Abstract
From 7% to 10% of all retinoblastomas and from 44% to 71% of familial retinoblastomas in developed countries are diagnosed in the neonatal period, usually through pre- or post-natal screening prompted by a positive family history and sometimes serendipitously during screening for retinopathy of prematurity or other reasons. In developing countries, neonatal diagnosis of retinoblastoma has been less common. Neonatal retinoblastoma generally develops from a germline mutation of RB1, the retinoblastoma gene, even when the family history is negative and is thus usually hereditary. At least one-half of infants with neonatal retinoblastoma have unilateral tumors when the diagnosis is made, typically the International Intraocular Retinoblastoma Classification (Murphree) Group B or higher, but most germline mutation carriers will progress to bilateral involvement, typically Group A in the fellow eye. Neonatal leukokoria usually leads to the diagnosis in children without a family history of retinoblastoma, and a Group C tumor or higher is typical in the more advanced involved eye. Almost all infants with neonatal retinoblastoma have at least one eye with a tumor in proximity to the foveola, but the macula of the fellow eye is frequently spared. Consequently, loss of reading vision from both eyes is exceptional. A primary ectopic intracranial neuroblastic tumor known as trilateral retinoblastoma is no more common after neonatal than other retinoblastoma. For many reasons, neonatal retinoblastoma may be a challenge to eradicate, and the early age at diagnosis and relatively small tumors do not guarantee the preservation of both eyes of every involved child. Oncology nurses can be instrumental in contributing to better outcomes by ensuring that hereditary retinoblastoma survivors receive genetic counseling, by referring families of survivors to early screening programs when they are planning for a baby, and by providing psychological and practical support for parents when neonatal retinoblastoma has been diagnosed.
Keywords: Germline mutation, neonatal cancer, prenatal diagnosis, retinoblastoma

Introduction
Neonatal cancer is defined as a malignant neoplasm that is diagnosed during the first 4 weeks of life (within 28 postnatal days) which, in the case of preterm birth, is equivalent to diagnosis at <44 weeks of gestational age.[1,2,3] These cancers likely arise during embryonic or fetal development from immature tissues because of intrinsic disturbances of cell proliferation and growth.[1] A mutation in the retinoblastoma gene RB1, usually inherited, is one defect that may lead to such an early disturbance and thus can initiate cancerogenesis.
Frequency of Neonatal Retinoblastoma
According to several series, retinoblastoma is responsible for 4%–29% of solid neonatal cancers (excluding hematological neoplasms and nonmalignant teratomas) that make up 1%–3% of childhood malignancies [Table 1]. Conversely, 7%–10% of retinoblastomas are diagnosed in neonates. The experience in the Ocular Oncology Service of the Helsinki University Hospital, a national referral center for retinoblastoma in Finland, is similar to these series: of 116 consecutive retinoblastomas diagnosed during the past three decades, 11 (9%; 95% confidence interval [CI], 5–16) were detected during the neonatal period. Given the estimated number of 8100 retinoblastomas diagnosed annually worldwide,[10,11] one could expect 570–810 of these tumors to be neonatal.
Table 1.
Frequency of neonatal retinoblastoma among solid cancers of childhood
| Country | Period | Pediatric cancer | Retinoblastoma | |||
|---|---|---|---|---|---|---|
| All (n) | Neonatal, n (%) | All (n) | Neonatal, n (%) | Percentage of neonatal cancer (95% CI) | ||
| Canada[4] | 1922-1982 | 3681 | 94 (2.6) | 148 | 17 (11.0) | 18 (11-27) |
| USA[5] | 1941-1991 | 32 | 3 | 9 (2-25) | ||
| Denmark[6] | 1943-1985 | 56 | 2 | 4 (0-12) | ||
| UK[7] | 1960-1989 | 54 | 5 | 9 (3-20) | ||
| USA[8] | 1962-1988 | 28 | 3 | 11 (2-28) | ||
| Turkey[9] | 1972-2000 | 21 | 3 | 14 (3-36) | ||
| USA[1] | 1980-1998 | 899 | 14 (1.6) | 55 | 4 (7.0) | 29 (8-58) |
CI: Confidence interval
The available data come from developed countries in which retinoblastomas are diagnosed at a median age of 1 month when a positive family history leads to screening that begins before or after birth, making most of these cases neonatal. In developing countries, the median age at diagnosis of familial retinoblastoma is 6 months.[12] Altogether, 23% of familial retinoblastoma in developing as opposed to 72% in developed countries were detected through screening, suggesting that neonatal diagnosis of retinoblastoma may be three times more frequent in the latter. Given that the majority of retinoblastomas is diagnosed in developing countries,[10,11] a more likely estimate may thus approach a third of the figures above or 190–270 neonatal retinoblastomas per year worldwide. These are still large numbers, and neonatal patients with retinoblastoma form a distinct subgroup that has certain typical characteristics in common.
