (13, 29). The in vivo efficacy of CAS was tested in diabetic ketoacidotic mice infected with R. oryzae. Intravenous treatment with AMB (0.5 mg/kg b.i.d.) or CAS (0.5, 2.5, or 5 mg/kg b.i.d.) was initiated 24 h after the mice were infected with 5 × 102 or 5 × 103 spores of R. oryzae. At 0.5 mg/kg b.i.d., CAS, but not AMB, improved the survival of mice infected with 5 × 102 spores of R. oryzae compared to that of the infected untreated mice (P = 0.049) (Fig. 2a). Eighty percent of the diabetic mice treated with CAS at 0.5 mg/kg/day were alive 10 days after infection, whereas 30% of the infected untreated mice were alive at that time. Surprisingly, higher doses of CAS (2.5 or 5 mg/kg b.i.d.) did not improve the rate of survival. Neither CAS nor AMB protected mice from the larger inoculum (5 × 103 spores) of R. oryzae (Fig. 2b).
FIG. 2.
Survival of diabetic mice infected with 5 × 102 (a) or 5 × 103 (b) spores of R. oryzae and treated with different regimens of AMB, CAS, or vehicle. Treatment was initiated at 24 h postinfection and was given twice daily for a total of 4 days. (a) n = 10 mice per group; (b) n = 12 mice per group. *, P < 0.05 for CAS (0.5 mg/kg b.i.d.) versus the results obtained with placebo; solid line, uninfected and untreated; ▪, infected and untreated; ▴, AMB at 0.5 mg/kg b.i.d.; ×, CAS at 0.5 mg/kg b.i.d.; □, CAS at 2.5 mg/kg b.i.d.; •, CAS at 5.0 mg/kg b.i.d.
Tissue fungal burden determined by qPCR but not CFU correlates with disease progression in diabetic mice infected with R. oryzae.
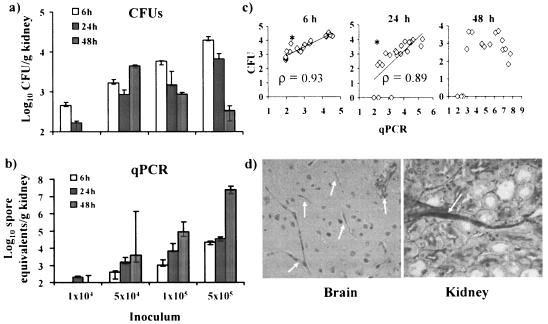
Because of the nature of hyphal growth of filamentous fungi, CFU determination may not accurately measure the fungal biomass in infected tissues (3). We therefore sought to develop a qPCR-based assay, previously used to measure the extent of A. fumigatus infection in mice (4), to quantitate the R. oryzae burden in murine tissues. We initially quantified the fungal burden in the kidney because the gross appearance of the kidneys in preliminary necropsies was more abnormal than that of the other organs (data not shown). At 6 h postinfection, there were strong correlations between the inoculum and the fungal burden measured by either CFU determination or qPCR (P < 0.0001 for both end points; Spearman rank sum correlation value [ρ] = 0.97 for CFU and 0.95 for qPCR) (Fig. 3). The results from the CFU and qPCR analyses strongly correlated with each other at this time point (P < 0.0001; ρ = 0.93) (Fig. 3c), consistent with the expectation that R. oryzae is predominantly present as spores and small germlings rather than long hyphae at 6 h postinfection. By 24 h after infection, the results of both CFU and qPCR analyses still correlated with the inocula and with each other, but to a slightly decreased degree (P < 0.0001 and ρ = 0.91 for both CFU and qPCR; P = 0.0001 and ρ = 0.89 for CFU versus qPCR). However, when the mycelial burden was measured at 48 h, by which time filamentation is expected to have occurred in vivo, there was no correlation between CFU values and the inocula (P = 0.2) or between the CFU and qPCR values (P = 0.1; ρ = 0.39). In contrast, the results of qPCR analysis still correlated strongly with the burden in tissue from the increasing inocula (P = 0.0001, ρ = 0.9) (Fig. 3). Infection in the kidneys progressed between 6 and 48 h postinfection at the three largest inocula (≥5 × 104 CFU/ml), but only when qPCR values (Fig. 3b) and not CFU values (Fig. 3a) were considered. Histopathology of the brains and kidneys of mice infected with 5 × 105 spores of R. oryzae confirmed the presence of active infection at 48 h, in line with the qPCR results (Fig. 3d).
