Abstract
Hepatocellular carcinoma (HCC) usually develops in chronically damaged liver. We investigated hepatic reserves during chemotherapy of patients with advanced HCC and compensated liver function to evaluate the effect on patients' outcomes of maintaining hepatic reserve after chemotherapy. We retrospectively reviewed the medical records of 190 patients with Child-Pugh A with advanced HCC who were treated with sorafenib or hepatic arterial infusion chemotherapy (HAIC). We investigated the Child-Pugh score and albumin-bilirubin grade for hepatic reserve, and evaluated the effect of the change in Child-Pugh scores on patients' outcomes. Subjects were treated with sorafenib (n = 59) or HAIC (n = 131). Of patients with Child-Pugh data, 66.7% maintained or improved their Child-Pugh score after 4 weeks. Treatment with HAIC was the only factor that significantly contributed to maintaining Child-Pugh scores after 4 weeks. The overall survival of patients with a higher Child-Pugh score after 4 weeks was shorter than that of patients whose Child-Pugh classification was unchanged. Multivariate analysis demonstrated that an increased Child-Pugh score after 4 weeks was one of the independent unfavorable prognostic factors. The change of hepatic reserve as a function of albumin-bilirubin grade did not significantly correlate with patients' outcomes. Maintaining the Child-Pugh score during chemotherapy benefits the outcomes of patients with advanced HCC, even those with sufficient hepatic reserve.
Keywords: Hepatocellular carcinoma, Hepatic reserve, Child-Pugh score, Hepatic arterial infusion chemotherapy, Sorafenib
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related mortality worldwide [1]. Surveillance of high-risk patients with hepatitis virus infection or liver cirrhosis who are at risk for HCC occurrence and new imaging techniques have enabled the detection of HCC at an early stage [2], and advances in therapeutic procedures have improved curability [3]. However, patients who receive curative treatment frequently experience multicentric recurrence and intrahepatic metastases. Consequently, recurrent HCC invades the intrahepatic vasculature or metastasizes to extrahepatic sites and becomes refractory to transarterial chemoembolization. Administration of sorafenib or hepatic arterial infusion chemotherapy (HAIC) to such patients benefits their survival [4,5].
Impaired hepatic reserve caused by advanced chronic liver disease is another crucial factor that significantly affects patients' outcomes [6]. For example, patients with more advanced stages of HCC may experience deterioration of hepatic reserve [7], possibly due to a progressive tumor burden directly affecting hepatic function, or impaired blood supply of the portal vein caused by vascular invasion damages. Although hepatic reserve before treatment affects the outcome of a patient with advanced HCC when subsequently treated with chemotherapy, there have been no relevant studies focusing on hepatic reserve during treatment of patients with sufficient hepatic reserve.
Therefore, the aim of the present study was to investigate the hepatic reserves after sorafenib treatment or HAIC of patients with advanced HCC and compensated liver function. We further analyzed the effect on patients' outcomes of maintaining hepatic reserve after chemotherapy that was associated with changes of hepatic reserve after administration of sorafenib or HAIC.
Materials and Methods
Patients
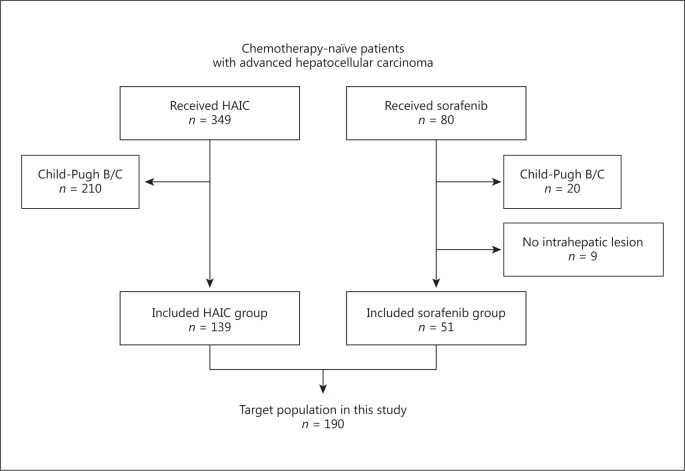
We studied chemotherapy-naïve patients with advanced HCC who were treated with sorafenib or HAIC at the Kanazawa University Hospital from March 2003 to December 2015. All patients underwent dynamic computed tomography or magnetic resonance imaging to diagnose and assess the extent of HCC. HCC was diagnosed according to the guidelines of the American Association for the Study of Liver Disease, and histological examination was performed if required [8]. If the radiological findings showed vascular invasion, multiple intrahepatic lesions, or both, patients were judged to be unsuitable for surgery, locoregional therapy, and transarterial chemoembolization. If patients met these criteria, further inclusion criteria were as follows: (1) without prior chemotherapy, (2) without cirrhosis or with Child-Pugh A cirrhosis, and (3) presence of intrahepatic lesions (Fig. 1). Patients with extrahepatic lesions were judged to be eligible for HAIC if the extrahepatic lesions were mild and not prognostic, e.g. small tumor burden, slowly growing tumor, and no effect of the tumor on the patient's symptoms.
Fig. 1.
Flow diagram of the study.