Diagnosis of Neonatal Retinoblastoma
Diagnosis before birth
With the help of prenatal genetic testing, when available, it is now possible to predict before birth whether or not a child will develop retinoblastoma when the mother or father carries a known germline mutation.[13,14] Should the prenatal test be positive, the eyes of the fetus can be screened either with ultrasonography or magnetic resonance imaging during the third trimester.[14,15,16,17]
In five small case series, prenatal screening identified retinoblastomas during pregnancy in 3 of 14 (21%) children who carried a germline mutation [Table 2], and in two cases, labor was thereafter induced to treat the tumor earlier, which is a controversial approach.[18] All three children had bilateral International Intraocular Retinoblastoma Classification (Murphree)[25,26] Group B tumors. In three of the remaining children, retinoblastoma was present upon birth, and in the others their first focus typically appeared during the first 6 weeks of postnatal life. Thus, prenatal as compared to prompt postnatal screening does not automatically lead to expedited management of retinoblastoma in predisposed children.
Table 2.
Retinoblastomas detected in selected infants who underwent screening
| Reference | Gestational week | Family history | Laterality and IIRC group at diagnosis | Details | |
|---|---|---|---|---|---|
| Birth | Diagnosis | ||||
| Prenatal screening for retinoblastoma in mutation carriers | |||||
| Paquette et al.[15] | N/A | 37 | Yes | Bilateral B/B | US, 2 mm thick tumor |
| Neriyanuri et al.[16] | 34 | 2 years* | Yes | Unilateral A | None detected at birth (13q - deletion) |
| Staffieri et al.[17] | 38 | 4/2 weeks* | Yes | Unilateral A | None detected at 1 day |
| 37 | 6/3 weeks* | Yes | Unilateral B | None detected at 3 days | |
| 40 | - | Yes | None | By 2 years, low penetrance mutation | |
| 36 | 5/1 weeks* | Yes | Unilateral A | None detected at 2 days | |
| 35 | 35 | Yes | Bilateral B/B | MRI, 6 mm×1.9 mm and 5 mm×1.2 mm tumor | |
| Shah et al.[14] | Term | 10 days* | Yes | Bilateral B/A | None detected at 3 days |
| Soliman et al.[19] | 39 | Birth | Yes | Bilateral C/B | None detected prenatally |
| 36 | 36 | Yes | Bilateral B/B | US, detected at 34 weeks | |
| 36 | Birth | Yes | Unilateral A | Detected at birth | |
| 37 | 17/14 weeks* | Yes | Unilateral A | None detected at birth | |
| 36 | 15/11 weeks* | Yes | Bilateral B/B | None detected at birth | |
| 38 | 5/3 weeks* | Yes | Bilateral A/A | None detected at birth | |
| Postnatal screening for retinopathy of prematurity | |||||
| Amino et al.[20] | 26 | 7 weeks/33* | No | Bilateral B/A | Dizygotic twin |
| 26 | 7 weeks/33* | No | Bilateral B/B | Dizygotic twin | |
| Picton et al.[21] | 32 | 3 weeks/35* | No | Bilateral | Fetal distress |
| Self et al.[22] | 26 | 8 weeks/34* | No | Bilateral B/B | Dizygotic twin, in vitro fertilization |
| Al-Abdi[23] | 28 | 4 weeks/32* | No | Bilateral | Dizygotic twin (c. 635delT) |
| Abramson et al.[24] | 26 | 12 weeks/38* | No | Unilateral B | Dizygotic twin |
Diagnosis in premature neonates
With widespread screening for retinopathy of prematurity, especially using wide-field fundus photography, retinoblastomas are occasionally detected in premature infants [Table 2], a disproportionate number of which have been dizygotic twins, suggesting reporting bias. In one series of 1450 infants screened in neonatal intensive care units across one state in India because their birth weight was 2000 g or less or they were born at 34 weeks of gestation or earlier, 2 (0.14%; 95% CI 0.02–0.50) retinoblastomas were found.[27] The published image of one neonate shows a multifocal, posterior pole, Group C retinoblastoma with focal vitreous seeding. Another series from China found one (0.13%; 95% CI 0.00–0.71) retinoblastoma among 779 infants who either were premature or small-for-date at full term.[28] It remains open whether all three children had multifocal and thus hereditary retinoblastomas.
These observations raise the possibility that retinoblastoma might be more common in premature and small-for-date neonates, because the predicted frequency of a positive screening examination in the absence of a positive family history would of course be no more than the usual 1 in 16,600 live-born infants,[29] rather than the observed 1 in 725–779 neonates in the Asian series.