FIG. 3.
qPCR measurement better reflects progression of R. oryzae infection in kidneys than CFU quantitation. Log10 CFU (a) and log10 spore equivalents (b) per gram of kidney tissue as functions of the inoculum at various time points after infection are shown. The limit of detection for the qPCR assay is 1.99 log10 spore equivalents per gram of kidney tissue. Data are displayed as medians ± interquartile ranges. (c) Log10 CFU (y axis) versus log10 spore equivalents (x axis) per gram of kidney tissue at various time points (n = 5 mice per group). ρ was determined by the Spearman rank sum test. *, P < 0.05. (d) Periodic acid-Schiff staining of brain and kidney tissues from diabetic mice infected for 48 h with 5 × 105 spores of R. oryzae. Arrows indicate R. oryzae hyphae in tissue. Magnifications, ×400.
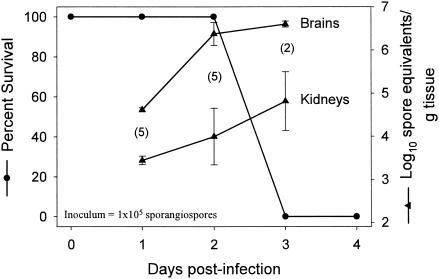
We assessed the tissue distribution and progression of R. oryzae disease in this model using qPCR. Mice infected with 5 × 104 spores of R. oryzae were euthanized 72 h postinfection; and the brains, kidneys, spleens, livers, and lungs were collected and evaluated for their fungal burdens. The heaviest fungal load in diabetic mice infected with R. oryzae 99-880 was in the brain; and secondary target organs included the kidney, spleen, liver, and lung (Table 1). In another study, animals infected with 105 spores were either monitored for survival or assessed for the fungal burden in their brains and kidneys by qPCR. The infections in both organs progressed between days 1 and 3 postinfection, and all deaths in the survival group occurred on day 3 postinfection (Fig. 4). The infections in the brains were consistently more severe than those in the kidneys, and an apparent peak of brain burden coincident with the onset of mortality was observed.
TABLE 1.
Distribution of R. oryzae burden in infected untreated diabetic mice as determined by qPCRa
| Tissue | Log10 spore equivalents/g of tissue |
|---|---|
| Brain | 6.60 ± 0.07 |
| Kidney | 4.81 ± 0.68 |
| Spleen | 3.84 ± 0.03 |
| Liver | 3.77 ± 0.34 |
| Lung | 3.30 ± 0.49 |
Mice (n = 5) were infected with 5 × 104 spores of R. oryzae and were euthanized at 72 h postinfection. Values are means ± standard errors.
FIG. 4.
Correlation between onset of mortality and increased fungal burden, as determined by qPCR. Diabetic mice were infected with 105 R. oryzae spores, and parallel groups of mice were either monitored for survival (n = 8) or euthanized for measurement of kidney and brain tissue fungal burdens. Tissue fungal burden values are presented as means ± standard errors of log10 spore equivalents per gram of tissue. Values in parentheses represent the numbers of mice used to determine the tissue fungal burdens.
Prophylactic treatment but not delayed therapy with CAS reduces the fungal burden.
Because CAS showed promising activity in protecting mice from R. oryzae infection, we investigated the effect of drug treatment on fungal burden. On the basis of our earlier results (Table 1), the brains and kidneys were chosen for assessment of the tissue burden by qPCR.
