Abstract
Objective:
Individuals with diabetes who develop cancer have a worse 5-year overall survival rate and are more likely to develop an infection and/or be hospitalized when compared to those without diabetes. Patients with diabetes and cancer receiving chemotherapy have an increased risk for developing glycemic issues. The relationship between chemotherapy and glycemic control is not completely understood. The aim of this study was to explore the relationship between glycemic control, symptoms, physical and mental function, development of adverse events, and chemotherapy reductions or stoppages in adults with Type 2 diabetes (T2D) and cancer.
Methods:
A prospective 12-week longitudinal cohort study recruited 24 adults with T2D, solid tumor cancer, or lymphoma receiving outpatient intravenous chemotherapy. Eighteen individuals completed baseline data and were included in the analysis. A comparative case analysis was performed to analyze the results.
Results:
Potential predictors of occurrence of an adverse event include sex (relative risk [RR] = 1.5), treatment with insulin (RR = 2.17), years with diabetes (RR = 3.85), and baseline glycated hemoglobin (HbA1c) (odds ratio [OR] = 1.67). Baseline body mass index (BMI) (OR = 1.16) and HbA1c (OR = 1.61) were potentially predictive of a chemotherapy stoppage.
Conclusions:
Level of glycemic control at the time an individual begins treatment for cancer appears to contribute to the occurrence of an adverse event, developing an infection and/or being hospitalized during treatment, and the increased risk of having a chemotherapy reduction or stoppage. Clinicians working with patients receiving chemotherapy for a solid tumor cancer who have pre-existing diabetes, need to be aware of how the patients glycemic level at the start of treatment may impact successful treatment completion.
Keywords: Cancer, chemotherapy, diabetes, glycemic control

Introduction
Currently, about 8%–18% of all cancer patients have preexisting diabetes.[1] Individuals with diabetes who develop cancer have a 42% increased risk of death, a 21% increased risk of recurrence, and a significantly worse 5-year overall and cancer-specific survival rate as compared to individuals with cancer who do not have diabetes.[1,2,3] Patients with diabetes and cancer who are receiving chemotherapy are at an increased risk for developing glycemic issues.[4,5,6] The relationship between chemotherapy and glycemic control is not completely understood. The purpose of this study was to explore the impact chemotherapy that had on glycemic control over a 12-week period in adults with Type 2 diabetes (T2D) and solid tumor or lymphoma cancer.
Hyperglycemia in cancer patients has been linked to the risk of developing a nonhematological clinical toxicity while being treated with chemotherapy.[7] Many chemotherapy agents have been linked to the development of hyperglycemia in patients without diabetes.[4] The combination of chemotherapy and corticosteroids that are commonly used during cancer treatment puts the patient at risk for developing hyperglycemia, which is a clinical toxicity that can have an impact on chemotherapy dose reductions, interruptions, or stoppages.[7,8] Poor glycemic control in cancer patients is associated with a more clinically aggressive cancer course and development of adverse events such as neutropenia, infections, and mortality.[9,10,11,12,13,14]
Most studies that have explored outcomes in cancer patients with diabetes have either been retrospective or prospective cohort studies using medical records or claims data[2,3,9,14] or cross-sectional.[15] Very few studies have explored outcomes longitudinally while including an assessment of the patient's glycemic status. The aim of this paper is to describe findings from a 12-week longitudinal study exploring the relationship between glycemic control and health-related outcomes of symptom severity and interference, physical and mental function, as well as the development of adverse events (infections, chemotherapy dose reductions or stoppages, and hospital admissions) in adults with solid tumor cancer or lymphoma and diabetes using comparative case analysis.
Methods
Design
A prospective longitudinal cohort study design was used to explore the relationship between glycemic control, symptoms, and chemotherapy dose adjustments in adults with solid tumor cancer or lymphoma and T2D. Due to the small sample size, data will be evaluated in an exploratory, hypothesis-generating manner using a comparative case analysis approach. Figure 1 shows the study flowchart.
Figure 1.
