Abstract
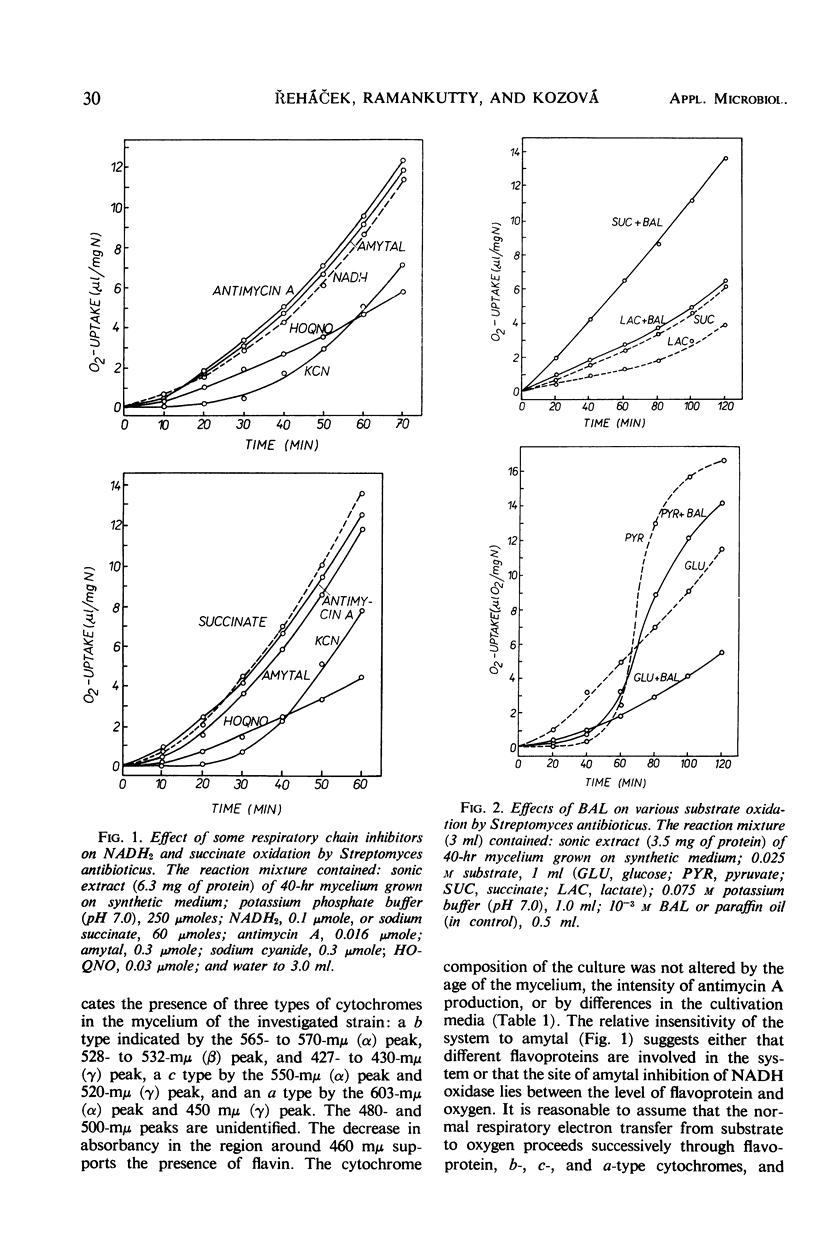
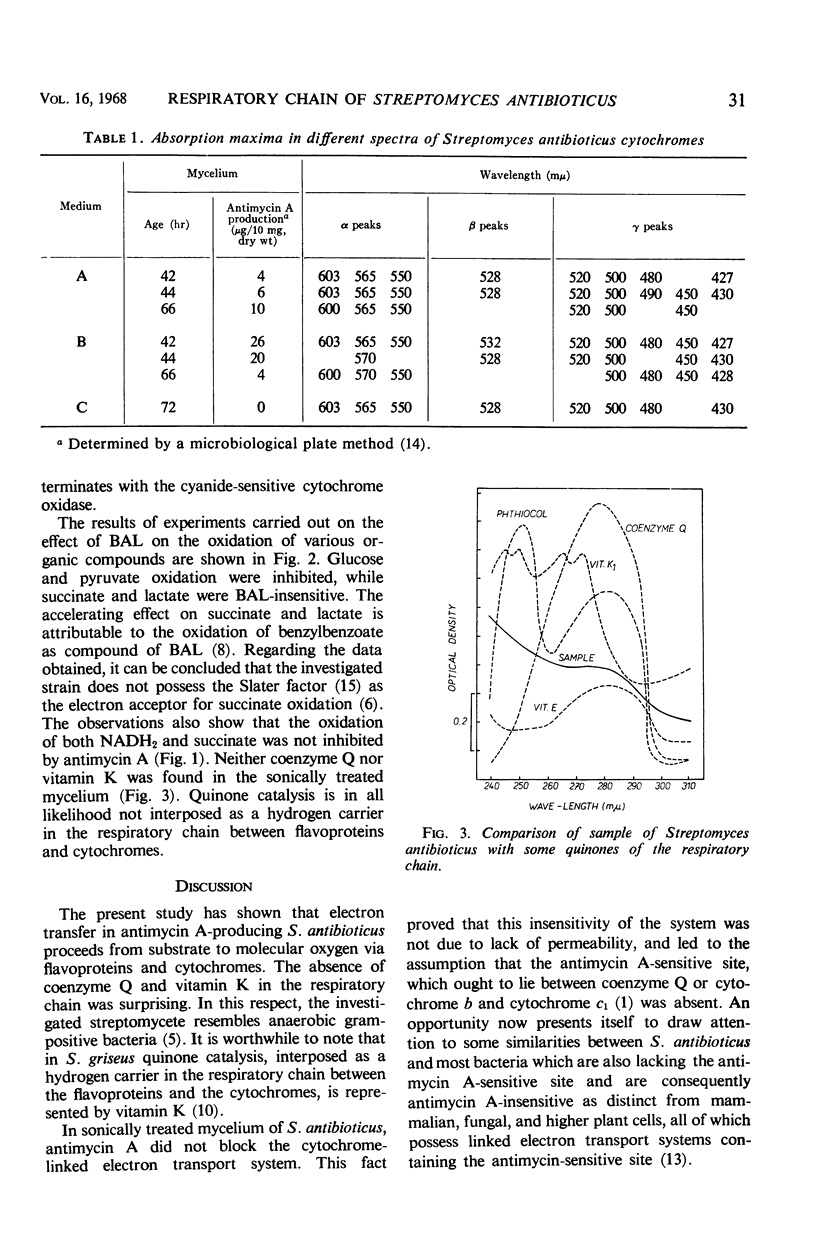
An active respiratory chain system was demonstrated in sonically treated mycelium of Streptomyces antibioticus, the producer of antimycin A. The respiratory electron transfer from substrate to oxygen proceeded successively through flavoprotein(s), b-, c-, and a-type cytochromes, and terminated with the cyanide-sensitive cytochrome oxidase. The cytochrome composition of the culture was not affected by the age of the mycelium, the intensity of antimycin A production, or differences in the media. Slater factor, coenzyme Q, and vitamin K were not interposed as hydrogen carriers in the respiratory chain between flavoproteins and cytochromes. The oxidation of reduced nicotinamide adenine dinucleotide and succinate was unaffected by antimycin A. Evidence is presented in support of the absence of the antimycin A-sensitive site from the electron transport system of S. antibioticus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASFORD R. E., TISDALE H. D., GLENN J. L., GREEN D. E. Studies on the terminal electron transport system. VII. Further studies on the succinic dehydrogenase complex. Biochim Biophys Acta. 1957 Apr;24(1):107–115. doi: 10.1016/0006-3002(57)90152-x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- GALE P. H., ERICKSON R. E., PAGE A. C., Jr, FOLKERS K. COENZYME Q. LI. NEW DATA ON THE DISTRIBUTION OF COENZYME Q IN NATURE. Arch Biochem Biophys. 1964 Jan;104:169–172. doi: 10.1016/s0003-9861(64)80051-5. