Abstract
Background
Currently, there is an extensive but highly inconsistent body of literature regarding donor adverse events (AEs) in haemapheresis. As the reports diverge with respect to types and grading of AEs, apheresis procedures and machines, the range of haemapheresis-related AEs varies widely from about 0.03% to 6.6%.
Methods
The German Society for Transfusion Medicine and Immunohaematology (DGTI) formed a ‘Haemapheresis Vigilance Working Party’ (Arbeitsgemeinschaft Hämapheresevigilanz; AGHV) to create an on-line registry for comprehensive and comparable AE assessment with all available apheresis devices in all types of preparative haemapheresis: plasmapheresis (PLS), plateletpheresis (PLT), red blood cell apheresis, all kind of leukaphereses (autologous/allogeneic blood stem cell apheresis, granulocyte apheresis, lymphocyte/monocyte apheresis) and all possible types of multi-component apheresis. To ensure the comparability of the data, the AGHV adopted the ‘Standard for Surveillance of Complications Related to Blood Donation’ from the International Society for Blood Transfusion in cooperation with the International Haemovigilance Network (IHN) and the American Association of Blood Banks for AE acquisition and automated evaluation. The registry is embedded in a prospective observational multi-centre study with a study period of 7 years.
Results
A preliminary evaluation encompassed the time period from January, 2012 to December, 2015. During this time, the system proved to be safe and stable. Out of approximately 345,000 haemaphereses 16,477 AEs were reported (4.9%) from 20 participating centres. The majority of AEs occurred in PLSs (63%), followed by PLT (34.5%) and SC (2.2%). Blood access injuries (BAI) accounted for about 55% of the supplied AEs, whereas citrate toxicity symptoms, vasovagal reactions and technical events (e.g. disposable leakages, software failures) rather equally affected haemaphereses at 8-15%. Out of 12,348 finalized AEs, 8,759 (70.1%) were associated with a procedure-related break-off, with BAI being the prevailing cause (5,463/8,759; 62.4%). An automated centre- and procedure-specific AE evaluation according to the latest IHN standard and AGHV pre-settings is available within a few minutes.
Conclusions
An on-line electronic platform for comprehensive assessment and centre-specific automated evaluation of AEs in haemaphereses has been developed and proved to be stable and safe over a period of 4 years.
Keywords: Adverse reactions, Haemapheresis, Haemovigilance, Plasmapheresis, Plateletpheresis, Stem cell collection, Donor safety, Adverse events
Introduction
Modern haemapheresis machines enable transfusion medicine specialists to rapidly provide a wide variety of different blood components for therapeutic use. These include therapeutic plasma, classical cellular blood components (i.e., red blood cell or platelet concentrates) and more advanced leucocyte preparations such as granulocyte concentrates, blood stem cell (SC) concentrates for haematopoietic stem cell transplantation (HSCT) or lymphocyte collections for treatment of relapsed haematological malignancies after HSCT [1,2,3,4]. Recently, multi-component apheresis has been introduced to increase the number of different blood components that can be obtained from a limited donor pool [5]. The number of preparative haemaphereses world-wide per year is unknown. However, in Germany alone in 2014 approximately 2.8 million plasmaphereses (PLSs) were done to obtain large quantities of plasma (ca. 2.1 million litres) as source material for industrial purposes or as therapeutic plasma. In addition, about 352,000 platelet concentrates from 178,000 plateletphereses (PLTs) as well as SC, granulocyte or lymphocyte components - in total around 11,000 units - were prepared for transfusion [6]. Thus complications related to haemapheresis procedures in Germany may add to several ten thousands adverse events (AEs) per year, even if they would occur in about 1-2% of the procedures only.
As preparative haemaphereses are done for more than 60 years [7], there is an extensive but inconsistent body of literature regarding donor reactions in haemapheresis [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. As shown in table 1, the number of AEs reported in the context of haemapheresis varies by two log steps from 0.025% [9] to 5.77% and 6.55% [10,11]. One explanation for this wide range is that some authors included mild, clinically insignificant donor reactions (e.g., small haematomas, short vasovagal reactions without faint, mild paresthesia [11,12,13,14]), whereas other reports referred to moderate and severe adverse events (SAEs) [14,15,16] or to SAEs only [9,17]. But even within the donor reaction grading ‘SAE’, the results may vary by 10-fold between 0.025% [9] and 0.24% [13]. These differences reflect the largely diverging study protocols within the literature that affect virtually all evaluation parameters. The numbers of evaluated aphereses ranged from approximately 5,000-20,000 [12,13,14,15,18] to beyond 1 million [9,11]. The AE categories encompassed blood access injuries (BAI) or vasovagal reactions (VVR) as a single evaluation parameter in a few papers [16,18,19,20], the combination of BAI, citrate toxicity (CT) and VVR in other studies [9,10,12,15, 17,21,23] and occasionally a more comprehensive selection including BAI, CT, VVR, donor compliance (DC) problems and technical (apheresis machine- or apheresis disposable-related) events [11,13]. Further largely diverging parameters refer to the choice of the apheresis machines and the apheresis procedures. Some authors report data from one specific apheresis procedure (e.g., PLS [11] or PLT [13,15]), whereas others presented AEs from different procedures simultaneously such as PLT, single or double red blood cell (RA1/RA2) apheresis or multi-component (MC) apheresis [9,14,18,20]. Taken these considerations in mind and given that haemapheresis is a versatile field with different types of apheresis machines and many different technical procedures, it is not surprising that the results obtained from AE studies showed a wide variance. Another very important point that impairs the comparability of the reported AE results was the long lasting absence of an internationally accepted standard for assessment and grading of AEs in blood donation. Consequently, 11 out of 14 groups developed own centre-specific systems for assessment and grading of AEs [10,11,13,14,15,16,19,20,21,22,23]. The remaining groups adopted a standard that had been developed by a scientific society [12] or applied a national (French) standard for donor haemovigilance [9,17]. The latter one, however, was designed to assess SAEs only, thereby limiting its explanatory power. Taken altogether, there were neither uniform study designs nor uniform definitions for AEs nor uniform definitions for the grading of the AEs so that a direct comparison of AE data between studies was impossible.
Table 1.