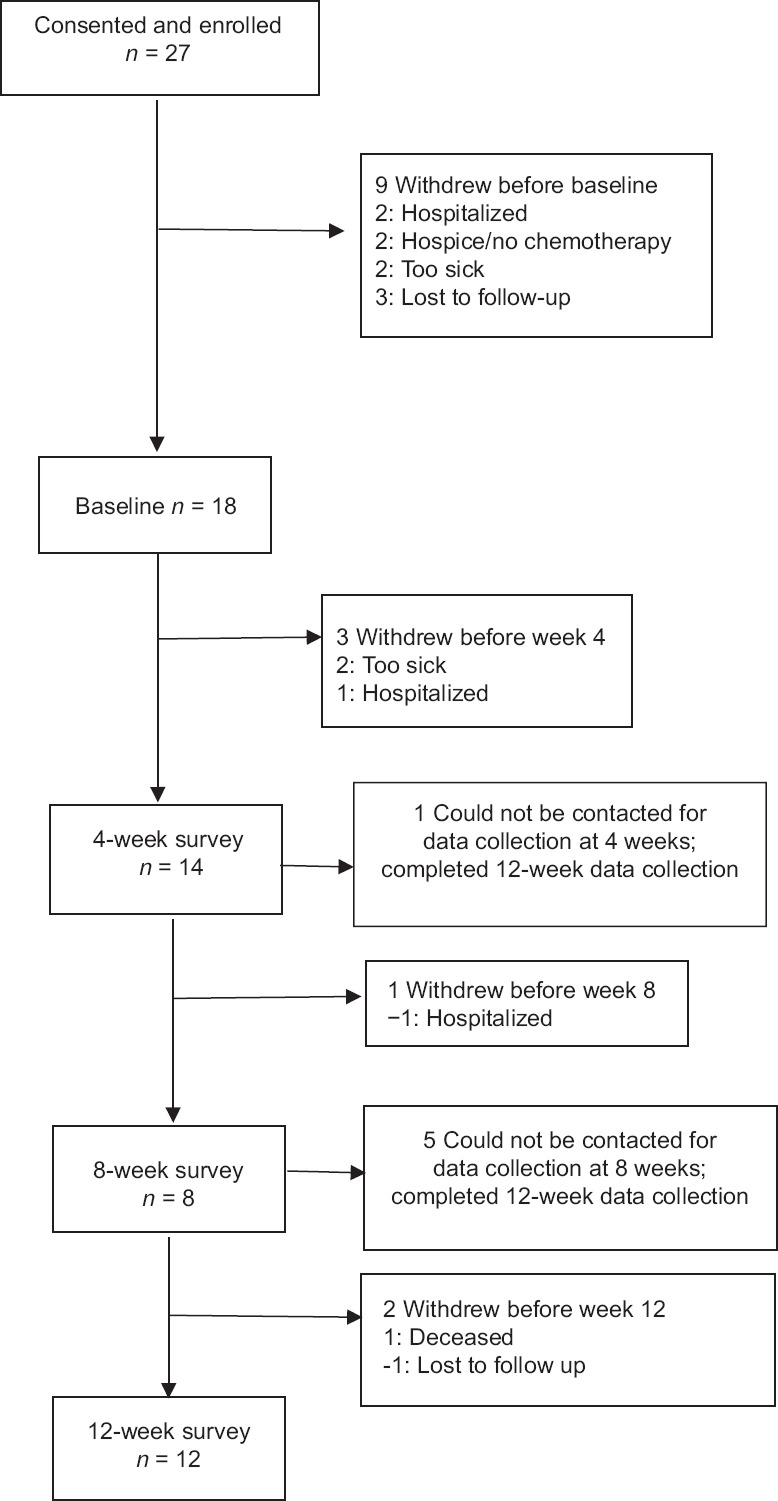
Study flowchart
Sample
Adults aged 21 years or older with a preexisting history of T2D, newly diagnosed with solid tumor cancer or lymphoma who were eligible and elected to receive outpatient intravenous chemotherapy were recruited for this study. In addition to having preexisting diabetes and solid tumor cancer or lymphoma, participants needed to be able to read, write, and speak English as well as have access to a telephone. Participants were excluded if they had a history of cognitive impairment, renal failure, Alzheimer's or Dementia, or a history of brain metastasis. Patients with Type 1 diabetes were also excluded from the study.
Ethical considerations
Participants were recruited from three different community cancer centers in Michigan after Institutional Review Board approval was received from Michigan State University and the participating cancer centers.
Data collection
Baseline interviews were obtained through the telephone within 48 h of obtaining consent. Participants were contacted at 4, 8, and 12 weeks following baseline for follow-up assessments. All data were collected by trained research assistants. In addition to self-report data, glycated hemoglobin (HbA1c) was obtained at baseline and 12 weeks with Bayer A1cNow. Medical chart audits were conducted to collect data regarding chemotherapy regimens, dose delays and reductions, and other cancer-specific information, i.e., stage, metastasis, etc.
Variables and instruments
Sociodemographics
Age, sex, education level, income status, marital status, race and ethnicity, living arrangements, and employment status were assessed by self-report.
Body mass index
Body mass index (BMI) was obtained through a medical chart audit.
Number of comorbidities
They were assessed using a modified Katz Comorbidity Questionnaire (KCQ). The KCQ is a 10-item self-report questionnaire, which asks participants if they have a specific condition. The KCQ has a test–retest reliability of 0.91.[16]
Diabetes-specific clinical characteristics
It included measurements for the duration of diabetes, type of medication used to treat their diabetes. Duration was obtained by asking participants how many months/years they have had diabetes. Type of diabetes medications was classified as oral, insulin, or both.
Cancer-specific clinical characteristics
It included type, stage of cancer, metastasis, and type of chemotherapy. Type of chemotherapy was classified as intravenous, oral, or both. Chemotherapy for this study was defined as being on an intravenous taxane, platinum-based, or alkylating agent.
Glycemic control
Glycemic control was defined according to the American Diabetes Association 2012 medical standards.[17] Individuals who had a HbA1c <7.0 were considered to be in control, and those ≥7.0 were classified not in control. HbA1c was measured on all participants at baseline and 12 weeks using the Bayer A1cNow monitor.