Treatment
Patients in the sorafenib group received 400 mg of sorafenib orally twice daily. Patients in the HAIC group received 5-fluorouracil as follows: 5-fluorouracil (330 mg/m2/day) was administered continuously on days 1–5 and days 8–12. Some patients received cisplatin injection (20 mg/m2/day) into the hepatic artery for 10 min before administration of 5-fluorouracil. Interferon-α-2b or pegylated interferon-α-2b was used at the physician's discretion. Pegylated interferon-α-2b (1.0 μg/kg) was administered subcutaneously on days 1, 8, 15, and 22, and interferon-α-2b (3 × 106 U) was administered intramuscularly 3 times each week. The administration of drugs was followed by a 14-day rest period. The implantation of a drug-delivery reservoir system was established before treatment as previously described [9].
Both treatments were temporarily interrupted, or the dose of the drugs was reduced according to toxicity. Treatment was subsequently continued until confirmation of tumor progression, unacceptable toxicity, a patient's refusal of treatment, or death.
Effects of Treatment
At each visit, a patient's Child-Pugh score was assessed according to physiological, laboratory, and radiological findings. The albumin-bilirubin (ALBI) grade, a simple assessment criterion for hepatic reserve, was calculated as previously reported [10]. We used dynamic computed tomography or dynamic magnetic resonance imaging every 4–6 weeks during treatment to assess the efficacy of treatment. The antitumor effects of treatment were assessed according to the Response Evaluation Criteria in Solid Tumors v1.1 [11]. The disease control rate was defined as the sum of the rates of complete response, partial response, and stable disease. Progression-free survival (PFS) was defined as the time from the start of treatment until the date of radiological progression and death. Overall survival (OS) was defined as the time from the start of treatment until death.
Data Collection
We reviewed patients' medical records and collected demographic, clinical, and laboratory data, which included age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), hepatitis virus status, hepatic reserve, consumption of branched-chain amino acids, imaging data (vascular invasion and extrahepatic lesions), analyses of tumor markers, and response to treatment. The Institutional Review Board at Kanazawa University approved the study, which was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Categorical variables were compared using the χ2 test when appropriate for univariate analysis, and logistic regression analysis was used to perform multivariate analysis. Cumulative survival was calculated using the Kaplan-Meier method, and differences were evaluated using the log-rank test. Only variables with statistically significant values determined using univariate analysis were subsequently evaluated using multivariate analysis with the Cox proportional hazards regression model. p < 0.1 and p < 0.05 were considered statistically significant in univariate analysis and multivariate analysis, respectively. All statistical analyses were performed using SPSS statistical software (v21.0; SPSS, Chicago, IL, USA).
Results
Characteristics of Patients
We retrospectively reviewed the records of 80 and 349 chemotherapy-naïve patients with advanced HCC who were treated with sorafenib or HAIC, respectively, at Kanazawa University Hospital between March 2003 and December 2015. Overall, 190 patients (51 treated with sorafenib [sorafenib group] and 139 treated with HAIC [HAIC group]) met the inclusion criteria (Fig. 1). Patients' characteristics are summarized in Table 1. We determined that 54.9 and 45.1% of patients in the sorafenib group and 46.8 and 53.2% of patients in the HAIC group had Child-Pugh scores of 5 and 6, respectively. Other factors associated with general condition, hepatic reserve, and tumors were similarly distributed between groups, except that extrahepatic metastases were observed more frequently in patients in the sorafenib group than in the HAIC group (49.0 vs. 17.3%; p < 0.001), and maximum tumor size of 50 mm or larger and tumor number of 6 or more were observed more frequently in patients in the HAIC group than in the sorafenib group (36.7 vs. 15.7%, p = 0.006; 74.1 vs. 58.8%, p = 0.042, respectively).
Table 1.
Demographic characteristic of the patients according to the type of treatment
| All (n = 190) | Treatment |
p valuea | ||
|---|---|---|---|---|
| sorafenib (n = 51) | HAIC (n = 139) | |||
| Age | ||||
| Median, years | 69 | 69 | 69 | |
| ≥69 years | 101 (53.2) | 27 (52.9) | 74 (53.2) | 0.97 |
| Male | 156 (82.1) | 45 (88.2) | 111 (79.9) | 0.18 |
| ECOG performance status | ||||
| 0 | 175 (92.1) | 46 (90.2) | 129 (92.8) | 0.55 |
| 1–3 | 15 (7.9) | 5 (9.8) | 10 (7.2) | |
| Hepatitis B virus surface antigen | 52 (27.4) | 16 (31.4) | 36 (25.9) | 0.45 |
| Hepatitis C virus antibody | 99 (52.1) | 24 (47.1) | 75 (54.0) | 0.40 |
| Child-Pugh score | ||||
| 5 | 93 (48.9) | 28 (54.9) | 65 (46.8) | 0.32 |
| 6 | 97 (51.1) | 23 (45.1) | 74 (53.2) | |
| Branched-chain amino acid | 30 (15.8) | 9 (17.6) | 21 (15.1) | 0.67 |
| Maximum tumor size ≥50 mm | 59 (31.1) | 8 (15.7) | 51 (36.7) | 0.006 |
| Tumor number ≥6 | 133 (70.0) | 30 (58.8) | 103 (74.1) | 0.042 |
| Vascular invasion | 75 (39.5) | 18 (35.3) | 57 (41.0) | 0.48 |
| Extrahepatic spread | 49 (25.8) | 25 (49.0) | 24 (17.3) | <0.001 |
| AFP ≥400 ng/mL | 69 (36.3) | 16 (31.4) | 53 (38.1) | 0.39 |
| Crossover second-line chemotherapy | 50 (26.3) | 28 (54.9) | 22 (15.8) | <0.001 |
Data are presented as n (%). ECOG, Eastern Cooperative Oncology Group; AFP, α-fetoprotein; HAIC, hepatic arterial infusion chemotherapy.