Mice were infected with 5 × 103 spores of R. oryzae, and treatment with AMB or CAS at 0.5 mg/kg b.i.d. was initiated for 2 days either 24 h prior to infection (prophylactic treatment) or 24 h after infection (delayed treatment). Compared to the outcome for untreated controls, prophylactic treatment with AMB or CAS significantly reduced the brain fungal burden by >1.7 log10 spore equivalents at 24 h following administration of the last dose of therapy (P ≤ 0.005) (Table 2). The fungal burdens in kidneys from the same animals trended to lower levels with AMB or CAS therapy (P = 0.15 and 0.1, respectively). The tissue fungal burdens of mice treated prophylactically were no longer different from those of the untreated controls by 48 h after administration of the last dose of CAS (Table 2). In contrast to prophylactic treatment, delayed therapy with AMB and CAS did not significantly reduce the tissue fungal burden in the kidneys or brain (data not shown).
TABLE 2.
Effect of prophylaxis with CAS or AMB on R. oryzae burden
| Treatment (dose [mg/kg b.i.d.]) | Log10 spore equivalents/g of tissue at the following times after last dosea:
|
|||
|---|---|---|---|---|
| 24 h
|
48 h
|
|||
| Kidney | Brain | Kidney | Brain | |
| Infected untreated | 4.33 ± 0.13 | 4.74 ± 0.11 | 3.81 ± 0.36 | 6.07 ± 0.39 |
| AMB (0.5) | 3.40 ± 0.42 | 2.26 ± 0.14b | 2.61 ± 0.37 | 5.72 ± 0.52 |
| CAS (0.5) | 3.56 ± 0.31 | 2.96 ± 0.23b | 3.67 ± 0.58 | 6.43 ± 0.32 |
Values are means ± standard errors (n = 7).
P < 0.05 compared to the results for the control.
DISCUSSION
There have been no prospective randomized trials to define the optimal antifungal therapy for zygomycosis, and AMB remains the only antifungal agent approved for the treatment of this infection (22, 35). However, given the unacceptably high rate of mortality from zygomycosis (>50%), clinicians are in dire need of novel therapeutic strategies for this disease. In the past decade, new therapies that may be useful for the treatment of zygomycosis have become available. For example, high-dose lipid formulations of AMB have been demonstrated to be efficacious in both a murine model of R. oryzae infection (21) and individual patients (15, 19). Studies have also suggested that the investigational triazole posaconazole may be effective (5, 6, 36). The potential activities of echinocandins against zygomycosis have remained relatively unexplored, due in part to their unimpressive in vitro activities against zygomycetes.
In this study, we demonstrated that the most common causative organism of zygomycosis, R. oryzae, possesses a genetic homolog of FKS, the molecular target of echinocandin and a subunit of GS. CAS was shown to inhibit R. oryzae GS activity. Given the presence of the target enzyme in R. oyrzae and the known difficulties of interpreting echinocandin MICs for molds (14, 29), we tested the efficacy of CAS at 0.5, 2.5, and 5 mg/kg b.i.d. for the treatment of zygomycosis in vivo. These doses were chosen on the basis of the findings of previous studies of Candida, Aspergillus, and Histoplasma infections (1, 4, 16, 17, 23, 24). The 0.5-mg/kg b.i.d. dose approximates the normal dose in humans (a 70-mg load, followed by 50 mg/day) (17). As well, the levels achievable in serum with the 2.5-mg/kg b.i.d. dose are approximately 20 to 30 μg/ml in mice (32) and therefore are in excess of the IC50 for the R. oryzae GS enzyme. Finally, because R. oryzae is notoriously resistant to antifungal therapy, we also tested a dose higher than that evaluated previously (5 mg/kg b.i.d.), and the dose was found to be nontoxic in our preliminary experiments (data not shown). We found that one CAS dosing regimen (0.5 mg/kg b.i.d.) improved the rate of survival of diabetic ketoacidotic mice infected with a small inoculum (5 × 102 spores) of R. oryzae. There was a surprising inverse dose-response relationship for CAS in this study, with higher doses failing to improve survival. The reason for this inverse dose-response relationship is unclear, but it may be a phenomenon similar to the Eagle effect seen with β-lactams and other antibiotics, in which extremely high concentrations of drug are less microbicidal than lower concentrations (25, 34). There have been other reports of an inverse dose-response relationship for the antifungal activities for echinocandins. For example, more than 15 years ago, cilofungin demonstrated worse in vitro activity against Candida spp. at higher concentrations (18). More recently, a similar in vitro phenomenon was described for CAS against C. albicans in the context of biofilm formation (30). Finally, Stevens et al. (33) have confirmed the phenomenon of a paradoxical increased growth of C. albicans at higher in vitro concentrations of CAS. The in vivo significance of these in vitro data is underscored by the findings from a murine model of CAS treatment for pulmonary aspergillosis (40) that are similar to our results with R. oryzae.