Symptoms (severity and interference)
Theywere assessed with a modified MD Anderson Symptom Inventory (MDASI). The MDASI is a 13-item questionnaire that assesses severity and interference of common symptoms associated with cancer. In addition to symptoms asked in the MDASI, patients were assessed for four common symptoms associated with diabetes: headache, faintness/dizziness, vision changes, and irritability/anxiousness. Patients were asked whether they had the symptom over the last week; if yes, they were asked to rate the severity of the symptom on a 0–10 scale, with 0 indicating a lack of presence of the symptom and 10 being as bad as they could imagine. Interference was measured from six different dimensions: interference with general activity, mood, work (including work around the house), relationship with other people, walking, and enjoyment in life. Participants were asked to rate interference for each area using a 0–10 scale, with 0 indicating no interference and 10 indicating complete interference. The MDASI is a proven, reliable, and valid instrument.[18]
Adverse events
Adverse events included hospitalizations, development of infections, and chemotherapy dose delays, reductions, or stoppages. Patients were considered to have a dose delay if they started their scheduled chemotherapy cycle more than 7 days from the originally scheduled date. Dose reductions were considered to have occurred if they received 80% or less than the originally recommended dose.
Statistical analysis
Due to the small sample size of this study, a comparative case study analysis was used to generate more generalizable information about causal questions.[19,20] As noted by Pickvance,[20] comparative case analyses can be defined by two features: (1) an interest in the explanatory question of why the observed similarities and differences between cases exist and (2) reliance on the collection of data on two or more cases, ideally according to a common framework.
As such, the data generated by this study were well suited to a comparative case analysis. A challenge in case study analysis can be to quantify what is meant by “similarities and differences between cases.” Here, statistical analyses were conducted in an exploratory manner for the purpose of identifying or refining causative hypotheses. Significance testing was not conducted in the traditional manner. Rather, for each analysis, metrics were created to identify associations and patterns in associations that merit additional investigation.
Statistical analyses were conducted to quantify associations as appropriate for the types of variables (i.e., categorical or continuous) used in each analysis as the dependent variable (DV) (outcome) and independent variable (IV) (predictor). Correspondingly, the following methods were used: (1) categorical DV, categorical IV: Fisher's exact test[21,22] and relative risk (RR) measures; (2) categorical DV, continuous IV: logistic regression analysis and odds ratios (ORs);[23] (3) continuous DV, categorical IV: GroupWise means and standard deviations; and (4) continuous DV, continuous IV: Pearson's correlation coefficients. RR and OR measures[24] were calculated to provide information for interpreting results of Fisher's exact test and logistic regression, respectively. RR represents the ratio of risks between the two groups represented in the IV or predictor variable. RRs can be easily interpreted (e.g., for an RR of 2, the risk of the outcome occurring in Group 1 is twice that of Group 2). An OR is more complex conceptually; it represents the ratio of the odds of the outcome occurring in one group versus the other as identified by the IV. R statistical software was used for all analyses[25] including the R packages Hmisc[26] and Pastecs.[27]
Results
Sample characteristics
Eighteen participants were included in this study. Of these, 12 completed the study. Participant characteristics are summarized in Table 1. Fifty-six percent of the participants were female (n = 10), and 44% (n = 8) were male. On average, participants were 63 years of age with a history of T2D for 10 years and three comorbidities. Twenty-eight percent (n = 5) were treated with insulin for their diabetes alone or in combination with an oral agent, and 47% (n = 8) were under control at baseline in terms of their HbA1c values. Cancer types were highly variable, Table 1 shows the distribution of cancer type across participants, and 28% (n = 5) of participants had metastasis.
Table 1.