χ2 test.
Treatment
When data collection terminated on 18 March, 2016, 177 patients (93.2%) had discontinued treatment, and 153 patients (80.5%) had died. The median follow-up was 12.9 months (range, 0.5–151.3 months). Both treatments were well tolerable, although 2 patients, 1 patient in each group, discontinued treatment because of liver failure. Both of them had numerous small HCCs in the liver, portal vein tumor thrombus and extrahepatic lesion, and their Child-Pugh scores were 6 before treatment. However, the liver function of a patient treated with HAIC deteriorated to Child-Pugh score 11 after 4 weeks, and she stopped HAIC on day 35. Another patient treated with sorafenib also developed impaired liver function with a Child-Pugh score of 9 after 8 weeks, and he stopped sorafenib on day 57. Both impaired liver functions were irreversible.
Response to Treatment
Responses to treatment are shown in Table 2. Objective responses to treatment were achieved by 5.9% (3/51) and 35.3% (49/139) of patients treated with sorafenib and HAIC, respectively. The disease control rates were 51.0 and 67.6% in the sorafenib and HAIC groups, respectively. Responsiveness to treatment in the HAIC group was better than that in the sorafenib group (objective response: p < 0.001, disease control rate: p = 0.035) (Table 2a). Changes of α-fetoprotein (AFP) are shown in Table 2b. The median AFP before treatment and 4 weeks after treatment start were 72 and 63.5 ng/mL, respectively, in the sorafenib group, whereas they were 74 and 47 ng/mL, respectively, in the HAIC group. AFP was 20 ng/mL or more before treatment among 30 (58.8%) and 95 patients (68.3%) treated with sorafenib and HAIC, respectively. AFP decreased by 50% or more after 4 weeks after treatment in only 1 (3.3%) patient of the sorafenib group, and 37 patients (38.9%) of the HAIC group (p < 0.001).
Table 2.
Response to treatment according to the type of treatment
| Treatment |
p valueb | ||
|---|---|---|---|
| sorafenib (n = 51) | HAIC (n = 139) | ||
| a Radiological assessmenta | |||
| Complete response | 0 (0%) | 12 (8.6%) | |
| Partial response | 3 (5.9%) | 37 (26.6%) | |
| Stable disease | 23 (45.1%) | 45 (32.4%) | |
| Progressive disease | 23 (45.1%) | 41 (29.5%) | |
| Objective response rate | 5.9% | 35.3% | <0.001 |
| Disease control rate | 51.0% | 67.6% | 0.035 |
| b Change of AFP | |||
| Before treatment, median | 72 | 74 | |
| After 4 weeks, median | 63.5 | 47 | |
| ≥50% decreased after 4 weeks/patients with ≥20 ng/mL before treatment | 1/30 (3.3%) | 37/95 (38.9%) | <0.001 |
AFP, α-fetoprotein; HAIC, hepatic arterial infusion chemotherapy.
According to RECIST v1.1.
χ2 test.
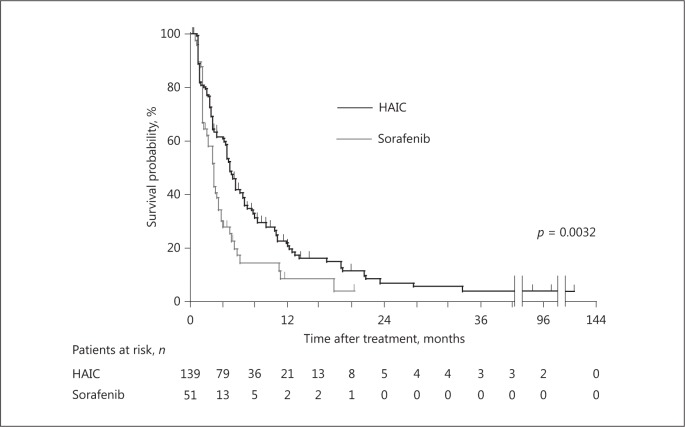
The median PFS of all patients was 4.4 months. The median PFS values of patients treated with HAIC and sorafenib were 4.8 and 2.8 months, respectively. The PFS of the patients treated with HAIC was better compared with that of patients treated with sorafenib (p = 0.0032) (Fig. 2).
Fig. 2.
Kaplan-Meier plots of progression-free survival (PFS) after treatment commencement. The median PFS was 4.8 months for patients treated with HAIC, which was significantly better compared with that of patients treated with sorafenib (p = 0.0032, log-rank test).
Effect of Chemotherapy on Child-Pugh Scores
Child-Pugh data after treatment were available for 183 (96.3%) and 162 patients (85.3%) at 4 and 12 weeks, respectively. The Child-Pugh scores of 122 patients (66.7%) were maintained or improved after 4 weeks (50.0 and 72.3%) among patients treated with sorafenib or HAIC, respectively (Table 3). Similarly, the Child-Pugh scores of 21 (47.7%) and 84 patients (71.2%) were unchanged or improved after 12 weeks among patients treated with sorafenib or HAIC, respectively. The Child-Pugh scores of more patients treated with HAIC were significantly maintained or improved after 4 and 12 weeks compared with those who were treated with sorafenib (p = 0.006 and p = 0.005, respectively) (Table 3). This tendency was also observed among the patients whose best response to treatment was complete response, partial response, or stable disease (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000472262).