As an additional marker of efficacy, we evaluated the impact of CAS therapy on the tissue burden of R. oryzae. During infection, R. oryzae propagates in the filamentous form through apical extension (38). The relationship between CFU and fungal mass is not well defined for most molds, which grow as multicellular mycelia in the organs of infected hosts (4, 38). We found that the R. oryzae CFU values in the kidneys of infected mice did not increase with time, and there was no correlation between the initial inoculum and the fungal load at a time when hyphal growth is prevalent in tissues (48 h postinfection). To circumvent this problem, we developed a qPCR-based assay to quantify R. oryzae in the tissues of infected mice. Previous work with A. fumigatus suggested that qPCR could be used to measure the progression of infection and the efficacy of antifungal therapy in animal models of disease (4). In contrast to CFU measurement, the fungal burden assessed by qPCR correlated with the increasing inoculum at all times tested and also temporally paralleled the rate of mortality. Our results indicate that qPCR values more accurately reflect the R. oryzae burden in tissue than CFU values beyond 24 h of infection in mice. Histopathology confirmed the presence of extensive hyphae in the kidneys and brains of mice infected with the largest inocula (5 × 105) at 48 h (Fig. 3d), concordant with the qPCR results and discordant with the CFU results. Therefore, the histopathology findings support the conclusion that qPCR is more accurate for quantitation of the burden of filamentous fungi in tissue beyond 24 h of infection. Of note, although the qPCR results accurately reflected the tissue fungal burden, only a small amount of tissue is actually assessed for fungal burden in the qPCR assay (<1 mg equivalent). As a result, the inoculum required to detect a qPCR signal (1 × 104) was 20-fold greater than the smallest inoculum required to cause death (5 × 102).
Prophylaxis with CAS decreased the burden of R. oryzae in the kidneys and brains when it was measured at 24 h posttreatment. However, this therapeutic effect was lost by 72 h posttreatment, at which point the burdens were equivalent in treated and untreated mice. The tissue fungal burden was assessed with inocula larger than those used to assess survival, due to the lower limit of detection of the qPCR assay, which required an inoculum of at least 104 spores to permit detection of the fungal burden. Hence, direct comparisons of the effects of CAS on survival and tissue fungal burden as assessed by qPCR could not be performed in this study.
One possible explanation for the limited activity of CAS is the requirement for a relatively high concentration of drug to inhibit R. oryzae GS. Echinocandins inhibit crude GS from susceptible fungi (A. fumigatus and C. albicans) with IC50s in the nanogram-per-milliliter range (10, 27), which is roughly 1,000-fold lower than the concentration of CAS required to inhibit R. oryzae GS synthesis by 50% (∼12 μg/ml). However, there was no trend toward superior efficacy with increasing CAS dose in our models of infection, suggesting that incremental increases in GS inhibition might not lead to greater susceptibility in vivo. The role of GS in R. oryzae cell wall metabolism is poorly understood. The fungus may form a functional wall in the absence of 1,3-β-d-glucan (i.e., GS may not be essential in R. oryzae), or synthesis may be important during one phase of apical growth and dispensable during another. A more thorough characterization of R. oryzae GS, and cell wall synthesis in general, is warranted.