AEs in haemaphereses: literature overview
| Reference | Design | Apheresis devices | Adverse events, categories | Grading – design | Apheresis procedures, types, n | Results |
||
|---|---|---|---|---|---|---|---|---|
| AE, % | grading: M, MOD, SAE | |||||||
| Danic et al., 2010 [9] | RS, multi-centre | n. a. | BAI, VVR, CT | French national agency ‘Afssaps’ | MC, 1,499,469 | 0.025% | SAE = 0.025% | |
| Eder et al., 2008 [10] | PS, single-centre | Amicus, Spectra, Trima | BAI, VVR, CT | centre-specific (ARC) | R2, 228,183; PLT 449,594, in total 677,777 | PLT 5.77%; R2 5.38% | MAJ = 0.45% | |
| Diekamp et al., 2014 [11] | PS, multi-centre | n. a. | BAI, VVR, CT, DC, TE | centre-specific (Haema) | PLS, 1,107,846 | 6.55% | M = 2.18% MOD = 0.27% SAE = 0.06%. |
|
| McLeod et al., 1998 [12] | PS, multi-centre | Fenwal CS3000, Haemonetics MCS, Spectra | BAI, VVR, CT | centre-specific (AABB) | PLT, 17,591; PLS, 1,353; GA, 588; others, 79; in total 19,611 | 2.18% | n.d. | |
| Despotis et al., 1999 [13] | RS, single-centre | Amicus, Fenwal CS3000, Spectra | BAI, VVR, CT, TE | centre-specific | PLT, 19,736 | 0.81% | SAE = 0.24% | |
| Yuan et al., 2008 [14] | RS, single-centre | Trima Accel | VVR, CT | centre-specific | MC, 11,333 | n.a. | MOD = 0.27% SAE = 0,20% |
|
| Yuan et al., 2010 [15] | RS, single-centre | Trima Accel | BAI, VVR, CT | centre-specific | PLT, 15,763 | n.a. | MOD+SAE = 0.37% | |
| Wiltbank et al., 2007 [16] | RS, multi-centre | Alyx, Amicus, MCS+, Spectra, Trima | VVR | centre-specific (UBS) | R2, 249,154; R1 40,870; PLT 90,082; in total 380,106 | n.a. | MOD = 0,14% SAE = 0.035% |
|
| Ounnoughene et al., 2013 [17] | RS, multi-centre | n. a. | BAI, VVR, CT | French national haemovigilance database ‘e-FIT’ | n.a. | n.a. | SAE = 0.18% | |
| Bueno et al., 2006 [18] | RS, single-centre | Trima, Amicus | BAI | n.d. | MC, 5,177 | 3.3% | n.d. | |
| Tomita et al., 2002 [19] | RS, single-centre | MCS 3P | VVR | n.d. | AD: males, n = 14,523; females, n = 6,722 | ♂1–2%, ♀4–5% | n.d. | |
| Kamel et al., 2010 [20] | RS, multi-centre | n.a. | VVR, delayed vs immediate | centre-specific (UBS) | R2, 164,179; PLT/P, 54,841; R1/PLT/PLS, 18,790; in total 237,810 | 0.18% | n.a. | |
| Crocco et al., 2009 [21] | RS, 2 centres | MCS+, PCS+, Trima Accel, always SN | VVR, CT | centre-specific | PLS, 38,647; PLT, 2,641; MC, 8,784; in total 50,072 | 0.63% | SAE ≤ 0.01% | |
AD = Apheresis donors; DC = donor compliance; GA = granulocyte apheresis; M = mild; MC = multi-component apheresis; MOD = moderate; n.a. = not available; n.d. = not done; PLT/PLS = plateletpheresis/plasmapheresis combined; PS = prospective; R1/R2 = single red cell apheresis/double red cell apheresis; R1/PLT/P = single red cell/platelet/plasma-apheresis, combined; RS = retrospective; SAE = severe adverse event; TE = technical events.
For these obvious reasons there is a need to define, to assess and to grade AE in haemapheresis in a manner that allows comparability. To accomplish these aims the scientific section ‘Preparative and Therapeutic Haemapheresis’ of the German Society for Transfusion Medicine and Immunohaematology (DGTI) formed a ‘Haemapheresis Vigilance Working Party’ (Arbeitsgemeinschaft Hämapheresevigilanz; AGHV) in 2008. The AGHV was assigned to create an easily accessible, easily usable and safe web-based registry for comprehensive and comparable AE assessment with all apheresis devices, regardless of the manufacturer, in all types of preparative haemapheresis: PLS, PLT, red blood cell apheresis (RA), all kind of leukaphereses (autologous and allogeneic blood SC apheresis, granulocyte (PMN) apheresis, lymphocyte/monocyte (MNC) apheresis) and all possible types of multicomponent (MC) apheresis. To ensure the comparability of the data, we adopted the ‘Standard for Surveillance of Complications Related to Blood Donation’, originally released in 2008, and its update, released in December, 2014 by the International Society of Blood Transfusion (ISBT) Working Group on Donor Vigilance in cooperation with the International Haemovigilance Network (IHN) and the American Association of Blood Banks for data acquisition and evaluation [24]. The registry was embedded in a prospective observational study entitled ‘Open Prospective Multicentre Long-Term Study for Assessment of Adverse Events in the Context of Haemaphereses by Means of an Internet-Based Haemapheresis Vigilance System’ that was financed in part by the DGTI and the Swiss Red Cross. The study was approved by the Ethics Committee and by the Data Protection Commissioner of Hanover Medical School. The study is registered in ClinicalTrials.gov under NCT01576237.
Internet-Based Haemapheresis Vigilance System
General Characteristics
The main purpose of this paper is the illustration of our method to assess and evaluate donor AEs in haemaphereses. In cooperation with Aix Scientifics®, Aachen, Germany, an on-line registry was programmed (now available in German, English and French) that consists of 6 HTML pages: a title page with a wide range of search functions and an access to system-related documents (such as the study curriculum, Ethics Committee approval and the user manual), two HTML pages for AE assessment, two pages for the AE evaluation programme and one page for centre-related administration purposes. The system secures that persons (operators, medical staff, physicians, centre administrators) of one specific participating centre do not have access to the data as well as to the evaluation of data of other participating centres. The system administrator and the administrators of the participating centres cooperate with regard to the restriction of persons (‘users’ such as donor physicians and operators) with access to the system. All individual users receive a password that is linked to a certain hierarchy. Operators who carry out the haemaphereses usually enter AE data into the haemapheresis vigilance system. They have accounts with the lowest hierarchy level that enables them to save AE data as a ‘draft’. Physicians who are responsible for haemapheresis-related donor care possess an account with a higher hierarchy level so that they are able to review and finalize the AE data of their specific centre. Finalized data only are included into the automated evaluation programme. The password of the centre-administrator allows starting the centre-specific evaluation programme so that the AE evaluation will be available together with anonymized data of all other participating centres (benchmark data).