However, we cannot dismiss the null hypothesis that the investigators were just lucky to serendipitously diagnose a retinoblastoma in premature neonates because in a third study, from the same Indian state, one (0.10%; 95% CI 0.00–0.54) retinoblastoma was likewise found among 1021 infants born at term with a birth weight over 2000 g (of whom 990 had been delivered by cesarean section in a single public hospital) who were photographically screened within 3 days from birth.[30] The image shows a solitary, equatorial, Group C retinoblastoma with focal cloud-like vitreous seeding. In another series of 3573 healthy Chinese children born at gestational week 37 or later and weighing at least 2500 g who were photographed within a week from birth, two (0.06%; 95% CI 0.01–0.20) infants were suspected of harboring a retinoblastoma or an astrocytic hamartoma.[31] The image of one child shows a potential unifocal Group A retinoblastoma.
Diagnosis in neonates born at term
Neonatal retinoblastoma after an uneventful pregnancy is typically diagnosed in a child who was born to parents with a positive family history for retinoblastoma and was screened after delivery. In a nationwide study in the Netherlands, 17 children were screened for familial retinoblastoma during the first 2 postnatal weeks and then every 2 weeks, and 12 tumors were detected during the 1st month of life.[32] Thus, 71% (95% CI 44–90) of Dutch familial retinoblastomas were detected in neonates. Of 16 consecutive Finnish infants screened because of a positive family history for retinoblastoma, 7 (44%; 95% CI 20-70) were diagnosed in the neonatal period [Figure 1] or whom 2 (29%) had leukokoria upon birth. A tumor of that size probably would have been detected in a prenatal scan [Figure 2a and b]. The remaining nine children developed their first tumor later.
Figure 1.
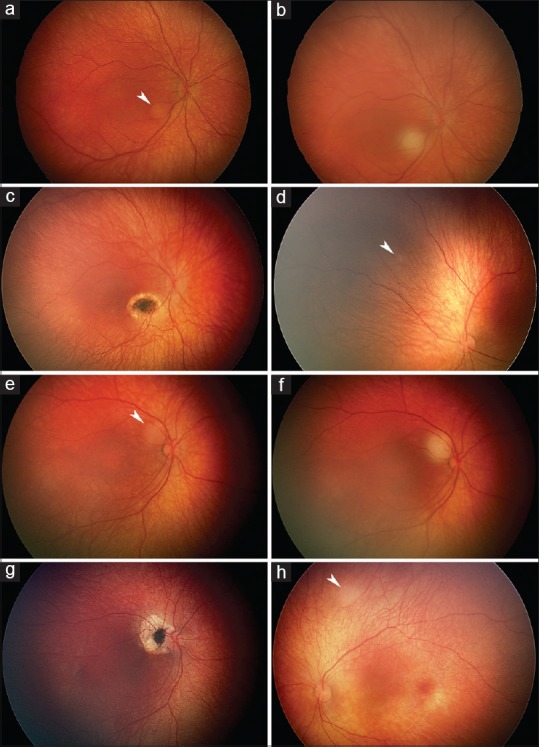
Neonatal retinoblastomas detected through screening of familial mutation carriers. Group B perifoveal 2 mm tumor as first detected during screening at the age of 10 days (a, arrowhead) and immediately after infrared diode laser transpupillary thermotherapy (b). At the age of 1 month, the tumor is replaced with a scar (c) and the first 1 mm Group A tumor is diagnosed in the fellow eye. (d, arrowhead). Group B perifoveal 1.5 mm tumor as first detected during screening at the age of 18 days (e, arrowhead) and immediately after transpupillary thermotherapy (f). At the age of 4 months, after additional thermotherapy sessions, the tumor is replaced with a scar. (g) and the first 1.5 mm Group A tumor is diagnosed in the fellow eye (h, arrowhead)
Figure 2.
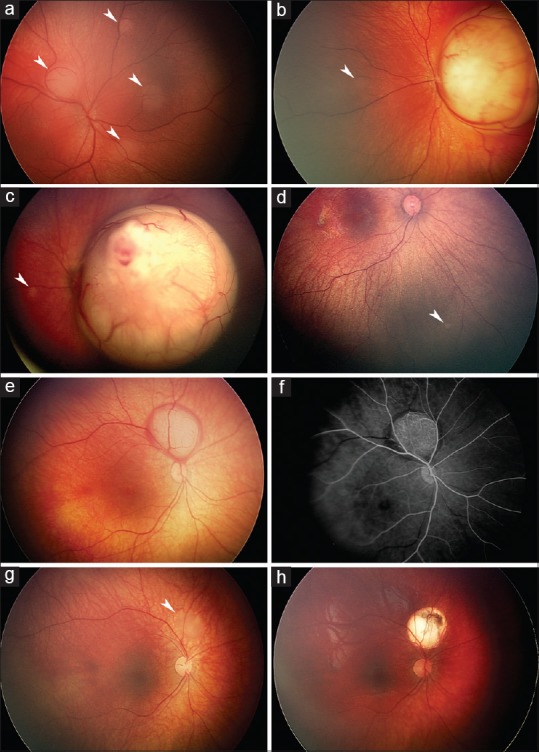
Neonatal retinoblastomas either causing leukokoria (a-d) or serendipitously detected through unrelated screening (e-h). Group B peripapillary 3 mm tumor and three smaller tumors detected during screening at the age of 3 days (a, arrowheads) with leukokoria already noted from a larger macular Group B retinoblastoma in a familial mutation carrier; a small second tumor is also visible (b, arrowhead). Group C macular tumor with focal vitreous seeding detected after leukokoria was noted at the age of 23 days; a small second tumor is also visible (c, arrowhead). At the age of 3 months, the first 0.5 mm Group A tumor is diagnosed in the fellow eye; meanwhile, a germline mutation that was not found in the parents was detected (d, arrowhead). Group B peripapillary 4.5 mm tumor surrounded by subretinal bleeding as detected during ocular screening during a neurologic workup at the age of 12 days (e). Fluorescein angiography shows prominent arterial circulation in the tumor (f) that regressed with chemoreduction (g, arrowhead) that was followed by infrared diode laser transpupillary thermotherapy (h).