Sample characteristics at baseline and 12 weeks
| Characteristic | n (%) | |
|---|---|---|
| Categorical variables | Baseline | 12 weeks |
| Sex | ||
| Female | 10 (56) | 6 (50) |
| Male | 8 (44) | 6 (50) |
| Race | ||
| White | 15 (83) | 10 (83) |
| African-American | 2 (11) | 1 (8) |
| Hispanic | 1 (6) | 1 (8) |
| Relationship status | ||
| Never married | 3 (17) | 2 (17) |
| Married | 7 (39) | 5 (42) |
| Divorced/separated | 2 (11) | 1 (8) |
| Widowed | 3 (17) | 2 (17) |
| Living together | 3 (17) | 2 (17) |
| Household income | ||
| ≤$24,999 | 3 (17) | 2 (17) |
| $25,000-$49,999 | 2 (11) | 1 (8) |
| $50,000-$99,999 | 5 (28) | 4 (33) |
| $100,000-$149,000 | 1 (6) | 1 (8) |
| Chose not to answer | 7 (39) | 4 (33) |
| Type of cancer | ||
| Breast | 2 (11) | 1 (8) |
| Colon | 2 (11) | 1 (8) |
| Lung | 2 (11) | 1 (8) |
| Bladder | 1 (6) | 1 (8) |
| Gynecologic (other than ovarian) | 1 (6) | 1 (8) |
| GI | 1 (6) | 0 |
| Lymphoma | 4 (22) | 4 (33) |
| Ovarian | 2 (11) | 2 (17) |
| Other | 3 (17) | 1 (8) |
| Metastasis | ||
| Yes | 5 (28) | 1 (8) |
| No | 13 (72) | 11 (92) |
| Glycemic control based on A1c | ||
| <7.0 | 8 (47) | 6 (50) |
| ≥7.0 | 9 (53) | 6 (50) |
| On insulin | ||
| Yes | 5 (28) | 3 (25) |
| No | 13 (72) | 9 (75) |
| Diabetes <5 years | ||
| Yes | 5 (28) | 4 (33) |
| No | 13 (72) | 8 (67) |
| Diabetes <10 years | ||
| Yes | 9 (50) | 6 (50) |
| No | 9 (50) | 6 (50) |
| Comorbidities | ||
| 0-1 | 5 (28) | 5 (42) |
| 2-4 | 6 (33) | 2 (16) |
| >5 | 7 (39) | 5 (42) |
| Continuous variables | n | Mean (SD) |
| Age | ||
| Baseline | 18 | 63 (11) |
| 12 weeks | 12 | 63 (12) |
| Years with diabetes | ||
| Baseline | 18 | 10 (9) |
| 12 weeks | 12 | 8 (7) |
| Total number of other comorbidities | ||
| Baseline | 18 | 3 (2) |
| 12 weeks | 12 | 3 (2) |
| Total symptom severity | ||
| Baseline | 18 | 50 (25) |
| 12 weeks | 12 | 47 (32) |
| Total symptom interference | ||
| Baseline | 18 | 39 (27) |
| 12 weeks | 12 | 33 (28) |
| Physical function | ||
| Baseline | 18 | 30 (6) |
| 12 weeks | 12 | 31 (9) |
| Emotional function | ||
| Baseline | 18 | 50 (10) |
| 12 weeks | 12 | 51 (10) |
| Cancer treatment impact on diabetes management | ||
| Baseline | Not measured | Not measured |
| 12 weeks | 12 | 32 (23) |
| Estimated daily average glucose (based on A1c) | ||
| Baseline | 17 | 166 (33) |
| 12 weeks | 12 | 171 (39) |
GI: Gastrointestinal, SD: Standard deviation
Glycemic control and chemotherapy dose reductions and stoppages
To summarize chemotherapy reductions and stoppages, an outcome variable was created and coded “1” or “yes” for all cases with a chemotherapy reduction and/or stoppage and “0” or “no” for all other cases. Associations between potential predictor variables and chemotherapy reductions or stoppages are summarized in Table 2 for categorical predictors and Table 3 for continuous predictors. As metrics for identifying associations that merit additional investigation, the following cutoffs were used: (1) for categorical predictor variables, RR >2 (or RR <0.5); (2) for continuous predictor variables, OR >1.5 (or OR <0.5), or P< 0.1. Based on these cutoffs, years with diabetes (<10 years vs. ≥10 years), baseline BMI, and baseline HbA1c are potential predictors of reductions and/or stoppages in chemotherapy.
Table 2.
Relative risk of categorical predictors on development of chemotherapy stoppages or reductions, infections, hospitalizations, and adverse events
| Predictor | Chemotherapy stoppage or reduction | Infection | Hospitalization | Adverse event | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No, n (%) | Yes, n (%) | RR | P | No, n (%) | Yes, n (%) | RR | P | No, n (%) | Yes, n (%) | RR | P | No, n (%) | Yes, n (%) | RR | P | |
| Sex | ||||||||||||||||
| Female | 6 (60) | 4 (40) | 1.00 | 7 (70) | 3 (30) | 0.34 | 6 (60) | 4 (40) | 1.00 | 5 (50) | 5 (50) | 0.37 | ||||