Data Safety
Data safety is performed according European, national, and state law. All data are transmitted via https connection to Aix Scientifics and are at least 128-bit-SSL-encrypted. Registered users only get access to the system. A password hierarchy automatically assigns users to data input only, to review and finalize data, or to administrative activities such as centre-related generation of apheresis profiles or centre-related data evaluation. The system secures that each centre has access to own data, but not to data of other centres.
Assessment of AEs
Apheresis Specifications
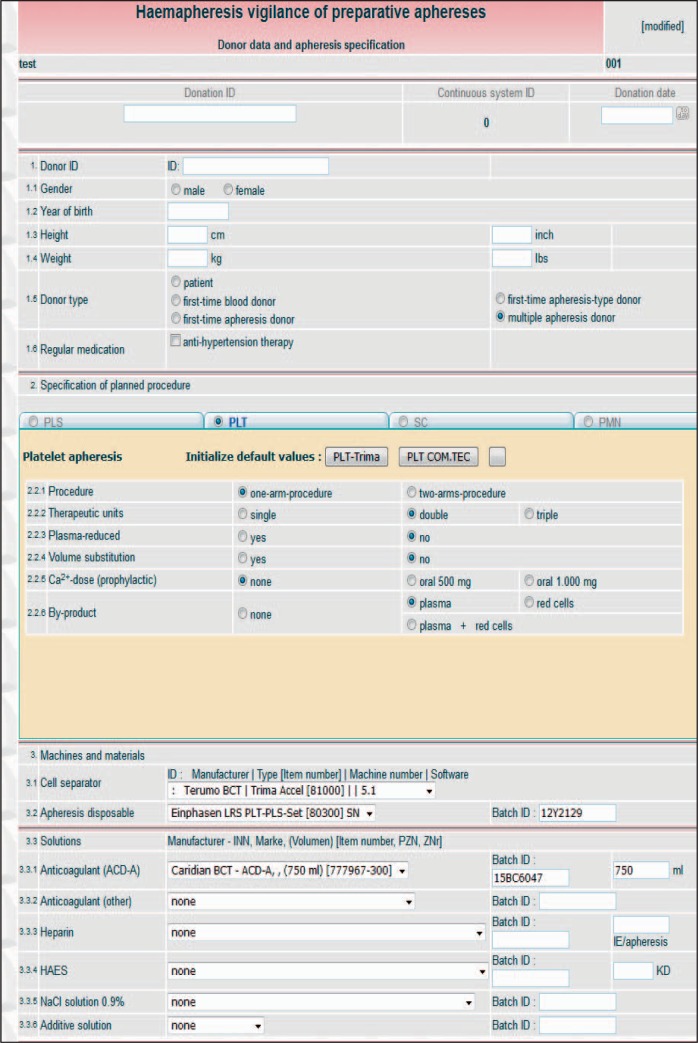
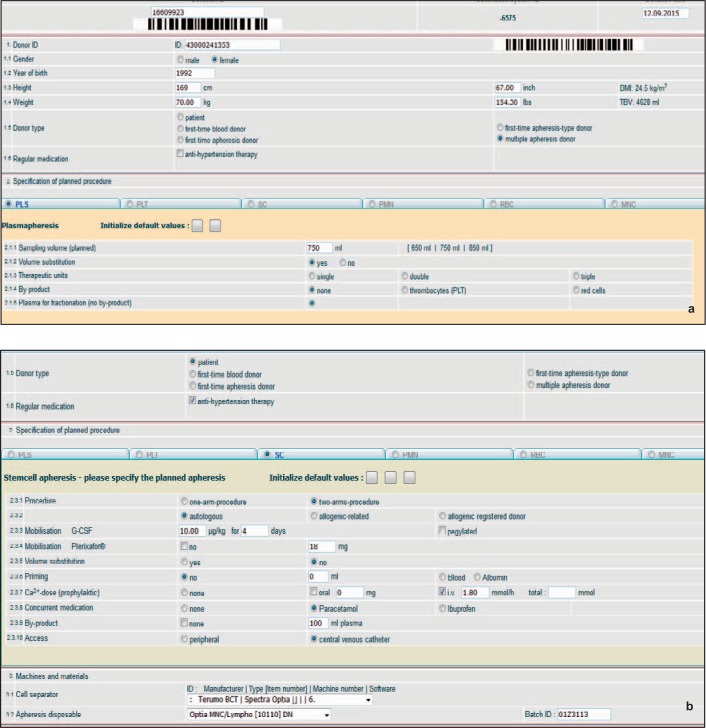
Following an appropriate password-controlled login, the user is guided to the HTML pages ‘Donor data and apheresis specification’ (fig. 1, 2) and ‘Complication data’ (fig. 3). The first page (fig. 1, overview) consists of an upper part that records donor variables. Donor data are restricted to a minimum including the specific donation number, a pseudonymised donor-ID (optional), gender, year of birth, height, body weight and donor type (e. g., first-time or repeat apheresis donor, see fig. 2a). No other donor data have to be given. The conversion of height (cm) to height (inch), of body weight (kg) to body weight (lbs) and the calculations for total donor blood volume (TBV) and donor body mass index (BMI) are automatically carried out by the system. To facilitate data input, the donation number and the donor-ID code can be entered by scanning barcodes. The middle and the lower part of the HTML page contain the specifications of the intended apheresis procedure that is involved in the AE. All types of preparative haemaphereses are available in form of electronic index cards: PLT (fig. 1), PLS (fig. 2a), SC (fig. 2b), haemaphereses for PMN, MNC and RA (not shown). If one of these electronic index cards is activated by a click into the corresponding round button, the card opens with all information to the planned apheresis procedure that may be relevant for an AE: e.g., single/double needle plateletpheresis procedures, volume replacement, CT prophylaxis and the number of target products (single-double-triple platelet units for transfusion) if they shall be routinely obtained in a PLT (fig. 1), or the intended plasma yield of 650-850 ml in a PLS (fig. 2a). Combinations of by-products can also be entered with a selection of plasma, of red cells or of plasma plus red cells, if they shall be obtained as a form a MC apheresis in single or double PLT (fig. 1). Technical data entry comprehends information to the apheresis device including machine and software identification, the lot numbers of the machine disposables (tubing sets and citrate/saline fluidities) and other specific variables such as the use of additive solution in PLT (fig. 1). The system is also able to include complex haemaphereses such as blood stem cell procedures (fig. 2b). This index card provides input positions that are unique for this type of apheresis: e.g., details to the donor (autologous or allogeneic), to the mobilization of the donor (G-CSF ± plerixafor), to priming procedures with red cells or albumin in case of paediatric SC, a centre-specific CT prophylaxis regimen, potential by-products and a central venous catheter as a possibility for blood access (fig. 2b).