Although it is considered unusual to diagnose a neonatal retinoblastoma in the absence of a positive family history,[33] series from various cancer centers have included 28%–76% of such cases [Table 3]. In a retrospective case series from New York, leukokoria led to diagnosis in 13% of neonatal retinoblastoma patients.[3] In Finland, 4 (36%) of 11 neonatal retinoblastomas were sporadic and diagnosed after noticing leukokoria at the age of 5–23 days [Figure 2b and c]. Three of the sporadic neonatal retinoblastomas progressed to bilateral disease and originated from new germline mutations [Figure 2d]. We diagnosed, however, one nonhereditary neonatal retinoblastoma in a child who was hypotonic upon birth and underwent ophthalmologic screening as part of a neurologic workup; a unilateral, unifocal retinoblastoma was detected at the age of 12 days. No germline mutation was detected, and no further tumors developed, suggesting nonhereditary retinoblastoma [Figure 2e-h].
Table 3.
Characteristics of neonatal retinoblastomas
| Country | Period | All (n) | Family history, n (%) | Laterality | Metastatic death, n (%) | |
|---|---|---|---|---|---|---|
| Unilateral*, n (%) | Bilateral*, n (%) | |||||
| Canada[4] | 1922-1982 | 17 | 4 (24) | 4 (24) | 13 (76) | 4 (24) |
| USA[8] | 1962-1988 | 3 | 3 (100) | N/A | N/A | N/A |
| Turkey[9] | 1972-2000 | 3 | N/A | 0 | 3 (100) | 3 (100) |
| USA[1] | 1980-1998 | 4 | N/A | 0 | 4 (100) | 2 (50) |
| USA[3] | N/A | 46 | 33 (72) | 26/4 (57/9) | 20/42 (43/91) | 4 (9) |
| Netherlands[32] | 1992-2004 | 12 | 12 (100)† | 5/1 (42/8) | 7/11 (58/92) | 0 |
| Finland | 1985-2015 | 11 | 7 (64) | 8/1 (73/9) | 3/10 (27/91) | 0 |
*Initially/eventually, †Restricted to familial patients. N/A: Not available
Characteristics of Neonatal Retinoblastoma
Family history
A common characteristic of neonatal retinoblastoma, as mentioned, is a positive family history [Table 3]. This is largely because the offspring of parents with a history of retinoblastoma, especially when they have tested positive for a mutation of the retinoblastoma gene, will be screened soon after birth and, sometimes, now already during pregnancy for an inherited tumor. Hence, when a neonatal tumor is present, it will be promptly diagnosed.
Bilateralization
A second common characteristic of neonatal retinoblastoma is that unilateral disease at the time of diagnosis is common [Table 2], but it has a very high rate of becoming bilateral. In the Dutch series, 5 (40%) of 12 neonatal, familial retinoblastomas were initially unilateral.[32] All except one (92%) progressed to bilateral disease within 5 months. In Finland, 7 (70%) of the 10 hereditary neonatal retinoblastomas were first unilateral, but all became bilateral within 4 months. In an earlier larger series from New York, 26 (57%) of 46 neonatal retinoblastomas were first unilateral, and 22 (85%) of them progressed to bilateral involvement.[3] Thus, probably about 50% of neonatal retinoblastoma will be unilateral when diagnosed, but the fellow eye will become involved in about 90% of cases before the age of 6 months.[32]
The remaining 10% of children who do not develop bilateral disease usually still have a positive family history[3,32] and likely carry a mutation characterized with lower penetrance and expressivity.[34] In the Retinoblastoma Referral Service of Siena, Italy, between 2000 and 2015, in 10 families with one parent who had a history of unilateral retinoblastoma, 7 (64%; 95% CI 31–89) of 11 affected children also developed a unilateral tumor that in one of them was a retinoma, whereas in 25 families with a bilateral tumor in one parent, only one (3%; 95% CI 0–18) of 29 affected children was diagnosed with unilateral retinoblastoma.