| Male | 4 (50) | 4 (50) | 1.25 | 3 (38) | 5 (63) | 2.08 | 4 (50) | 4 (50) | 1.25 | 2 (25) | 6 (75) | 1.5 | ||||
| A1c <7 at baseline | ||||||||||||||||
| No | 6 (67) | 3 (33) | 0.53 | 0.35 | 4 (44) | 5 (56) | 2.22 | 0.33 | 4 (44) | 5 (56) | 1.48 | 0.64 | 3 (33) | 6 (67) | 1.33 | 0.64 |
| Yes | 3 (38) | 5 (63) | -- | -- | 6 (75) | 2 (25) | 5 (63) | 3 (38) | 4 (50) | 4 (50) | ||||||
| A1c <7 at 12 weeks | ||||||||||||||||
| No | 4 (57) | 3 (43) | 0.54 | 0.29 | 4 (57) | 3 (43) | 2.14 | 0.58 | 4 (57) | 3 (43) | 2.14 | 0.58 | 3 (43) | 4 (57) | 1.43 | 1.00 |
| Yes | 1 (20) | 4 (80) | 4 (80) | 1 (20) | 4 (80) | 1 (20) | 3 (60) | 2 (40) | ||||||||
| On insulin | ||||||||||||||||
| No | 7 (54) | 6 (46) | 1.00 | 9 (69) | 4 (31) | 0.12 | 9 (69) | 4 (31) | 0.12 | 7 (54) | 6 (46) | 0.10 | ||||
| Yes | 3 (60) | 2 (40) | 0.87 | 1 (20) | 4 (80) | 2.60 | 1 (20) | 4 (80) | 2.60 | 0 (0) | 5 (100) | 2.17 | ||||
| Diabetes <5 years | ||||||||||||||||
| Yes | 2 (40) | 3 (60) | 1.56 | 0.61 | 4 (80) | 1 (20) | 0.37 | 0.31 | 4 (80) | 1 (20) | 0.37 | 0.31 | 4 (80) | 1 (20) | 0.26 | 0.047 |
| No | 8 (62) | 5 (38) | 0.64 | 6 (46) | 7 (54) | 2.69 | 6 (46) | 7 (54) | 2.69 | 3 (23) | 10 (77) | 3.85 | ||||
| Diabetes <10 years | ||||||||||||||||
| Yes | 3 (33) | 6 (67) | 3.00 | 0.15 | 6 (67) | 3 (33) | 0.60 | 0.64 | 4 (44) | 5 (56) | 1.67 | 0.64 | 4 (44) | 5 (56) | 0.83 | 1.00 |
| No | 7 (78) | 2 (22) | 0.33 | 4 (44) | 5 (56) | 1.67 | 6 (67) | 3 (33) | 0.60 | 3 (33) | 6 (67) | 1.20 | ||||
*A metric of RR >1.5 or <0.67 was used to identify categorical predictors that merit additional investigation. These items are bolded in the table. RR: Relative risk
Table 3.
Odds ratios of baseline continuous predictors on development of chemotherapy stoppages, infections, hospitalizations, and adverse events (n=18)
| Predictor | Chemotherapy stoppage or reduction | Infection | Hospitalization | Adverse event | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5% LCL | 97.5% UCL | OR | P | 2.5% LCL | 97.5% UCL | OR | P | 2.5% LCL | 97.5% UCL | OR | P | 2.5% LCL | 97.5% UCL | OR | P | |
| Mental health | 0.94 | 1.15 | 1.04 | 0.50 | 0.95 | 1.17 | 1.05 | 0.38 | 0.82 | 1.02 | 0.92 | 0.16 | 0.86 | 1.06 | 0.96 | 0.43 |
| Physical health | 0.75 | 1.07 | 0.91 | 0.29 | 0.90 | 1.27 | 1.06 | 0.50 | 0.89 | 1.24 | 1.04 | 0.63 | 0.88 | 1.23 | 1.03 | 0.69 |
| Symptom severity | 0.97 | 1.05 | 1.01 | 0.64 | 0.94 | 1.02 | 0.99 | 0.47 | 0.95 | 1.03 | 0.99 | 0.64 | 0.95 | 1.03 | 0.99 | 0.69 |
| Symptom interference | 0.97 | 1.04 | 1.00 | 0.83 | 0.96 | 1.03 | 1.00 | 0.86 | 0.96 | 1.04 | 1.00 | 0.95 | 0.96 | 1.04 | 1.00 | 0.98 |
| BMI | 1.01 | 1.47 | 1.19 | 0.06 | 0.86 | 1.13 | 0.99 | 0.83 | 0.84 | 1.11 | 0.97 | 0.66 | 0.82 | 1.09 | 0.95 | 0.48 |
| HbA1c | 0.66 | 5.15 | 1.61 | 0.33 | 0.87 | 9.17 | 2.26 | 0.15 | 0.66 | 5.15 | 1.61 | 0.33 | 0.65 | 6.47 | 1.67 | 0.35 |
| Years with diabetes | 0.79 | 1.04 | 0.93 | 0.26 | 1.00 | 1.38 | 1.13 | 0.10 | 0.87 | 1.09 | 0.98 | 0.71 | 0.99 | 1.47 | 1.15 | 0.15 |
| Number of comorbidities | 0.50 | 1.41 | 0.89 | 0.63 | 0.70 | 1.79 | 1.11 | 0.66 | 0.78 | 2.07 | 1.24 | 0.38 | 0.77 | 2.08 | 1.23 | 0.39 |
*Metric of ORs >1.5 or <0.5 or P<0.1 was used to identify continuous predictor variables that merit additional investigation. BMI: Body mass index, ORs: Odds ratios, HbA1c: Glycated hemoglobin, LCL: Lower confidence limit, UCL: Upper confidence limit
Glycemic control, symptom severity, and interference; physical and mental function
Total symptom severity (TSS), total symptom interference (TSI), physical health, and mental health were all evaluated as continuous outcome variables. Physical and mental health was measured at baseline and week 12. TSS and TSI were measured at baseline and weeks 4, 8, and 12; however, due to low sample sizes in weeks 4 and 8, results from baseline and week 12 only are presented.