Table 3.
Correlation of Child-Pugh scores with the type of treatment at 4 and 12 weeks after treatment commencement
| All | Treatment |
p valuea | ||
|---|---|---|---|---|
| sorafenib | HAIC | |||
| Four weeks after treatment commenced | ||||
| Evaluable | 183 | 46 | 137 | |
| Maintained or improved | 122 (66.7%) | 23 (50.0%) | 99 (72.3%) | 0.006 |
| Deteriorated | 61 (33.3%) | 23 (50.0%) | 38 (27.7%) | |
| Twelve weeks after treatment commenced | ||||
| Evaluable | 162 | 44 | 118 | |
| Maintained or improved | 105 (64.8%) | 21 (47.7%) | 84 (71.2%) | 0.005 |
| Deteriorated | 57 (35.2%) | 23 (52.3%) | 34 (28.8%) | |
HAIC, hepatic arterial infusion chemotherapy.
χ2 test.
Univariate analysis revealed that Child-Pugh scores before treatment, extrahepatic spread, and treatment correlated significantly with unchanged Child-Pugh scores (p = 0.037, p = 0.020, and p = 0.0056, respectively), and multivariate logistic regression analysis revealed that HAIC was the only variable that was significantly associated with unchanged Child-Pugh scores after 4 weeks (hazard ratio [HR], 2.119; p = 0.044) (Table 4). HAIC was also one of the contributing factors to maintaining Child-Pugh scores at 12 weeks (HR, 2.852) as well as Child-Pugh score 6 before treatment (HR, 3.086), maximum tumor size <50 mm (HR, 3.417), and best response to treatment of complete response, partial response, or stable disease (HR, 2.364) (online suppl. Table 2).
Table 4.
Factors that contributed to maintaining Child-Pugh scores at 4 weeks
| n | Maintaining Child-Pugh scores, % | Univariate p valuea | Hazard ratio (95% CI) | Multivariate p valueb | |
|---|---|---|---|---|---|
| Age | |||||
| ≥69 years | 97 | 66.0 | 0.83 | ||
| <69 years | 86 | 67.4 | |||
| Gender | |||||
| Male | 149 | 66.4 | 0.89 | ||
| Female | 34 | 67.6 | |||
| ECOG performance status | |||||
| 0 | 169 | 68.0 | 0.17 | ||
| 1–3 | 14 | 50.0 | |||
| Hepatitis B virus surface antigen | |||||
| Positive | 51 | 70.6 | 0.48 | ||
| Negative | 132 | 65.2 | |||
| Hepatitis C virus antibody | |||||
| Positive | 94 | 64.9 | 0.60 | ||
| Negative | 89 | 68.5 | |||
| Child-Pugh score | |||||
| 6 | 91 | 73.9 | 0.037 | 1.898 (0.996–3.610) | 0.052 |
| 5 | 92 | 59.3 | |||
| Branched-chain amino acid | |||||
| Positive | 28 | 57.1 | 0.25 | ||
| Negative | 155 | 68.4 | |||
| Maximum tumor size | |||||
| <50 mm | 126 | 67.5 | 0.73 | ||
| ≥50 mm | 57 | 64.9 | |||
| Number of tumors | |||||
| <6 | 54 | 64.8 | 0.73 | ||
| ≥6 | 129 | 67.4 | |||
| Vascular invasion | |||||
| Negative | 110 | 69.1 | 0.39 | ||
| Positive | 73 | 63.0 | |||
| Extrahepatic lesion | |||||
| Negative | 139 | 71.2 | 0.020 | 1.861 (0.883–3.923) | 0.10 |
| Positive | 44 | 52.2 | |||
| AFP | |||||
| <400 ng/mL | 116 | 69.0 | 0.39 | ||
| ≥400 ng/mL | 67 | 62.7 | |||
| Treatment | |||||
| HAIC | 137 | 72.3 | 0.0056 | 2.119 (1.021–4.399) | 0.044 |
| Sorafenib | 46 | 50.0 | |||
| Best response to treatmentc | |||||
| Complete response, partial response, or stable disease | 119 | 69.7 | 0.23 | ||
| Progressive disease or not evaluable | 64 | 60.9 |
AFP, α-fetoprotein; HAIC, hepatic arterial infusion chemotherapy; ECOG, Eastern Cooperative Oncology Group.
log-rank test.
Cox proportional hazards regression model.
Based on RECIST v1.1.