Fig. 1.
Donor data and apheresis specification HTML page: overview, displaying data entry possibilities for donor and apheresis variables (here: a double PLT/PLS multi-component procedure).
Fig. 2.
a Data entry in PLS: donor data and selected procedure variables. b Specific features for data entry in SC apheresis. Example of an autologous SC apheresis. The patient received G-CSF plus plerixafor as mobilization regimen. A central venous catheter was chosen as blood access. Routine CT prophylaxis as well as routine plasma collection as a by-product of the procedure can be easily entered, either individually or as a default, if a specific SC apheresis procedure is routinely applied.
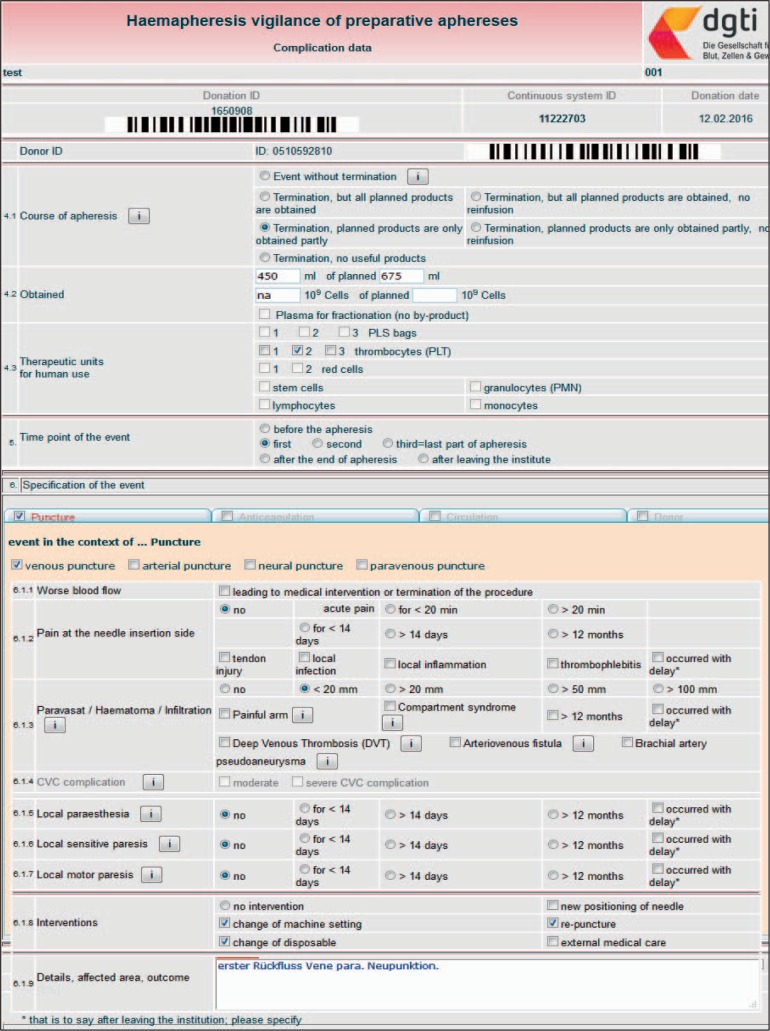
Fig. 3.
Adverse event data HTML page: overview, displaying data entry possibilities for outcome variables and AE specification. Here: a complex BAI in a triple PLT procedure. The first venepuncture developed a haematoma within a few minutes after the start of PLT. After the break-off the machine was newly equipped with a second tubing kit and a second venepuncture was done (given as ‘re-puncture’ and ‘change of disposable’ in section ‘6.1.8 Interventions’). The second PLT was successful, but due to donor time shortage two out of three planned units of apheresis platelets were obtained only (given in line 4.1 ‘Termination, planned products are obtained partly’ and in line 4.3 ‘Therapeutic units for human use’).
Apheresis Procedure Defaults
As shown in figure 1, many variables characterise apheresis procedures that are routinely done in a specific apheresis centre. For instance, a routine PLT may comprehend 16-18 positions for data entry (fig. 1) because each variable may influence the type, the frequency and/or the severity of AEs. The specifications of other haemaphereses exhibit a similar extent (PLS, fig. 2a) or are even more complex (SC, fig. 2b). To simplify and accelerate the data entry for apheresis procedures, the centre administrator is enabled to generate electronic apheresis profiles with a large number of defaults that characterise a routine apheresis procedure in a specific centre. A skilful use of this feature reduces the number of operator-associated mandatory data entries from 15-20 per apheresis type to just two positions: the lot numbers for the apheresis tubing set and for the ACD-A solution. Of note, apheresis profiles with a large number of defaults can be generated for all types of apheresis procedures, even for procedures as complex as SC aphereses.
Assessment of Complications
The Complication Data HTML page (fig. 3, overview) consists of an upper part that records the course of the apheresis. This section states whether the AE was associated with a procedure break-off and at which point in time the AE occurred (fig. 2). Moreover, the course of the AE apheresis is specified with respect to products for transfusion that could be obtained despite the break-off and with respect to a possible re-infusion of the whole blood / anticoagulant mixture that was located in the tubing set when the break-off occurred. The lower part of the Complication Data HTML page contains the specifications of the apheresis-related AE. All types of AEs are available in form of electronic index cards: AE in the context of ‘puncture’ (BAI), ‘anticoagulation’ CT), ‘circulation’ (VVR), ‘donor events’, (DEs), ‘technical events’ (TEs) and ‘miscellaneous events’ (MEs). If one of these electronic index cards is activated by a click into the corresponding square click box, a tripartite submenu opens with the structure: symptoms typically associated with the AE that entitled the index card, possible interventions of the apheresis operator or donor physician to treat the AE and a free-text area for a brief comment to the AE (optional). The BAI (fig. 3) and VVR (fig. 4a) symptoms are given in a way that all features of the latest IHN Standard for Surveillance of Complications Related to Blood Donation are represented [24]. Although they are not a topic of the IHN standard, we also included DEs (not shown) and TEs. DEs comprise blood count abnormalities (e g. leucocytosis, low platelet counts) and donor compliance problems that might cause a break-off. TEs include a variety of machine-, software- or disposable-related shortcomings because they occasionally cause significant difficulties and/or break-offs during haemaphereses (fig. 4b).