Tumor stage
The tumor in the first involved or the more advanced eye of a child with neonatal retinoblastoma is usually of a higher grade than Group A, typically assigned to Group B because of tumor proximity to the foveola or the optic disk rather than because of its size. The tumor in the first involved eye is also typically of a higher grade than it will be in the fellow eye that becomes involved later, which almost always will be diagnosed with a Group A retinoblastoma because of close screening at short intervals after diagnosis of the first eye.
In this regard, one child in the Dutch series[32] had a Group A tumor in the first involved or the more advanced eye, nine (75%) tumors were Group B, and one each was Group C and E (we reclassified tumors reported as Group A but involving the macula as Group B). Only one child had advanced disease in both eyes (Group C and E). In all children but one (with a Group B tumor), the fellow eye had, or later developed, a Group A tumor (reported as Group A without macular involvement). In Finland, all seven children diagnosed by screening had a Group B tumor in at least one eye in 5 instances so classified solely because of tumor location but in 2 cases also because of its size. Two of them had a Group B tumor in their fellow eye, and the remaining five children later developed a Group A tumor in the fellow eye. The three children without a family history had more advanced Group C, D, and E retinoblastomas and later developed a Group A tumor in the fellow eye.
Vision prognosis
The third characteristic of neonatal retinoblastoma is macular involvement, which is typically unilateral. In the Netherlands, 10 (83%) of 12 infants with a positive family history had an uninvolved macula in one eye and one child in both eyes.[32] Nine (75%) of the children maintained 20/30 or better vision in their better eye. In Finland, 8 (80%) of 10 children with hereditary neonatal retinoblastoma had one involved macula, and in all eight initially uninvolved fellow eyes, the tumor developed outside the macula. Moreover, all children maintained 20/25 vision or better in the better eye. Thus, in more than 75% of children with neonatal retinoblastoma, the macula and good vision likely will be spared in at least one eye.
On the other hand, one eye is equally likely to have compromised vision. In New York, mostly before modern chemoreduction era, 10 (38%) of 26 initially involved eyes of unilateral patients and 7 (18%) of 38 eyes of bilaterally involved children were lost.[3] In the Dutch series of 12 familial neonatal retinoblastomas, also largely before the chemoreduction era, one eye was enucleated and four children ended up with finger counting or worse vision in their more advanced eye.[32] Four (33%) children retained 20/30 vision or better in their worse eye. In the Finnish series, the 3 Group C to E eyes of patients without a family history eventually were enucleated. All seven children with a positive family history and a Group B eye with a macular tumor in their worse eye ended up with 20/400 to counting fingers vision in that eye, but none needed to be enucleated.
Tendency for local relapse
A fourth characteristic feature is that standard chemotherapy protocols that generally are effective in curing retinoblastoma in part may fail more often in neonatal retinoblastoma because of the lack of significant vascular supply in smaller tumor foci. Moreover, the neonatal fundus is typically lightly pigmented and thus transpupillary thermotherapy may not produce enough heat to kill a small focus that in an older child would be eradicated [Figure 3]. These tumors, some of which may still be lower grade lesions more akin to retinocytomas, consequently may need chemothermotherapy[35,36] or a radioactive plaque to be controlled. The preliminary experience in the Retinoblastoma Referral Service of Sienna suggests that ophthalmic artery chemosurgery may be more efficient in destroying such small tumors. In addition, neonatal retinoblastomas frequently seem to develop early vitreous seeding even when the tumor is still relatively small.[27,30] This increases the likelihood of a vitreous relapse. Finally, children with neonatal retinoblastoma quite often develop new tumors after or even during systemic chemotherapy. All these potential pitfalls make neonatal retinoblastoma a challenge to manage in spite of the typically early diagnosis and earlier than average tumor stage.[18]
Figure 3.
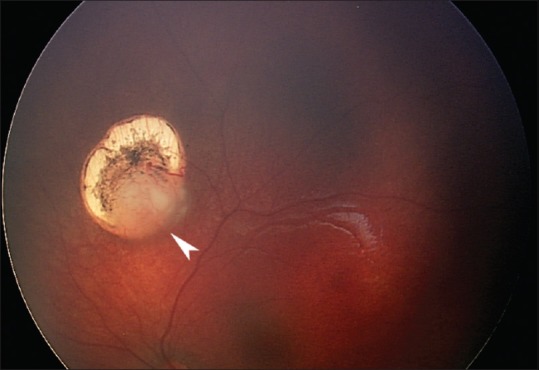
Recurrence of a neonatal retinoblastoma (arrowhead) several months following clinically complete initial regression obtained with infrared diode laser transpupillary thermotherapy
Special Issues
Trilateral neonatal retinoblastoma
In a single-center series of 46 children with neonatal retinoblastoma, two (4%; 95% CI 1–15) later developed a trilateral pineal retinoblastoma.[3] In a recent systematic review and meta-analysis, 9 of 174 (5%; 95% CI 2–10) trilateral retinoblastomas later developed to children who had had a neonatal retinoblastoma.[37] Both percentages are comparable with the general 4.1% (95% CI 2–7) risk of trilateral retinoblastoma developing in children with hereditary retinoblastoma, according to the same meta-analysis. No trilateral retinoblastoma was diagnosed during the neonatal period although 2 (1%; 95% CI 0–4), one pineal and one suprasellar, were detected at the age of 1 month and likely could have been detected in the neonatal period, had an earlier scan been performed. Both children had synchronous bilateral retinoblastomas, and at least one had a positive family history for retinoblastoma.