Impact of Maintaining the Child-Pugh Score on Patients' Outcomes
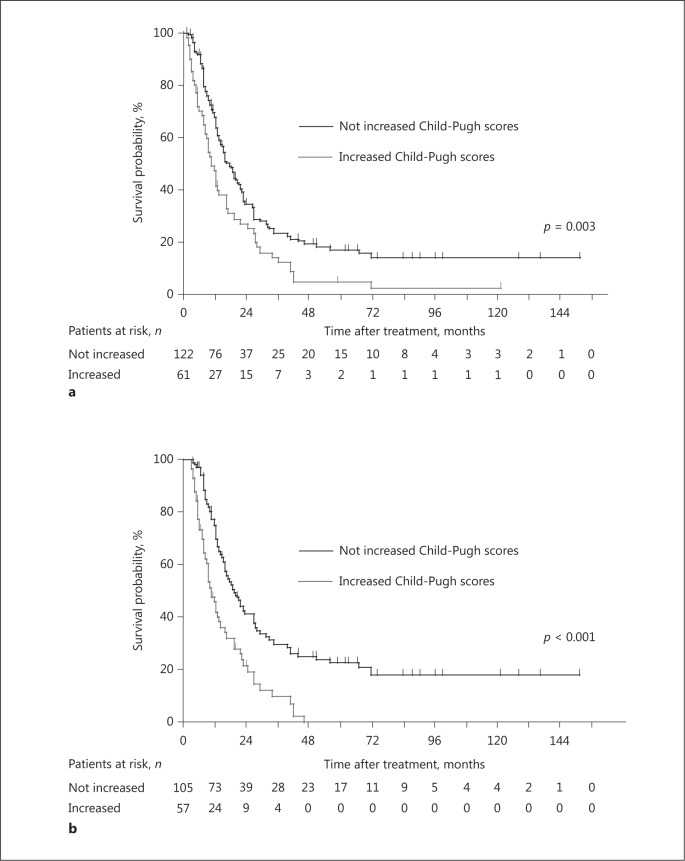
The median OS of all patients was 14.5 months. The median OS values of patients with an increased Child-Pugh score and those with unchanged or improved Child-Pugh scores after 4 weeks were 10.7 and 17.7 months, respectively. The OS of the patients with increased Child-Pugh scores after 4 weeks was shorter compared with those of patients with unchanged or improved Child-Pugh scores (p = 0.003) (Fig. 3a), and the difference was more remarkable 12 weeks after treatment commenced (p < 0.001) (Fig. 3b). These differences were more apparent in the subgroups with good prognoses such as the elderly, patients negative for hepatitis B virus surface antigen, patients with Child-Pugh score = 5, absence of vascular invasion, absence of extrahepatic lesions, or tumor control except AFP (Table 5).
Fig. 3.
Kaplan-Meier plots of overall survival (OS) after treatment commencement. a The median OS was 17.7 months for patients with not increased Child-Pugh scores 4 weeks after treatment, which was significantly better compared with that of patients with increased Child-Pugh scores (p = 0.003, log-rank test). b Similarly, the median OS was 19.4 months for patients with not increased hepatic reserve 12 weeks after treatment, which was significantly better compared with that of patients with increased Child-Pugh scores (p < 0.001, log-rank test).
Table 5.
Effect of maintaining Child-Pugh scores on patients’ outcomes in each subgroup 4 weeks after treatment commencement
| n | Median overall survival, months | Hazard ratio (95% CI) | p valuea | |
|---|---|---|---|---|
| Age | ||||
| <69 years | 86 | 13.1 | 1.281 (0.785–2.091) | 0.32 |
| ≥69 years | 97 | 16.1 | 2.120 (1.336–3.365) | 0.001 |
| Gender | ||||
| Female | 34 | 12.0 | 1.646 (0.749–3.619) | 0.21 |
| Male | 149 | 15.6 | 1.654 (1.142–2.396) | 0.008 |
| ECOG performance status | ||||
| 1–3 | 14 | 7.1 | 1.199 (0.363–3.955) | 0.77 |
| 0 | 169 | 15.5 | 1.601 (1.125–2.278) | 0.009 |
| Hepatitis B virus surface antigen | ||||
| Positive | 51 | 12.2 | 1.265 (0.662–2.415) | 0.48 |
| Negative | 132 | 16.2 | 1.832 (1.237–2.712) | 0.003 |
| Hepatitis C virus antibody | ||||
| Negative | 89 | 12.4 | 1.663 (1.025–2.699) | 0.039 |
| Positive | 94 | 16.6 | 1.739 (1.090–2.773) | 0.020 |
| Child-Pugh score | ||||
| 6 | 92 | 13.0 | 1.407 (0.851–2.326) | 0.18 |
| 5 | 91 | 15.6 | 1.996 (1.251–3.187) | 0.004 |
| Branched-chain amino acid intake | ||||
| Positive | 28 | 12.0 | 1.232 (0.546–2.778) | 0.62 |
| Negative | 155 | 15.2 | 1.684 (1.163–2.440) | 0.006 |
| Vascular invasion | ||||
| Positive | 73 | 9.0 | 1.468 (0.888–2.429) | 0.13 |
| Negative | 110 | 19.2 | 1.777 (1.134–2.784) | 0.012 |
| Extrahepatic lesion | ||||
| Positive | 44 | 7.7 | 1.256 (0.661–2.390) | 0.49 |
| Negative | 139 | 17.2 | 1.624 (1.089–2.422) | 0.017 |
| AFP | ||||
| ≥400 ng/mL | 67 | 10.5 | 2.069 (1.215–3.523) | 0.0079 |
| <400 ng/mL | 116 | 18.6 | 1.439 (0.934–2.217) | 0.099 |
| Treatment procedure | ||||
| Sorafenib | 46 | 13.0 | 1.195 (0.620–2.301) | 0.59 |
| HAIC | 137 | 15.6 | 1.709 (1.144–2.552) | 0.009 |
| Best response to treatment | ||||
| Progressive disease or not evaluable | 64 | 7.7 | 1.458 (0.869–2.445) | 0.15 |
| Complete response, partial response, or stable disease | 119 | 22.0 | 1.667 (1.072–2.593) | 0.023 |
AFP, α-fetoprotein; HAIC, hepatic arterial infusion chemotherapy; ECOG, Eastern Cooperative Oncology Group.