Fig. 4.
a Assessment of vasovagal reactions. Break-off 45 min after the start of the apheresis due to a mild, but therapy-refractory VVR. b Assessment of technical events. Technical AE, here version in German. The event was automatically graded as ‘severe’. In severe reactions the user who finalizes the AE has to state the imputability of the AE to the apheresis procedure.
Automated Grading of AEs
The haemapheresis vigilance system classifies symptoms as ‘mild’, ‘moderate’ or ‘severe’ without any user interaction according to an internal algorithm that includes symptom severity as well as staff interventions. To illustrate the function of this algorithm, we here present three examples: An uncomplicated haemapheresis-related small haematoma with a diameter of 20-50 mm is graded as a ‘mild’ symptom. However, if the donor requires external medical help outside the apheresis unit for haematoma treatment (e.g. because a branch of the brachial artery was hurt), it is classified as a ‘severe’ complication. CT symptoms responding quickly to calcium orally are considered to be ‘mild’. If intravenous (i.v.) calcium is required however, the grading increases to ‘moderate’, or even to severe if relatively large amounts of i.v. calcium (≥3 ampoules) have to be administered. An uncomplicated loss of consciousness (LOC) on-side the apheresis unit, without any further harm for the donor, is graded as ‘mild’, if it lasts less than 60 s, and as ‘moderate’ in case of a LOC > 60 s. However, a LOC outside the apheresis unit, even if it happens without any harm for the donor, is considered to be a ‘severe’ reaction as it potentially threatens a donor to a much higher degree than the same reaction on-side the unit.
Automated Evaluation of AEs
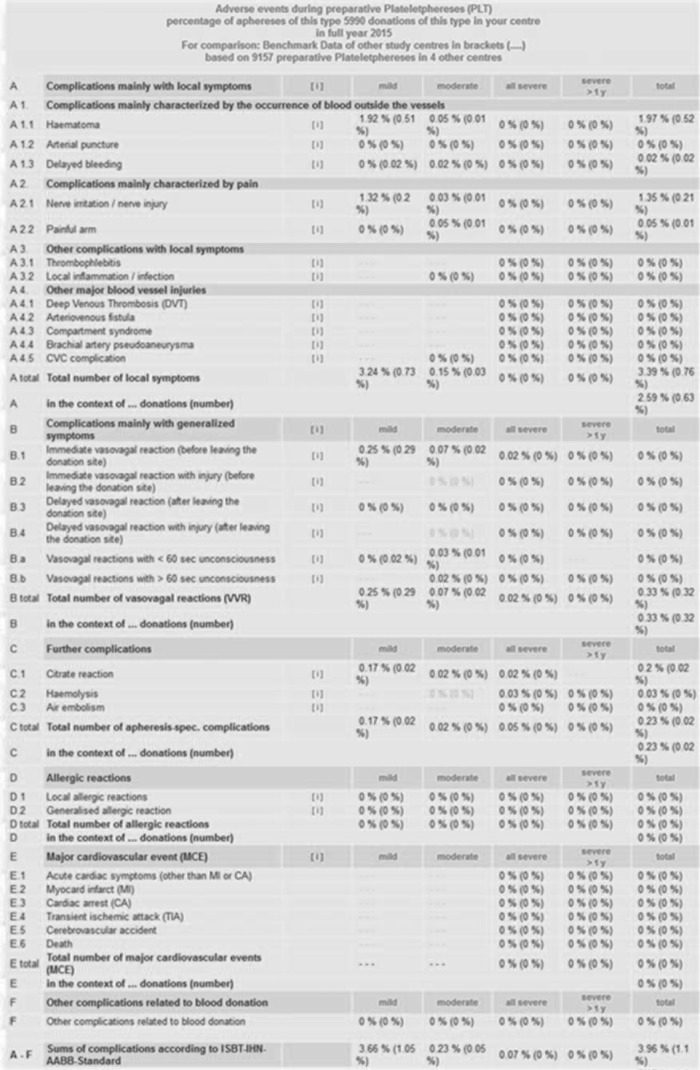
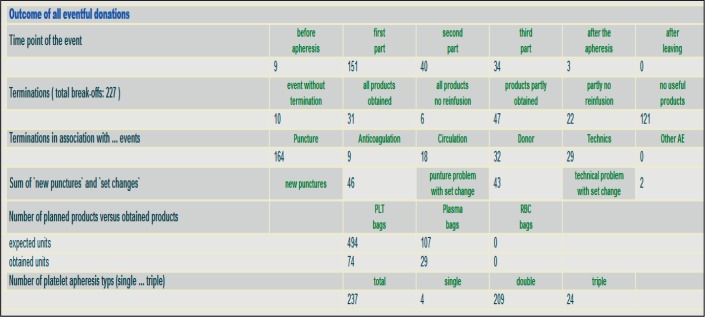
The centre administrator is qualified to start the evaluation programme for his specific centre. The AEs are evaluated for each type of apheresis procedure separately (e. g. PLT or PLS or SC apheresis etc.). Evaluations are possible for a time span ranging from 1 month to 1 calendar year. The AE results emerge either as absolute numbers or as relative numbers (percent values). Data evaluation follows the IHN standard (December 2014) and the AGHV pre-settings (June 2016). IHN standard evaluation data are shown in the sequence and order of the IHN standard (upper part of the ‘Evaluation Data’ HTML page, from A (Local Symptoms) to F (Other AE), see fig. 5). The specific AGHV evaluation pre-settings are given on the lower part of the Evaluation Data HTML page, see figure 6. In this part the data are presented in the order SAE, break-offs, planned blood products versus obtained blood products despite break-off, and demographic donor data. The evaluation process takes place within approximately 60 s for the absolute AE numbers as well as for the relative AE numbers (percent calculations including the corresponding benchmark results that are given as percent values in brackets. For example, mild VVR from 5,990 combined PLT/PLS in Hanover in 2015 occurred at a rate of 0.25% compared to 0.29% as benchmark result from 9,157 PLT procedures that were performed in four other participating centres in 2015 (fig. 5, see line B total: Total number of vasovagal reactions). A detail for the depth of the evaluation according to the AGHV pre-settings is given in figure 6. This kind of automated evaluation does not only comprise break-offs but also reasons for break-offs (puncture-, CT(anticoagulation)-, circulation-related etc.), the type of PLT procedures that were involved in break-offs (single, double or triple PLT) and a comparison between the numbers of planned blood products at start of PLT versus the numbers of blood products that could be obtained at the time when the PLT had to be terminated.