In summary, we demonstrated that R. oryzae possesses an FKS homolog and contains membrane-associated GS activity which can be inhibited by CAS, albeit at relatively high concentrations. Despite unimpressive MICs, therapy with CAS at 0.5 mg/kg b.i.d. resulted in significant improvement in survival during murine zygomycosis caused by a small inoculum of R. oryzae. CAS given prophylactically reduced the tissue fungal burden early in the infection, but the protective benefit of CAS was lost with a larger inoculum or at later time points. Finally, assessment of the organ fungal burden by a qPCR assay was superior to CFU measurement for monitoring the progression of R. oryzae infection. Although these results do not support a role for CAS as monotherapy against R. oryzae, its potential for use in combination therapy with a polyene or azole merits investigation.
Acknowledgments
This study was supported by a research and educational grant from Merck & Company and a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund to A.S.I. A.S.I. is also supported by grant RO3 AI054531 from the National Institute of Allergy and Infectious Diseases. B.S. is supported by Public Health Service grant KO8 AI060641-01.
We thank Jennifer Nielsen Kahn and Ming-Jo Hsu for help with the GS assays and Jennifer Anderson for design of the PKG-specific primers and probe.
The research described in this report was conducted in part at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
REFERENCES
- 1.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman, J., A. Ibrahim, J. Anderson, P. Liberator, V. Avanessian, J. J. Edwards, and C. Douglas. 2002. Quantitation of mycelial fungal pathogens by PCR, abstr. M-910. Abstr. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C.
- 4.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannaoui, E., J. F. Meis, D. Loebenberg, and P. E. Verweij. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dannaoui, E., J. Meletiadis, J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 51:45-52. [DOI] [PubMed] [Google Scholar]
- 7.Del Poeta, M., M. C. Cruz, M. E. Cardenas, J. R. Perfect, and J. Heitman. 2000. Synergistic antifungal activities of bafilomycin A(1), fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning, D. W. 1997. Echinocandins and pneumocandins—a new antifungal class with a novel mode of action. J. Antimicrob. Chemother. 40:611-614. [DOI] [PubMed] [Google Scholar]
- 9.Douglas, C. M. 2001. Fungal beta(1,3)-d-glucan synthesis. Med. Mycol. 39(Suppl. 1):55-66. [DOI] [PubMed] [Google Scholar]
- 10.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas, C. M., J. A. Marrinan, W. Li, and M. B. Kurtz. 1994. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-β-d-glucan synthase. J. Bacteriol. 176:5686-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards, J., Jr. 1989. Zygomycosis, p. 1192-1199. In P. Hoeprich and M. Jordan (ed.), Infectious disease, 4th ed. J. B. Lippincott Co., Philadelphia, Pa.
- 13.Espinel-Ingroff, A. 1999. Problems of antifungal in vitro testing in Aspergillus fumigatus. Contrib. Microbiol. 2:139-148. [DOI] [PubMed] [Google Scholar]
- 14.Espinel-Ingroff, A. 2003. Utility of mould susceptibility testing. Curr. Opin. Infect. Dis. 16:527-532. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez, C. E., D. R. Couriel, and T. J. Walsh. 1997. Disseminated zygomycosis in a neutropenic patient: successful treatment with amphotericin B lipid complex and granulocyte colony-stimulating factor. Clin. Infect. Dis. 24:192-196. [DOI] [PubMed] [Google Scholar]
- 16.Graybill, J. R., R. Bocanegra, L. K. Najvar, S. Hernandez, and R. A. Larsen. 2003. Addition of caspofungin to fluconazole does not improve outcome in murine candidiasis. Antimicrob. Agents Chemother. 47:2373-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajdu, R., R. Thompson, J. G. Sundelof, B. A. Pelak, F. A. Bouffard, J. F. Dropinski, and H. Kropp. 1997. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall, G. S., C. Myles, K. J. Pratt, and J. A. Washington. 1988. Cilofungin (LY121019), an antifungal agent with specific activity against Candida albicans and Candida tropicalis. Antimicrob. Agents Chemother. 32:1331-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbrecht, R., V. Letscher-Bru, R. A. Bowden, S. Kusne, E. J. Anaissie, J. R. Graybill, G. A. Noskin, B. A. Oppenheim, E. Andres, and L. A. Pietrelli. 2001. Treatment of 21 cases of invasive mucormycosis with amphotericin B colloidal dispersion. Eur. J. Clin. Microbiol. Infect. Dis. 20:460-466. [DOI] [PubMed] [Google Scholar]
- 20.Hussain, S., N. Salahuddin, I. Ahmad, I. Salahuddin, and R. Jooma. 1995. Rhinocerebral invasive mycosis: occurrence in immunocompetent individuals. Eur. J. Radiol. 20:151-155. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim, A. S., V. Avanessian, B. Spellberg, and J. E. Edwards, Jr. 2003. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob. Agents Chemother. 47:3343-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim, A. S., J. E. J. Edwards, and S. G. Filler. 2003. Zygomycosis, p. 241-251. In W. E. Dismukes, P. G. Pappas, and J. D. Sobel (ed.), Clinical mycology. Oxford University Press, New York, N.Y.