Metastatic retinoblastoma
Synchronous intraocular and metastatic disease at the time of birth has been reported once.[3] In the older New York series, 4 (9%) of the 46 neonatally diagnosed patients died of metastases.[3] No metastases were mentioned in the Dutch study.[32] In Finland, one of the 11 children with neonatal retinoblastoma died in a traffic accident but none developed metastases.
The role of the oncology nurse
Oncology and other nurse specialists who meet survivors of hereditary retinoblastoma can be instrumental in maintaining the current favorable survival rates. Patients who had bilateral retinoblastoma or unilateral retinoblastoma with a germline RB1 mutation need to be advised about the late effects of their genetic predisposition to themselves and their family. The former includes the possibility of developing a second primary neoplasm.[38] The latter is even more important. Since retinoblastoma arises almost exclusively in early childhood, is dominantly inherited, and is rapidly growing, it is imperative that all survivors of hereditary retinoblastoma are made aware of the role of prenatal genetic diagnosis and, if such testing is either not possible or is declined, of the importance of having the eyes of their neonate screened in the prenatal period or promptly after birth.[19] Oncology nurses play a key role whenever they meet a retinoblastoma survivor through checking that such education is not forgotten.
Oncology nurses benefit of being knowledgeable not only about the genetics of hereditary forms of childhood cancer, such as retinoblastoma, but also about psychological issues involved both in genetic testing and in testing-guided screening.[39] Cultural and religious beliefs and family values about health care and cancer vary. In case the survivor of hereditary retinoblastoma is planning for a baby or is pregnant, creating an atmosphere for open dialog, and asking the right questions to probe for any parental concern regarding screening may be especially important to elicit participation in early detection of retinoblastoma.[40]
Parents, especially mothers of infants with bilateral retinoblastoma, are at increased risk of parenting stress when treatment is ongoing.[40] The oncology nurse can provide educational and psychological support to alleviate it, which is especially important when a neonatal retinoblastoma has been diagnosed. Parenting stress is more intense if the child has visual impairment from the tumor.[40] This is an excellent reason to pursue prompt screening and early treatment when the time of delivery of a neonate at risk is approaching, to minimize chances of poor vision. Furthermore, it is useful for the oncology nurse to familiarize oneself with any community group and other available outside resources so as to know where to refer parents when they need additional information and support, especially is the child has become visually impaired.
Children with neonatal retinoblastoma are especially challenging to manage, and their families need a lot of support. As shown in this review, these children are nevertheless likely to survive and retain reading vision in at least one eye, especially if their parents have been educated to take full advantage of existing prenatal genetic counseling and proper neonatal screening. Oncology nurses often play an important role in the multidisciplinary team managing neonatal retinoblastoma patients and survivors of hereditary retinoblastoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by the Friends of the Blind and the Helsinki University Central Hospital Research Fund (TYH2017218), Helsinki, Finland.