Fig. 5.
Automated evaluation according to IHN-standard 2014. Data from Hanover Medical School 2015 as an example for the function of the system. The design of the evaluation follows the sequence and order of the AE as enlisted in the IHN standard 2014. The percent values without brackets represent the AE results for PLT (usually double PLT plus plasma or triple PLT). For comparison the system also provides AE percent values in brackets. These benchmark values arise from 9,157 PLTs that were done in 4 other centres in 2015.
Fig. 6.
Automated evaluation according to AGHV standard (here: break-off during PLT, detail). Data from Hanover Medical School 2015 as an example for the function of the system. The automated evaluation does not only comprehend break-offs, but also reasons for break-offs (puncture-, citrate toxicity (anticoagulation)-, circulation-related etc.), the type of PLT procedures that were involved in the break-off (single, double or triple PLT) and a comparison between the numbers of planned blood products at start of PLT versus the numbers of blood products that had been already obtained when the PLT had to be terminated.
Preliminary Multi-Centre Study Results
To give the reader an impression of the power and quality of the collected data and of the performance of the system, here we present a short overview of data entry from all centres that participate in the multi-centre trial. From January 2012 to December 2015 the on-line haemapheresis vigilance system collected 16,744 AEs out of approximately 345,000 haemaphereses (4.9%). The majority of the AEs occurred in preparative PLSs (63%, see table 2), followed by PLTs at 34.5% and blood SC aphereses at 2.2%. BAIs account for about 55% of the supplied AEs. DEs (e.g., blood count irregularities, lipaemic plasma, termination of the procedure at the request of the donor), anticoagulation (CT) symptoms or circulation complications equally affected haemaphereses at about 11-15%, whereas TEs (e.g., disposable leakages, hardware or software crash) narrowed 10% (table 3). Out of 12,348 finalized AEs, 8,759 (70.1%) were associated with a break-off of the procedure (table 4). Interestingly, CT-related AEs had the lowest relative break-off rate, indicating that this type of AE is easier to manage than other complications.
Table 2.
On-line haemapheresis vigilance system: allocation of AEs to type of apheresis procedure
| Apheresis procedure | Frequency | Percent |
|---|---|---|
| PLS | 10,565 | 63.03 |
| PLT | 5,769 | 34.52 |
| SC | 363 | 2.17 |
| MNC | 36 | 0.22 |
| Erythrocytes | 10 | 0.06 |
| PMN | 1 | 0.01 |
| Total | 16,744 | 100.00 |
Table 3.
On-line haemapheresis vigilance system: Kind and frequency of AEs
| Type of AE | Frequency, n | Frequency, % |
|---|---|---|
| Blood access | 9,372 | 54.5 |
| CT | 1,894 | 11.0 |
| Circulation | 1,955 | 11.4 |
| Des | 2,527 | 14.7 |
| TEs | 1,427 | 8.3 |
| MEs | 17 | 0.1 |
| Total* | 17,192* | 100.0 |
Including 478 aphereses with ‘double events’ (e.g., a ‘late’ haematoma at 60 min after the start of the haemapheresis due to ‘motorical restlessness’ (= uncontrolled arm movements) of the donor: blood access injury (event 1) plus donor compliance problem (event 2, ‘double events’).
Table 4.
On-line haemapheresis vigilance system: Procedure-related break-offs during haemaphereses
| Type of AE | Finalized* | Break-off |
||
|---|---|---|---|---|
| n | % | |||
| Blood access | 6,955 | 5,463 | 78,5 | |
| CT | 1,534 | 161 | 10,5 | |
| Circulation | 1,385 | 1,002 | 72,3 | |
| DEs | 1,535 | 1,425 | 92,8 | |
| TEs | 937 | 707 | 75,5 | |
| Total | 12,348 | 8,759 | 70,1 | |
Including finalized AE only, i.e., data accepted by a physician or another person with a higher password hierarchy and responsibility for donor care.
Discussion
International as well as German regulations prescribe to assess and to evaluate donor complications in blood donation carefully [25,26]. Our on-line haemapheresis vigilance system was designed as a modern solution for assessment of these complications in the versatile field of preparative haemapheresis. The system is able to display virtually every routine apheresis procedure regardless which apheresis machine is used, which type of cells is collected or which combination of products (cellular products or plasma plus cellular blood components) are obtained (fig. 1, 2). With respect to complication data, our system is able to assess all possible AEs in a way that the IHN Standard for Surveillance of Complications Related to Blood Donation is followed. As shown in figure 3, we have recently revised our system so that the 2nd edition of the IHN standard (effective from December 2014) with, e.g., rare but dangerous puncture-related symptoms such as deep venous thrombosis, arterio-venous fistula and brachial artery pseudoaneurysm and all other changes could be included. Another attractive feature of our on-line system is the automated evaluation. Here again the latest version of the IHN standard was followed. This is shown by the arrangement of the symptoms shown on the upper part the evaluation HTML page (fig. 5). The symptoms strictly follow the nomenclature and the order of the IHN standard 2014. A further strength of the system is the possibility to exactly assess whether an apheresis procedure had to be prematurely terminated (fig. 3, table 4). This is an important specification as it makes a difference whether a procedure had to be finished a few minutes after the start of the apheresis (without any products obtained) or shortly before the intended completion of the procedure (maybe with all planned products obtained, fig. 3).