Associations between continuous potential predictor variables and the continuous outcomes listed above were evaluated using Pearson's correlation coefficients (r). A metric of | r|>0.5 was used to identify potential predictor variables that merit additional investigation. Age was positively associated with baseline mental health (r = 0.61) and mental health at week 12 (r = 0.70). Number of comorbidities was negatively associated with baseline mental health (r = –0.56). Baseline and week 12 BMI were negatively associated with week 12 mental health (r = –0.58 and r = –0.68, respectively) and positively associated with week 12 TSI (r = 0.54 and r = 0.55, respectively). HbA1c measured at week 12 was also negatively associated with week 12 mental health.
To evaluate for associations between potential categorical predictors and each of the continuous outcomes (i.e., TSS, TSI, physical health, and mental health), means and standard deviations were calculated for each group identified by the categorical predictors [Table 4]. Noteworthy, differences between group means include the following: (1) Sex appears to be associated with TSS and TSI at baseline and TSI and week 12, with higher symptom scores corresponding to females; (2) Level of HbA1c control at baseline does not appear to influence measures of TSS or TSI at baseline although in-control levels at baseline generally correspond to higher levels of TSS and TSI at week 12. Other noteworthy associations include the potential influence of insulin treatment on TSI (higher average measures in the group treated with insulin at both baseline and week 12) and mental health (lower measures at week 12 for those receiving insulin versus those not receiving insulin). Patients with diabetes <5 years appeared to have decreased measures of TSS and TSI from baseline to week 12 although the same pattern was not apparent for patients with diabetes ≥5 years. The same pattern was observed with the 10-year cutoff for diabetes. In addition, average TSS and TSI scores appear to increase with higher numbers of comorbidities baseline, while average baseline mental health scores appear to decrease with increasing numbers of comorbidities.
Table 4.
Categorical predictors of symptoms and function
| Predictor | x̄ (SD) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | HbA1c <7.0 at baseline | Cancer Mets | Insulin | Diabetes <5 years | Diabetes <10 years | Comorbidity | |||||||||
| Female | Male | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | 0-1 | 2-4 | ≥5 | |
| Total symptom severity | |||||||||||||||
| Baseline | 62 (23) | 36 (20) | 49 (20) | 46 (26) | 47 (18) | 47 (37) | 49 (33) | 51 (24) | 59 (21) | 47 (27) | 55 (22) | 46 (29) | 44 (28) | 50 (30) | 55 (22) |
| 12 weeks | 49 (31) | 45 (36) | 62 (31) | 32 (27) | 40 (NA) | 40 (30) | 50 (35) | 46 (33) | 39 (32) | 51 (33) | 43 (36) | 50 (30) | 50 (36) | 17 (5) | 56 (30) |
| Total symptom interference | |||||||||||||||
| Baseline | 54 (25) | 21 (17) | 35 (20) | 36 (25) | 35 (18) | 40 (30) | 47 (36) | 36 (24) | 50 (22) | 35 (28) | 43 (21) | 35 (32) | 33 (25) | 42 (33) | 41 (26) |
| 12 weeks | 39 (34) | 27 (23) | 41 (24) | 24 (32) | 17 (NA) | 34 (29) | 49 (37) | 27 (25) | 24 (29) | 37 (29) | 29 (28) | 36 (31) | 32 (31) | 15 (1) | 40 (31) |
| Physical function | |||||||||||||||
| Baseline | 28 (7) | 32 (4) | 30 (6) | 31 (6) | 32 (4) | 29 (7) | 28 (7) | 30 (6) | 28 (7) | 31 (6) | 29 (6) | 31 (6) | 30 (5) | 30 (7) | 29 (7) |
| 12 weeks | 28 (7) | 35 (9) | 30 (9) | 33 (9) | 26 (NA) | 32 (9) | 28 (4) | 32 (10) | 27 (9) | 33 (8) | 28 (7) | 34 (10) | 34 (12) | 27 (2) | 30 (7) |
| Mental function | |||||||||||||||
| Baseline | 46 (10) | 55 (7) | 52 (9) | 49 (11) | 50 (7) | 50 (11) | 46 (12) | 51 (9) | 53 (8) | 49 (11) | 51 (10) | 49 (11) | 56 (8) | 52 (8) | 44 (10) |
| 12 weeks | 50 (12) | 53 (8) | 52 (8) | 51 (13) | 50 (NA) | 52 (11) | 44 (14) | 54 (8) | 56 (7) | 49 (11) | 53 (7) | 49 (13) | 55 (9) | 58 (7) | 45 (10) |
Corresponding samples sizes for each grouping are shown in Table 1. NA: SD could not be calculated based on sample size of 1, HbA1c: Glycated hemoglobin, SD: Standard deviation
Glycemic control and development of adverse events
Adverse events were characterized by three different dichotomous variables: (1) infections, (2) hospitalizations, and (3) one or more adverse events (i.e., infections, hospitalizations, and/or death). Each variable was coded “1” or “yes” if the adverse event of interest occurred and “0” or “no” if not. Associations between potential predictor variables and adverse events are summarized in Table 2 for categorical predictors and Table 3 for continuous predictors.