Cox proportional hazards regression model.
Univariate analyses revealed that 10 of the 16 selected variables were significantly associated with OS. They were as follows: sex, ECOG PS, consumption of branched-chain amino acids, maximum tumor size, vascular invasion, extrahepatic lesion, serum AFP levels, treatment procedure, and response to treatment as well as unchanged Child-Pugh scores. Multivariate analysis revealed that an increased Child-Pugh score after 4 weeks was an independent unfavorable predictive factor for OS (HR, 1.425) as well as consumption of branched-chain amino acids (HR, 1.873), maximum tumor size of 50 mm or larger (HR, 2.319), best response to treatment of complete, partial response, or stable disease (HR, 3.150) (Table 6).
Table 6.
Factors that influenced patients’ outcomes
| n | Median over-all survival, months | Univariate p valuea | Hazard ratio (95% CI) | Multi-variate p valueb | |
|---|---|---|---|---|---|
| Age | |||||
| <69 years | 89 | 13.1 | 0.83 | ||
| ≥69 years | 101 | 16.1 | |||
| Gender | |||||
| Female | 34 | 12.0 | 0.058 | 1.026 (0.653–1.612) | 0.91 |
| Male | 156 | 15.6 | |||
| ECOG performance status | |||||
| 1–3 | 15 | 7.1 | 0.0075 | 1.891 (0.954–3.747) | 0.068 |
| 0 | 175 | 15.5 | |||
| Hepatitis B virus surface antigen | |||||
| Positive | 52 | 12.2 | 0.16 | ||
| Negative | 138 | 16.2 | |||
| Hepatitis C virus antibody | |||||
| Negative | 91 | 12.4 | 0.11 | ||
| Positive | 99 | 16.6 | |||
| Child-Pugh score | |||||
| 6 | 97 | 13.0 | 0.49 | ||
| 5 | 93 | 15.6 | |||
| Branched-chain amino acid | |||||
| Positive | 30 | 12.0 | 0.086 | 1.873 (1.157–3.033) | 0.011 |
| Negative | 160 | 15.2 | |||
| Maximum tumor size | |||||
| ≥50 mm | 59 | 8.1 | <0.001 | 2.319 (1.476–3.643) | <0.001 |
| <50 mm | 131 | 18.5 | |||
| Number of tumors | |||||
| ≥6 | 133 | 15.6 | 0.47 | ||
| <6 | 57 | 12.4 | |||
| Vascular invasion | |||||
| Positive | 75 | 9.0 | <0.001 | 1.145 (0.780–1.681) | 0.49 |
| Negative | 115 | 19.2 | |||
| Extrahepatic lesion | |||||
| Positive | 49 | 7.7 | <0.001 | 1.313 (0.862–2.000) | 0.20 |
| Negative | 141 | 17.2 | |||
| AFP | |||||
| ≥400 ng/mL | 69 | 10.5 | 0.0032 | 1.233 (0.836–1.820) | 0.29 |
| <400 ng/mL | 121 | 18.6 | |||
| Treatment | |||||
| Sorafenib | 51 | 13.0 | 0.077 | 1.240 (0.800–1.922) | 0.34 |
| HAIC | 139 | 15.6 | |||
| CPS after 4 weeks | |||||
| Deteriorated | 61 | 10.7 | 0.0028 | 1.425 (1.005–2.021) | 0.047 |
| Maintained or improved | 122 | 17.7 | |||
| Best response to treatmentc | |||||
| Progressive disease or not evaluable | 70 | 7.7 | <0.001 | 3.150 (2.174–4.566) | <0.001 |
| Complete response, partial response, or stable disease | 120 | 22.0 | |||
| Crossover second-line chemotherapy | |||||
| Positive | 50 | 15.5 | 0.25 | ||
| Negative | 140 | 14.1 |
AFP, α-fetoprotein; HAIC, hepatic arterial infusion chemotherapy; ECOG, Eastern Cooperative Oncology Group.
log-rank test.
Cox proportional hazards regression model.
Based on RECIST v1.1.