- 23.Ju, J. Y., C. Polhamus, K. A. Marr, S. M. Holland, and J. E. Bennett. 2002. Efficacies of fluconazole, caspofungin, and amphotericin B in Candida glabrata-infected p47phox−/− knockout mice. Antimicrob. Agents Chemother. 46:1240-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohler, S., L. J. Wheat, P. Connolly, C. Schnizlein-Bick, M. Durkin, M. Smedema, J. Goldberg, and E. Brizendine. 2000. Comparison of the echinocandin caspofungin with amphotericin B for treatment of histoplasmosis following pulmonary challenge in a murine model. Antimicrob. Agents Chemother. 44:1850-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo, N., K. Kuwahara-Arai, H. Kuroda-Murakami, E. Tateda-Suzuki, and K. Hiramatsu. 2001. Eagle-type methicillin resistance: new phenotype of high methicillin resistance under mec regulator gene control. Antimicrob. Agents Chemother. 45:815-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnarao, T. V., and J. N. Galgiani. 1997. Comparison of the in vitro activities of the echinocandin LY303366, the pneumocandin MK-0991, and fluconazole against Candida species and Cryptococcus neoformans. Antimicrob. Agents Chemother. 41:1957-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon-Chung, K. J., and J. E. Bennett. 1992. Mucormycosis, p. 524-559. In Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 28a.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Approved standard M38-A. NCCLS, Wayne, Pa.
- 29.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30:251-255. [DOI] [PubMed] [Google Scholar]
- 30.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandhu, P., X. Xu, P. J. Bondiskey, S. K. Balani, M. L. Morris, Y. S. Tang, A. R. Miller, and P. G. Pearson. 2004. Disposition of caspofungin, a novel antifungal agent, in mice, rats, rabbits, and monkeys. Antimicrob. Agents Chemother. 48:1272-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stratton, C. W., C. Liu, H. B. Ratner, and L. S. Weeks. 1987. Bactericidal activity of deptomycin (LY146032) compared with those of ciprofloxacin, vancomycin, and ampicillin against enterococci as determined by kill-kinetic studies. Antimicrob. Agents Chemother. 31:1014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugar, A. M. 1995. Agent of mucormycosis and related species, p. 2311-2321. In G. Mandell, J. Bennett, and R. Dolin (ed.), Principles and practices of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 36.Sun, Q. N., L. K. Najvar, R. Bocanegra, D. Loebenberg, and J. R. Graybill. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob. Agents Chemother. 46:2310-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang, C. M., J. Cohen, and D. W. Holden. 1992. An Aspergillus fumigatus alkaline protease mutant constructed by gene disruption is deficient in extracellular elastase activity. Mol. Microbiol. 6:1663-1671. [DOI] [PubMed] [Google Scholar]
- 38.Waldorf, A. R., C. Halde, and N. A. Vedros. 1982. Murine model of pulmonary mucormycosis in cortisone-treated mice. Sabouraudia 20:217-224. [DOI] [PubMed] [Google Scholar]
- 39.Waldorf, A. R., N. Ruderman, and R. D. Diamond. 1984. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J. Clin. Investig. 74:150-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]