References
- 1.Halperin EC. Neonatal neoplasms. Int J Radiat Oncol Biol Phys. 2000;47:171–8. doi: 10.1016/s0360-3016(00)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Moore SW, Satgé D, Sasco AJ, Zimmermann A, Plaschkes J. The epidemiology of neonatal tumours. Report of an international working group. Pediatr Surg Int. 2003;19:509–19. doi: 10.1007/s00383-003-1048-8. [DOI] [PubMed] [Google Scholar]
- 3.Abramson DH, Du TT, Beaverson KL. (Neonatal) retinoblastoma in the first month of life. Arch Ophthalmol. 2002;120:738–42. doi: 10.1001/archopht.120.6.738. [DOI] [PubMed] [Google Scholar]
- 4.Campbell AN, Chan HS, O'Brien A, Smith CR, Becker LE. Malignant tumours in the neonate. Arch Dis Child. 1987;62:19–23. doi: 10.1136/adc.62.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue H, Horwitz JR, Smith MB, Lally KP, Black CT, Cangir A, et al. Malignant solid tumors in neonates: A 40-year review. J Pediatr Surg. 1995;30:543–5. doi: 10.1016/0022-3468(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 6.Børch K, Jacobsen T, Olsen JH, Hirsch F, Hertz H. Neonatal cancer in Denmark 1943-1985. Pediatr Hematol Oncol. 1992;9:209–16. doi: 10.3109/08880019209016588. [DOI] [PubMed] [Google Scholar]
- 7.Parkes SE, Muir KR, Southern L, Cameron AH, Darbyshire PJ, Stevens MC. Neonatal tumours: A thirty-year population-based study. Med Pediatr Oncol. 1994;22:309–17. doi: 10.1002/mpo.2950220503. [DOI] [PubMed] [Google Scholar]
- 8.Crom DB, Wilimas JA, Green AA, Pratt CB, Jenkins JJ 3rd, Behm FG. Malignancy in the neonate. Med Pediatr Oncol. 1989;17:101–4. doi: 10.1002/mpo.2950170206. [DOI] [PubMed] [Google Scholar]
- 9.Büyükpamukçu M, Varan A, Tanyel C, Senocak ME, Gögüs S, Akyüz C, et al. Solid tumors in the neonatal period. Clin Pediatr (Phila) 2003;42:29–34. doi: 10.1177/000992280304200105. [DOI] [PubMed] [Google Scholar]
- 10.Kivelä T. The epidemiological challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–31. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 11.Usmanov RH, Kivelä T. Predicted trends in the incidence of retinoblastoma in the Asia-Pacific Region. Asia Pac J Ophthalmol (Phila) 2014;3:151–7. doi: 10.1097/APO.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 12.Chantada GL, Dunkel IJ, Qaddoumi I, Antoneli CB, Totah A, Canturk S, et al. Familial retinoblastoma in developing countries. Pediatr Blood Cancer. 2009;53:338–42. doi: 10.1002/pbc.21970. [DOI] [PubMed] [Google Scholar]
- 13.Lau CS, Choy KW, Fan DS, Yu CB, Wong CY, Lam DS, et al. Prenatal screening for retinoblastoma in Hong Kong. Hong Kong Med J. 2008;14:391–4. [PubMed] [Google Scholar]
- 14.Shah PK, Sripriya S, Narendran V, Pandian AJ. Prenatal genetic diagnosis of retinoblastoma and report of RB1 gene mutation from India. Ophthalmic Genet. 2016;37:430–3. doi: 10.3109/13816810.2015.1107595. [DOI] [PubMed] [Google Scholar]
- 15.Paquette LB, Miller D, Jackson HA, Lee T, Randolph L, Murphree AL, et al. In utero detection of retinoblastoma with fetal magnetic resonance and ultrasound: Initial experience. AJP Rep. 2012;2:55–62. doi: 10.1055/s-0032-1316465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neriyanuri S, Raman R, Rishi P, Govindasamy K, Ramprasad VL, Sharma T. Prenatal genetic diagnosis of retinoblastoma – Clinical correlates on follow-up. Indian J Ophthalmol. 2015;63:741–2. doi: 10.4103/0301-4738.170979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staffieri SE, McGillivray G, Elder JE, Bristowe A, Cole S, McKenzie JD, et al. Managing fetuses at high risk of retinoblastoma: Lesion detection on screening MRI. Prenat Diagn. 2015;35:174–8. doi: 10.1002/pd.4514. [DOI] [PubMed] [Google Scholar]
- 18.Gombos DS. Retinoblastoma in the perinatal and neonatal child. Semin Fetal Neonatal Med. 2012;17:239–42. doi: 10.1016/j.siny.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Soliman SE, Dimaras H, Khetan V, Gardiner JA, Chan HS, Héon E, et al. Prenatal versus postnatal screening for familial retinoblastoma. Ophthalmology. 2016;123:2610–7. doi: 10.1016/j.ophtha.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Amino K, Ichioka I, Ichioka H, Matsubara K, Lin YW, Ohta S. Retinoblastoma in dizygotic twins born as extremely low birth weight infants. Jpn J Ophthalmol. 1992;36:310–4. [PubMed] [Google Scholar]
- 21.Picton SV, Keeble J, Holden V, Errington J, Boddy AV, Veal GJ. Therapeutic monitoring of carboplatin dosing in a premature infant with retinoblastoma. Cancer Chemother Pharmacol. 2009;63:749–52. doi: 10.1007/s00280-008-0787-6. [DOI] [PubMed] [Google Scholar]