Impact of the ALBI Grade on Patients' Outcomes
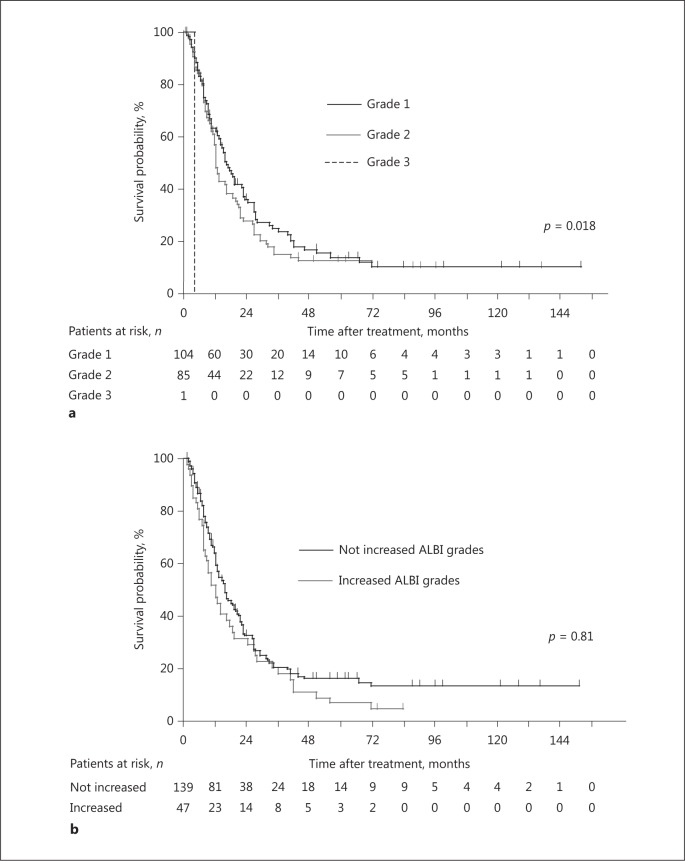
We used the ALBI grade as well as the Child-Pugh score to evaluate the effect of a patient's hepatic reserve on outcome. OS was stratified according to ALBI grade at the start of treatment (p = 0.018) (Fig. 4a). However, there was no significant difference in median OS times (16.2 vs. 13.0 months, respectively, p = 0.81) between patients with and without an unfavorable ALBI grade after 4 weeks (Fig. 4b).
Fig. 4.
Kaplan-Meier plots of overall survival (OS) after treatment commencement. a OS was stratified according to ALBI grade when treatment commenced, and median OS times were 16.5, 12.4, and 4.2 months for patients with ALBI grades 1, 2, and 3 (p = 0.018, log-rank test). b OS did not differ between patients with not increased ALBI grades and patients with increased ALBI grades after 4 weeks (median OS times, 16.2 and 13.0 months, respectively, p = 0.81, log-rank test).
Discussion
The most important insight provided by the current study is that mild deterioration of hepatic reserve during chemotherapy had an unfavorable effect on a patient's prognosis, even in patients with well-preserved hepatic function before treatment. This result was interesting because it was independent of an antitumor effect as revealed by multivariate analysis. Our recent study of patients with Child-Pugh B found that an improved Child-Pugh score during chemotherapy is a favorable prognostic factor [7], which is reasonable because hepatic failure is often fatal. In contrast, HAIC and sorafenib are well-tolerated by patients with sufficient hepatic reserve [12,13], and in the present study there were only 3 patients whose Child-Pugh scores rose to ≥9 after 4 weeks of treatment and 2 patients due to liver failure, suggesting that this result was not caused by lethal hepatic failure. Although we did not definitively identify the reason for these findings, the duration of survival after sorafenib treatment contributes to patients' OS [14], in part, because of increased availability of further treatment options [15,16]. The present study suggests that a minor change in Child-Pugh score after 4 weeks might have a greater effect on outcome than our assumption related to patients with sufficient hepatic reserve. Although ALBI grade consisting of only 2 factors was simple and useful for the prediction of the patients' outcome before treatment, Child-Pugh score consisting of 5 factors seemed to be more sensitive than ALBI grade to evaluate the change of hepatic reserve contributing patients' outcome during chemotherapy. Therefore, when planning a treatment strategy, we should consider evaluating hepatic reserve using Child-Pugh score as well during the administration of chemotherapy.
Another unique aspect of the present study was that we compared sorafenib with HAIC, focusing on the change of hepatic reserve. Sorafenib has shown survival benefit compared with placebo for patients with advanced HCC in a phase III trial [4], and previous reports compared the outcomes of sorafenib and HAIC treatments. However, the clinical benefits of these treatments for patients with advanced HCC were assessed only in the context of prolonging survival, and it remains unclear which treatment is more useful for patients with advanced HCC [17,18]. The results of this study demonstrated that HAIC maintained the Child-Pugh scores of patients and had a greater antitumor effect compared with sorafenib. Moreover, the antitumor effect did not significantly affect the Child-Pugh score, because the percentage of patients with unchanged Child-Pugh scores did not differ significantly according to antitumor effect, which suggested that the action of sorafenib blocking tumor cell proliferation and angiogenesis may have some effect on the hepatic damage, although to our knowledge there have been no reports describing that sorafenib worsened the hepatic reserve, and then, further investigation is needed to confirm the damage to normal liver cells by sorafenib.
We further show that there was no significant difference in OS between the 2 treatment groups in multivariate analysis, and the PFS of the patients treated with HAIC was better compared with that of the patients who were treated with sorafenib. This discrepancy was partially because more patients treated with sorafenib also received HAIC as the crossover second-line chemotherapy, and the subsequent therapy especially targeting intrahepatic lesions was suggested to be effective even after systemic chemotherapy, sorafenib [15,16]. Hepatic reserve closely correlates with the quality of life of patients with HCC [19], and we further showed that it contributed to patients' outcome. The subgroup analysis shown in Table 5 indicated that HAIC may replace sorafenib to treat patients with advanced HCC who were supposed to be long-term survivors because it is relatively more important to maintain hepatic reserve as well as to control tumor in such patients. However, we could not assess this assumption because of the lack of quality of life data for the patients studied here.