Potential categorical predictors of infections over the duration of the study were sex (males were observed to be more likely to develop infections), control of HbA1c levels at baseline and week 12, whether the patient's diabetes medication regimen included insulin, and years with diabetes at both cutoffs (i.e., <5 years vs. ≥5 years, <10 years vs. ≥10 years). Potential continuous predictors of infections were baseline HbA1c and total years with diabetes.
Hospitalization during the study may be predicted by the categorical variables HbA1c control at week 12, treatment of diabetes using insulin alone or in combination with an oral agent, and years with diabetes at both cutoffs. The continuous variable HbA1c at baseline may also be predictive of hospitalization during the study.
Potential predictors of the occurrence of one or more adverse events (i.e., infection, hospitalization, and/or death) include the categorical variables sex (with males being more likely to experience an adverse event), treatment with insulin alone or in combination with an oral agent, and years with diabetes (5-year cutoff only), as well as the continuous predictor baseline HbA1c.
Comparison of differences between men and women
Sex was identified as a possible contributing variable to chemotherapy stoppage and adverse events across the board. In each case, men were more likely to experience the chemotherapy stoppage or adverse events than women. Additional comparisons were performed between men and women to try and identify factors that may have contributed to this difference. Men in the study were generally older, with an average age of 67 compared to the female average age of 59, and had more years with diabetes (12, compared to 8 for women). Men had an average of 2.5 comorbidities versus 4.1 for females. Men's BMIs were lower at baseline and 12 weeks, and their HbA1c levels were generally higher at baseline, but not at 12 weeks. Baseline TSS and TSI varied by sex although less so with week 12 TSS and TSI, with males having general lower TSS and TSI than females. Physical and mental health also appears to be associated with sex, with males having a generally higher physical function at baseline and week 12 and higher mental function at baseline, although not week 12.
Fifty percent of women and 62.5% of men were in a relationship. Ten percent of women and 12.5% of men lived alone. Thirty percent of women were on insulin, while 25% of men were on insulin. Women had the following cancer types: breast (2), colon (1), lung (1), gynecologic (GYN) other than ovarian (1), GYN ovarian (2), and other (3). Men's cancer types included colon (1), lung (1), bladder (1), gastrointestinal GI (1), and lymphoma (4). Twenty percent of women had metastases as compared to 37.5% of men.
Discussion
The findings from this study identified potential factors, which may contribute to the development of these adverse events and chemotherapy reduction and delays. Level of glycemic control at the time an individual begins treatment for a cancer appears to be a factor that contributes to the development of an adverse event, specifically the likelihood an individual would develop an infection, and/or be hospitalized while receiving cancer treatment, as well as the increased risk of having a chemotherapy reduction or stoppage. Prior work by Brunello and Kapoor[7] did not find a link between hyperglycemia and hospitalizations; however, this was a retrospective study utilizing chart audit, and glycemic status was determined by averaging random blood glucose levels that were obtained while the patient was receiving treatment. Further research needs to be done to determine the actual role baseline glycemic levels play with regard to the development of infections and hospitalizations. Clinicians need to be aware of the increased risk in patients who are not in good glycemic control at the time they begin treatment.
The length of time (longer amount of time) an individual had diabetes and those on insulin were also found to increase the risk for development of infections and/or an adverse event in adults with T2D and a solid tumor cancer or lymphoma. The risk of complications associated with diabetes increases, the longer the patient has diabetes.[28] More research is needed to understand this relationship, including the role of insulin resistance and other pathophysiologic changes that occur over time in individuals with T2D- and cancer-related outcomes.
One factor that consistently appeared to be potentially related to the development of adverse events and the likelihood of having a chemotherapy stoppage or reduction was being male. The males in this study tended to be older, had diabetes longer, and were more likely to have higher A1c levels when compared to the females in the study. Men tended to have the factors that potentially increased the risk for development of these adverse events. Studies have shown differences in outcomes related to sex in individuals with T2D.[29] More research needs to be conducted in how sex plays a role in the development of adverse events and complications associated with cancer treatment in individuals with T2D.
This study did not find a difference in the level of TSS and interference based on the level of glycemic control. This could be due to the fact that patients may experience different levels of symptom severity and interference for specific symptoms, when total scores do not show this variation. Future research needs to explore difference based on specific symptoms, specifically those that are commonly shared by both diabetes and cancer, i.e., neuropathy, pain, and fatigue. Glycemic control may play a role in the level of severity experienced for each of these symptoms.
The findings of this study are limited by the fact that the study sample size was small with high levels of attrition and missing data. In addition, the number of confounding variables that can lead to dose reductions and development of adverse events that may not have been addressed by this study is also a limiting factor. However, trends and potential factors, which may impact outcomes in patients with diabetes and cancer, can be drawn based on this comparative case analysis. Additional limitation of this study is related to the diverse types of cancer that were included; even though all patients had solid tumor cancer or lymphoma, treatment regimens for cancer including type of chemotherapy, frequency of administration, and dosing of the chemotherapy will vary. Future studies need to consider these factors and potentially focus on patients who receive similar treatment regimens. The amount and types of data collected did allow for this type of analysis to occur.