- 22.Self J, Bush K, Baral VR, Puvanachandra N, Puddy VF, Hodgkins P. Bilateral retinoblastoma presenting at retinopathy of prematurity screening. Arch Dis Child Fetal Neonatal Ed. 2010;95:F292. doi: 10.1136/adc.2009.178780. [DOI] [PubMed] [Google Scholar]
- 23.Al-Abdi SY. A case of bilateral retinoblastoma with a novel mutation presenting at retinopathy of prematurity screening. Saudi Med J. 2012;33:680. [PubMed] [Google Scholar]
- 24.Abramson DH, Schefler AC, Beaverson KL, Rollins IS, Ruddat MS, Kelly CJ. Rapid growth of retinoblastoma in a premature twin. Arch Ophthalmol. 2002;120:1232–3. doi: 10.1001/archopht.120.9.1232. [DOI] [PubMed] [Google Scholar]
- 25.Murphree AL. Intraocular retinoblastoma: The case for a new group classification. Ophthalmol Clin North Am. 2005;18:41–53, viii. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Novetsky DE, Abramson DH, Kim JW, Dunkel IJ. Published international classification of retinoblastoma (ICRB) definitions contain inconsistencies – An analysis of impact. Ophthalmic Genet. 2009;30:40–4. doi: 10.1080/13816810802452168. [DOI] [PubMed] [Google Scholar]
- 27.Jayadev C, Vinekar A, Bauer N, Mangalesh S, Mahendradas P, Kemmanu V, et al. Look what else we found – Clinically significant abnormalities detected during routine ROP screening. Indian J Ophthalmol. 2015;63:373–7. doi: 10.4103/0301-4738.159859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo R, Liu J, Hu P, Cheng SS, Shi BZ, Zhu JH, et al. Results of 779 cases of neonatal fundus screening and risk factors for neonatal fundus diseases. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:1197–201. [PubMed] [Google Scholar]
- 29.Seregard S, Lundell G, Svedberg H, Kivelä T. Incidence of retinoblastoma from 1958 to 1998 in Northern Europe: Advantages of birth cohort analysis. Ophthalmology. 2004;111:1228–32. doi: 10.1016/j.ophtha.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Vinekar A, Govindaraj I, Jayadev C, Kumar AK, Sharma P, Mangalesh S, et al. Universal ocular screening of 1021 term infants using wide-field digital imaging in a single public hospital in India – A pilot study. Acta Ophthalmol. 2015;93:e372–6. doi: 10.1111/aos.12685. [DOI] [PubMed] [Google Scholar]
- 31.Li LH, Li N, Zhao JY, Fei P, Zhang GM, Mao JB, et al. Findings of perinatal ocular examination performed on 3573, healthy full-term newborns. Br J Ophthalmol. 2013;97:588–91. doi: 10.1136/bjophthalmol-2012-302539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imhof SM, Moll AC, Schouten-van Meeteren AY. Stage of presentation and visual outcome of patients screened for familial retinoblastoma: Nationwide registration in the Netherlands. Br J Ophthalmol. 2006;90:875–8. doi: 10.1136/bjo.2005.089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plotsky D, Quinn G, Eagle R, Jr, Shields J, Granowetter L. Congenital retinoblastoma: A case report. J Pediatr Ophthalmol Strabismus. 1987;24:120–3. doi: 10.3928/0191-3913-19870501-05. [DOI] [PubMed] [Google Scholar]
- 34.Taylor M, Dehainault C, Desjardins L, Doz F, Levy C, Sastre X, et al. Genotype-phenotype correlations in hereditary familial retinoblastoma. Hum Mutat. 2007;28:284–93. doi: 10.1002/humu.20443. [DOI] [PubMed] [Google Scholar]
- 35.Lumbroso L, Doz F, Urbieta M, Levy C, Bours D, Asselain B, et al. Chemothermotherapy in the management of retinoblastoma. Ophthalmology. 2002;109:1130–6. doi: 10.1016/s0161-6420(02)01053-9. [DOI] [PubMed] [Google Scholar]
- 36.Kivelä T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118:1689, 1689–e1-6. doi: 10.1016/j.ophtha.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 37.de Jong MC, Kors WA, de Graaf P, Castelijns JA, Moll AC, Kivelä T. The incidence of trilateral retinoblastoma: A systematic review and meta-analysis. Am J Ophthalmol. 2015;160:1116–26. doi: 10.1016/j.ajo.2015.09.009. e5. [DOI] [PubMed] [Google Scholar]
- 38.Temming P, Viehmann A, Arendt M, Eisele L, Spix C, Bornfeld N, et al. Pediatric second primary malignancies after retinoblastoma treatment. Pediatr Blood Cancer. 2015;62:1799–804. doi: 10.1002/pbc.25576. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald DJ, Lessick M. Hereditary cancers in children and ethical and psychosocial implications. J Pediatr Nurs. 2000;15:217–25. doi: 10.1053/jpdn.2000.8044. [DOI] [PubMed] [Google Scholar]
- 40.Nagayoshi M, Hirose T, Toju K, Suzuki S, Okamitsu M, Omori T, et al. Parenting stress related to raising infants receiving treatment for retinoblastoma. Psychooncology. 2016;25:1507–11. doi: 10.1002/pon.4062. [DOI] [PubMed] [Google Scholar]