The administration of branched-chain amino acid effectively maintains the Child-Pugh score during chemotherapy of patients with advanced HCC [20,21]. In contrast, the present study did not detect a significant effect of consumption of branched-chain amino acids on maintaining Child-Pugh scores. This apparent discrepancy may be explained by the retrospective nature of this study of patients with Child-Pugh A, the small population that received branched-chain amino acids, and bias introduced by the backgrounds of the groups. Therefore, a prospective trial with a sufficient number of patients is required to confirm the effectiveness of branched-chain amino acids or other factors in maintaining hepatic reserve of during chemotherapy of patients with advanced HCC.
Conclusions
In conclusion, maintaining Child-Pugh scores contributed to a more favorable outcome of patients with advanced HCC, even if they had sufficient hepatic reserve when chemotherapy commenced. HAIC may therefore have greater potential for maintaining Child-Pugh scores compared with sorafenib.
Disclosure Statement
The authors declare that they have no conflict of interest.
Supplementary Material
Supplementary data
Supplementary data
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita T, Kitao A, Matsui O, Hayashi T, Nio K, Kondo M, et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology. 2014;60:1674–1685. doi: 10.1002/hep.27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, Park CH, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–1250. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita T, Arai K, Sunagozaka H, Ueda T, Terashima T, Yamashita T, et al. Randomized, phase II study comparing interferon combined with hepatic arterial infusion of fluorouracil plus cisplatin and fluorouracil alone in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:281–290. doi: 10.1159/000334439. [DOI] [PubMed] [Google Scholar]
- 6.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Terashima T, Yamashita T, Arai K, Kawaguchi K, Kitamura K, Yamashita T, et al. Response to chemotherapy improved hepatic reserve for patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis. Cancer Sci. 2016;107:1263–1269. doi: 10.1111/cas.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J, Sheman M, Practice Guidelines Committee, American Association for the study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 9.Terashima T, Yamashita T, Iida N, Yamashita T, Nakagawa H, Arai K, et al. Blood neutrophil to lymphocyte ratio as a predictor in patients with advanced hepatocellular carcinoma treated with hepatic arterial infusion chemotherapy. Hepatol Res. 2015;45:949–959. doi: 10.1111/hepr.12436. [DOI] [PubMed] [Google Scholar]
- 10.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko S, Ikeda K, Matsuzaki Y, Furuse J, Minami H, Okayama Y, et al. Safety and effectiveness of sorafenib in Japanese patients with hepatocellular carcinoma in daily medical practice: interim analysis of a prospective postmarketing all-patient surveillance study. J Gastroenterol. doi: 10.1007/s00535-016-1173-5. DOI: 10.1007/s00535-016-1173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terashima T, Yamashita T, Arai K, Sunagozaka H, Kitahara M, Nakagawa H, et al. Feasibility and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma after sorafenib. Hepatol Res. 2014;44:1179–1185. doi: 10.1111/hepr.12266. [DOI] [PubMed] [Google Scholar]
- 14.Terashima T, Yamashita T, Takata N, Nakagawa H, Toyama T, Arai K, et al. Post-progression survival and progression-free survival in patients with advanced hepatocellular carcinoma treated by sorafenib. Hepatol Res. 2016;46:650–656. doi: 10.1111/hepr.12601. [DOI] [PubMed] [Google Scholar]
- 15.Terashima T, Yamashita T, Horii R, Arai K, Kawaguchi K, Kitamura K, et al. Potential efficacy of therapies targeting intrahepatic lesions after sorafenib treatment of patients with hepatocellular carcinoma. BMC Cancer. 2016;16:338. doi: 10.1186/s12885-016-2380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano M, Tanaka M, Kuromatsu R, Nagamatsu H, Satani M, Niizeki T, et al. Alternative treatments in advanced hepatocellular carcinoma patientes with progressive disease after sorafenib treatment: a prospective multicenter cohort study. Oncotarget. doi: 10.18632/oncotarget.10794. DOI: 10.18632/oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaoka T, Aikata H, Hyogo H, Morio R, Morio K, Hatooka M, et al. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J Dig Dis. 2015;16:505–512. doi: 10.1111/1751-2980.12267. [DOI] [PubMed] [Google Scholar]
- 18.Shiozawa K, Watanabe M, Ikehara T, Kogame M, Matsui T, Okano N, et al. Comparison of sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: a propensity score matching study. Hepatogastroenterology. 2014;61:885–891. [PubMed] [Google Scholar]
- 19.Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Lin HC, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer System. Hepatology. 2013;57:112–119. doi: 10.1002/hep.25950. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Chen Y, Wang X, Li H, Zhang H, Gong J, et al. Efficacy and safety of oral branched-chain amino acid supplementataion in patients undergoing interventions for hepatocellular carcinoma: a meta-analysis. Nutr J. 2015;14:67. doi: 10.1186/s12937-015-0056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanekawa T, Nagai H, Kanayama M, Sumino Y. Importance of branched-chain amino acids in patients with liver cirrhosis and advanced hepatocellular carcinoma receiving hepatic arterial infusion chemotherapy. Cancer Chemother Pharmacol. 2014;75:899–909. doi: 10.1007/s00280-014-2564-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data