The Open Prospective Multi-Centre Long-Term Study for Assessment of AEs in Haemaphereses via an On-Line Haemapheresis Vigilance System that underlies the system was designed as a 7-year observational study. As some participating centres needed the first months in 2012 to start data assessment, the study will be presumably closed by June 30, 2019. A comprehensive and detailed publication of all study data is planned for 2020. Here we present a small subset of preliminary data, just to demonstrate the potential and the power of the system. From January 2012 to December 2015 a total of 16,744 complications out of approximately 345,000 haemaphereses (4.9%) were entered into the system by all participating centres. This value is in good accordance to other reports on donor complications in apheresis with a similarly large body of data [16,20]. Preparative PLSs were most often affected (63%), followed by PLTs (34.5%, table 5). This is not surprising as both PLSs and PLTs account for the apheresis techniques that are most often applied in Germany [6]]. Of note, our system will probably assess more SAEs than previously reported [13,14,15,23]. This is, at least in part, due to assessment of technical (e.g., disposable leakage) and donor characteristics typically associated with deferral from haemapheresis (e.g., blood count abnormalities). The latter ones are usually not part of donor haemovigilance systems but are also important to assess.
Table 5.
Composition of the on-line haemapheresis vigilance system
| HTML page | Functions | Examples, see figure |
|---|---|---|
| Title page | start of data entry, search functions, access to system-related documents | n.s. |
| Apheresis specification | donor and procedure variables (overview) | 1 |
| procedure variables (here plasmapheresis, stem cell apheresis) | 2a,b | |
| Complication data | outcome and AE specification variables (overview) | 3 |
| blood access injury (‘puncture’) | 3 | |
| vasovagal reaction (‘circulation’) | 4a | |
| technical problem (‘technics’) | 4b | |
| Centre administration | default entry, start of evaluation | n.s. |
| Apheresis numbers | apheresis numbers, procedure-specific | n.s. |
| Evaluation | evaluation according IHN 2014 | 5 |
| Evaluation | evaluation according AGHV 2016 | 6 |
n.s. = Not shown.
From the very beginning, our on-line haemapheresis vigilance system was aimed to fulfil three different purposes:
- First and clearly most important, to provide a platform for comprehensive and easy assessment and automated evaluation of donor complications.
- Second, to deliver basic information for staff education. For example, haematoma are not only a question of donor safety but may also trigger a training course for single staff members or for the whole operator group.
- Third, to numeralize potential economic losses that are associated with premature break-offs of haemaphereses. A break-off of a whole blood donation may charge a blood donation service with a relatively small amount in the range of EUR 10.00, if the blood bag system is considered. In contrast, a break-off in a haemapheresis will charge the apheresis unit with costs far beyond EUR 100.00, if the apheresis disposable for a PLT is considered.
Thus our on-line system provides assessment and evaluation tools to support apheresis units in the versatile and complex field of haemapheresis.
Conclusions and Prospects
To our knowledge, we present here the first web-based system for assessment of AEs in haemaphereses that is based on the IHN Standard for Surveillance of Complications Related to Blood Donation. The system is in operation since January 2012. It is working continuously and stable during an operation time of more than 4 years. The system is going to be continuously developed. This is shown by the recent integration of the IHN standard 2014 update. It is comprehensive as all kind of preparative haemaphereses including all conceivable product combinations, even in terms of MC apheresis, and all types of AEs can be supplied. It provides a reliable grading of AE as it works with an internal algorithm that excludes a subjective operator-derived bias, thereby ensuring the comparability of the data. The system presents a unique and highly automated evaluation tool that offers a quick and comprehensive analysis of the centre-specific haemapheresis-related AEs within 2-3 min. The evaluation tool has adopted the IHN standard, includes a benchmark function for comparison of AE data within the system and offers additional features such as break-off evaluation.
Our on-line haemapheresis vigilance platform is intended as a gradually growing system. Further developments may include the creation of interfaces to automate the import of, e.g., donor data or apheresis specifications and to establish data transfer to regulatory authorities in case of SAEs. Furthermore, in addition to the now available IHNstandard evaluation, other automated evaluation modes are being developed. These evaluation modes will be based on specific parameters (e.g., evaluation of VVR or CT in context with, e.g., donor body weight, gender and apheresis procedure) and will allow an even more detailed automated AE evaluation in haemaphereses.
Our on-line system offers a ‘low-threshold’ opportunity to assess and evaluate apheresis-related complications. Low threshold means that simple registration, predominantly self-explaining items and, as a non-profit project, reasonable costs facilitate the participation to the system. After appropriate registration the on-line access allows every centre active in preparative aphereses from everywhere in the world to enter data into our system and to benefit from an automated evaluation according to internationally accepted standards. Theoretically, on-line registries like ours have the potential to overcome the variety of haemovigilance studies (as shown in table 1) with their widely incongruent and conflicting results.
Disclosure Statement
The authors certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in this manuscript (e.g., employment, consultancies, board membership, stock ownership, honoraria). The research or project support for this multi-centre trial was as follows: The study is, at least in part, financed by grants from the German Society for Transfusion Medicine and Immunohaematology (DGTI) and the Swiss Red Cross (SRC).
Acknowledgement
The authors thank the staff members, in particular operators, donor physicians and centre administrators, of the participating centres for their efforts in data collection and for their help and support in the improvement of the on-line haemapheresis vigilance system. Currently (August, 2016) the following centres participate in the study: Bavarian Red Cross (Centre Munich), German Red Cross Lower Saxony, Thuringia, Oldenburg and Bremen (centres Dessau and Oldenburg), German Red Cross North-East (centres Chemnitz, Cottbus, Dresden, Plauen, Potsdam, Zwickau), Swiss Red Cross Luzern, Blood Donation Services from/within University Clinics Basel, Erlangen, Hanover (Hanover Medical School), Münster and Vienna.
References
- 1.Heuft HG, Moog R, Fischer EG, Zingsem J, German and Austrian Plateletpheresis Study Group Donor safety in triple plateletpheresis: results from the German and Austrian Plateletpheresis Study Group multicenter trial. Transfusion. 2013;53:211–220. doi: 10.1111/j.1537-2995.2012.03714.x. [DOI] [PubMed] [Google Scholar]
- 2.Thorausch K, Schulz M, Bialleck H, Luxembourg B, Seifried E, Bonig H. Granulocyte collections: comparison of two apheresis systems. Transfusion. 2013;53:3262–3268. doi: 10.1111/trf.12197. [DOI] [PubMed] [Google Scholar]
- 3.Brauninger S, Bialleck H, Thorausch K, Felt T, Seifried E, Bonig H. Allogeneic donor peripheral blood stem cell apheresis: prospective comparison of two apheresis systems. Transfusion. 2012;52:1137–1145. doi: 10.1111/j.1537-2995.2011.03414.x. [DOI] [PubMed] [Google Scholar]
- 4.Heuft H-G, Goudeva L, Martens J, Krettek U, Priesner C, Aleksandrova K, Blasczyk R. T-cell collections from healthy donors for therapeutic use. Transfus Med Hemother. 2015;42((suppl 1)):54. (abstract). [Google Scholar]
- 5.Picker SM, Radojska SM, Gathof BS. Evaluation of concurrent collection of in-line filtered platelets and packed red blood cells by multicomponent apheresis with three last-generation apparatuses. Vox Sang. 2006;91:47–55. doi: 10.1111/j.1423-0410.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 6.Report to the Withdrawal, Production, Importation, Exportation and Therapeutic Application of Blood Components according §21 German Transfusion Law (in German). www.pei.de/DE/infos/meldepflichtige/meldung-blutprodukte-21-transfusionsgesetz/berichte/berichte-21tfg-node.html (last accessed October 26, 2016).