Conclusion
This study contributes to the science by identifying potential factors that may contribute to the development of complications, i.e., infections and adverse events in patients with cancer and chemotherapy. Clinicians working with patients receiving chemotherapy for a solid tumor cancer who have pre-existing diabetes, need to be aware of how the patients glycemic level at the start of treatment may impact successful treatment completion. It is essential that diabetes and oncology providers collaborate from the beginning to develop a plan of care that will ensure good glycemic control while the patients are receiving chemotherapy, improve cancer- and diabetes-related outcomes, and increase the chance of survival in this unique population. The results of this study may also assist future investigators in narrowing their focus when exploring the relationship between glycemic control and outcomes in patients with cancer.
Financial support and sponsorship
This study was supported by a grant from the Walther Cancer Foundation.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Postoperative mortality in cancer patients with preexisting diabetes: Systematic review and meta-analysis. Diabetes Care. 2010;33:931–9. doi: 10.2337/dc09-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA. 2008;300:2754–64. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh HC, Platz EA, Wang NY, Visvanathan K, Helzlsouer KJ, Brancati FL. A prospective study of the associations between treated diabetes and cancer outcomes. Diabetes Care. 2012;35:113–8. doi: 10.2337/dc11-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershey DS, Bryant AL, Olausson J, Davis ED, Brady VJ, Hammer M. Hyperglycemic-inducing neoadjuvant agents used in treatment of solid tumors: A review of the literature. Oncol Nurs Forum. 2014;41:E343–54. doi: 10.1188/14.ONF.E343-E354. [DOI] [PubMed] [Google Scholar]
- 5.Hammer MJ, Motzer SA, Voss JG, Berry DL. Glycemic control among older adult hematopoietic cell transplant recipients. J Gerontol Nurs. 2010;36:40–50. doi: 10.3928/00989134-20091207-99. [DOI] [PubMed] [Google Scholar]
- 6.Hammer MJ, Voss JG. Malglycemia and cancer: Introduction to a conceptual model. Oncol Nurs Forum. 2012;39:E275–87. doi: 10.1188/12.ONF.E275-E287. [DOI] [PubMed] [Google Scholar]
- 7.Brunello A, Kapoor R, Extermann M. Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol. 2011;34:292–6. doi: 10.1097/COC.0b013e3181e1d0c0. [DOI] [PubMed] [Google Scholar]
- 8.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: A large population based analysis. Int J Cancer. 2007;120:1986–92. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–40. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 10.Fuji S, Kim SW, Mori S, Fukuda T, Kamiya S, Yamasaki S, et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation. 2007;84:814–20. doi: 10.1097/01.tp.0000296482.50994.1c. [DOI] [PubMed] [Google Scholar]
- 11.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol. 2003;157:1092–100. doi: 10.1093/aje/kwg100. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: A case-control study. Dig Dis Sci. 2008;53:2486–94. doi: 10.1007/s10620-008-0264-4. [DOI] [PubMed] [Google Scholar]
- 13.Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, et al. Diabetes mellitus and breast cancer outcomes: A systematic review and meta-analysis. J Clin Oncol. 2011;29:40–6. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–6. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershey DS, Given B, Given C, Von Eye A, You M. Diabetes and cancer: Impact on health-related quality of life. Oncol Nurs Forum. 2012;39:449–57. doi: 10.1188/12.ONF.449-457. [DOI] [PubMed] [Google Scholar]
- 16.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Goodrick D. Comparative Case Studies. [Last accessed on 2017 Jan 18]. Available from: http://www.devinfolive.info/impact_evaluation/ie/img/downloads/Comparative_Case_Studies_ENG.pdf .
- 20.Pickvance C. The Four Varieties of Comparative Analysis: The Case of Environmental Regulation. Converence on Small and Large-N Comparative Solutions, University of Sussex. 2005 Sep 22-23; [Google Scholar]
- 21.Agresti A. Wiley Series in Probability and Statistice: Catergorical Data Anaylsis. 3rd ed. Somerset, US: Wiley; 2013. [Google Scholar]
- 22.Fischer RA. On the interpretation of X 2 from contigency tables, and the calculation of P. J R Stat Soc. 1922;85:87–94. [Google Scholar]
- 23.Hosmer DW, Lemeshow S, Strudivant RX. Applied Logistic Regression. 3rd ed. Hoboken, New Jersey: John Wiley and Sons Inc; 2013. [Google Scholar]
- 24.Bonita R, Beaglehole R, Kjellstrom T. Basic Epidemiology. 2nd ed. WHO Press, Geneva: World Health Organization; 2006. [Google Scholar]
- 25.A Language and Environment for Statistitcal Computing [Computer Program] Vienna, Austria: R Foundation for Sttistical Computing; 2015. [Google Scholar]
- 26.Hmisc: Harrell Miscellaneous [Computer Program]. R Package Version 3.17-2. 2016 [Google Scholar]
- 27.Pastecs: Package for Analysis of SPace-Time Ecological Series [Computer Program] 2014 [Google Scholar]
- 28.Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57:2465–74. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 29.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]