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., ENGLE L. P. VITAMIN K COMPOUNDS IN BACTERIA THAT ARE OBLIGATE ANAEROBES. Science. 1964 Dec 4;146(3649):1307–1309. doi: 10.1126/science.146.3649.1307. [DOI] [PubMed] [Google Scholar]
- HATEFI Y. COENZYME Q (UBIQUINONE). Adv Enzymol Relat Areas Mol Biol. 1963;25:275–328. doi: 10.1002/9780470122709.ch5. [DOI] [PubMed] [Google Scholar]
- INOUE Y. The metabolism of Streptomyces griseus. IV. The terminal pathway of the respiration of Streptomyces griseus. J Antibiot (Tokyo) 1958 May;11(3):109–115. [PubMed] [Google Scholar]
- JACKSON F. L., LIGHTBOWN J. W. Inhibition of dihydrocozymase-oxidase activity of heart-muscle preparations and of certain cell-free bacterial preparations by 2-heptyl-4-hydroxyquinoline N-oxide. Biochem J. 1958 May;69(1):63–67. doi: 10.1042/bj0690063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NIEDERPRUEM D. J., HACKETT D. P. Respiratory chain of Streptomyces. J Bacteriol. 1961 Apr;81:557–563. doi: 10.1128/jb.81.4.557-563.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMACHANDRAN S., GOTTLIEB D. Mode of action of antibiotics. II. Specificity of action of antimycin A and ascosin. Biochim Biophys Acta. 1961 Oct 28;53:396–402. doi: 10.1016/0006-3002(61)90451-6. [DOI] [PubMed] [Google Scholar]
- SLATER E. C. The components of the dihydrocozymase oxidase system. Biochem J. 1950 Apr;46(4):484–499. doi: 10.1042/bj0460484. [DOI] [PMC free article] [PubMed] [Google Scholar]