- 7.Tullis JL. Separation and purification of leucocytes and platelets. Blood. 1952;7:891–896. [PubMed] [Google Scholar]
- 8.Maurício R, de Sousa G, Seghatchian J. What's happening: an overview of potential adverse reactions associated with apheresis technology. Transfus Apher Sci. 2005;33:351–356. doi: 10.1016/j.transci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Danic B, Lefort C. Serious adverse effects of blood collection (in French) Transfus Clin Biol. 2010;17:301–305. doi: 10.1016/j.tracli.2010.09.164. [DOI] [PubMed] [Google Scholar]
- 10.Eder AF, Dy BA, Kennedy JM, Notari Iv EP, Strupp A, Wissel ME, Reddy R, Gibble J, Haimowitz MD, Newman BH, Chambers LA, Hillyer CD, Benjamin RJ. The American Red Cross donor hemovigilance program: complications of blood donation reported in 2006. Transfusion. 2008;48:1809–1819. doi: 10.1111/j.1537-2995.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 11.Diekamp U, Gneißl J, Rabe A, Kießig ST. Donor hemovigilance during preparatory plasmapheresis. Transfus Med Hemother. 2014;41:123–133. doi: 10.1159/000357991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLeod BC, Price TH, Owen H, Ciavarella D, Sniecinski I, Randels MJ, Smith JW. Frequency of immediate adverse effects associated with apheresis donation. Transfusion. 1998;38:938–943. doi: 10.1046/j.1537-2995.1998.381098440858.x. [DOI] [PubMed] [Google Scholar]
- 13.Despotis GJ, Goodnough LT, Dynis M, Baorto D, Spitznagel E. Adverse events in platelet apheresis donors: a multivariate analysis in a hospital-based program. Vox Sang. 1999;77:24–32. doi: 10.1159/000031070. [DOI] [PubMed] [Google Scholar]
- 14.Yuan S, Gornbein J, Smeltzer B, Ziman AF, Lu Q, Goldfinger D. Risk factors for acute, moderate to severe donor reactions associated with multicomponent apheresis collections. Transfusion. 2008;48:1213–1219. doi: 10.1111/j.1537-2995.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 15.Yuan S, Ziman A, Smeltzer B, Lu Q, Goldfinger D. Moderate and severe adverse events associated with apheresis donations: incidences and risk factors. Transfusion. 2010;50:478–486. doi: 10.1111/j.1537-2995.2009.02443.x. [DOI] [PubMed] [Google Scholar]
- 16.Wiltbank TB, Giordano GF. The safety profile of automated collections: an analysis of more than 1 million collections. Transfusion. 2007;47:1002–1005. doi: 10.1111/j.1537-2995.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 17.Ounnoughene N, Sandid I, Carlier M, Joussemet M, Ferry N. The blood donors' haemovigilance in France (in French) Transfus Clin Biol. 2013;20:182–192. doi: 10.1016/j.tracli.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Bueno JL, Castro E, García F, Barea L, González R. Hematomas in multicomponent apheresis: searching for related factors. Transfusion. 2006;46:2184–2191. doi: 10.1111/j.1537-2995.2006.01050.x. [DOI] [PubMed] [Google Scholar]
- 19.Tomita T, Takayanagi M, Kiwada K, Mieda A, Takahashi C, Hata T. Vasovagal reactions in apheresis donors. Transfusion. 2002;42:1561–1566. doi: 10.1046/j.1537-2995.2002.00241.x. [DOI] [PubMed] [Google Scholar]
- 20.Kamel H, Tomasulo P, Bravo M, Wiltbank T, Cusick R, James RC, Custer B. Delayed adverse reactions to blood donation. Transfusion. 2010;50:556–565. doi: 10.1111/j.1537-2995.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 21.Crocco I, Franchini M, Garozzo G, Gandini AR, Gandini G, Bonomo P, Aprili G. Adverse reactions in blood and apheresis donors: experience from two Italian transfusion centres. Blood Transfus. 2009;7:35–38. doi: 10.2450/2008.0018-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosencher J, Zuily S, Varenne O, Spaulding C, Weber S. Acute myocardial infarction secondary to platelet apheresis in a 57-year healthy donor. Int J Cardiol. 2011;150:119–120. doi: 10.1016/j.ijcard.2010.02.069. [DOI] [PubMed] [Google Scholar]
- 23.Song EY, Yoon JH, Lee JW, Park CW, Kwon SW, Kim DW, Lim YA, Kim HO, Han KS. Establishment of a national on-line registry for apheresis in Korea. Transfus Apher Sci. 2008;38:93–100. doi: 10.1016/j.transci.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Working Group on Donor Vigilance Standard for Surveillance of Complications Related to Blood Donation. Released in December 2014 by the International Society of Blood Transfusion (ISBT) in cooperation with the International Haemovigilance Network and the American Association of Blood Banks. www.isbtweb.org/fileadmin/user_upload/2_Donor-Standard-Definitions.pdf (last accessed October 26, 2016).
- 25.Richtlinie 2002/98/EG des europäischen Parlaments und des Rates vom 27. Januar 2003 zur Festlegung von Qualitäts- und Sicherheitsstandards für die Gewinnung, Testung, Verarbeitung, Lagerung und Verteilung von menschlichem Blut und Blutbestandteilen und zur Änderung der Richtlinie 2001/83/EG. Veröffentlicht im Amtsblatt der europäischen Union vom 08.02.2003
- 26.Gesetz über den Verkehr mit Arzneimitteln. www.gesetze-im-internet.de/amg_1976/63i.html (last accessed October 26, 2016).